Synthesis and DNase I Inhibitory Properties of New Squaramides
Abstract
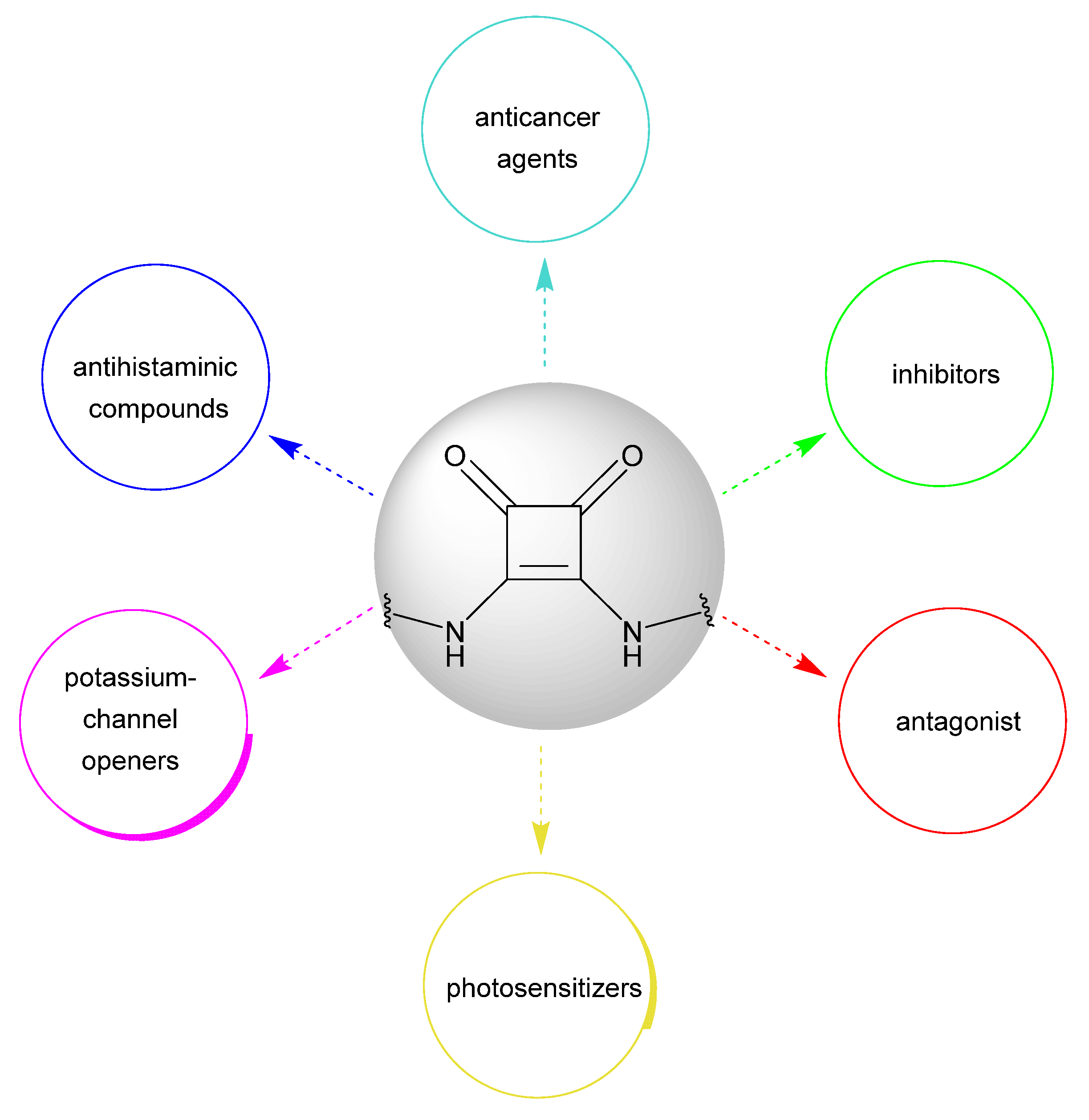
1. Introduction
2. Results and Discussion
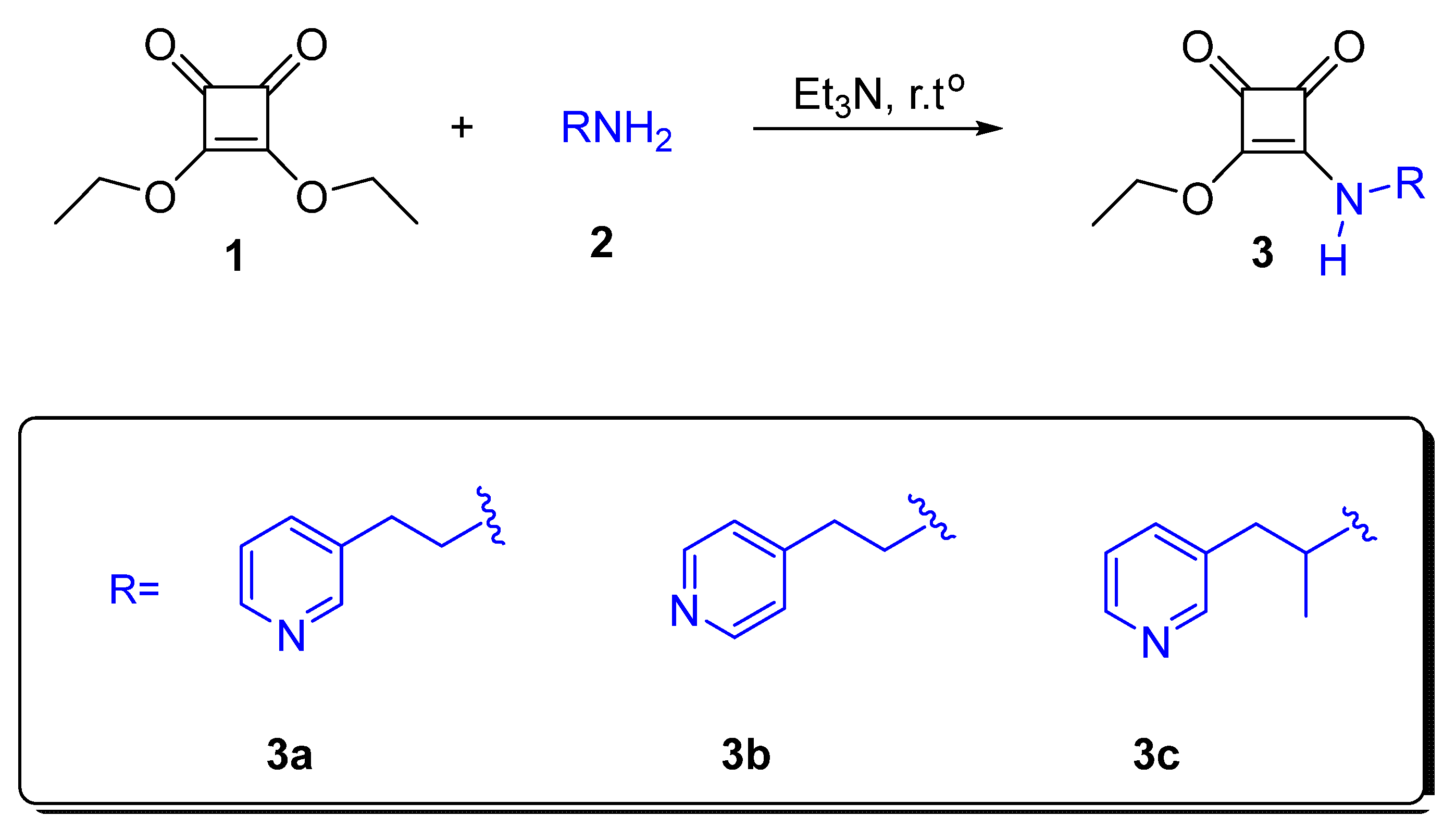
2.1. Chemistry
2.2. Structure Characterization
2.2.1. Spectral Analysis
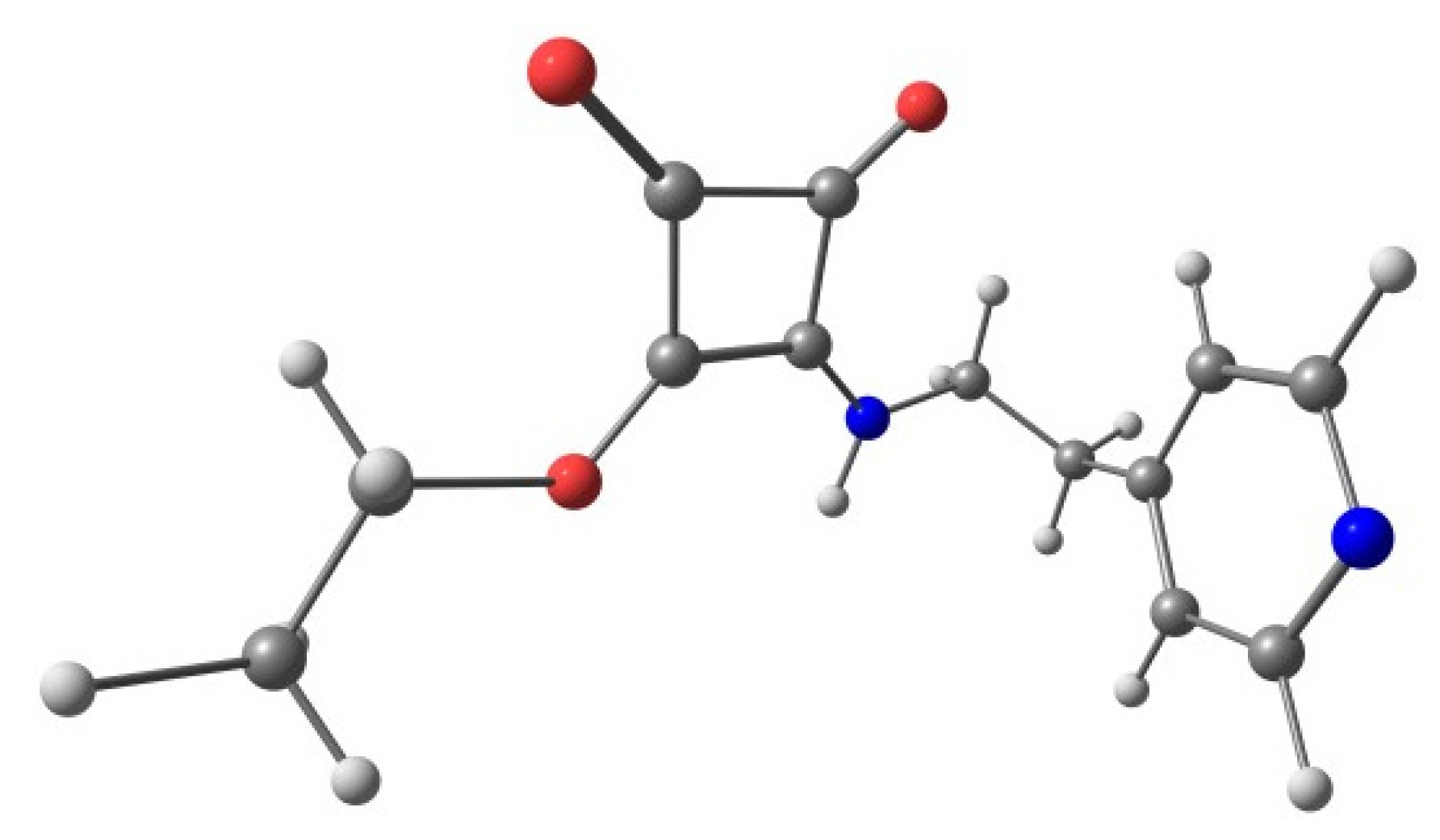
2.2.2. Crystal Structure Analysis
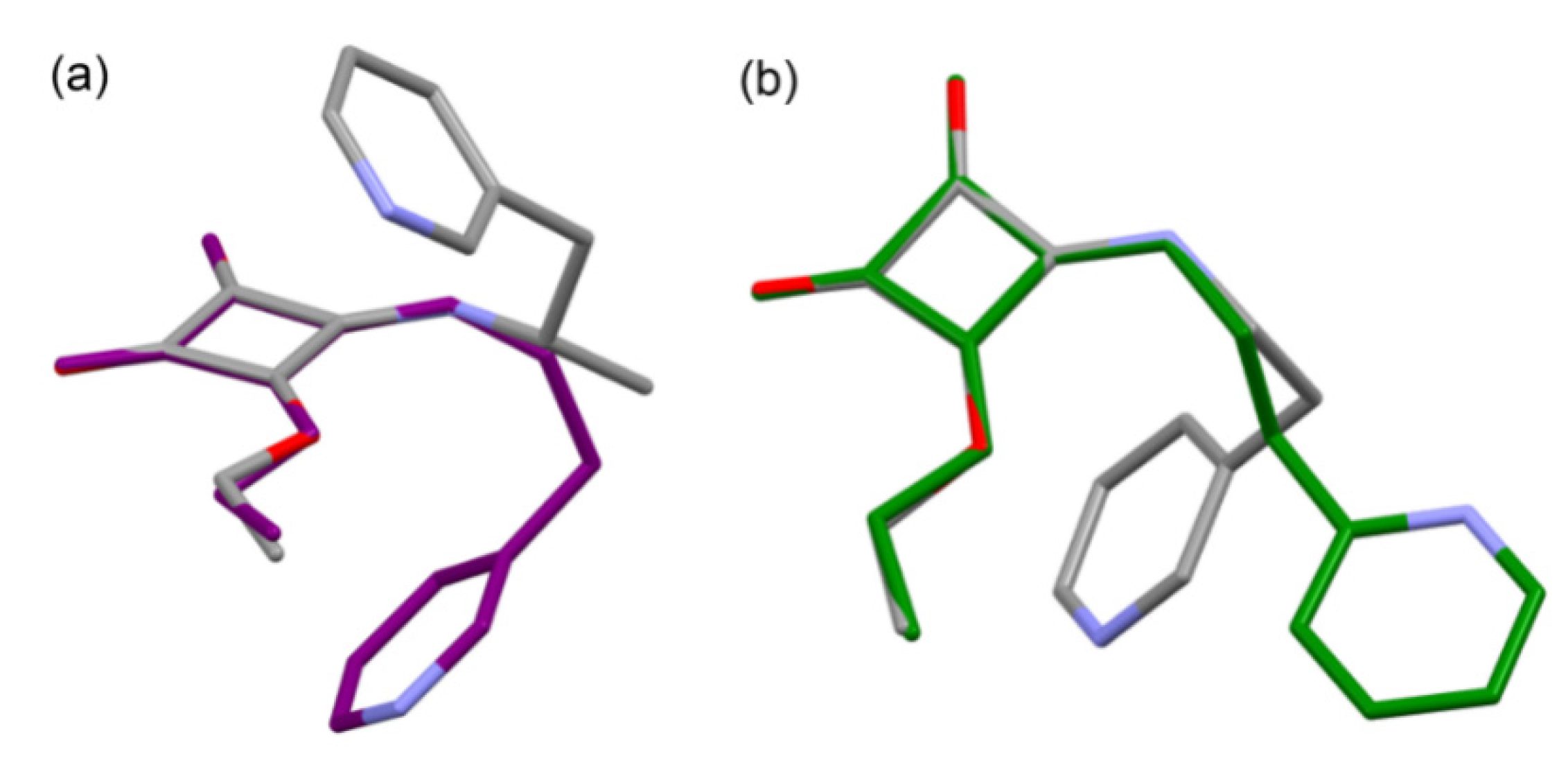
2.2.3. Quantum Chemical Modeling
2.3. ADME Screening
2.4. Biological Evaluation
2.4.1. Antiproliferative Activity
2.4.2. DNase I and XO inhibition
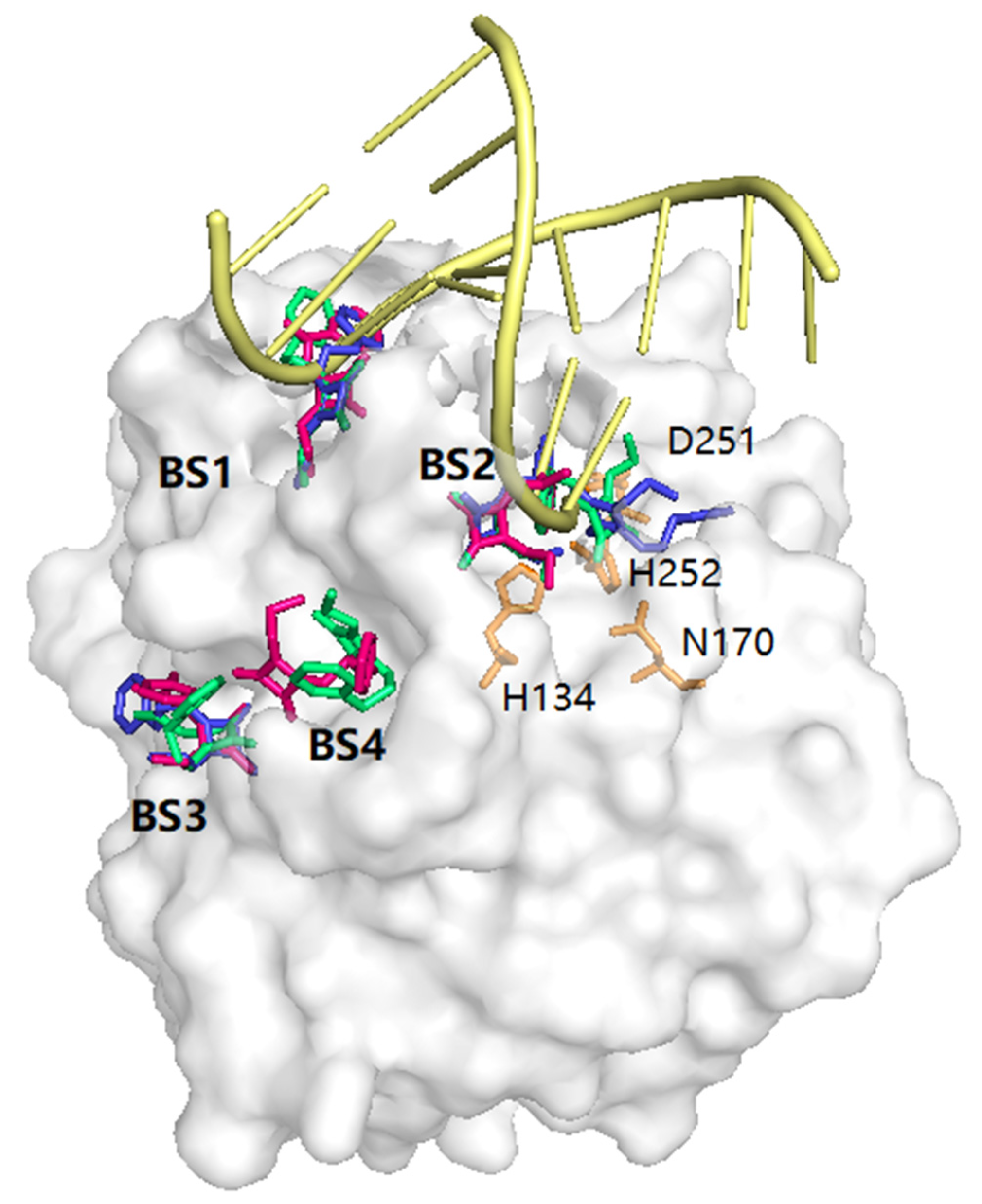
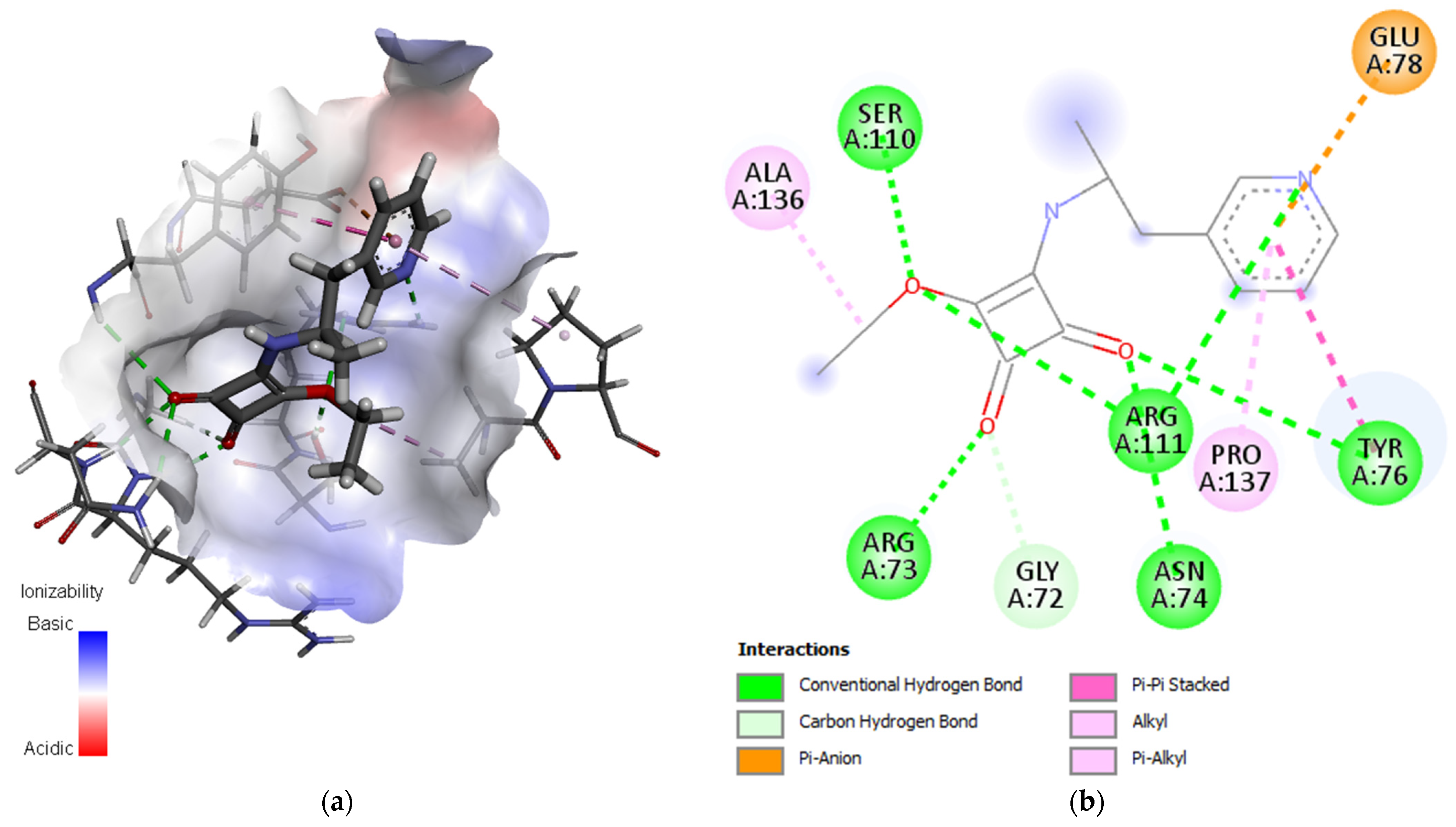
3. Molecular Docking Study
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cai, X.J.; Li, Z.; Chen, W.H. Synthesis, Anion Recognition and Transmembrane Anion-Transport Properties of Squaramides and Their Derivatives. Mini Rev. Org. Chem. 2018, 15, 148–156. [Google Scholar] [CrossRef]
- Agnew-Francis, K.A.; Williams, C.M. Squaramides as Bioisosteres in Contemporary Drug Design. Chem. Rev. 2020, 120, 11616–11650. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Liebeskind, L.S. Synthesis and Evaluation of Novel Proton Exchange Membranes Containing the Semisquaric Acid Group. Macromol. Rapid Commun. 2010, 31, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Basak, D.; Versek, C.; Toscano, D.T.; Christensen, S.; Tuominen, M.T.; Venkataraman, D. Anhydrous Proton Conductivities of Squaric Acid Derivatives. Chem. Commun. 2012, 48, 5922–5924. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Squaramide Based Organocatalysts in Organic Transformations. Curr. Org. Chem. 2016, 20, 2996–3013. [Google Scholar] [CrossRef]
- Marchetti, L.; Kumawat, K.; Mao, N.; Stephens, J.; Elmes, R. Versatility of Squaramides: From Supramolecular Chemistry to Chemical Biology. Chem 2019, 5, 1398–1485. [Google Scholar]
- Hu, L.; Yan, Z.; Xu, H. Advances in synthesis and application of near-infrared absorbing squaraine dyes. RSC Adv. 2013, 3, 7667–7676. [Google Scholar] [CrossRef]
- Xia, G.; Wang, H. Squaraine Dyes: The Hierarchical Synthesis and Its Application in Optical Detection. J. Photochem. Photobiol. C 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Rostami, A.; Colin, A.; Li, X.Y.; Chudzinski, M.G.; Lough, A.J.; Taylor, M.S. N, N′-Diarylsquaramides: General, High-Yielding Synthesis and Applications in Colorimetric Anion Sensing. J. Org. Chem. 2010, 75, 3983–3992. [Google Scholar]
- Rostami, A.; Guérin, G.; Taylor, M.S. Structure—Activity Relationships for Anion-Responsive Poly(Squaramides): Support for an Analyte-Induced Noncovalent Polymer Cross-Linking Mechanism. Macromolecules 2013, 46, 6439–6450. [Google Scholar]
- ClinicalTrials.gov. Efficacy and Safety Study of Navarixin (MK-7123) in Combination with Pembrolizumab (MK-3475) in Adults with Selected Advanced/Metastatic Solid Tumors (MK-7123-034). Identifier NCT03473925. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03473925 (accessed on 28 November 2022).
- ClinicalTrials.gov. Long-Term Study of the Effects of Navarixin (SCH 527123, MK- 7123) in Participants with Moderate to Severe COPD (MK-7123-019). Identifier NCT01006616. 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01006616 (accessed on 28 November 2022).
- Kinney, W.A.; Abou-Gharbia, M.; Garrison, D.T.; Schmid, J.; Kowal, D.M.; Bramlett, D.R.; Miller, T.L.; Tasse, R.P.; Zaleska, M.M.; Moyer, J.A. Design and synthesis of [2-(8,9-dioxo-2,6-diazabicyclo [5.2.0]non-1-en-2-yl)-ethyl]phosphonic acid (EAA-090), a potent N-methyl-d-aspartate antagonist, via the use of 3-cyclobutene-1,2-dione as an achiral α-amino acid bioisostere. J. Med. Chem. 1998, 41, 236–246. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study Evaluating EAA-090 in Adult Outpatients with Neuropathic Pain Associated with Diabetic Neuropathy. Identifier NCT00073034. 2004. Available online: https://clinicaltrials.gov/ct2/show/NCT00073034 (accessed on 28 November 2022).
- Losol, E.; Șentürk, N. Squaric acid dibutyl ester for the treatment of alopecia areata: A retrospective evaluation. Dermatol. Ther. 2021, 34, e14726. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, M.; Daga, A.; Cantoni, C.; Lunardi, C.; Millo, R.; Puccetti, A. DNase I mediates internucleosomal DNA degradation in human cells undergoing drug-induced apoptosis. Eur. J. Immunol. 2001, 31, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Samejima, K.; Earnshaw, W.C. Trashing the genome: The role of nucleases during apoptosis. Nat. Rev. Mol. Cell. Biol. 2005, 6, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Schatzmann-Turhani, D.; Labudova, O.; Yeghiazaryan, K.; Rink, H.; Hauser, E.; Cairns, N.; Lubec, G. Overexpression of DNAse I in brain of patients with Down syndrome. In The Molecular Biology of Down Syndrome; Springer-Verlag Wien: Vienna, Austria, 1999; pp. 353–362. [Google Scholar] [CrossRef]
- Nepali, K.; Agarwal, A.; Sapra, S.; Mittal, V.; Kumar, R.; Banerjee, U.C.; Gupta, M.K.; Satti, N.K.; Suri, O.P.; Dhar, K.L. N-(1,3-Diaryl-3-oxopropyl)-amides as a new template for xanthine oxidase inhibitors. Bioorg. Med. Chem. 2011, 19, 5569–5576. [Google Scholar] [PubMed]
- Dhiman, R.; Sharma, S.; Singh, G.; Nepali., K.; Bedi, P.M. Design and synthesis of aza-flavones as a new class of xanthine oxidase inhibitors. Arch. Pharm. 2013, 346, 7–16. [Google Scholar]
- Tietze, L.F.; Arlt, M.; Beller, M.; Glüsenkamp, K.-H.; Jähde, E.; Rajewsky, M.F. Anticancer Agents, 15. Squaric Acid Diethyl Ester: A New Coupling Reagent for the Formation of Drug Biopolymer Conjugates. Synthesis of Squaric Acid Ester Amides and Diamides. Chem. Ber. 1991, 124, 1215–1221. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Prohens, R.; Portell, A.; Font-Bardia, M.; Bauzá, A.; Frontera, A. A combined crystallographic and theoretical study of weak intermolecular interactions in crystalline squaric acid esters and amides. Cryst. Eng. Comm. 2017, 19, 3071–3077. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, U.D. Molecular properties that influence the oral 658 bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug. Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.C.; Rollison, H.; Johansson, S.; Kanebratt, K.P.; Lambert, C.; Vishwanathan, K.; Andersson, T.B. Managing the Risk of CYP3A Induction in Drug Development: A Strategic Approach. Drug Metab Dispos. 2017, 45, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Konstantinov, S.; Eibl, H.; Berger, M. BCR-ABL influences the antileukaemic effcacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–374. [Google Scholar] [CrossRef]
- Kolarevic, A.; Yancheva, D.; Kocic, G.; Smelcerovic, A. Deoxyribonuclease inhibitors. Eur. J. Med. Chem. 2014, 88, 101–111. [Google Scholar] [CrossRef]
- Kolarević, A.; Ilić, B.S.; Anastassova, N.; Mavrova, A.T.; Yancheva, D.; Kocić, G.; Šmelcerović, A. Benzimidazoles as novel deoxyribonuclease I inhibitors. J. Cell. Biochem. 2018, 119, 8937–8948. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Dimov, S.; Yancheva, D.; Kolarević, A.; Ilić, B.S.; Kocić, G.; Šmelcerović, A. Synthesis and DNase I inhibitory properties of some 5,6,7,8-tetrahydrobenzo [4,5]thieno [2,3-d]pyrimidines. Bioorg. Chem. 2018, 80, 693–705. [Google Scholar] [CrossRef]
- Ilić, B.S.; Kolarević, A.; Kocić, G.; Šmelcerović, A. Ascorbic acid as DNase I inhibitor in prevention of male infertility. Biochem. Biophys. Res. Commun. 2018, 498, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Bondžić, B.P.; Džambaski, Z.; Kolarević, A.; Đorđević, A.; Anderluh, M.; Šmelcerović, A. Synthesis and DNase I inhibitory properties of new benzocyclobutane-2,5-diones. Future Med. Chem. 2019, 11, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Kolarević, A.; Pavlovic, A.; Djordjevic, A.; Lazarevic, J.; Savic, S.; Kocic, G.; Anderluh, M.; Smelcerovic, A. Rutin as deoxyribonuclease I inhibitor. Chem. Biodivers. 2019, 16, e1900069. [Google Scholar] [CrossRef] [PubMed]
- Kolarević, A.; Ilić, B.S.; Kocić, G.; Džambaski, Z.; Šmelcerović, A.; Bondžić, B.P. Synthesis and DNase I inhibitory properties of some 4-thiazolidinone derivatives. J. Cell. Biochem. 2019, 120, 264–274. [Google Scholar] [CrossRef]
- Šmelcerović, A.; Zivkovic, A.; Ilić, B.S.; Kolarević, A.; Hofmann, B.; Steinhilber, D.; Stark, H. 4-(4-Chlorophenyl) thiazol-2-amines as pioneers of potential neurodegenerative therapeutics with anti-inflammatory properties based on dual DNase I and 5-LO inhibition. Bioorg. Chem. 2020, 95, 103528. [Google Scholar] [CrossRef]
- Ilić, B.S.; Gajić, M.; Bondžić, B.P.; Džambaski, Z.; Kocić, G.; Šmelcerović, A. Deoxyribonuclease I Inhibitory Properties, Molecular Docking and Molecular Dynamics Simulations of 1-(Pyrrolidin-2-yl)propan-2-one Derivatives. Chem. Biodivers. 2021, 18, e2000996. [Google Scholar] [CrossRef]
- Gajić, M.; Ilić, B.S.; Bondžić, B.P.; Džambaski, Z.; Kojić, V.V.; Jakimov, D.S.; Kocić, G.; Šmelcerović, A. 1,2,3,4-Tetrahydroisoquinoline Derivatives as a Novel Deoxyribonuclease I Inhibitors. Chem. Biodivers. 2021, 18, e2100261. [Google Scholar] [CrossRef]
- Gajić, M.; Džambaski, Z.; Ilić, B.S.; Kocić, G.; Bondžić, B.P.; Šmelcerović, A. Synthesis and analysis of 4-oxothiazolidines as potential dual inhibitors of deoxyribonuclease I and xanthine oxidase. Chem. Biol. Interact. 2021, 345, 109536. [Google Scholar] [CrossRef]
- Gajic, M.; Knez, D.; Sosič, I.; Mravljak, J.; Meden, A.; Košak, U.; Leitzbach, L.; George, S.; Hofmann, B.; Zivkovic, A.; et al. Repurposing of 8-Hydroxyquinoline-Based Butyrylcholinesterase and Cathepsin B Ligands as Potent Nonpeptidic Deoxyribonuclease I Inhibitors. ChemMedChem 2022, 17, e202100694. [Google Scholar] [CrossRef]
- Lahm, A.; Suck, D. DNase I-induced DNA conformation: 2 Å Structure of a DNase I-octamer complex. J. Molec. Biol. 1991, 222, 645–667. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Oefner, C.; Suck, D. Crystallographic refinement and structure of DNase I at 2 A resolution. J. Mol.Biol. 1986, 192, 605–632. [Google Scholar] [CrossRef]
- Guéroult, M.; Picot, D.; Abi-Ghanem, J.; Hartmann, B.; Baaden, M. How cations can assist DNase I in DNA binding and hydrolysis. PLoS Comput. Biol. 2010, 6, e1001000. [Google Scholar]
- Jones, S.J.; Worrall, A.F.; Connolly, B.A. Site-directed mutagenesis of the catalytic residues of bovine pancreatic deoxyribonuclease I. J. Mol. Biol. 1996, 264, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys.Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Bruker. APEX3 Crystallography Software Suite; Bruker AXS, Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Smelcerovic, Z.; Veljkovic, A.; Kocic, G.; Yancheva, D.; Petronijevic, Z.; Anderluh, M.; Smelcerovic, A. Xanthine oxidase inhibitory properties and anti-inflammatory activity of 2-amino-5-alkylidene-thiazol-4-ones. Chem. Biol. Interact. 2015, 229, 73–81. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Cryst. 2002, 40, 82–92. [Google Scholar]
- Zoete, V.; Daina, A.; Bovigny, C.; Michielin, O. SwissSimilarity: A Web Tool for Low to Ultra High Throughput Ligand-Based Virtual Screening. J. Chem. Inf. Model. 2016, 56, 1398–1404. [Google Scholar] [CrossRef]


| Geometric Parameters | 3a | 3b | |
|---|---|---|---|
| Bond lengths (Å) | Theor. | Exp. | Theor. |
| C=O | 1.206 | 1.208 | 1.214 |
| C=O | 1.206 | 1.222 | 1.215 |
| C=C | 1.390 | 1.397 | 1.394 |
| C-N | 1.337 | 1.319 | 1.338 |
| Angles (o) | |||
| C-C-N | 133.6 | 132.5 | 133.7 |
| C-N-C | 122.9 | 124.3 | 123.7 |
| Compound | 3a | 3c |
|---|---|---|
| Empirical formula | C13H14N2O3 | C14H16N2O3 |
| Formula weight | 246.26 | 260.12 |
| Temperature/K | 290.00 | 290 |
| Crystal system | monoclinic | triclinic |
| Space group | P21/n | P-1 |
| a/Å | 7.2704(5) | 7.2913(4) |
| b/Å | 12.0914(7) | 9.7897(5) |
| c/Å | 14.6534(7) | 9.9532(5) |
| α/° | 90 | 79.847(2) |
| β/° | 100.592(2) | 78.133(2) |
| γ/° | 90 | 84.349(2) |
| Volume/Å3 | 1266.22(13) | 682.96(6) |
| Z | 4 | 4 |
| ρcalcg/cm3 | 1.292 | 1.266 |
| μ/mm−1 | 0.093 | 0.090 |
| F(000) | 520.0 | 276.0 |
| Crystal size/mm3 | 0.5 × 0.4 × 0.4 | 0.25 × 0.18 × 0.16 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.398 to 50.686 | 4.236 to 50.674 |
| Index ranges | −8 ≤ h ≤ 8, −14 ≤ k ≤ 14, −17 ≤ l ≤ 15 | −8 ≤ h ≤ 8, −11 ≤ k ≤ 11, −11 ≤ l ≤ 11 |
| Reflections collected | 7264 | 19858 |
| Independent reflections | 2208 [Rint = 0.0460, Rsigma = 0.0435] | 2485 [Rint = 0.0675, Rsigma = 0.0502] |
| Data/restraints/parameters | 2208/0/165 | 2485/0/178 |
| Goodness-of-fit on F2 | 1.038 | 1.067 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0438, wR2 = 0.0948 | R1 = 0.0713, wR2 = 0.1718 |
| Final R indexes [all data] | R1 = 0.0674, wR2 = 0.1059 | R1 = 0.0893, wR2 = 0.2070 |
| Largest diff. peak/hole/e Å−3 | 0.16/−0.14 | 0.46/−0.34 |
| CCDC deposition | 2213349 | 2213350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruseva, N.; Sbirkova-Dimitrova, H.; Atanasova, M.; Marković, A.; Šmelcerović, Ž.; Šmelcerović, A.; Bakalova, A.; Cherneva, E. Synthesis and DNase I Inhibitory Properties of New Squaramides. Molecules 2023, 28, 538. https://doi.org/10.3390/molecules28020538
Ruseva N, Sbirkova-Dimitrova H, Atanasova M, Marković A, Šmelcerović Ž, Šmelcerović A, Bakalova A, Cherneva E. Synthesis and DNase I Inhibitory Properties of New Squaramides. Molecules. 2023; 28(2):538. https://doi.org/10.3390/molecules28020538
Chicago/Turabian StyleRuseva, Nina, Hristina Sbirkova-Dimitrova, Mariyana Atanasova, Ana Marković, Žaklina Šmelcerović, Andrija Šmelcerović, Adriana Bakalova, and Emiliya Cherneva. 2023. "Synthesis and DNase I Inhibitory Properties of New Squaramides" Molecules 28, no. 2: 538. https://doi.org/10.3390/molecules28020538
APA StyleRuseva, N., Sbirkova-Dimitrova, H., Atanasova, M., Marković, A., Šmelcerović, Ž., Šmelcerović, A., Bakalova, A., & Cherneva, E. (2023). Synthesis and DNase I Inhibitory Properties of New Squaramides. Molecules, 28(2), 538. https://doi.org/10.3390/molecules28020538







