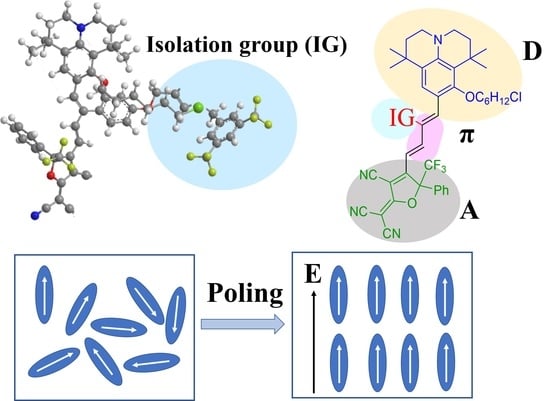
Systematic Study on Nonlinear Optical Chromophores with Improved Electro-Optic Activity by Introducing 3,5-Bis(trifluoromethyl)benzene Derivative Isolation Groups into the Bridge
(This article belongs to the Section Materials Chemistry)
Abstract
1. Introduction
2. Results and Discussion
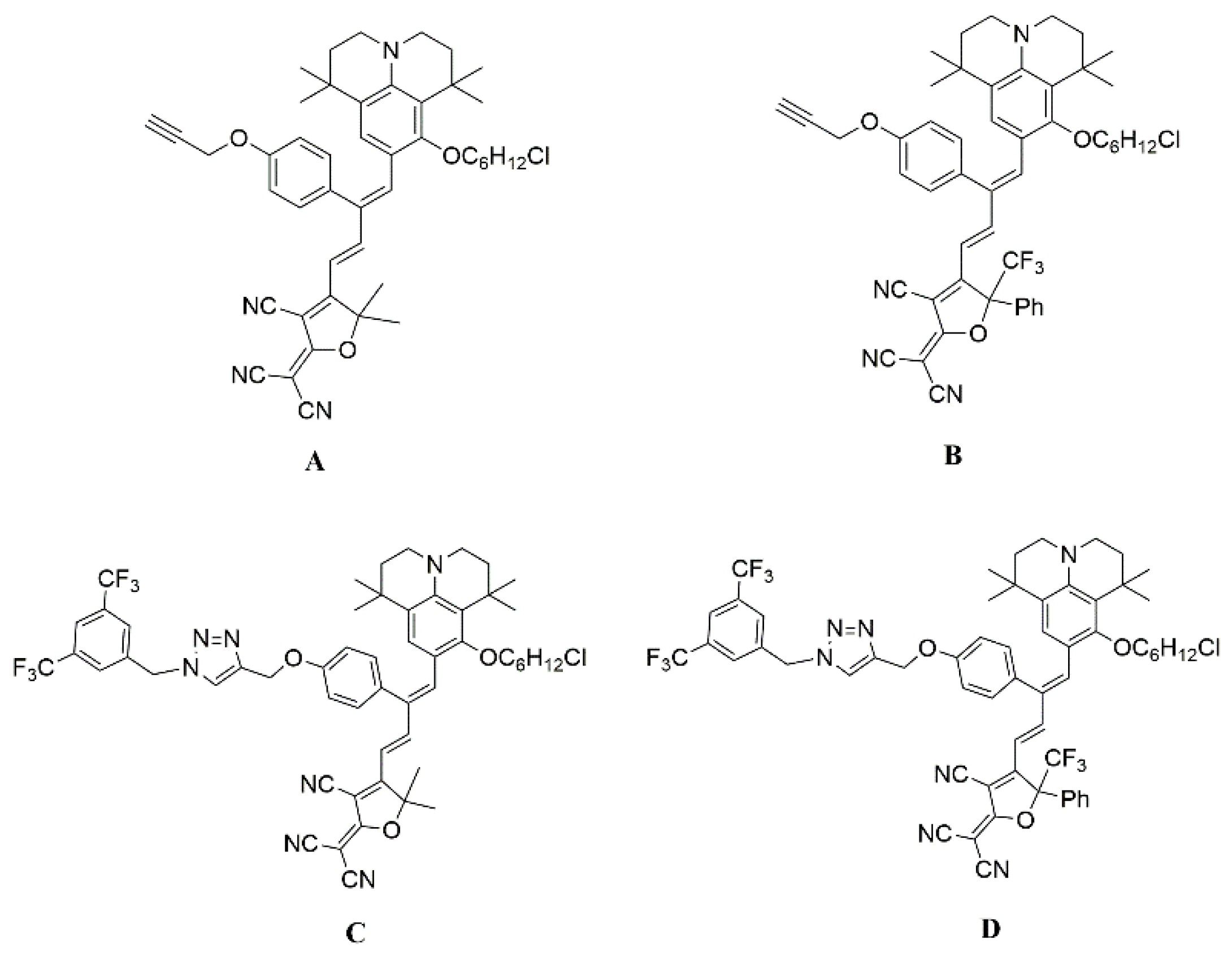
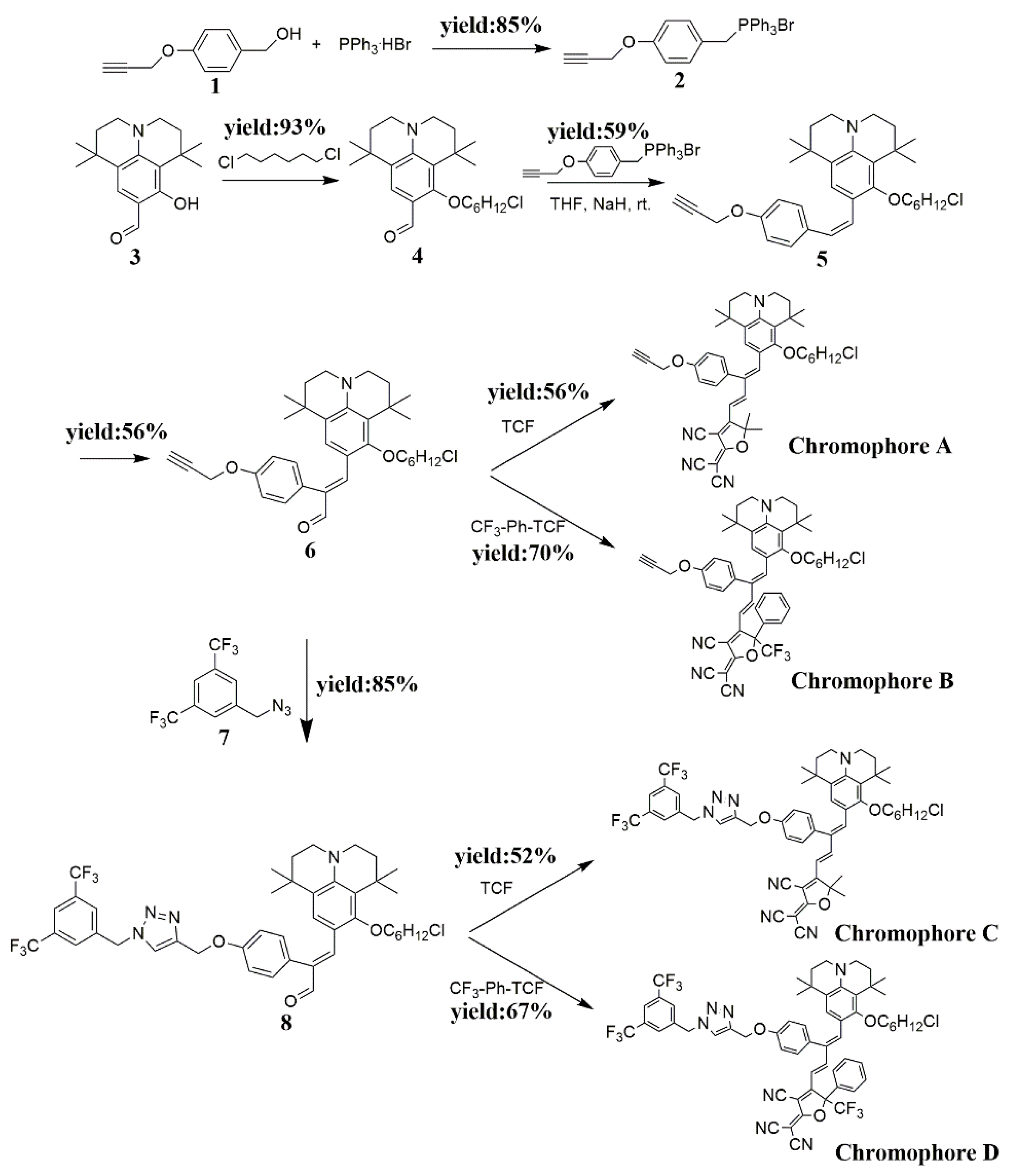
2.1. Synthesis and Characterization
2.2. Thermal Stability
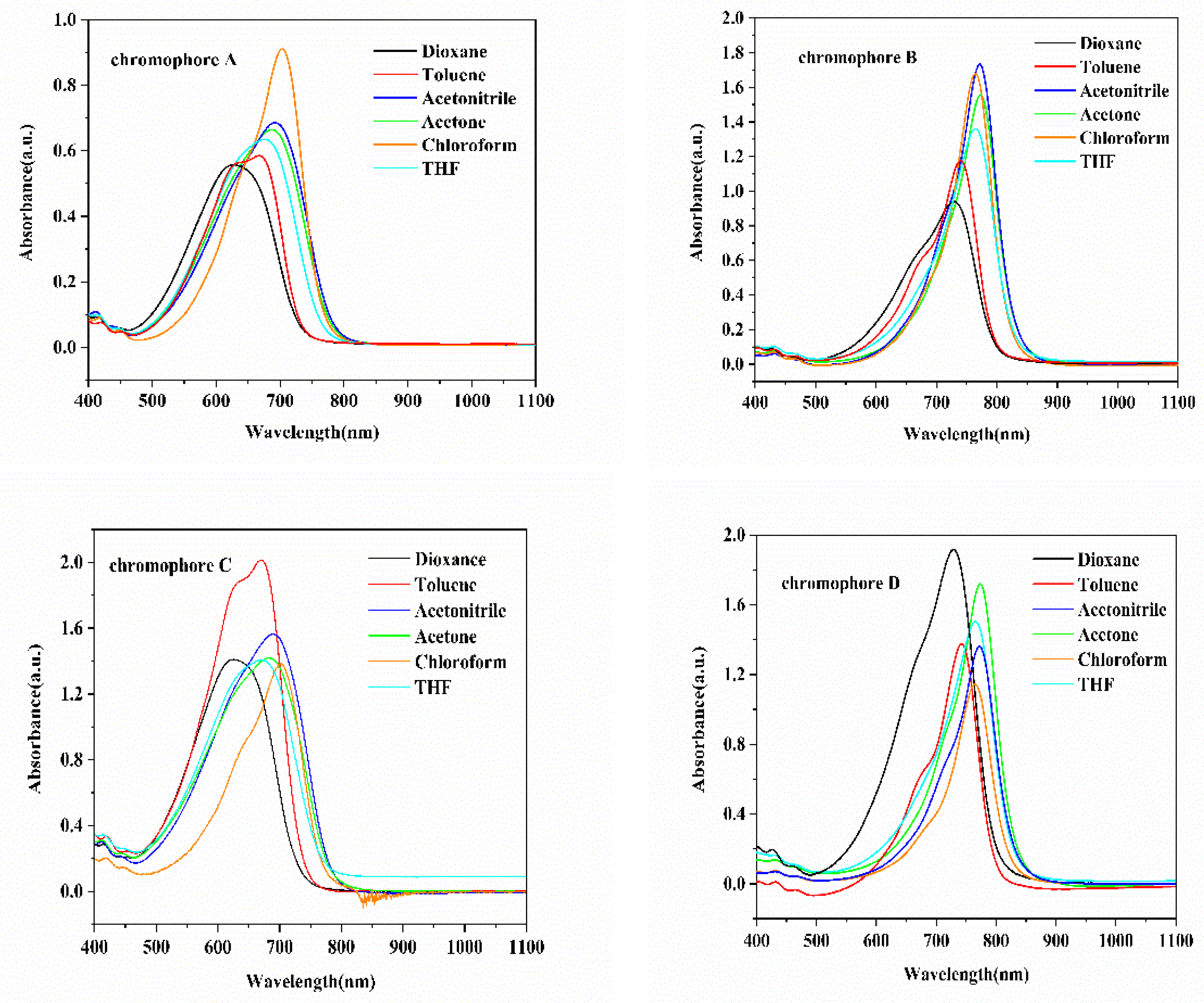
2.3. Optical Properties
2.4. Theoretical Calculations
2.5. Electric Field Poling and EO Property Measurements
3. Experiments
3.1. Materials
3.2. Measurements and Instrumentation
3.3. Synthesis of Compound
3.4. Synthesis of Compound 2
3.5. Synthesis of Compound 6
3.6. Synthesis of Chromophore A
3.7. Synthesis of Chromophore B
3.8. Synthesis of Compound 8
3.9. Synthesis of Chromophore C
3.10. Synthesis of Chromophore D
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bonjour, R.; Burla, M.; Abrecht, F.C.; Welschen, S.; Hoessbacher, C.; Heni, W.; Gebrewold, S.A.; Baeuerle, B.; Josten, A.; Salamin, Y.; et al. Plasmonic phased array feeder enabling ultra-fast beam steering at millimeter waves. Opt. Express 2016, 24, 25608–25618. [Google Scholar] [CrossRef] [PubMed]
- Burla, M.; Hoessbacher, C.; Heni, W.; Hafffner, C.; Fedoryshyn, Y.; Werner, M.; Watanabe, T.; Massler, H.; Elder, D.L.; Leuthold, J. 500 GHz plasmonic Mach-Zehnder modulator enabling sub-THz microwave photonics. APL Photon. 2019, 4, 056106. [Google Scholar] [CrossRef]
- Alloatti, L.; Palmer, R.; Diebold, S.; Pahl, K.P.; Chen, B.Q.; Dinu, R.; Fournier, M.; Fedeli, J.M.; Zwick, T.; Freude, W.; et al. 100 GHz silicon–organic hybrid modulator. Light Sci. Appl. 2014, 3, e173. [Google Scholar] [CrossRef]
- Salamin, Y.; Benea-Chelmus, I.-C.; Fedoryshyn, Y.; Heni, W.; Elder, D.L.; Dalton, L.R.; Faist, J.; Leuthold, J. Compact and ultra-efficient broadband plasmonic terahertz field detector. Nat. Commun. 2019, 10, 5550. [Google Scholar] [CrossRef] [PubMed]
- Ummethala, S.; Harter, T.; Koehnle, K.; Li, Z.; Muehlbrandt, S.; Kutuvantavida, Y.; Kemal, J.; Marin-Palomo, P.; Schaefer, J.; Tessmann, A.; et al. THz-to-optical conversion in wireless communications using an ultra-broadband plasmonic modulator. Nat. Photonics 2019, 13, 519–524. [Google Scholar] [CrossRef]
- Haffner, C.; Heni, W.; Fedoryshyn, Y.; Niegemann, J.; Melikyan, A.; Elder, D.L.; Baeuerle, B.; Salamin, Y.; Josten, A.; Koch, U.; et al. All-plasmonic Mach–Zehnder modulator enabling optical high-speed communication at the microscale. Nat. Photonics 2015, 9, 525–529. [Google Scholar] [CrossRef]
- Liu, D.P.; Tang, J.; Meng, Y.; Li, W.; Zhu, N.H.; Li, M. Ultra-low Vpp and high-modulationdepth InP-based electro-optic microring modulator. J. Semicond 2021, 42, 082301. [Google Scholar] [CrossRef]
- He, M.B.; Xu, M.Y.; Ren, Y.X.; Jian, J.; Ruan, Z.; Xu, Y.; Gao, S.; Sun, S.; Wen, X.; Zhou, L.; et al. High-performance hybrid silicon and lithium niobate Mach–Zehnder modulators for 100 Gbit s−1 and beyond. Nat. Photonics 2019, 13, 359. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, C.R.; Pan, A.; Ding, Y.; Wang, X.; Qu, Z.; Wei, J.; Liu, Y.; Zeng, C.; Xia, J. Photonic devices based on thin-film lithium niobate on insulator. J. Semicond. 2021, 42, 041304. [Google Scholar] [CrossRef]
- Xu, M.Y.; Cai, X.L. Advances in integrated ultra-wideband electro-optic modulators. Opt. Express 2022, 30, 7253. [Google Scholar] [CrossRef]
- Kim, K.; Choi, J.Y.; Kim, T.; Cho, S.H.; Chung, H.J. A role for graphene in silicon-based semiconductor devices. Nature 2011, 479, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.T.; Liu, J.Y.; Li, C.B.; Chen, Z.; Bo, S.H. Organic electro-optic polymer materials and organic-based hybrid electro-optic modulators. J. Semicond. 2022, 43, 101301. [Google Scholar] [CrossRef]
- Lu, G.W.; Hong, J.X.; Qiu, F.; Spring, A.M.; Kashino, T.; Oshima, J.; Ozawa, M.; Nawata, H.; Yokoyama, S. High-temperature-resistant silicon-polymer hybrid modulator operating at up to 200 Gbit s−1 for energy-efficient datacentres and harsh-environment applications. Nat. Comm. 2020, 11, 1–9. [Google Scholar]
- Ayata, M.; Fedoryshyn, Y.; Heni, W.; Baeuerle, B.; Josten, A.; Zahner, M.; Koch, U.; Salamin, Y.; Hoessbache, C.; Haffner, C.; et al. High-speed plasmonic modulator in a single metal layer. Science 2017, 358, 630–632. [Google Scholar] [CrossRef]
- Haffner, C.; Chelladurai, D.; Fedoryshyn, Y.; Josten, A.; Baeuerle, B.; Heni, W.; Watanabe, T.; Cui, T.; Cheng, B.; Saha, S.; et al. Low-loss plasmon-assisted electro-optic modulator. Nature 2018, 556, 483–488. [Google Scholar] [CrossRef]
- Koch, U.; Uhl, C.; Hettrich, H.; Fedoryshyn, Y.; Hoessbacher, C.; Heni, W.; Baieuerle, B.; Bitachon, B.I.; Josten, A.; Ayata, M.; et al. A monolithic bipolar CMOS electronic–plasmonic high-speed transmitter. Nat. Elec. 2020, 3, 338–345. [Google Scholar] [CrossRef]
- Kiennger, C.; Kutuvantavida, Y.; Eleder, D.L.; Wolf, S.; Zwickel, H.; Blaicher, M.; Kemal, J.D.; Lauermann, M.; Randel, S.; Freude, W.; et al. Ultra-high electro-optic activity demonstrated in a silicon-organic hybrid modulator. Optica 2018, 5, 739–748. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, X.Y.; Chen, Z.; Liu, F.G.; Huo, F.Y.; Bo, S.H.; Qiu, L.; Zhen, Z. Improved electro-optical property by introducing stronger acceptor to thermal stablechromophores using modified julolidine as donor. Dye. Pigment. 2019, 167, 245–254. [Google Scholar] [CrossRef]
- Xu, H.J.; Elder, D.L.; Johnson, L.E.; Heni, W.; Coene, Y.; Leo, E.; Destraz, M.; Meier, N.; Ghinst, W.; Hammond, S.R.; et al. Design and synthesis of chromophores with enhanced electro-optic activities in both bulk and plasmonic–organic hybrid devices. Mater. Horiz. 2022, 9, 261–270. [Google Scholar] [CrossRef]
- Benea-chelmus, I.C.; Salamin, Y.; Settembrini, F.F.; Fedoryshyn, Y.; Heni, W.; Elder, D.L.; Dalton, L.R.; Leuthold, J.; Faist, J. Electro-optic interface for ultrasensitive intracavity electric field measurements at microwave and terahertz frequencies. Optica 2020, 7, 498–505. [Google Scholar] [CrossRef]
- Bo, S.H.; Li, Y.; Liu, T.T.; Huo, F.Y.; Xiao, H.Y.; Zhang, H.; Chen, Z. Systematic study on the optimization of a bis(N,N-diethyl)aniline based NLO chromophore via a stronger electron acceptor, extended p-conjugation and isolation groups. J. Mater. Chem. C 2022, 10, 3343–3352. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Chen, X.Y.; Zeng, Z.Y.; Liang, Z.W.; Shi, L.; Huang, Z.L.; Bo, S.H.; Liu, F.G.; Wang, J.H. A modifiable double donor based on bis(N-ethyl-N-hydroxyethyl)aniline for organic optical nonlinear chromophores. Mater. Chem. Front. 2022, 6, 1079–1090. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, A.R.; Xiao, H.Y.; Huo, F.Y.; Zhen, Z.; Liu, X.H.; Bo, S.H. Tailoring the chemical structures and nonliear optical properties of julolidinyl-based chromophores by molecular engineering. Dye. Pigment. 2020, 173, 107876. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Liu, J.H.; Luo, T.Y.; Li, Z.B.; Liao, J.F.; Zhang, W.J.; Zhang, L.; Liu, F.G. Electro-optic crosslinkable chromophores with ultrahigh electro-optic coefficients and long-term stability. Chem. Sci. 2022, 13, 13393–13402. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Elder, D.L.; Johnson, L.E.; Coene, Y.; Hammond, S.R.; Ghinst, W.; Clays, K.; Dalton, L.R. Electro-optic activity in excess of 1000 pm V−1 achieved via theory-guided organic chromophore design. Adv. Mater. 2021, 33, 2104174. [Google Scholar] [CrossRef]
- Liu, J.; Gao, W.; Kityk, I.V.; Liu, X.; Zhen, Z. Optimization of Polycyclic Electron-Donors Based on Julolidinyl Structure in Push–Pull Chromophores for Second Order NLO Effects. Dye. Pigment. 2015, 122, 74–84. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Achelle, S.; Bureš, F.; le Poul, P.; Barsella, A.; Kvashnin, Y.A.; Rusinov, G.L.; Guen, F.R.; Chupakhin, O.N.; Charushin, V.N. Synthesis, Photophysical and Nonlinear Optical Properties of [1,2,5]Oxadiazolo [3,4-b]Pyrazine-Based Linear Push-Pull Systems. J. Photochem. Photobiol. A Chem. 2021, 404, 112900. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Ye, J.-T.; Zhang, Y.; Zhao, Y.-Y.; Qiu, Y.-Q. A Thorough Understanding of the Nonlinear Optical Properties of BODIPY/Carborane/Diketopyrrolopyrrole Hybrid Chromophores: Module Contribution, Linear Combination, One-/Two-Dimensional Difference and Carborane’s Arrangement. J. Mater. Chem. C 2019, 7, 7531–7547. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, C.; Huo, F.; He, W.; Cao, A. Progress in the Enhancement of Electro-Optic Coefficients and Orientation Stability for Organic Second-Order Nonlinear Optical Materials. Dye. Pigment. 2020, 181, 108509. [Google Scholar] [CrossRef]
- Zhang, D.; Zou, J.; Chen, W.L.; Yiu, S.M.; Tse, M.K.; Luo, J.D.; Jen, A.K.Y. Efficient, Stable, and Scalable Push–Pull Heptamethines for Electro-Optics. Chem. Mater. 2022, 34, 3683–3693. [Google Scholar] [CrossRef]
- Chen, P.Y.; Zhang, H.Y.; Han, M.M.; Cheng, Z.Y.; Peng, Q.; Li, Q.Q.; Li, Z. Janus molecules: Large second-order nonlinear optical performance, good temporal stability, excellent thermal stability and spherical structure with optimized dendrimer structure. Mater. Chem. Front. 2018, 2, 1374–1382. [Google Scholar] [CrossRef]
- Zang, X.B.; Liu, G.C.; Li, Q.Q.; Li, Z.A.; Li, Z. A Correlation Study between Dendritic Structure and Macroscopic Nonlinearity for Second-Order Nonlinear Optical Materials. Macromolecules 2020, 53, 4012–4021. [Google Scholar] [CrossRef]
- Gao, L.; Li, B.; Yi, H.; Cui, J.; Yang, L.; Song, Y.; Yang, H.-R.; Zhou, L.; Fang, S. Nonlinear Optical Properties of Pyrene Derivatives Based on a Donor–Acceptor Structure and Its Polyurethane Composites. ACS Omega 2022, 7, 27959–27968. [Google Scholar] [CrossRef]
- Liu, F.G.; Yang, Y.H.; Wang, H.R.; Liu, J.L.; Hu, C.L.; Huo, F.Y.; Bo, S.H.; Zhen, Z.; Liu, X.H.; Qiu, L. Comparative studies on structure-nonlinearity relationships in a series of novel second-order nonlinear optical chromophores with different aromatic amine donors. Dye. Pigment. 2015, 120, 347–356. [Google Scholar] [CrossRef]
- Dalton, L.; Sullivan, R.P.A.; Bale, D.H.; Olbricht, B.C. Theory-inspired nano-engineering of photonic and electronic materials: Noncentrosymmetric charge-transfer electro-optic materials. Solid-State Electron. 2007, 51, 1263–1277. [Google Scholar] [CrossRef]
- Hu, C.L.; Liu, F.G.; Zhang, H.; Huo, F.Y.; Yang, Y.H.; Wang, H.R.; Xiao, H.Y.; Chen, Z.; Liu, J.L.; Qiu, L.; et al. Synthesis of novel nonlinear optical chromophores: Achieving excellent electro-optic activity by introducing benzene derivative isolation groups into the bridge. J. Mater. Chem. C 2015, 3, 11595–11604. [Google Scholar] [CrossRef]
- Kopach, M.E.; Murray, M.M.; Braden, T.M. Improved synthesis of 1-(azidomethyl)-3,5-bis-(trifluoromethyl)benzene: Development of batch and microflow azide processes. Org. Process Res. Devepment 2009, 13, 152–160. [Google Scholar] [CrossRef]
- He, M.; Leslie, T.M.; Sinicropi, J.A. α-hydroxy ketone precursors leading to a novel class of electro-optic acceptors. Chem. Mater. 2002, 14, 2393–2400. [Google Scholar] [CrossRef]
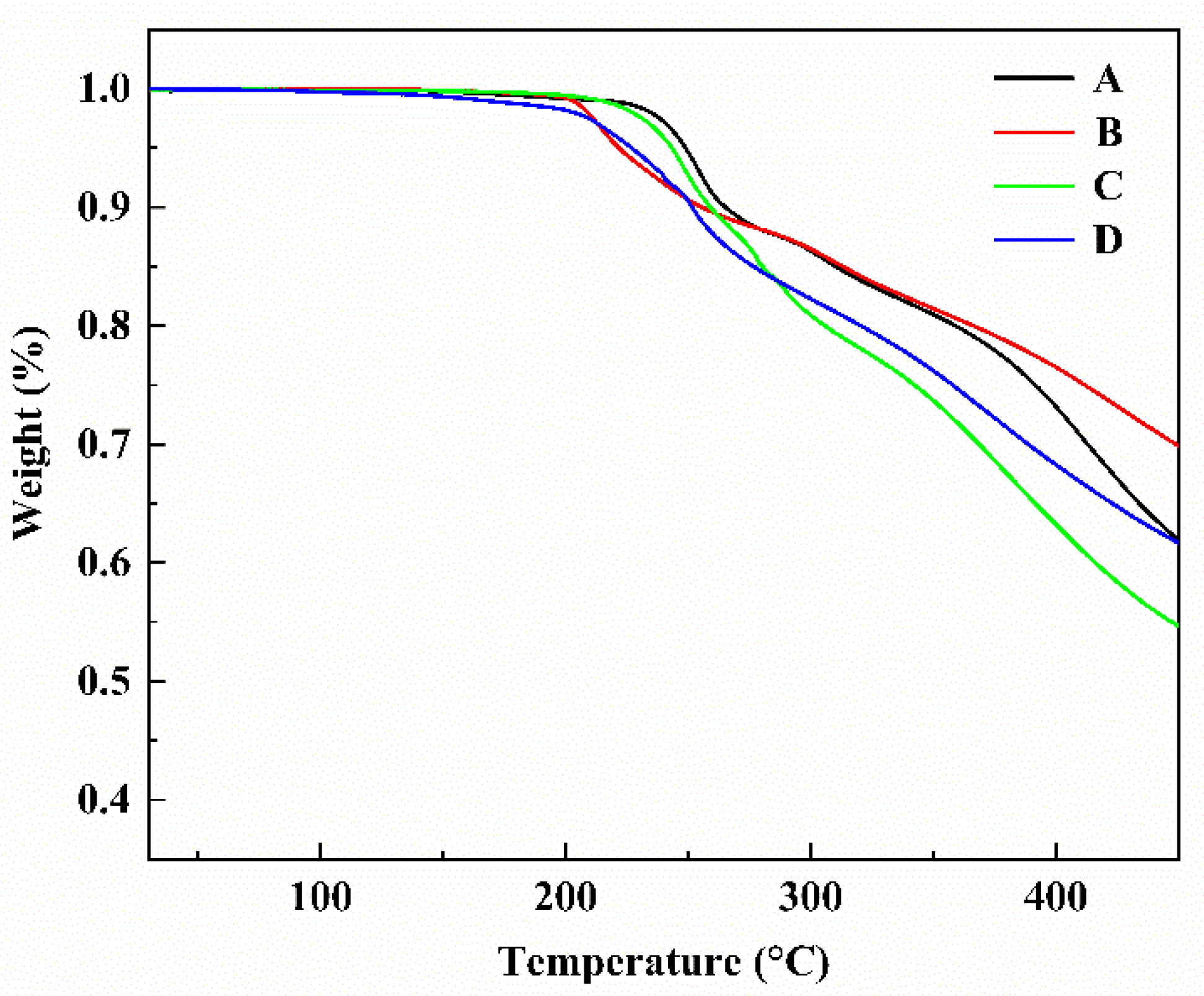


| Chromophore | Td (°C) | Tg (°C) |
|---|---|---|
| Chromophore A | 249 | 98.2 |
| Chromophore B | 221 | 90.4 |
| Chromophore C | 243 | 83.5 |
| Chromophore D | 226 | 80.2 |
| Chromophore | λmax a/ε | λmax b/ε | λmax c/ε | λmax d/ε | λmax e/ε | λmax f/ε | Δλmax g |
|---|---|---|---|---|---|---|---|
| (nm) | (nm) | (nm) | (nm) | (nm) | (nm) | (nm) | |
| Chromophore A | 666/11.6 | 702/18.2 | 674/12.6 | 625/11.2 | 685/13.2 | 693/13.8 | 77 |
| Chromophore B | 739/9.7 | 764/13.9 | 764/11.4 | 730/7.8 | 774/12.9 | 772/14.4 | 44 |
| Chromophore C | 670/16.8 | 702/11.6 | 671/11.7 | 625/11.7 | 683/11.8 | 690/13.0 | 77 |
| Chromophore D | 742/11.4 | 765/9.4 | 766/12.6 | 728/15.9 | 774/14.2 | 772/11.2 | 46 |
| Chromophores | EHOMO/eV | ELUMO/eV | ΔE/eV | max/10−30 esu | μ (D) |
|---|---|---|---|---|---|
| A | −6.10 | −2.08 | 4.01 | 225 | 23.8 |
| B | −6.14 | −2.19 | 3.95 | 241 | 24.0 |
| C | −6.16 | −2.10 | 4.06 | 243 | 20.9 |
| D | −6.19 | −2.20 | 3.99 | 255 | 21.3 |
| Chromophores | with 15 wt% | with 25 wt% | with 35 wt% |
|---|---|---|---|
| A | 13 pm/V | 18 pm/V | 15 pm/V |
| B | 17 pm/V | 28 pm/V | 18 pm/V |
| C | 23 pm/V | 32 pm/V | 38 pm/V |
| D | 29 pm/V | 43 pm/V | 54 pm/V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Huo, F.; Ge, C.; Li, Y.; He, J.; Zheng, H.; He, Q.; Zhao, Y.; Chen, Z.; Bo, S. Systematic Study on Nonlinear Optical Chromophores with Improved Electro-Optic Activity by Introducing 3,5-Bis(trifluoromethyl)benzene Derivative Isolation Groups into the Bridge. Molecules 2023, 28, 488. https://doi.org/10.3390/molecules28020488
Liu T, Huo F, Ge C, Li Y, He J, Zheng H, He Q, Zhao Y, Chen Z, Bo S. Systematic Study on Nonlinear Optical Chromophores with Improved Electro-Optic Activity by Introducing 3,5-Bis(trifluoromethyl)benzene Derivative Isolation Groups into the Bridge. Molecules. 2023; 28(2):488. https://doi.org/10.3390/molecules28020488
Chicago/Turabian StyleLiu, Tongtong, Fuyang Huo, Changqing Ge, Ya Li, Jing He, Han Zheng, Qian He, Yinsen Zhao, Zhuo Chen, and Shuhui Bo. 2023. "Systematic Study on Nonlinear Optical Chromophores with Improved Electro-Optic Activity by Introducing 3,5-Bis(trifluoromethyl)benzene Derivative Isolation Groups into the Bridge" Molecules 28, no. 2: 488. https://doi.org/10.3390/molecules28020488
APA StyleLiu, T., Huo, F., Ge, C., Li, Y., He, J., Zheng, H., He, Q., Zhao, Y., Chen, Z., & Bo, S. (2023). Systematic Study on Nonlinear Optical Chromophores with Improved Electro-Optic Activity by Introducing 3,5-Bis(trifluoromethyl)benzene Derivative Isolation Groups into the Bridge. Molecules, 28(2), 488. https://doi.org/10.3390/molecules28020488