Isotopic and Elemental Fingerprint of Edible Egg Parts—The Health Risk Assessment Based on Potentially Toxic Elements Content
Abstract
1. Introduction
2. Results and Discussion
2.1. Isotopic Fingerprint of Egg Samples
2.2. Content of Macro-Elements, Micro-Elements, and Trace Elements in Egg White and Yolk Samples
2.2.1. The Main Macro-Elements, Micro-Elements, and Trace Elements (in mg/kg Fresh Sample) in Egg White
2.2.2. The Main Macro-Elements, Micro-Elements and Trace Elements (in mg/kg Fresh Sample) in Egg Yolk
2.3. Developing of PLS–DA Models
2.3.1. The Classification According to Hen’s Growing System—Egg White Samples
2.3.2. The Classification According to Hen’s Growing System—Egg Yolk Samples
2.4. Human Health Risk Assessment and Estimation of Non-Carcinogenic Risk
3. Materials and Methods
3.1. Samples Description
3.2. Samples Preparation and Stable Isotopic Analysis
3.3. Samples Digestion Procedure and Elemental Analysis
3.4. Data Analysis
3.5. Human Health Risk Assessment and Estimation of Non-Carcinogenic Risk
3.5.1. Estimated Daily Intake (EDI) of Heavy Metals through Eggs Consumption
3.5.2. Estimation of Non-Carcinogenic Risks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Demirulus, H. The Heavy Metal Content in Chicken Eggs Consumed in Van Lake Territory. Ekoloji 2013, 22, 19–25. [Google Scholar] [CrossRef]
- Available online: https://www.internationalegg.com/resource/global-egg-production-continues-to-grow (accessed on 19 December 2022).
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and Egg-Derived Foods: Effects on Human Health and Use as Functional Foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef]
- Giucă, A.-D.; Necula, D.M. The evolution of egg production and consumption at the level of Romania in the period 2016–2020. In Agrarian Economy and Rural Development—Realities and Perspectives for Romania. International Symposium, 12th ed.; The Research Institute for Agricultural Economy and Rural Development (ICEADR): Bucharest, Romania, 2021; pp. 62–67. [Google Scholar]
- Bay, L.J.; Harn Chan, J.S.; Walczyk, T. Optimization of analytical strategies by Monte Carlo simulation: A case study in eggs for tracing their geographical origin using stable isotope signatures. Forensic Chem. 2018, 11, 32–37. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y. Application and Preparation Progress of Stable Isotope Reference Materials in Traceability of Agricultural Products. Crit. Rev. Anal. Chem. 2021, 51, 742–753. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef]
- Cristea, G.; Voica, C.; Feher, I.; Puscas, R.; Magdas, D.A. Isotopic and elemental characterization of Romanian pork meat in corroboration with advanced chemometric methods: A first exploratory study. Meat Sci. 2022, 189, 108825. [Google Scholar] [CrossRef]
- Rogers, K.M. Stable Isotopes as a Tool to Differentiate Eggs Laid by Caged, Barn, Free Range, and Organic Hens. J. Agric. Food Chem. 2009, 57, 4236–4242. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Bendell-Young, L.I.; Elliott, J.E. Linking contaminant profiles to the diet and breeding location of American dippers using stable isotopes. J. Appl. Ecol. 2004, 41, 502–512. [Google Scholar] [CrossRef]
- Shaheen, N.; Ahmed, M.K.; Islam, M.S.; Habibullah-Al-Mamun, M.; Tukun, A.B.; Islam, S.; Rahim, A.T.M.A. Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’ foodstuffs (meat, milk and egg) in Bangladesh. Environ. Sci. Pollut. Res. 2016, 23, 7794–7806. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8340-4. [Google Scholar] [CrossRef]
- Saeed, A.A.M.; Kassem, A.S.; Hassan, M.M. Chemical Analysis of Some Essential Trace Elements in Hen’s Eggs: A Comparative Study. Inter. J. Sci. Eng. Res. 2021, 12, 188–193. [Google Scholar]
- Abd-Elghany, S.M.; Mohammed, M.A.; Abdelkhalek, A.; Saad, F.S.S.; Sallam, K.I. Health Risk Assessment of Exposure to Heavy Metals from Sheep Meat and Offal in Kuwait. J. Food Prot. 2020, 83, 503–510. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood from Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef]
- Bonsignore, M.; Salvagio Manta, D.; Oliveri, E.; Sprovieri, M.; Basilone, G.; Bonanno, A.; Falco, F.; Traina, A.; Mazzola, S. Mercury in fishes from Augusta Bay (southern Italy): Risk assessment and health implication. Food Chem. Toxicol. 2013, 56, 184–194. [Google Scholar] [CrossRef]
- Atique Ullah, A.K.M.; Afrin, S.; Hosen, M.M.; Musarrat, M.; Ferdoushy, T.; Nahar, Q.; Quraishi, S.B. Concentration, source identifcation, and potential human health risk assessment of heavy metals in chicken meat and egg in Bangladesh. Environ. Sci. Pollut. Res. 2022, 29, 22031–22042. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://thisnzlife.co.nz/from-yolk-to-shell-the-curious-process-of-how-a-hen-produces-an-egg/ (accessed on 13 December 2022).
- Available online: https://layinghens.hendrix-genetics.com/en/articles/egg-day-oviposition-explained/ (accessed on 15 December 2022).
- WHO. Principles and methods for the risk assessment of chemicals in Food. Environ Health Criteria 240. In IPCS (International Program on Chemical Safety). Dietary Exposure Assessment of Chemicals in Food; WHO Press: Geneva, Switzerland, 2009; pp. 6-1–6-60. [Google Scholar]
- EC (European Commission). Regulation (EC) N 178/2002 of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Comm. L 2002, 31, 1–24. [Google Scholar]
- Kolics, B.; Kolics, É.; Solti, I.; Bacsi, Z.; Taller, J.; Specziár, A.; Mátyás, K. Lithium Chloride Shows Effectiveness against the Poultry Red Mite (Dermanyssus gallinae). Insects 2022, 13, 1005. [Google Scholar] [CrossRef]
- Mızrak, C.; Yenice, E.; Cany, M.; Yıldırım, U.; Atik, Z. Effects of dietary boron on performance, egg production, egg quality and some bone parameters in layer hens. S. Afr. J. Anim. Sci. 2010, 40, 257–264. [Google Scholar] [CrossRef][Green Version]
- USEPA (U.S. Environmental Protection Agency). Risk assessment guidance for superfund. In Human Health Evaluation Manual Part A, Interim Final, Vol. I.; United States Environmental Protection Agency: Washington, DC, USA, 1989. [Google Scholar]
- JECFA. World Health Organization, Food and Agriculture Organization of the United Nations & Joint FAO/WHO Expert Committee on Food Additives. Meeting (73rd: 2010: Geneva, Switzerland). Evaluation of certain food additives and contaminants: Seventy-third [73rd] report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization. 2011. Available online: https://apps.who.int/iris/handle/10665/44515 (accessed on 15 December 2022).
- Fakhri, Y.; Khaneghah, A.M.; Hadiani, M.R.; Keramati, H.; Pouya, R.H.; Moradi, B.; Severino da Silva, B. Non-carcinogenic risk assessment induced by heavy metals content of the bottled water in Iran. Toxin Rev. 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Dehelean, A.; Cristea, G.; Puscas, R.; Hategan, A.R.; Magdas, D.A. Assigning the Geographical Origin of Meat and Animal Rearing System Using Isotopic and Elemental Fingerprints. App. Sci. 2022, 12, 12391. [Google Scholar] [CrossRef]
- Denadai, J.C.; Ducatti, C.; Sartori, J.R.; Pezzato; Móri, C.A.C.; Gottmann, R.; Mituo, M.A.O.; Bordinhon, A.M. The traceability of animal meals in layer diets as detected by stable carbon and nitrogen isotope analyses of eggs. Braz. J. Poult. Sci. 2008, 10, 189–194. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for stable isotope ratio analysis 2013 (IUPAC). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Griboff, J.; Wunderlin, D.A.; Monferran, M.V. Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J. 2017, 130, 236–244. [Google Scholar] [CrossRef]
- Giri, S.; Kumar Singh, A. Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt, India. Arch. Environ. Occup. Health. 2017, 74, 161–170. [Google Scholar] [CrossRef]
- Hashemi, M.; Sadeghi, A.; Saghi, M.; Aminzare, M.; Raeisi, M.; Rezayi, M.; Tavakoly Sany, S.B. Health Risk Assessment for Human Exposure to Trace Metals and Arsenic via Consumption of Hen Egg Collected from Largest Poultry Industry in Iran. Biol. Trace Elem. Res. 2019, 188, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S. Tuna fish and common kilka: Health risk assessment of metal pollution through consumption of canned fish in Iran. J. Consum. Prot. Food Saf. 2017, 12, 157–163. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Li, X.; Yuan, Y. Heavy metal levels in kiwifruit orchard soils and trees and its potential health risk assessment in Shaanxi, China. Environ. Sci. Pollut. Res. 2016, 23, 14560–14566. [Google Scholar] [CrossRef]
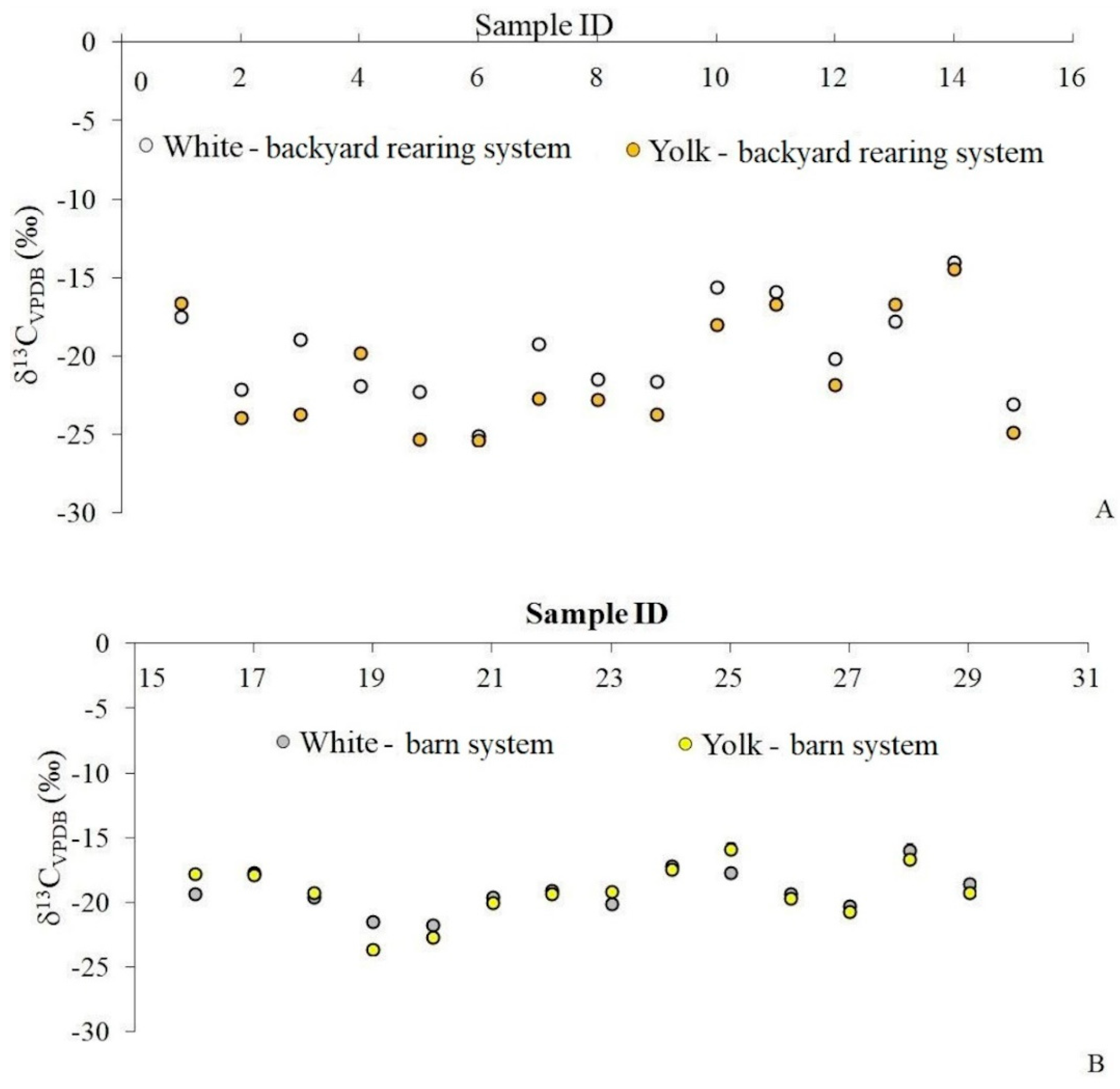
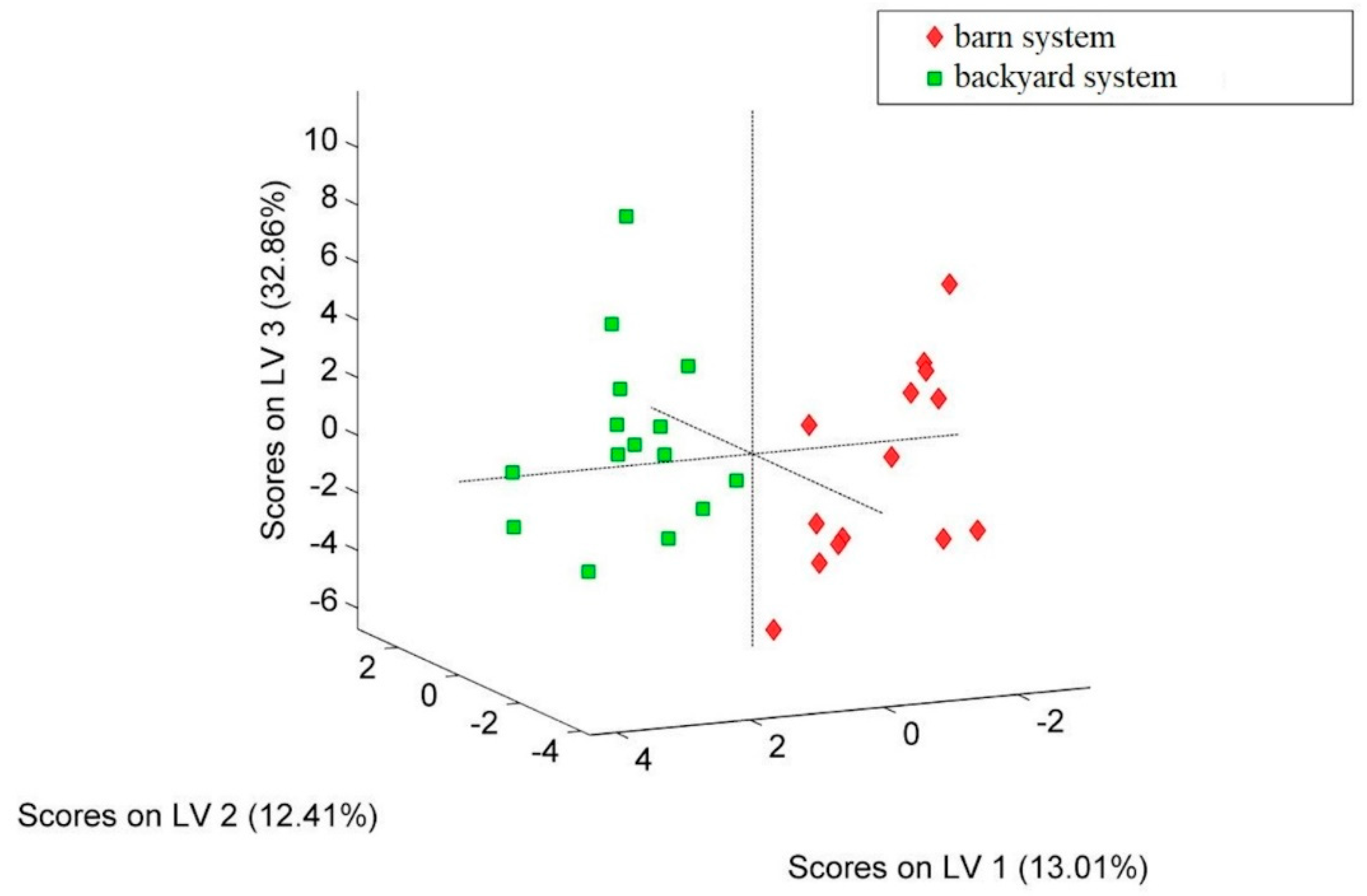

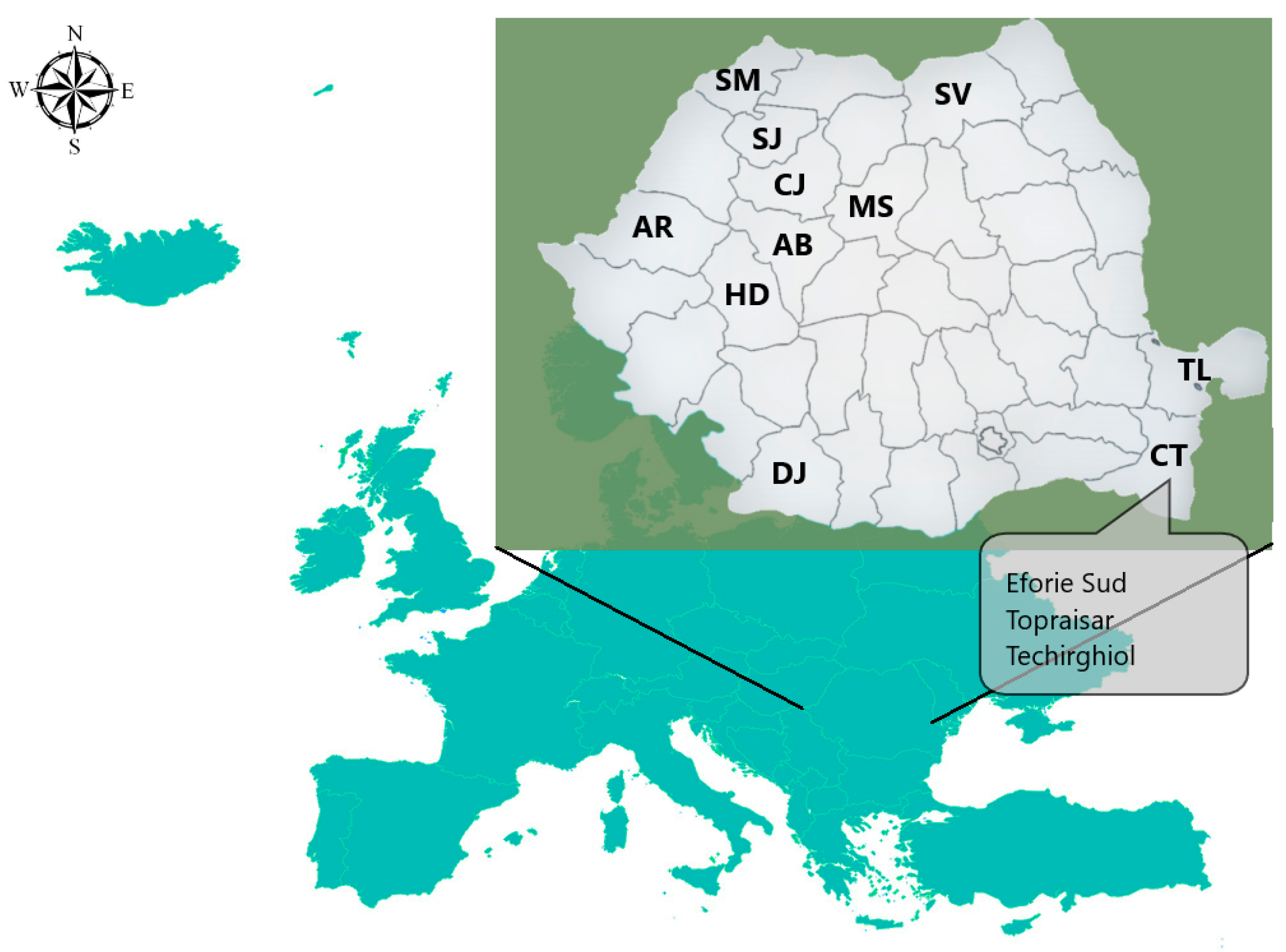
| Element | Egg White | Egg Yolk | ||||||
|---|---|---|---|---|---|---|---|---|
| Backyard | Barn | Backyard | Barn | |||||
| Value | ||||||||
| min | max | min | max | min | max | min | max | |
| δ13C (‰) | −25.1 | −14.0 | −21.8 | −15.9 | −25.3 | −14.4 | −23.7 | −15.9 |
| Li (mg/kg) * | 0.02 | 2.37 | 0.002 | 0.03 | 0.01 | 0.50 | 0.001 | 0.04 |
| B (mg/kg) * | 0.07 | 0.74 | 0.17 | 0.43 | 0.002 | 0.42 | 0.08 | 0.67 |
| Mg (mg/kg) * | 67.2 | 171.4 | 56.9 | 164.0 | 128.9 | 233.9 | 110.7 | 214.8 |
| K (mg/kg) * | 492.5 | 2010.8 | 424.7 | 1211.3 | 912.3 | 1634.3 | 752.7 | 1298.8 |
| Ca (mg/kg) * | 18.0 | 209.0 | 17.4 | 69.0 | 888.3 | 1704.9 | 963.8 | 1803.0 |
| Mn (mg/kg) * | 0.01 | 0.07 | 0.01 | 0.02 | 0.53 | 4.20 | 0.60 | 1.96 |
| Fe (mg/kg) * | 0.66 | 9.82 | 0.30 | 1.93 | 74.57 | 148.75 | 72.10 | 112.52 |
| Co (μg/kg) * | 0.12 | 4.70 | 0.40 | 2.47 | 6.94 | 36.28 | 2.40 | 12.11 |
| Zn (mg/kg) * | 0.002 | 1.05 | 0.03 | 0.21 | 18.85 | 46.32 | 19.95 | 36.07 |
| Rb (mg/kg) * | 0.26 | 1.56 | 0.58 | 1.90 | 0.38 | 1.23 | 0.69 | 1.64 |
| Sr (mg/kg) * | 0.06 | 0.36 | 0.02 | 0.10 | 0.78 | 3.10 | 0.37 | 0.74 |
| Mo (μg /kg) * | 0.29 | 23.00 | 4.36 | 13.97 | 6.07 | 128.04 | 69.70 | 246.40 |
| Ba (mg/kg) * | 0.01 | 0.10 | 0.003 | 0.06 | 0.96 | 17.79 | 0.40 | 3.42 |
| La (μg /kg) * | 0.03 | 1.79 | 0.02 | 0.41 | 0.20 | 1.44 | 0.19 | 8.50 |
| Ce (μg /kg) * | 0.03 | 1.81 | 0.02 | 0.57 | 0.20 | 3.78 | 0.17 | 5.39 |
| Pb (mg/kg) * | 0.001 | 0.59 | 0.002 | 0.06 | 0.03 | 0.17 | 0.02 | 0.06 |
| Sample Type | Growing System | Na | Mg | K | Ca | Li | B | Sc | Ti | V | Cr | Mn | Fe | Ni | Cu | Zn | Se | Rb | Sr |
| white | backyard | 1748.27 | 108.35 | 838.00 | 49.67 | 0.31 | 0.24 | 0.02 | 0.22 | 0.09 | 0.35 | 0.02 | 2.98 | 0.03 | 0.19 | 0.18 | 0.10 | 0.60 | 0.16 |
| barn | 1812.17 | 116.23 | 798.19 | 42.40 | 0.01 | 0.30 | 0.02 | 0.23 | 0.09 | 0.42 | 0.01 | 1.36 | 0.03 | 0.20 | 0.08 | 0.10 | 1.01 | 0.06 | |
| yolk | backyard | 887.12 | 180.08 | 1292.95 | 1350.70 | 0.11 | 0.22 | 0.22 | 1.50 | 0.32 | 3.28 | 1.34 | 114.27 | 0.28 | 2.40 | 30.67 | 0.60 | 0.75 | 1.70 |
| barn | 830.47 | 164.24 | 1028.51 | 1364.54 | 0.01 | 0.33 | 0.19 | 1.00 | 0.30 | 3.16 | 1.21 | 94.06 | 0.28 | 2.35 | 28.00 | 0.56 | 1.06 | 0.61 | |
| Sample type | growing system | Pd | Ba | Co | Zr | Nb | Mo | Ag | La | Ce | Pr | Gd | Pt | As | Cd | Sn | Sb | Pb | |
| white | backyard | 0.03 | 0.03 | 0.001 | 0.005 | 0.0003 | 0.005 | 0.01 | 0.0002 | 0.0003 | 0.0002 | 0.10 | 0.005 | 0.02 | 0.001 | 0.03 | 0.0005 | 0.05 | |
| barn | 0.03 | 0.01 | 0.001 | 0.01 | 0.0003 | 0.01 | 0.01 | 0.0001 | 0.0001 | 0.0003 | 0.04 | 0.005 | 0.03 | 0.001 | 0.04 | 0.0004 | 0.01 | ||
| yolk | backyard | 0.23 | 5.22 | 0.017 | 0.05 | 0.001 | 0.08 | 0.07 | 0.0006 | 0.0009 | 0.0010 | 0.13 | 0.02 | 0.05 | 0.005 | 0.23 | 0.0023 | 0.06 | |
| barn | 0.22 | 1.13 | 0.008 | 0.07 | 0.001 | 0.12 | 0.04 | 0.0016 | 0.0007 | 0.0006 | 0.10 | 0.03 | 0.05 | 0.006 | 0.31 | 0.0020 | 0.03 |
| Sample Code | Origin | Cr | Mn | Fe | Co | Ni | Cu | Zn | Se | Cd | Pb | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg-1 | Tulcea | 1.02 | 0.30 | 40.23 | 0.003 | 0.06 | 0.73 | 8.11 | 0.19 | 0.003 | 0.03 | 0.02 |
| Egg-2 | Tulcea | 1.86 | 0.37 | 41.64 | 0.005 | 0.14 | 1.08 | 13.35 | 0.30 | 0.004 | 0.03 | 0.02 |
| Egg-3 | Constanta | 1.22 | 0.22 | 31.50 | 0.004 | 0.10 | 1.00 | 7.68 | 0.14 | 0.004 | 0.37 | 0.02 |
| Egg-4 | Constanta | 1.56 | 0.45 | 45.02 | 0.008 | 0.10 | 1.04 | 11.22 | 0.33 | 0.004 | 0.03 | 0.03 |
| Egg-5 | Eforie Sud | 1.57 | 0.50 | 49.01 | 0.006 | 0.16 | 1.09 | 11.49 | 0.16 | 0.003 | 0.07 | 0.03 |
| Egg-6 | Topraisar | 2.06 | 0.60 | 65.39 | 0.013 | 0.22 | 1.44 | 14.74 | 0.55 | 0.004 | 0.05 | 0.06 |
| Egg-7 | Techirghiol | 1.62 | 0.38 | 56.01 | 0.007 | 0.06 | 1.31 | 11.44 | 0.23 | 0.003 | 0.03 | 0.03 |
| Egg-8 | Alba | 1.93 | 0.51 | 53.26 | 0.009 | 0.20 | 1.17 | 13.26 | 0.43 | 0.001 | 0.03 | 0.02 |
| Egg-9 | Alba | 1.50 | 0.51 | 50.99 | 0.005 | 0.14 | 1.11 | 11.87 | 0.39 | 0.003 | 0.02 | 0.03 |
| Egg-10 | Cluj | 1.36 | 0.28 | 32.48 | 0.006 | 0.11 | 1.13 | 11.23 | 0.59 | 0.003 | 0.02 | 0.04 |
| Egg-11 | Salaj | 1.97 | 1.69 | 53.00 | 0.015 | 0.17 | 1.27 | 16.00 | 0.31 | 0.001 | 0.02 | 0.05 |
| Egg-12 | Salaj | 1.65 | 0.37 | 54.80 | 0.008 | 0.09 | 1.04 | 13.77 | 0.36 | 0.003 | 0.03 | 0.04 |
| Egg-13 | Salaj | 0.76 | 0.92 | 53.78 | 0.013 | 0.23 | 0.98 | 18.67 | 0.14 | 0.001 | 0.03 | 0.04 |
| Egg-14 | Mures | 1.46 | 0.37 | 34.23 | 0.006 | 0.15 | 0.76 | 10.23 | 0.31 | 0.003 | 0.01 | 0.02 |
| Egg-15 | Suceava | 1.27 | 0.74 | 51.14 | 0.005 | 0.05 | 0.93 | 12.58 | 0.09 | 0.001 | 0.02 | 0.05 |
| Egg-16 | Satu Mare | 1.51 | 0.79 | 39.75 | 0.004 | 0.19 | 1.10 | 11.98 | 0.29 | 0.003 | 0.01 | 0.03 |
| Egg-17 | Satu Mare | 1.70 | 0.74 | 42.62 | 0.003 | 0.15 | 1.36 | 13.90 | 0.30 | 0.004 | 0.02 | 0.02 |
| Egg-18 | Satu Mare | 1.54 | 0.77 | 42.50 | 0.003 | 0.13 | 1.38 | 14.45 | 0.29 | 0.003 | 0.01 | 0.03 |
| Egg-19 | Arad | 1.36 | 0.58 | 38.63 | 0.004 | 0.10 | 0.91 | 11.25 | 0.33 | 0.003 | 0.01 | 0.03 |
| Egg-20 | Arad | 1.57 | 0.59 | 46.04 | 0.004 | 0.07 | 1.12 | 12.76 | 0.17 | 0.003 | 0.01 | 0.03 |
| Egg-21 | Arad | 1.27 | 0.75 | 33.19 | 0.005 | 0.09 | 0.89 | 11.27 | 0.15 | 0.003 | 0.01 | 0.05 |
| Egg-22 | Hunedoara | 1.50 | 0.24 | 34.31 | 0.002 | 0.10 | 0.86 | 9.19 | 0.33 | 0.003 | 0.01 | 0.04 |
| Egg-23 | Hunedoara | 1.60 | 0.36 | 36.06 | 0.004 | 0.14 | 1.05 | 10.73 | 0.39 | 0.003 | 0.03 | 0.05 |
| Egg-24 | Hunedoara | 1.79 | 0.32 | 40.75 | 0.006 | 0.16 | 1.21 | 11.02 | 0.37 | 0.004 | 0.01 | 0.05 |
| Egg-25 | Hunedoara | 1.65 | 0.35 | 36.81 | 0.003 | 0.20 | 1.14 | 12.06 | 0.26 | 0.003 | 0.01 | 0.04 |
| Egg-26 | Hunedoara | 1.67 | 0.29 | 38.67 | 0.003 | 0.13 | 1.08 | 10.12 | 0.13 | 0.003 | 0.01 | 0.04 |
| Egg-27 | Hunedoara | 1.46 | 0.26 | 34.22 | 0.003 | 0.15 | 0.91 | 8.05 | 0.29 | 0.004 | 0.05 | 0.04 |
| Egg-28 | Dolj | 1.66 | 0.50 | 44.89 | 0.003 | 0.14 | 0.95 | 12.60 | 0.42 | 0.003 | 0.01 | 0.03 |
| Egg-29 | Greece | 0.92 | 0.33 | 29.69 | 0.001 | 0.02 | 0.86 | 8.07 | 0.24 | 0.002 | 0.01 | 0.01 |
| RfD * | 0.003 | 0.14 | 0.7 | 0.0003 | 0.02 | 0.04 | 0.3 | 0.005 | 0.001 | 0.004 | 0.0003 | |
| No. | Origin | EDI (μg/kg/Body Weight/Day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Fe | Co | Ni | Cu | Zn | Se | As | Cd | Pb | ||
| 1 | Tulcea (n = 2) | 0.822 | 0.192 | 23.390 | 0.0024 | 0.056 | 0.516 | 6.131 | 0.141 | 0.011 | 0.0019 | 0.016 |
| 2 | Constanta (n = 2) | 0.796 | 0.191 | 21.865 | 0.0033 | 0.056 | 0.581 | 5.400 | 0.133 | 0.014 | 0.0021 | 0.112 |
| 3 | Eforie Sud (n = 1) | 0.900 | 0.285 | 28.003 | 0.0032 | 0.089 | 0.624 | 6.565 | 0.093 | 0.017 | 0.0016 | 0.041 |
| 4 | Topraisar (n = 1) | 1.178 | 0.344 | 37.366 | 0.0074 | 0.127 | 0.821 | 8.425 | 0.312 | 0.033 | 0.0020 | 0.027 |
| 5 | Techirghiol (n = 1) | 0.927 | 0.217 | 32.007 | 0.0040 | 0.036 | 0.746 | 6.538 | 0.130 | 0.020 | 0.0020 | 0.016 |
| 6 | Satu Mare (n = 3) | 0.905 | 0.436 | 23.787 | 0.0020 | 0.097 | 0.703 | 7.396 | 0.169 | 0.014 | 0.0017 | 0.008 |
| 7 | Arad (n = 3) | 0.800 | 0.365 | 22.449 | 0.0023 | 0.048 | 0.581 | 6.860 | 0.142 | 0.018 | 0.0017 | 0.008 |
| 8 | Hunedoara (n = 6) | 0.921 | 0.173 | 21.031 | 0.0017 | 0.068 | 0.545 | 5.691 | 0.205 | 0.026 | 0.0019 | 0.012 |
| 9 | Alba (n = 2) | 0.981 | 0.292 | 29.786 | 0.0038 | 0.098 | 0.650 | 7.179 | 0.233 | 0.016 | 0.0015 | 0.013 |
| 10 | Cluj (n = 1) | 0.776 | 0.162 | 18.560 | 0.0032 | 0.064 | 0.647 | 7.179 | 0.339 | 0.025 | 0.0011 | 0.014 |
| 11 | Salaj (n = 3) | 0.835 | 0.568 | 30.778 | 0.0067 | 0.073 | 0.660 | 8.505 | 0.191 | 0.027 | 0.0016 | 0.015 |
| 12 | Mures (n = 1) | 0.832 | 0.214 | 19.562 | 0.0032 | 0.083 | 0.434 | 5.843 | 0.175 | 0.010 | 0.0019 | 0.007 |
| 13 | Dolj (n = 1) | 0.948 | 0.285 | 25.652 | 0.0017 | 0.082 | 0.541 | 7.201 | 0.238 | 0.018 | 0.0006 | 0.006 |
| 14 | Suceava (n = 1) | 0.726 | 0.422 | 29.222 | 0.0030 | 0.030 | 0.530 | 7.189 | 0.051 | 0.031 | 0.0014 | 0.013 |
| 15 | Greece (n = 1) | 0.526 | 0.186 | 16.963 | 0.0006 | 0.014 | 0.489 | 4.611 | 0.134 | 0.007 | 0.001 | 0.008 |
| PTDI | 3 | 140 | 800 | 500 | 5 | 500 | 1000 | 5 | 2.14 | 0.8 | 3.57 | |
| No. | Origin | THQ of Individual Elements | ∑THQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Cd | Pb | HI | ||
| 1 | Tulcea (n = 2) | 0.274 | 0.001 | 0.033 | 0.008 | 0.003 | 0.013 | 0.020 | 0.036 | 0.028 | 0.002 | 0.004 | 0.423 |
| 2 | Constanta (n = 2) | 0.265 | 0.001 | 0.031 | 0.011 | 0.003 | 0.015 | 0.018 | 0.045 | 0.027 | 0.002 | 0.028 | 0.446 |
| 3 | Eforie Sud (n = 1) | 0.300 | 0.002 | 0.040 | 0.011 | 0.004 | 0.016 | 0.022 | 0.058 | 0.019 | 0.002 | 0.010 | 0.483 |
| 4 | Topraisar (n = 1) | 0.393 | 0.002 | 0.053 | 0.025 | 0.006 | 0.021 | 0.028 | 0.110 | 0.062 | 0.002 | 0.007 | 0.709 |
| 5 | Techirghiol (n = 1) | 0.309 | 0.002 | 0.046 | 0.013 | 0.002 | 0.019 | 0.022 | 0.066 | 0.026 | 0.002 | 0.004 | 0.510 |
| 6 | Satu Mare (n = 3) | 0.302 | 0.003 | 0.034 | 0.007 | 0.005 | 0.018 | 0.025 | 0.045 | 0.034 | 0.002 | 0.002 | 0.476 |
| 7 | Arad (n = 3) | 0.267 | 0.003 | 0.032 | 0.008 | 0.002 | 0.015 | 0.023 | 0.059 | 0.028 | 0.002 | 0.002 | 0.440 |
| 8 | Hunedoara (n = 6) | 0.307 | 0.001 | 0.030 | 0.006 | 0.003 | 0.014 | 0.019 | 0.085 | 0.041 | 0.002 | 0.003 | 0.511 |
| 9 | Alba (n = 2) | 0.327 | 0.002 | 0.043 | 0.013 | 0.005 | 0.016 | 0.024 | 0.053 | 0.047 | 0.002 | 0.003 | 0.534 |
| 10 | Cluj (n = 1) | 0.259 | 0.001 | 0.027 | 0.011 | 0.003 | 0.016 | 0.024 | 0.085 | 0.068 | 0.002 | 0.003 | 0.498 |
| 11 | Salaj (n = 3) | 0.278 | 0.004 | 0.044 | 0.022 | 0.004 | 0.017 | 0.028 | 0.089 | 0.038 | 0.001 | 0.004 | 0.529 |
| 12 | Mures (n = 1) | 0.277 | 0.002 | 0.028 | 0.011 | 0.004 | 0.011 | 0.019 | 0.032 | 0.035 | 0.002 | 0.002 | 0.423 |
| 13 | Dolj (n = 1) | 0.316 | 0.002 | 0.037 | 0.006 | 0.004 | 0.014 | 0.024 | 0.059 | 0.048 | 0.002 | 0.002 | 0.512 |
| 14 | Suceava (n = 1) | 0.242 | 0.003 | 0.042 | 0.010 | 0.001 | 0.013 | 0.024 | 0.102 | 0.010 | 0.001 | 0.003 | 0.451 |
| 15 | Greece (n = 1) | 0.175 | 0.001 | 0.024 | 0.002 | 0.001 | 0.012 | 0.015 | 0.023 | 0.027 | 0.001 | 0.002 | 0.285 |
| RfD values (mg kg−1 day−1) | 0.003 | 0.14 | 0.7 | 0.0003 | 0.02 | 0.04 | 0.3 | 0.0003 | 0.005 | 0.001 | 0.004 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, G.; Dehelean, A.; Puscas, R.; Hategan, A.R.; Magdas, D.A. Isotopic and Elemental Fingerprint of Edible Egg Parts—The Health Risk Assessment Based on Potentially Toxic Elements Content. Molecules 2023, 28, 503. https://doi.org/10.3390/molecules28020503
Cristea G, Dehelean A, Puscas R, Hategan AR, Magdas DA. Isotopic and Elemental Fingerprint of Edible Egg Parts—The Health Risk Assessment Based on Potentially Toxic Elements Content. Molecules. 2023; 28(2):503. https://doi.org/10.3390/molecules28020503
Chicago/Turabian StyleCristea, Gabriela, Adriana Dehelean, Romulus Puscas, Ariana Raluca Hategan, and Dana Alina Magdas. 2023. "Isotopic and Elemental Fingerprint of Edible Egg Parts—The Health Risk Assessment Based on Potentially Toxic Elements Content" Molecules 28, no. 2: 503. https://doi.org/10.3390/molecules28020503
APA StyleCristea, G., Dehelean, A., Puscas, R., Hategan, A. R., & Magdas, D. A. (2023). Isotopic and Elemental Fingerprint of Edible Egg Parts—The Health Risk Assessment Based on Potentially Toxic Elements Content. Molecules, 28(2), 503. https://doi.org/10.3390/molecules28020503








