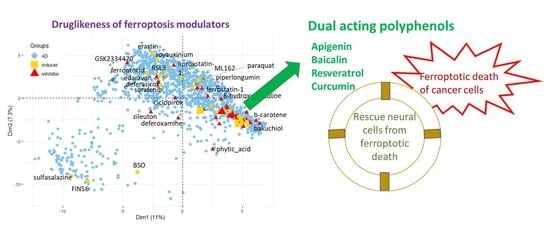
Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products
Abstract
1. Introduction
2. Ferroptosis—Three Main Factors
3. Ferroptosis as a Potential Therapeutical Target
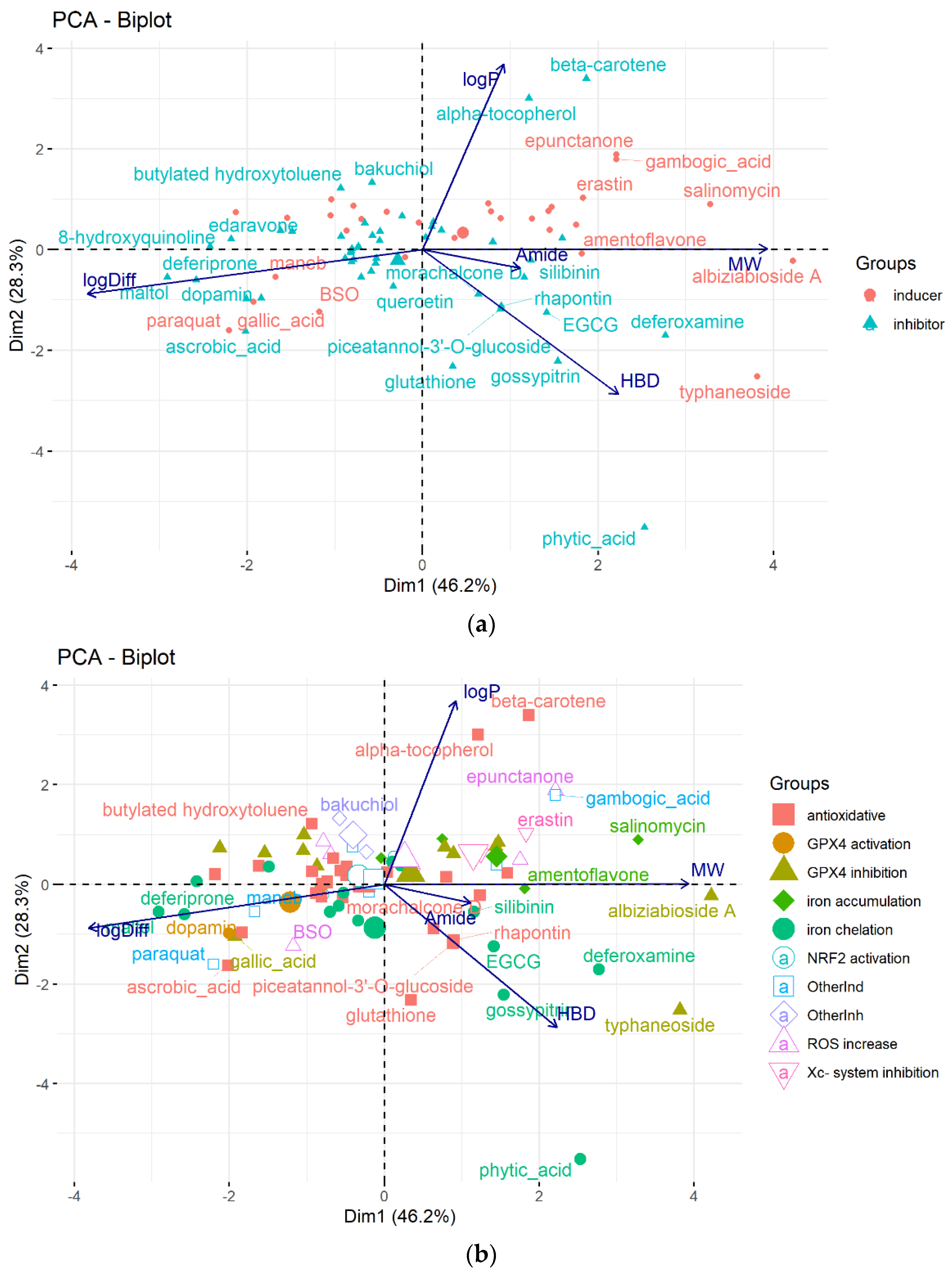
4. Chemical Aspects of Ferroptosis Modulators
5. Biological Aspects of Ferroptosis Modulators
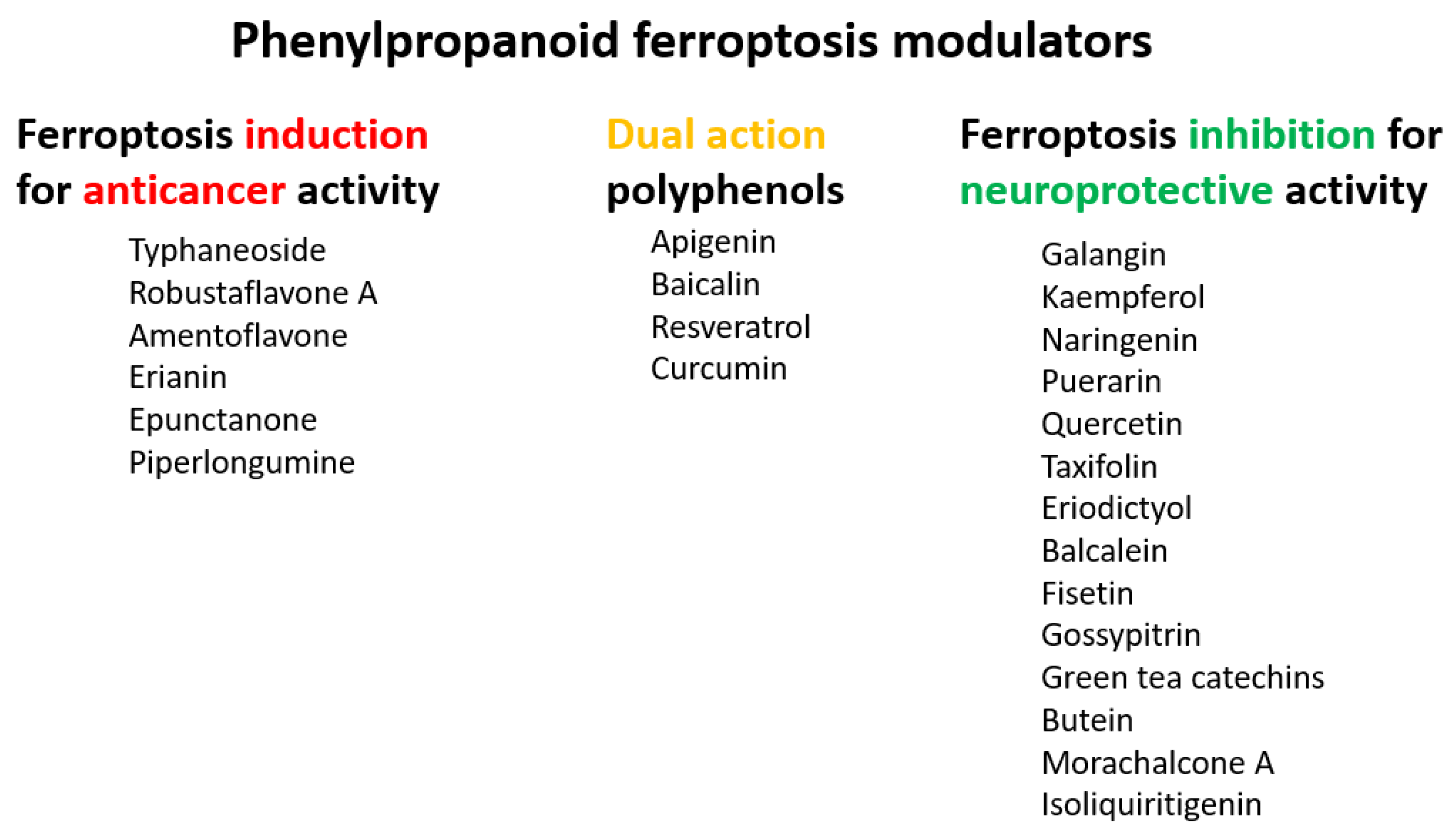
5.1. Ferroptosis Inducers
5.2. Ferroptosis Inhibitors
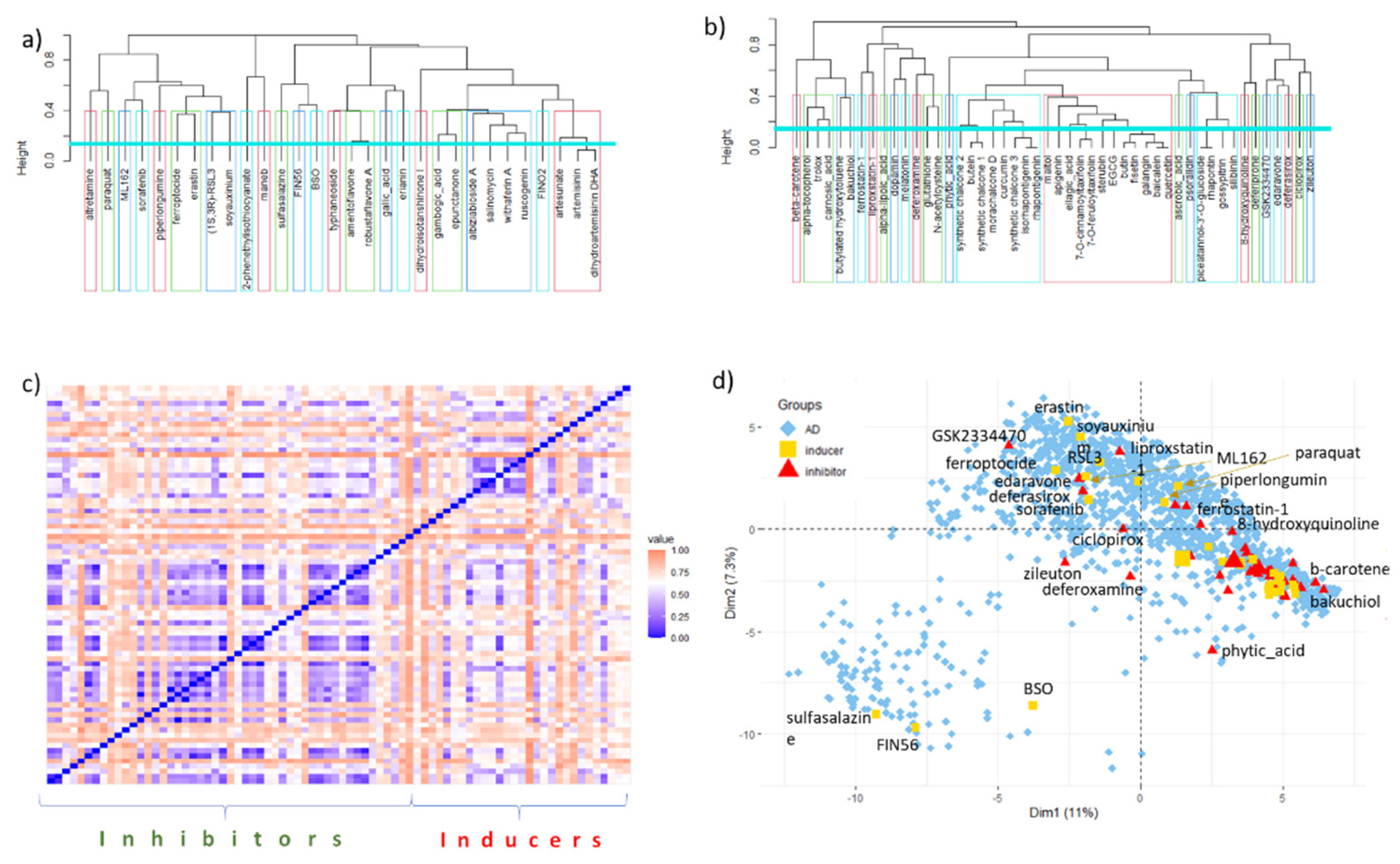
6. Data Set and Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schioth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann. Oncol. 2020, 31, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Venkata, K.C.N.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Stepanic, V.; Gasparovic, A.C.; Troselj, K.G.; Amic, D.; Zarkovic, N. Selected Attributes of Polyphenols in Targeting Oxidative Stress in Cancer. Curr. Top. Med. Chem. 2015, 15, 496–509. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Greco, G.; Catanzaro, E.; Fimognari, C. Natural Products as Inducers of Non-Canonical Cell Death: A Weapon against Cancer. Cancers 2021, 13, 304. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Zeitler, H.; Vetter, H.; Kraft, K. Synergy research: Vitamins and secondary plant components in the maintenance of the redox-homeostasis and in cell signaling. Phytomedicine 2009, 16, 2–16. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.S.; Ma, J.F.; Wang, H.; Wang, F.D. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wang, X.N.; Pang, Y.Z. Phytochemicals Targeting Ferroptosis: Therapeutic Opportunities and Prospects for Treating Breast Cancer. Pharmaceuticals 2022, 15, 1360. [Google Scholar] [CrossRef]
- de Souza, I.; Ramalho, M.C.C.; Guedes, C.B.; Osawa, I.Y.A.; Monteiro, L.K.S.; Gomes, L.R.; Rocha, C.R.R. Ferroptosis Modulation: Potential Therapeutic Target for Glioblastoma Treatment. Int. J. Mol. Sci. 2022, 23, 6879. [Google Scholar] [CrossRef]
- Lawless, M.S.; Waldman, M.; Fraczkiewicz, R.; Clark, R.D. Using Cheminformatics in Drug Discovery. In New Approaches to Drug Discovery; Nielsch, U., Fuhrmann, U., Jaroch, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–168. [Google Scholar]
- Davidson, A.J.; Wood, W. Igniting the spread of ferroptotic cell death. Nat. Cell Biol. 2020, 22, 1027–1029. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Gao, M.H.; Yi, J.M.; Zhu, J.J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X.J. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef]
- Andreini, C.; Putignano, V.; Rosato, A.; Banci, L. The human iron-proteome. Metallomics 2018, 10, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Vanden Berghe, T. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, W.K.; Bae, K.H.; Lee, S.C.; Lee, E.W. Lipid Metabolism and Ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; IngoId, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Steegmann-Olmedillas, J.L. The role of iron in tumour cell proliferation. Clin. Transl. Oncol. 2011, 13, 71–76. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Kang, R.; Kroemer, G.; Tang, D.L. The tumor suppressor protein p53 and the ferroptosis network. Free. Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef]
- Ratan, R.R. The Chemical Biology of Ferroptosis in the Central Nervous System. Cell Chem. Biol. 2020, 27, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen-peroxide by human tumor-cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Lin, Z.; Pavlides, S.; Whitaker-Menezes, D.; Pestell, R.G.; Howell, A.; Sotgia, F. Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis the seed and soil also needs “fertilizer”. Cell Cycle 2011, 10, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. H2O2: A Dynamic Neuromodulator. Neuroscientist 2011, 17, 389–406. [Google Scholar] [CrossRef]
- Fujihara, K.M.; Zhang, B.N.Z.; Clemons, N.J. Opportunities for Ferroptosis in Cancer Therapy. Antioxidants 2021, 10, 986. [Google Scholar] [CrossRef]
- Luo, L.X.; Wang, H.; Tian, W.; Li, X.L.; Zhu, Z.; Huang, R.M.; Luo, H. Targeting ferroptosis-based cancer therapy using nanomaterials: And. Theranostics 2021, 11, 9937–9952. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Klein, V.G.; Bond, A.G.; Craigon, C.; Lokey, R.S.; Ciulli, A. Amide-to-Ester Substitution as a Strategy for Optimizing PROTAC Permeability and Cellular Activity. J. Med. Chem. 2021, 64, 18082–18101. [Google Scholar] [CrossRef]
- Weiland, A.; Wang, Y.M.; Wu, W.H.; Lan, X.; Han, X.N.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef]
- Feunang, Y.D.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 20. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Fan, X.P.; Zhao, M.Y.; Zhu, P. The Relationship between Ferroptosis and Tumors: A Novel Landscape for Therapeutic Approach. Curr. Gene Ther. 2019, 19, 117–124. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yu, C.C.; Luo, M.; Cen, C.; Qiu, J.L.; Zhang, S.Z.; Hu, K.M. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 16. [Google Scholar] [CrossRef]
- Guo, J.P.; Xu, B.F.; Han, Q.; Zhou, H.X.; Xia, Y.; Gong, C.W.; Dai, X.F.; Li, Z.Y.; Wu, G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid.-Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Stepanić, V.; Kujundžić, R.N.; Trošelj, K.G. Epigenome, Cancer Prevention and Flavonoids and Curcumin. In Epigenetics and Epigenomics; Payne, C.J., Ed.; IntechOpen: London, UK, 2014; p. 220. [Google Scholar]
- Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.I.; Murphy, M.E.; George, D.L. Targeting ErbB3 and Cellular NADPH/NADP(+)Abundance Sensitizes Cutaneous Melanomas to Ferroptosis Inducers. ACS Chem. Biol. 2022, 17, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Huang, Z.X.; Chen, G.Q.; Sheng, F.; Zheng, Y.S. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem. Biophys. Res. Commun. 2019, 516, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.C.; Liu, W.L.; Kang, F.H.; Zou, Z.X.; Xu, K.P.; Xu, P.S.; Tan, G.S. Identification of a new natural biflavonoids against breast cancer cells induced ferroptosis via the mitochondrial pathway. Bioorg. Chem. 2021, 109, 11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.C.; Liu, W.L.; Xu, P.S.; Tan, G.S. Cytotoxic effects of the biflavonoids isolated from Selaginella trichoclada on MCF-7 cells and its potential mechanism. Bioorg. Med. Chem. Lett. 2022, 56, 5. [Google Scholar] [CrossRef]
- Xiong, X.F.; Tang, N.; Lai, X.D.; Zhang, J.L.; Wen, W.L.; Li, X.J.; Li, A.G.; Wu, Y.H.; Liu, Z.H. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Wang, H.J.; Wang, N.N.; Peng, H.; Wang, J.; Li, Y.H.; Liu, M.D.; Li, H.; Zhang, Y.; et al. Amentoflavone suppresses cell proliferation and induces cell death through triggering autophagy-dependent ferroptosis in human glioma. Life Sci. 2020, 247, 10. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.B.; Feng, J.; Yan, L.L.; Sun, Y.T.; Liu, S.P.; Xiang, Y.; Zhang, M.M.; Pan, T.; Chen, X.Y.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, X.; Wang, W.; Zhai, L.; Sun, X.; Feng, J.; Duan, T.; Zhang, M.; Pan, T.; Yan, L.; et al. Natural Product Erianin Inhibits Bladder Cancer Cell Growth by Inducing Ferroptosis via NRF2 Inactivation. Front. Pharmacol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Fotso, G.W.; Ngnintedo, D.; Kuete, V.; Ngadjui, B.T.; Keumedjio, F.; Andrae-Marobela, K.; Efferth, T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine 2018, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.K.; Langford, T.F.; Sikes, H.D. Using Sensors and Generators of H2O2 to Elucidate the Toxicity Mechanism of Piperlongumine and Phenethyl Isothiocyanate. Antioxid. Redox Signal. 2016, 24, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Cheung, P.C.K. Gene expression profile analysis of gallic acid-induced cell death process. Sci. Rep. 2021, 11, 17. [Google Scholar] [CrossRef]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 14. [Google Scholar] [CrossRef]
- Tang, H.M.; Cheung, P.C.K. Gallic Acid Triggers Iron-Dependent Cell Death with Apoptotic, Ferroptotic, and Necroptotic Features. Toxins 2019, 11, 492. [Google Scholar] [CrossRef]
- An, P.J.; Gu, D.H.; Gao, Z.G.; Fan, F.Y.; Jiang, Y.; Sun, B.W. Hypoxia-augmented and photothermally-enhanced ferroptotic therapy with high specificity and efficiency. J. Mat. Chem. B 2020, 8, 78–87. [Google Scholar] [CrossRef]
- Zhang, P.S.; Hou, Y.; Zeng, J.F.; Li, Y.Y.; Wang, Z.H.; Zhu, R.; Ma, T.C.; Gao, M.Y. Coordinatively Unsaturated Fe3+ Based Activatable Probes for Enhanced MRI and Therapy of Tumors. Angew. Chem.-Int. Edit. 2019, 58, 11088–11096. [Google Scholar] [CrossRef]
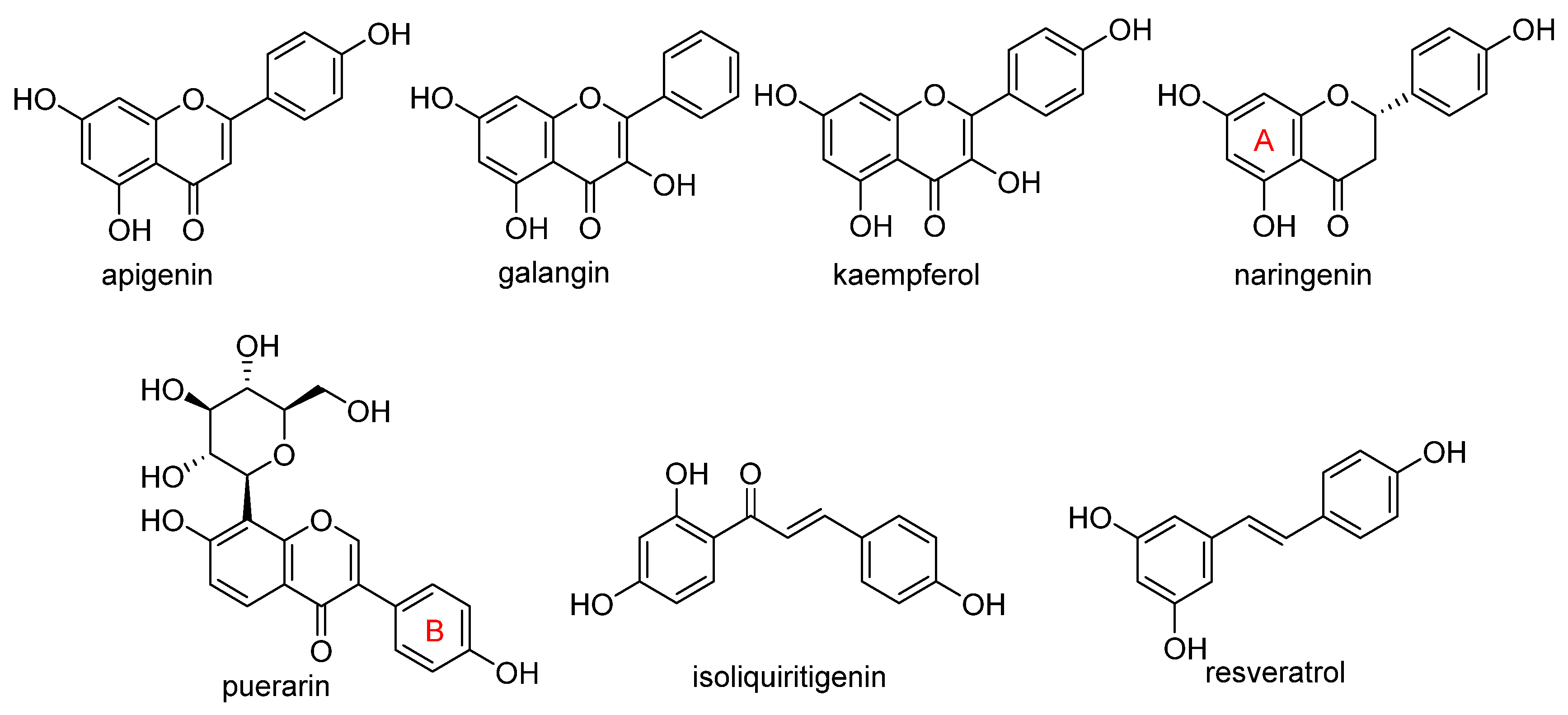
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Shao, C.W.; Yuan, J.W.; Liu, Y.N.; Qin, Y.J.; Wang, X.A.; Gu, J.; Chen, G.Q.; Zhang, B.; Liu, H.K.; Zhao, J.; et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad. Sci. USA 2020, 117, 10155–10164. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 15. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Naqishbandi, A.M.; Efferth, T. Cytotoxicity and apoptosis induction by Fumaria officinalis extracts in leukemia and multiple myeloma cell lines. J. Ethnopharmacol. 2021, 266, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Rong, G.; Liu, Y.; Huang, W.; He, D.; Lu, R. Delivery of apigenin-loaded magnetic Fe2O3/Fe3O4@mSiO2 nanocomposites to A549 cells and their antitumor mechanism. Mater. Sci. Eng. C 2021, 120, 111719. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, Z.H.; Zhu, S.; Cheng, M.J.; Ju, Y.T.; Ren, L.; Yang, G.L.; Min, D.Y. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 2021, 264, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhai, Y.Y.; Chen, J.J.; Xu, X.F.; Wang, H.M. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Xu, S.J.; Wu, B.X.; Zhong, B.Y.; Lin, L.Q.; Ding, Y.N.; Jin, X.; Huang, Z.W.; Lin, M.Y.; Wu, H.L.; Xu, D.P. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef]
- Xu, B.Y.; Wang, H.D.; Chen, Z. Puerarin Inhibits Ferroptosis and Inflammation of Lung Injury Caused by Sepsis in LPS Induced Lung Epithelial Cells. Front. Pediatr. 2021, 9, 7. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Yang, D.Y.; Wang, T.C.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 13. [Google Scholar] [CrossRef]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, G.; Tang, Y.H.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; Abd El-Hack, M.E.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Li, X.C.; Zeng, J.Y.; Liu, Y.P.; Liang, M.S.; Liu, Q.R.; Li, Z.; Zhao, X.J.; Chen, D.F. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.J.; Mei, G.B.; Zhao, Y.; Chen, L.; Liu, J.J.; Tang, Y.H.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic beta Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Gunesch, S.; Hoffmann, M.; Kiermeier, C.; Fischer, W.; Pinto, A.F.M.; Maurice, T.; Maher, P.; Decker, M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox Biol. 2020, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Currais, A.; Liang, Z.B.; Pinto, A.; Maher, P. Old age-associated phenotypic screening for Alzheimer’s disease drug candidates identifies sterubin as a potent neuroprotective compound from Yerba santa. Redox Biol. 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.J.; Zheng, X.R.; Liu, Q.L.; Du, Q.; Lai, Y.J.; Liu, S.Q. Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol. Med. 2022, 28, 20. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.C.; Song, X.X.; Sun, X.F.; Huang, J.; Zhong, M.Z.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D.L. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.Q.; Jia, J.N.; Sun, Q.Y.; Zhou, H.H.; Jin, W.L.; Mao, X.Y. Baicalein Exerts Neuroprotective Effects in FeCl3-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front. Pharmacol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.Y.; Li, H.J.; Zhang, X.X.; Liu, X.L.; Song, Y.Q. Baicalein inhibits RL S3-induced ferroptosis in melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, X.W.; Pang, L.J.; Che, Y.J.; Qi, X. Baicalin suppresses autophagy-dependent ferroptosis in early brain injury after subarachnoid hemorrhage. Bioengineered 2021, 12, 7794–7804. [Google Scholar] [CrossRef]
- Kong, N.; Chen, X.Y.; Feng, J.; Duan, T.; Liu, S.P.; Sun, X.N.; Chen, P.; Pan, T.; Yan, L.L.; Jin, T.; et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B 2021, 11, 4045–4054. [Google Scholar] [CrossRef]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants 2020, 9, 1113. [Google Scholar] [CrossRef]
- Becquer-Viart, M.A.; Gonzalez-Yaque, J.; Fonseca-Fonseca, L.A.; Nunez-Figueredo, Y.; Andreu, G.L.P. Antioxidant and neuroprotective effects of gossypitrin, a flavonoid from Talipariti elatum, against chemical hypoxia-induced PC12 cell death. J. Pharm. Pharmacogn. Res. 2018, 6, 72–80. [Google Scholar]
- Becquer-Viart, M.A.; Armentero-Lopez, A.; Alvarez-Alminaque, D.; Fernandez-Acosta, R.; Matos-Peralta, Y.; D’Vries, R.F.; Marin-Prida, J.; Pardo-Andreu, G.L. Gossypitrin, A Naturally Occurring Flavonoid, Attenuates Iron-Induced Neuronal and Mitochondrial Damage. Molecules 2021, 26, 3364. [Google Scholar] [CrossRef] [PubMed]
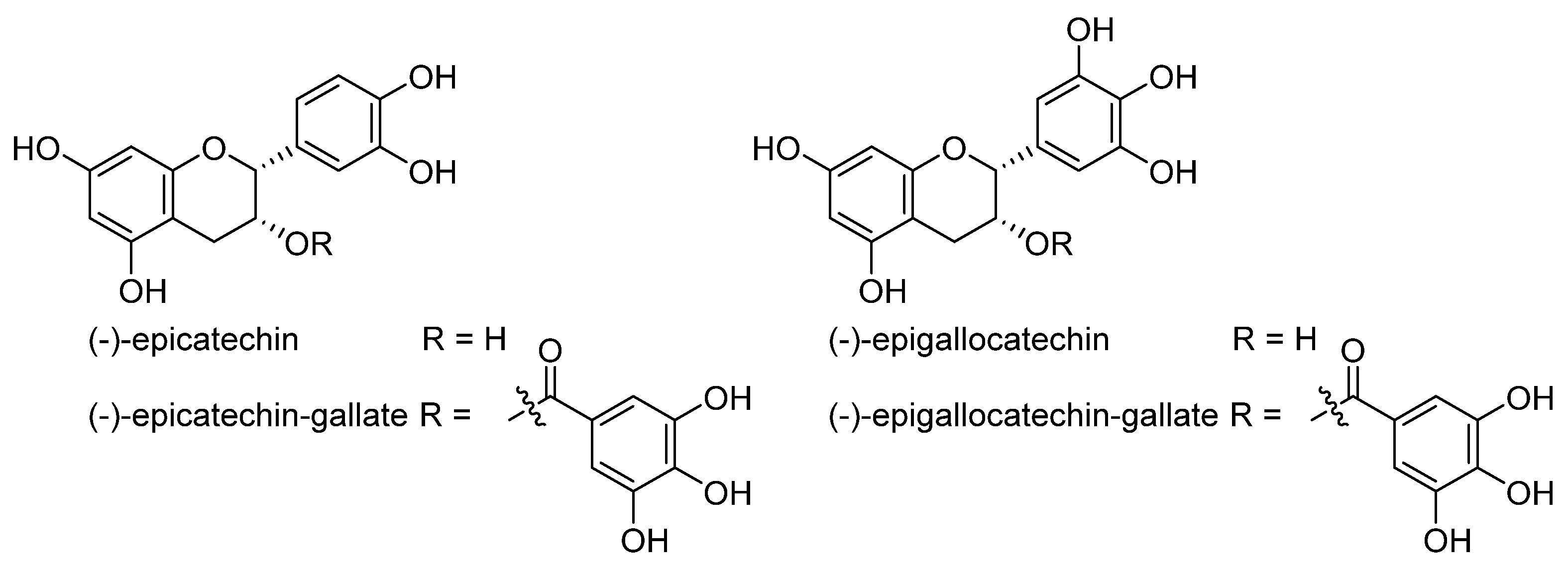
- Matic, S.; Takac, M.J.M.; Barbaric, M.; Lucic, B.; Troselj, K.G.; Stepanic, V. The Influence of In Vivo Metabolic Modifications on ADMET Properties of Green Tea Catechins-In Silico Analysis. J. Pharm. Sci. 2018, 107, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Ahmad, R.S.; Sultan, M.T.; Qayyum, M.M.N.; Naz, A. Green Tea and Anticancer Perspectives: Updates from Last Decade. Crit. Rev. Food Sci. Nutr. 2015, 55, 792–805. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yokozawa, T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002, 40, 1745–1750. [Google Scholar] [CrossRef]
- Chang, C.F.; Cho, S.; Wang, J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann. Clin. Transl. Neurol. 2014, 1, 258–271. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, Y.; Chen, L.; Duan, Y.Z.; Kuang, X.J.; Peng, Z.; Li, C.H.; Li, Y.H.; Xiao, Y.; Jin, H.; et al. EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats. Transl. Neurosci. 2020, 11, 173–181. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (-)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef]
- Mu, M.; Wang, Y.L.; Zhao, S.S.; Li, X.L.; Fan, R.R.; Mei, L.; Wu, M.; Zou, B.W.; Zhao, N.; Han, B.; et al. Engineering a pH/Glutathione-Responsive Tea Polyphenol Nanodevice as an Apoptosis/Ferroptosis-Inducing Agent. ACS Appl. Bio Mater. 2020, 3, 4128–4138. [Google Scholar] [CrossRef]
- Banoth, R.K.; Thatikonda, A. A Review on Natural Chalcones an Update. Int. J. Pharm. Sci. Res. 2020, 11, 546–555. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L. Potential pharmacological uses of chalcones: A patent review (from June 2011-2014). Expert Opin. Ther. Patents 2015, 25, 351–366. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Dong, X.Y.; Wang, Y.; Deng, Y.L.; Li, B.; Dai, R.J. On the role of synthesized hydroxylated chalcones as dual functional amyloid-beta aggregation and ferroptosis inhibitors for potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 166, 11–21. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.C.; Cai, R.X.; Ren, Z.W.; Zhang, A.Z.; Deng, F.D.; Chen, D.F. Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules 2020, 25, 674. [Google Scholar] [CrossRef]
- Wen, L.R.; Shi, D.D.; Zhou, T.; Tu, J.M.; He, M.; Jiang, Y.M.; Yang, B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020, 315, 11. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, H.J.; Xiao, Q.; Li, L.; Zhong, X.; Zhang, J.; Wang, F.; Li, G.S.; Wang, L.; Li, Y. Isoliquiritigenin attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. Ren. Fail. 2021, 43, 1551–1560. [Google Scholar] [CrossRef]
- Yaseen, A.; Yang, F.; Zhang, X.; Li, F.; Chen, B.; Faraag, A.H.I.; Wang, M.K.; Shen, X.F.; Wang, L. Ferroptosis inhibitory constituents from the fruits of Cullen corylifolium. Nat. Prod. Res. 2021, 35, 5364–5368. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H.G. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.J.; Chen, S.N.; Cai, J.; Ban, Y.Y.; Peng, Q.; Zhou, Y.; Zeng, Z.Y.; Peng, S.P.; Li, X.L.; et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 18. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, J.C.; Zhang, W.N.; Yu, J.Q.; Zhuang, C.L. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free. Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Jiang, L.P.; Chen, H.B.; Wei, S.; Yao, K.; Sun, X.C.; Yang, G.; Jiang, L.J.; Zhang, C.; Wang, N.N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L.L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 2022, 808, 9. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.S.; Duan, L.N.; Yang, Y.N.; Liu, W.; Zhang, Y.; Zhou, L.G.; Su, S.Y.; Lo, P.C.; Cai, J.Y.; Gao, L.Q.; et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021, 13, 3827–3840. [Google Scholar] [CrossRef]
- Lee, J.; You, J.H.; Kim, M.S.; Roh, J.L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020, 37, 12. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 15. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Garcia-Caballero, C.; Palomino-Antolin, A.; Rubio-Navarro, A.; Vazquez-Carballo, C.; Herencia, C.; Martin-Sanchez, D.; Farre-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. Faseb J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.M.; Li, R.Y.; Zhou, L.G.; Zhang, Y.; Duan, L.N.; Su, S.Y.; Li, M.; Wang, Q.; Chen, T.K.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.L.; Chen, X.; Yan, Y.X.; Wan, N.S.; Chen, Q.Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef]
- Li, R.H.; Zhang, J.; Zhou, Y.F.; Gao, Q.; Wang, R.; Fu, Y.R.; Zheng, L.W.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhu, W.J.; Peng, Y.J.; Cheng, S.D. Curcumin reverses the sunitinib resistance in clear cell renal cell carcinoma (ccRCC) through the induction of ferroptosis via the ADAMTS18 gene. Transl. Cancer Res. 2021, 10, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrian-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 1 January 2021).
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. Data Warrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. J. Chem Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]


| Inducer Name | 2D Structure | Mode of Action | logP | BBB |
|---|---|---|---|---|
| (1S,3R)-RSL3 | 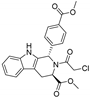 | GPX4 ↓ | 3.6 | low |
| 2-phenethylisothiocyanate | 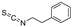 | GPX4 ↓ | 3.4 | high |
| gallic acid | 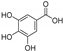 | GPX4 ↓ | 0.7 | low |
| FIN56 | 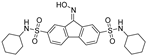 | GPX4 ↓ | 4.7 | low |
| albiziabioside A | 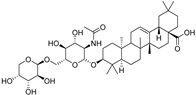 | GPX4 ↓ | 4.5 | low |
| dihydroisotanshinone I | 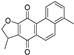 | GPX4 ↓ | 3.7 | high |
| withaferin A |  | GPX4 ↓ | 3.2 | high |
| ML162 | 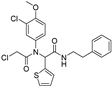 | GPX4 ↓ | 4.4 | low |
| altretamine | 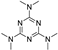 | GPX4 ↓ | 2.5 | high |
| typhaneoside | 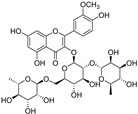 | GPX4 ↓ | −0.8 | low |
| artemisinin |  | iron ↑; GPX4 ↓ | 2.4 | high |
| dihydroartemisinin | 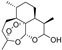 | iron ↑; GPX4 ↓ | 2.2 | high |
| artesunate |  | iron ↑ | 2.3 | low |
| ruscogenin | 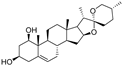 | iron ↑ | 4.5 | high |
| salinomycin |  | iron ↑ | 5.4 | low |
| amentoflavone |  | iron ↑ | 4.5 | low |
| BSO |  | ROS ↑ | −2.1 | low |
| FINO2 |  | ROS ↑ | 4.0 | high |
| epunctanone |  | ROS ↑ | 7.7 | low |
| piperlongumine |  | ROS ↑ | 1.9 | high |
| robustaflavone A |  | ROS ↑ | 4.6 | low |
| sorafenib | 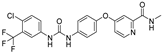 | Xc‾ system ↓ | 5.1 | low |
| erastin | 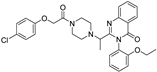 | Xc‾ system ↓ | 3.8 | low |
| sulfasalazine | 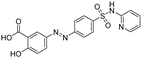 | Xc‾ system ↓ | 3.1 | low |
| ferroptocide |  | Other | 2.1 | low |
| maneb | 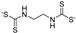 | other | 0.2 | low |
| gambogic acid |  | other | 7.4 | low |
| paraquat | 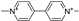 | other | −6.3 | low |
| soyauxinium |  | other | 0.4 | high |
| erianin |  | other | 3.4 | high |
| Inhibitor Name | Structure | Mode of Action | logP | BBB |
|---|---|---|---|---|
| butylatedhydroxytoluene | 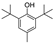 | antioxidative | 5.5 | high |
| ferrostatin-1 | 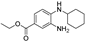 | antioxidative | 3.7 | high |
| α-tocopherol | 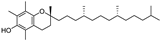 | antioxidative | 11.5 | high |
| β-carotene |  | antioxidative | 11.6 | high |
| glutathione |  | antioxidative | −3.4 | low |
| N-acetylcysteine | 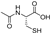 | antioxidative | −0.6 | low |
| ascorbic acid | 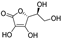 | antioxidative | −1.6 | low |
| edaravone | 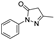 | antioxidative | 1.3 | high |
| GSK2334470 |  | antioxidative | 4.3 | low |
| liproxstatin-1 | 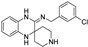 | antioxidative | 3.3 | high |
| trolox | 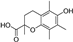 | antioxidative | 2.9 | low |
| α-lipoic acid |  | antioxidative; NRF2 ↑ | 2.7 | low |
| 7-O-cinnamoyltaxifolin | 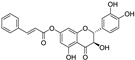 | antioxidative | 3.7 | low |
| 7-O-feruloyltaxifolin | 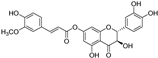 | antioxidative | 3.1 | low |
| butein | 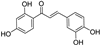 | antioxidative | 2.8 | low |
| butin | 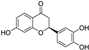 | antioxidative | 1.9 | low |
| isorhapontigenin | 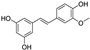 | antioxidative | 3.0 | high |
| morachalcone D | 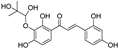 | antioxidative | 1.7 | low |
| piceatannol-3′-O-glucoside |  | antioxidative | 0.4 | low |
| rhapontigenin | 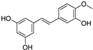 | antioxidative | 3.1 | low |
| rhapontin | 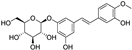 | antioxidative | 0.6 | low |
| synthetic chalcone 1 2 | 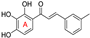 | antioxidative | 3.8 | low |
| Baicalein 2 | 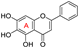 | antioxidative; 15-LOX ↓ | 3.0 | low |
| dopamine | 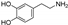 | GPX4 ↑ | −0.3 | low |
| galangin | 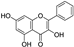 | GPX4 ↑ | 2.7 | low |
| apigenin | 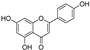 | GPX4 ↑ | 2.9 | low |
| silibinin | 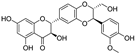 | iron chelation | 1.8 | low |
| deferoxamine | 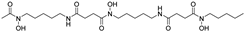 | iron chelation | −1.3 | low |
| phytic acid |  | iron chelation | −11.2 | high |
| 8-hydroxyquinoline |  | iron chelation | 2.1 | high |
| ciclopirox olamine |  | iron chelation | 2.5 | high |
| deferasirox |  | iron chelation | 3.8 | low |
| deferiprone | 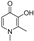 | iron chelation | −0.6 | high |
| maltol | 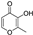 | iron chelation | 0.0 | high |
| quercetin | 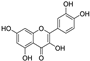 | iron chelation | 2.0 | low |
| curcumin |  | iron chelation | 3.0 | high |
| EGCG |  | iron chelation | 2.2 | low |
| ellagic acid | 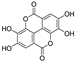 | iron chelation | 1.9 | low |
| gossypitrin |  | iron chelation | −0.7 | low |
| sterubin | 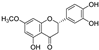 | iron chelation | 2.0 | low |
| fisetin | 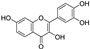 | iron chelation; NRF2 ↑ | 2.2 | low |
| carnosic acid | 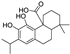 | NRF2 ↑ | 4.5 | low |
| melatonin | 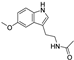 | NRF2 ↑ | 1.7 | high |
| zileuton | 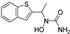 | 5-LOX ↓ | 1.9 | high |
| bakuchiol | 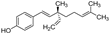 | other | 5.7 | high |
| psoralidin |  | other | 4.1 | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanić, V.; Kučerová-Chlupáčová, M. Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products. Molecules 2023, 28, 475. https://doi.org/10.3390/molecules28020475
Stepanić V, Kučerová-Chlupáčová M. Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products. Molecules. 2023; 28(2):475. https://doi.org/10.3390/molecules28020475
Chicago/Turabian StyleStepanić, Višnja, and Marta Kučerová-Chlupáčová. 2023. "Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products" Molecules 28, no. 2: 475. https://doi.org/10.3390/molecules28020475
APA StyleStepanić, V., & Kučerová-Chlupáčová, M. (2023). Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products. Molecules, 28(2), 475. https://doi.org/10.3390/molecules28020475