Abstract
The synthesis, structural, phonon, optical, and magnetic properties of two hybrid organic-inorganic chlorides with monoprotonated methylhydrazinium cations (CH3NH2NH2+, MHy+), [CH3NH2NH2]CdCl3 (MHyCdCl3), and [CH3NH2NH2]CuCl3 (MHyCuCl3), are reported. In contrast to previously reported MHyMIICl3 (MII = Mn2+, Ni2+, and Co2+) analogues, neither compound undergoes phase transitions. The MHyCuCl3 has a crystal structure familiar to previous crystals composed of edge-shared 1D chains of the [CuCl5N] octahedra. MHyCuCl3 crystallizes in monoclinic P21/c symmetry with MHy+ cations directly linked to the Cu2+ ions. The MHyCdCl3 analogue crystallizes in lower triclinic symmetry with zig-zag chains of the edge-shared [CdCl6] octahedra. The absence of phase transitions is investigated and discussed. It is connected with slightly stronger hydrogen bonding between cations and the copper–chloride chains in MHyCuCl3 due to the strong Jahn–Teller effect causing the octahedra to elongate, resulting in a better fit of cations in the accessible space between chains. The absence of structural transformation in MHyCdCl3 is due to intermolecular hydrogen bonding between two neighboring MHy+ cations, which has never been reported for MHy+-based hybrid halides. Optical investigations revealed that the bandgaps in Cu2+ and Cd2+ analogues are 2.62 and 5.57 eV, respectively. Magnetic tests indicated that MHyCuCl3 has smeared antiferromagnetic ordering at 4.8 K.
1. Introduction
Hybrid organic–inorganic materials (mostly halides) have received a great deal of attention in recent years due to their enormous application potential in the field of high tech industry, particularly as materials for electronic and optoelectronic devices [1,2]. Many of them exhibit controllable optical [3], electric [4], ferroelectric [5,6,7], switchable dielectric [6], and magnetic [8] properties.
Hydrazine is an interesting small inorganic molecule capable of forming an onium or double onium cation that can be incorporated into crystal structures [9,10]. Compounds containing onium hydrazine cations (Hy+) are well known and have been the focus of many investigations [9,10]. The degree of methylation of a hydrazine molecule affects its chemical properties and ability to bond in the crystal lattice. Simple organic methylhydrazinium cation (MHy+) has just been recognized as an object of interest due to its small enough size to form a three-dimensional (3D) organic–inorganic perovskites [11,12,13,14,15]. Only four organic cations have so far matched the size and shape parameters required to form a 3D perovskite architecture with divalent metal ions. Next to MHy+, these include the methylammonium (MA+) [16,17,18,19,20], formamidinium (FA+) [16,18,20,21,22], and aziridinium (AZ+) [23] cations.
There is also a class of halogenide perovskites of larger monovalent metals (MI = Na, K, Rb, Cs) that may form a 3D network with the general formula AMIX3·0.5H2O, where A stands for bivalent organic cation. In such networks, the bigger dodecahedral gap accommodates larger organic cations, such as dabconium, methyldabconium, 3-aminopyrrolidinium, piperazinium, or methylpiperazinium cations [24,25,26,27].
Recent research has shown that MHy+-containing hybrid coordination polymers may also form low-dimensional counterparts, such as layered (2D) [28] or chain (1D) [29,30] architectures. The most intriguing aspect is that the MHy+ ligand may interact differently with inorganic metal–ligand subnetworks in the accessible space: (i) organic cation can simply fill the available space and connect with an inorganic network of metal–ligand octahedra [MIIX6] through N–H···X (X = oxygen, halide) hydrogen bonds (HBs), as reported in [MHy]MII(HCOO)3 (MII = Mn2+, Mg2+, Fe2+, Zn2+) [13], [MHy]Mn(H2PO2)3 [31], or in MHyPbI3 [29]; (ii) organic cation can be strongly bound with the subnetwork of inorganic octahedra, resulting in additional short N···MII contacts, as reported in MHyPbX3 (X = Cl−, Br−) [14,15]; (iii) one of the N atoms can directly be in the first coordination sphere of the metal, forming non-uniform octahedra of the [MIIX5N] type, which has so far only been found for the MHyMIICl3 (MII = Mn2+, Co2+, Ni2+) crystals [30]. The third type of rare coordination has also been found for hybrid coordination polymers with Hy+, (Hy)3MnX5 (X = Cl−, Br−) [32], and 1,1,1-trimethylhydrazinium (Me3Hy+) [33] cations.
In this study, we synthesized new phases of the hybrid chlorides MHyMIICl3 that include Cu2+ and Cd2+ ions. We undertook a comprehensive physicochemical examination to determine why the structural features, including coordination type and interactions of MHy+ cations with the metal–chloride framework, of those two analogues vary from other known counterparts. The goal of this work is also to understand why Cu2+ and Cd2+ analogues do not exhibit phase transitions (PTs) when compared to other members of this family of chlorides.
2. Results and Discussion
2.1. Structural Properties
MHyCuCl3 adopted monoclinic P21/c symmetry. It is yet another example of hybrid MHyMIICl3 compounds with MII = Co2+, Ni2+, Mn2+ reported to date [30], in which the terminal N atom of MHy+ is a co-creator of MII first coordination sphere. In other words, [CuCl5N] octahedra were formed. The octahedra were arranged by edge-sharing, parallel chains propagating along the [010] direction (Figure 1a). The P21/c phase was isostructural to the low temperature (measured at 100–120 K) phases of Co2+, Ni2+, and Mn2+ analogues [30]. All atoms occupied general positions of C1 site symmetry. The Cu–Cl distances were 2.2691(10)–2.815(1) Å, while the Cu–N bond length was equal to 2.061(3) Å. The Cu–Cl distances had a much wider range (~0.55 Å) than their Co2+ (0.06 Å), Ni2+ (0.02 Å), and Mn2+ (0.07 Å) counterparts [30]. Indeed, an axial elongation of the octahedra was observed (Figure S1), pointing out the presence of the Jahn–Teller effect, characteristic of Cu2+ compounds with octahedral geometries [34]. The MHy+ cations were positionally ordered and anchored in the structure by several N–H···Cl HBs (green dashed lines in Figure 1a,b). Both terminal and middle NH2 groups interacted with chlorine ion acceptors from neighboring chains, stabilizing the crystal structure in [100] and [001] directions (with donor-acceptor (D···A) distances of 3.426(3) Å and 3.269(3) Å, respectively). The HBs within the chains were also present with D···A distances of 3.157(3)–3.631(3) Å. The intermolecular interactions lead to angular and (combined with Jahn–Teller effect) bond length distortion of the octahedra, as indicated by octahedral angle variance and bond length distortion values of σ2 = 20.7 deg2 and Δ = 0.1225, respectively. Both values were calculated using the VESTA program [35].
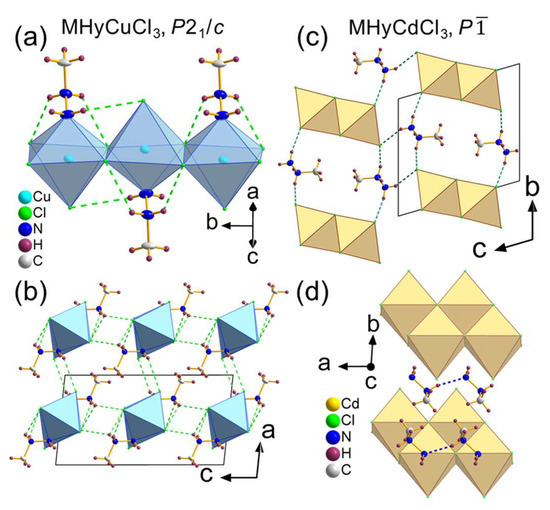
Figure 1.
Crystal structures of (a,b) MHyCuCl3 and (c,d) MHyCdCl3; dashed lines represent hydrogen bonds: N–H···Cl (green) and N–H···N (blue).
The second newly obtained compound reported herein, i.e., MHyCdCl3, crystallized in triclinic, centrosymmetric P symmetry. The motif of MHyCdCl3 consisted of inorganic [CdCl3−]∞ double chains propagating along the [100] direction, separated by the MHy+ cations. All atoms adopted C1 site symmetry. The chains were composed of edge-sharing octahedra with Cd–Cl distances of 2.523(1)–2.712(1) Å. The MHy+ cations were anchored via N–H···Cl (Figure 1c) and N–H···N (Figure 1d) HBs with D···A distances of 3.168(4)–3.384(4) and 2.975(5) Å, respectively. It is worth noting that both the N1 and N2 atoms of MHy+ were engaged in creating a 3D network of HBs. An interplay between inorganic and organic constituents affected the octahedra symmetry, expressed in σ2 and Δ values of 17.8 deg2 and 0.021, respectively. Analogous atomic alignment, i.e., with 1D edge-sharing double chains, was reported for MHyPbI3 [29]. Higher symmetry of MHyPbI3 (monoclinic, P21/c) is associated with the presence of larger metal cations and halide anions, which leads to enlarged interatomic distances and, therefore, weaker HBs and less distorted octahedra (σ2 = 6.32 deg2). Analogous alignment was also reported for [C3H7N2S]CdCl3, where C3H7N2S+ is the 2-amino-4,5-dihydro-3H+-1,3-thiazolium cation [36].
The phase purity of the MHyCdCl3 bulk sample was confirmed by a good match of its PXRD pattern (Rexp = 1.70, Rprof = 6.26, wRprof = 11.22, GOF = 6.6) with the simulated one based on the single crystal structure (Figure 2). The measured PXRD pattern of the MHyCuCl3 bulk sample was also in good agreement (Rexp = 1.52, Rprof = 5.44, wRprof = 9.85, GOF = 6.5) with the calculated one based on the single-crystal data. The Pawley refinement method was used to obtain the fitted profiles. The PXRD analysis results also revealed the negligible presence of another phase (CuCl2·H2O) in an amount of about 2%; the peak from the additional phase is marked with an asterisk in Figure 2.
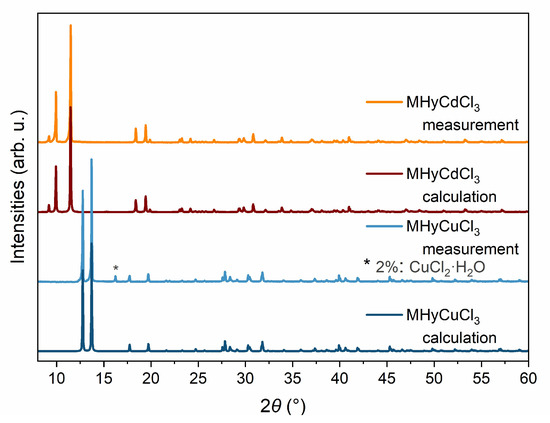
Figure 2.
The comparison of the experimental and calculated using Pawley method PXRD patterns for MHyCdCl3 and MHyCuCl3 samples showing the quality of phase purity; * the additional phase is marked with an asterisk.
2.2. Phonon Properties
Table S1 defines and lists the 24 internal (13A′ + 11A″) and 6 external (3A′ + 3A″) vibrational modes of the free MHy+ ion with Cs symmetry. The presence of two MHy+ ions in the primitive cell doubles the number of modes corresponding to MHy+ with the factor group symmetry Ci in the triclinic MHyCdCl3 crystal with Z = 2. As a result, the internal and external modes are increased to 48 (24Ag + 24Au) and 12 (6Ag + 6Au), respectively. Since the number of modes corresponding to MHy+ cations with the C2h symmetry is increased by 4 times in the monoclinic MHyCuCl3 crystal with Z = 4, the number of modes corresponding to MHy+ cations with the C2h symmetry is 96 (24Ag + 24Au + 24Bg + 24Bu) and 24 (6Ag + 6Au + 6Bg + 6Bu), respectively. Similar considerations apply to metal cations MII and chloride ligands Cl−, which have 6 (3Ag + 3Au) and 18 (9Ag + 9Au) modes in the triclinic MHyCdCl3 crystal, respectively, and 12 (3Ag + 3Au + 3Bg + 3Bu) and 36 (9Ag + 9Au + 9Bg + 9Bu) modes in the monoclinic MHyCuCl3 crystal.
To summarize, the total number of expected vibrational modes for MHyCdCl3 is 84 (42Ag + 42Au), which includes 81 optical (42Ag + 39Au) and 3 acoustic (3Au), and 168 (42Ag + 42Au + 42Bg + 42Bu) for MHyCuCl3, which includes 165 optical (42Ag + 41Au + 42Bg + 40Bu) and 3 acoustic. Because g-type modes are only Raman-active and u-type modes are only IR (infrared)-active, the number of expected bands in the Raman (IR) spectrum of MHyCdCl3 is 42 (39). These values are 84 and 81 for the MHyCuCl3 analogue, respectively. The number of observed bands in the room-temperature (RT) spectra is lower than expected (Figure 3, Table S2). This effect is caused by the overlapping of closely spaced bands caused by the low factor group (Davydov) splitting.
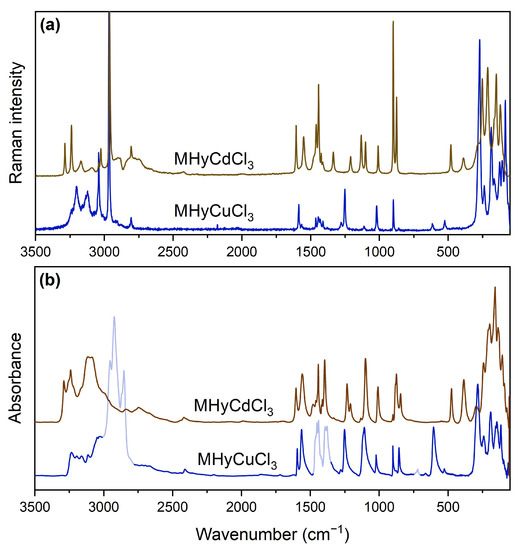
Figure 3.
Raman (a) and IR (b) spectra of MHyMIICl3 (MII = Cd2+, Cu2+); transparent sections of the MHyCuCl3 IR spectrum on panel (b) indicate the absorption ranges of the medium used (nujol).
The proposed assignment, presented in Table S2, is based on a comparison with literature sources, including MHyMIICl3 (MII = Co2+, Ni2+, Mn2+) [30], 3D and 2D lead halides comprising the MHy+ cation [12,29,37], MHyMn(H2PO2)3 [31], and MHyMII(HCOO)3 (MII = Mg2+, Fe2+, Mn2+, and Zn2+) [13], as well as density functional calculations (DFT) calculations performed for the MHy+ ion [31]. The majority of IR and Raman bands corresponding to the internal vibrations of MHy+ are observed in typical ranges as reported in the literature.
The IR and Raman spectra of MHyCuCl3 are qualitatively very similar to those of MHyMIICl3 (MII = Co2+, Ni2+, Mn2+) [30]. The differences involving wavenumber up- or downshifts reaching a few cm−1 for MHyCuCl3 are mainly due to the higher mass, different ionic radius, the strength of HBs, and Jahn–Teller effect activity of Cu2+ ions in an octahedral configuration. The most pronounced variations were observed for the so-called MHy+-cage mode, which can be correlated with the parameter defined as the space available for cation per formula unit VZ [30]. This mode was described as a torsional mode with a strong sensitivity to ligand type due to coupling with the inorganic cage via HBs bonds [37], which is similar to the behavior of the MA+-cage mode, which has been initially reported for the MAPbX3 (X = Br−, I−) hybrids [38]. In our previous paper concerning MHyMIICl3 (MII = Co2+, Ni2+, Mn2+), the MHy+-cage mode clearly correlates to the ionic radius of MII and metal electronegativity, i.e., it was observed as an IR band at 515 cm−1 for MHyMnCl3, 563 cm−1 for MHyCoCl3, and at 595 cm−1 for MHyNiCl3 [30]. Taking into account the IR and Raman spectra of MHyCdCl3 and MHyCuCl3, we assigned the MHy+-cage mode to IR bands at 386 and 606 cm−1, as well as Raman bands at 389 and 614 cm−1, respectively (see Table S2). The correlation of these values with the ionic radius is higher for MHyCuCl3 and much lower for MHyCdCl3. This is not surprising considering that MHy+ is not a direct ligand for the metal cation in the MHyMIICl3 series. As a result, the torsional vibrational energy is predicted to differ.
Another interesting feature that can be analyzed using vibrational spectroscopy is the strength of HBs. The positions of IR and Raman bands corresponding to stretching vibrations of both NH2 and NH2+ groups for the MHyCuCl3 analogue have very similar energies in comparison to the previously studied MHyMIICl3 (MII = Co2+, Ni2+, Mn2+) series. However, for MHyCuCl3, most of the bands observed above 3000 cm−1 have the lowest positions among these four isostructural analogues. It proves our SCXRD analysis, showing that the MHyCuCl3 analogue creates the shortest D···A contacts and thus forms the strongest hydrogen bonds.
As mentioned before, the vibrational spectra of MHyCdCl3 differ most strongly in this region, suggesting that the HB network has different properties. Crystallographic data showed that the MHy+ cations do not enter the first coordination sphere of Cd2+ ions, therefore, they have more freedom in the metal–chloride framework. This leads to the formation of direct, short, and strong N–H···N HBs between MHy+ cations, which has never been reported for an MHy+ cation so far. We think that these stronger contacts are responsible for the formation of the broad bands observed in spectra above 2750 cm−1, which are absent for other representatives of the MHyMIICl3 group.
2.3. Optical Properties
The diffuse reflectance spectra of MHyMIICl3 (MII = Cd2+, Cu2+) are shown in Figure 4. In the low-wavelength range, MHyCdCl3 exhibits an intense absorption band with a maximum at 212 nm and a weaker band at 275 nm, similar to FACd(H2PO2)3 (FA+ = formamidinium) [39] and [TPrA]Cd(dca)3 (TPrA+ = tetrapropylammonium, dca− = dicyanamide) [40]. MHyCuCl3 has a much wider low-wavenumber band, with maxima at 249, 330, 379, and 410 nm previously assigned to the ligand-to-metal charge transfer from Cl− ligand to Cu2+ centers for (C5H14N2)[CuCl4] (C5H14N22+ = diprotonated 1,4-diazacycloheptane), (N(CH3)4)CdX3:Cu2+ (N(CH3)4+ =tetramethylammonium, X = Cl−, Br−), and MA2CuClxBr4-x (MA+ = methylammonium) [41,42,43]. The diffuse reflectance spectrum of MHyCuCl3 also has a very broad absorption band with a maximum of about 850 nm, which is caused by Cu2+ d-d transitions in the D4h coordination centers [41,43,44]. The presence of this band for MHyCuCl3, which is composed of CuCl6 octahedra, indicates a strong Jahn–Teller effect causing octahedra elongation, as confirmed by SCXRD studies (see Figure S1).
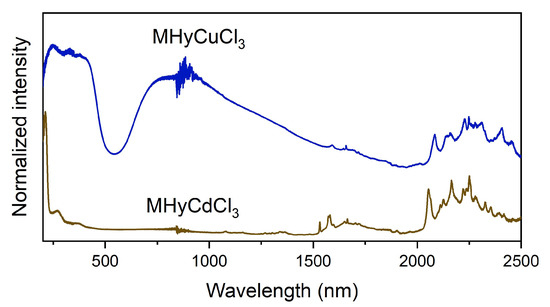
Figure 4.
Diffuse reflectance spectra of MHyMIICl3 (MII = Cd2+, Cu2+).
Both spectra show the presence of several bands above 1500 nm that corresponds to MHy+ overtones and combinations of vibrational modes.
The diffuse reflectance spectra of MHyMIICl3 (MII = Cd2+, Cu2+) were further recalculated to determine the bandgap energy using the Kubelka–Munk function F(R) = (1 − R)2/2R, where R is the reflectance. The results are presented in Figure S2, and the obtained bandgap energies are 2.62 and 5.57 eV for MHyCuCl3 and MHyCdCl3, respectively. The obtained values are within the ranges reported previously for (C5H14N2)[CuCl4] (2.56 eV) [41], FACd(H2PO2)3 (5.42 eV) [39], and [TPrA]Cd(dca)3 (5.02 eV) [40].
2.4. Magnetic Properties
Figure 5 summarizes the results of the magnetic property measurements carried out for MHyCuCl3, which are very similar to those obtained for MHyMnCl3 [30]. As can be inferred from panel (a), the compound with copper exhibited paramagnetic behavior in almost the entire temperature range studied, and its magnetic susceptibility χ(T) could be described by the Curie–Weiss law χ(T) = C/(T − θp) down to a few kelvins with the least-squares fitting parameters C = 0.42(2) emu mol−1 K (Curie constant) and θp = −1.7(4) K (Curie–Weiss temperature); see the thick solid line in Figure 5a. The effective magnetic moment μeff derived from the Curie constant C was about 1.83(1) μB, which was lower than μeff = 3.55 μB expected for a free Cu2+ ion with the 3d9 electron configuration (i.e., for S = 1/2, L = 2, J = 5/2, gJ = 1.2) and only slightly higher than the spin-only magnetic moment of 1.73 μB (i.e., calculated for S = 1/2, L = 0, J = 1/2, gJ = 2), suggesting a non-negligible, yet still very small, orbital contribution to μeff. The estimated value of the magnetic moment was, in turn, very close to the averaged experimental value of μeff reported for paramagnetic salts containing non-interacting Cu2+ ions, i.e., 1.83 μB. Moreover, the fitted value of C was nearly the same as the RT value of the product χT, i.e., 0.41 emu mol−1 K, and the negative sign of θp, which indicated the presence of antiferromagnetic correlations in MHyCuCl3, was in full agreement with the concave shape of the χT(T) curve observed down to the lowest temperature studied (see Figure 5a, right axis).
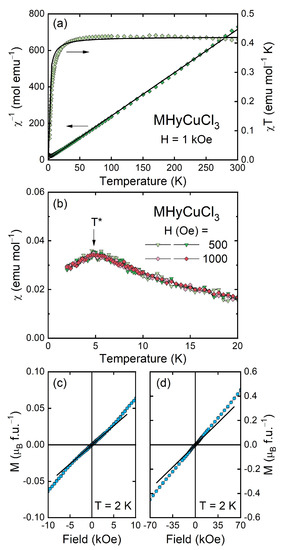
Figure 5.
(a) χ−1(T) and χT(T) (left and right axis, respectively) of MHyCuCl3 plotted with the fitted Curie–Weiss formula (solid curves). (b) χ(T) measured in the ZFC and FC regimes (bright and dark symbols, respectively); the arrow marks a characteristic temperature T*. (c,d) M vs. H plotted in two different field ranges; solid lines show linear M(H) dependence in low fields.
At low temperatures, MHyCuCl3 exhibited a broad anomaly in the temperature variation of the magnetic susceptibility with a maximum at T* = 4.8 K (Figure 5b), being very similar in shape to that found in its counterpart with Mn2+, yet much smaller [30]. In addition, here, χ(T) was independent of the magnetic field H (at least in low fields) and showed no difference between the curves taken in the ZFC and FC regimes, which suggests antiferromagnetic ordering. The antiferromagnetic-like character of the observed anomaly was confirmed by the linear field dependence of the magnetization (Figure 5c,d), exhibiting only a small change in its slope of about 3 kOe. In the highest field applied (70 kOe), magnetization M is far from any saturation and achieves a value corresponding to only 0.45 μB, which is much smaller than μord = 3 μB and 1 μB expected for full and spin-only magnetic moment of Cu2+, respectively.
2.5. Effect of Cd2+ and Cu2+ on the Properties
Cd2+ and Cu2+ coordination chemistry significantly differs from Ni2+, Co2+, and Mn2+, which has a significant influence on the structures of MHyCdCl3 and MHyCuCl3 in comparison to previously described compounds. To begin, unlike the other metals discussed, Cd2+ has a d10 close-shell electronic configuration. Additionally, according to Shannon [45], the ionic radius of Cd2+ is greater (95 pm) than that of Ni2+, Co2+, Mn2+, and Cu2+ (69–83 pm). Because of these features, the metal cation in MHyCdCl3 is coordinated by six chloride anions, and the MHy+ cation is excluded from the first coordination sphere. MHyCdCl3 has a triclinic structure, with the MHy+ cations occupying the voids in the metal–chloride framework. Furthermore, MHy+ cations are stacked in chains that are linked by stronger intermolecular HB.
The distinct behavior of Cd2+ and Cu2+ analogues has previously been observed for other hybrids. Among the AmMII(HCOO)3 (Am+ = NH4+; MII = Cd2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, and Zn2+) formates [46], only AmCd(HCOO)3 and AmCu(HCOO)3 members adopt the orthorhombic symmetry, which is lower compared to other hexagonal family members. Furthermore, as the sole representative, the AmCd(HCOO)3 crystal exhibits no PT and adopts a perovskite-like topology [47] in contrast to other members, which have chiral-like crystal architecture.
A similar outcome effect has been observed for [DMA]MII(HCOO)3 (DMA+ = dimethylammonium; MII = Cd2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, and Zn2+) formates [46], where only [DMA]Cu(HCOO)3 and [DMA]Cd(HCOO)3 do not undergo PTs. However, the structural symmetry of [DMA]Cd(HCOO)3 is hexagonal in this example, as it is for all members except for [DMA]Cu(HCOO)3, which crystallizes in the lower orthorhombic phase. In this case, the lack of PT has been attributed to the large size of the cavity occupied by DMA+ and weak HB contacts between cations and the cadmium-formate framework [48].
To determine the reason for the absence of PTs in the MHyCdCl3 and MHyCuCl3, we compared the estimated structural parameters presented in Table 1. As one can see, the tolerance factor (TF) is insufficient to explain this phenomenon, as the values for MHyCdCl3 and MHyCuCl3 are comparable to those reported for MHyMIICl3 (MII = Mn2+, Co2+, Ni2+) [30]. A detailed examination of σ2 and Δ, which characterize the framework flexibility, reveals that the Jahn–Teller effect prevents the emergence of PT. This elongation also causes the shortest Cu–Cu distances between octahedral chains propagating along the [100] and [001] directions to be 6.988(1) and 6.505(1) Å, respectively. The MII–MII distances or MHyMIICl3 (MII = Mn2+, Co2+, Ni2+) are comparable along the [001] direction (6.489(1)–6.580(1) Å) but much longer along the [100] direction (7.156(1)–7.338(1) Å) [30]. The closest Cu–Cu distances (3.601(1)–3.661(1) Å) along the chain are comparable or slightly higher than the MII–MII distances reported for MII = Mn2+, Co2+, Ni2+, which ranged from 3.455(1) to 3.630(1) Å [30]. Furthermore, among the other members, the Cu–N bond is the shortest (2.061(3) Å) and contributes the most covalent bonding (2.116(3), 2.194(2), and 2.365(2) Å for Ni2+, Co2+, and Mn2+, respectively). The substantially elongated [CuCl5N] octahedra change the available space for MHy+ cations and allow them to fit better in the accessible void, resulting in a slightly stronger network of HBs. This alignment in the metal-halide network might possibly be due to the ordered state of the MHy+ cations, which has been reported for MHyMIICl3 (MII = Mn2+, Co2+, Ni2+) in the low-temperature (LT) phase [30].

Table 1.
Comparison of tolerance factors (TFs), octahedral angle variance σ2, and bond length distortion Δ for MHyMIICl3.
The inability of Cd2+ ions to bind MHy+ also makes these cations better fit into the existing spaces in the network and can get close enough to form a HB between two adjacent cations. The existence of this unique contact, which is stronger than the HBs normally formed between organic cations and ligands, efficiently prevents cation disorder and allows the crystal to adopt lower triclinic symmetry with double edge-connected zig-zag octahedral chains, which is impossible for Ni2+, Mn2+, and Co2+ transition metal cations.
3. Materials and Methods
3.1. Synthesis
Methylhydrazine (98%, Sigma-Aldrich, Saint Louis, MS, USA), hydrochloric acid (35–38%, Avantor Performance Materials, Gliwice, Poland), cadmium(II) (98%, Sigma-Aldrich, Saint Louis, MS, USA), and copper(II) (98%, Sigma-Aldrich, Saint Louis, MS, USA) chlorides were obtained commercially and used without additional purification.
In order to grow MHyMIICl3 (MII = Cu2+, Cd2+) crystals, 1 mmol of MIICl2 was digested in hydrochloric acid. The solution was then dropwise treated with methylhydrazine (1.5 mmol, 0.2 mL). The resulting mixture was left undisturbed at RT in order to slowly evaporate the solvent. After 7–30 days, the colorless and green crystals of MHyCdCl3 and MHyCuCl3, respectively, were harvested from the solution and air-dried.
3.2. Single-Crystal and Powder X-ray Diffraction
Single-crystal X-ray diffraction (SCXRD) experiments were carried out with MoKα radiation using an Xcalibur four-circle diffractometer (Oxford Diffraction, Abingdon, UK), an Atlas CCD detector, and graphite-monochromated MoKα radiation. Absorption was corrected by multi-scan methods using CrysAlis PRO 1.171.39.46 (Rigaku Oxford Diffraction, 2018, Tokyo, Japan). Empirical absorption correction using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm, was applied. Hydrogen atoms were initially placed based on the local geometry and refined using a riding model. The crystal structure was solved in Olex2 1.5 [49] using SHELXT-2014/4 [50] and refined with SHELXL-2018/3 [51]. The unit cell of MHyCuCl3 (monoclinic, P21/c with a = 6.9879(5) Å, b = 7.2032(5) Å, c = 13.0105(9) Å, β = 96.022(7)°, V = 651.3(1) Å3, Z = 4) was chosen with respect to the analysis of diffraction pattern and systematic extinction rules (Figure S3). MHyCdCl3 (triclinic, P with a = 3.8660(1) Å, b = 9.3519(9) Å, c = 10.0790(3) Å, α = 106.146(6)°, β = 90.080(3)°, γ = 93.734(5)°, V = 349.21(4) Å3, Z = 2) was treated as a two-domain non-merohedral twin with twin fraction (BASF) equal to 0.5358(11). Experimental details and selected geometric parameters are presented in Tables S3–S5. The main components of the crystal structures of both compounds are presented in Figure S4. The CIF files of reported structures can be found in the CCDC Database with deposition numbers 2047529 for MHyCdCl3 and 2047531 for MHyCuCl3.
Powder X-ray diffraction (PXRD) experiments were performed in the reflection mode on a PANalytical X’Pert diffractometer (Almelo, The Netherlands) equipped with a a PIXcel solid-state linear detector using Ni filtered CuKα radiation (λ = 1.54184 Å). The X-ray diffraction patterns were generated at 30 mA and 40 kV. For the processing of the PXRD data, the program X’Pert High Score Plus (PANalytical, Almelo, The Netherlands) was involved [52].
3.3. Spectroscopic Measurements
IR spectra in the range of 4000–400 cm−1 (mid-IR) were measured using a Nicolet iS50 infrared spectrometer (Waltham, MA, USA) using a KBr pellet for MHyCdCl3 and a nujol suspension for MHyCuCl3 due to the reactivity of the compound with KBr. The far-IR spectra in the 400–50 cm−1 were measured as a nujol suspension on the polyethylene plate for both compounds. The spectral resolution was set to 2 cm−1.
Raman spectra in the 3500–50 cm−1 range with 2 cm−1 resolution were measured using a Bruker FT MultiRAM spectrometer (Billerica, MA, USA) equipped with the YAG:Nd laser operating at 1064 nm.
The absorption spectra in the back-scattering mode in the UV-VIS range were measured using an Agilent Cary 5000 spectrophotometer (Santa Clara, CA, USA) equipped with a PryingMantis™ diffuse reflectance accessory.
3.4. Magnetic Measurements
Magnetization of randomly oriented single crystals of MHyCuCl3 was measured using a commercial Quantum Design MPMS XL magnetometer (San Diego, CA, USA) from RT down to 2 K and in applied magnetic fields up to 70 kOe. The diamagnetic background coming from a sample holder was found to be weak and negligible in comparison to the signal coming from the samples; hence its subtraction was omitted. Moreover, no diamagnetization corrections were made to the data reported here.
4. Conclusions
The MHyMIICl3 (MII = Cd2+, Cu2+) hybrid organic–inorganic compounds were synthesized using a conventional technique of crystallization from solution by gradual evaporation. The MHyCuCl3 analogue crystallizes in the monoclinic P21/c symmetry, according to the single-crystal X-ray diffraction measurement. The structure is similar to previously reported MII = Mn2+, Ni2+, and Co2+ structures in that it is made up of edge-sharing 1D chains of the [CuCl5N] octahedra running in the [010] direction. MHy+ cations in MHyCuCl3 are effectively coordinated by metal ions, as in the three analogues mentioned. MHyCuCl3, as a single representative, on the other hand, experiences no PT, and the MHy+ cations are ordered at RT. The absence of PTs was attributed to the significant Jahn–Teller effect, which was supported by single-crystal X-ray diffraction and diffuse reflectance measurements. The elongation of the [CuCl5N] octahedra results in a better fit of organic cations in the accessible space as well as a considerably stronger network of HBs that prevent the disorder. Magnetic measurements revealed that MHyCuCl3 exhibits only smeared antiferromagnetic ordering at roughly 4.8 K and no ferromagnetic correlations up to the highest field investigated. Optical studies confirmed a strong Jahn–Teller distortion and revealed a bandgap of 2.62 eV for this material.
The cadmium analogue differs significantly from the other representatives in the MHyMIICl3 class of hybrids. The MHyCdCl3 compound crystallizes in triclinic symmetry, and its crystals are comprised of double zig-zag chains that propagate along the [100] direction. The MHy+ cations are ordered at RT, and the MHyCdCl3 crystal, like MHyCuCl3, does not experience PTs. In this case, the absence of structural transition was attributed to the presence of a unique HB contact between two neighboring MHy+ cations, which inhibits cation disorder. This type of contact is possible because, uniquely in this case, the organic cations are not included in the first coordination sphere of the Cd2+ ion. According to optical investigations, MHyCdCl3 has a bandgap energy of 5.57 eV.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020473/s1, Figure S1: Single [CuCl5N] octahedron labeled with atoms and bond lengths in Å; elongated Pb-Cl distances (marked in red and bold) indicate the Jahn–Teller effect; Figure S2: The energy band gap estimation for MHyMIICl3 (Cd, Cu) crystals using the Kubelka–Munk method; Figure S3: Reciprocal space reconstruction of (a) the h0l and (b) the h1l layers in MHyCuCl3; Figure S4: The main components of the crystal structures of (a) MHyCuCl3, and (b) MHyCdCl3 with vibrational ellipsoids at the 50% probability level; Table S1: Factor group analysis for MHyCdCl3 and MHyCuCl3 crystals; Table S2: Assignment of IR and Raman bands for MHyCdCl3 and MHyCuCl3; Table S3: Experimental and refinement details of MHyCdCl3 and MHyCuCl3; Table S4: Selected geometric parameters of MHyCdCl3 and MHyCuCl3 (Å, °); Table S5: Selected hydrogen bond parameters of MHyCdCl3 and MHyCuCl3.
Author Contributions
Conceptualization, J.A.Z. and M.P.; methodology, J.A.Z.; validation, J.A.Z., D.D., D.A.K., A.P. and M.P.; investigation, J.A.Z., D.D., D.A.K. and A.P.; resources, J.A.Z.; data curation, M.P.; writing—original draft preparation, J.A.Z., D.D., D.A.K. and A.P.; writing—review and editing, J.A.Z. and M.P.; visualization, J.A.Z., D.D., D.A.K. and A.P.; supervision, M.P.; project administration, J.A.Z.; funding acquisition, J.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science Centre, Poland (Narodowe Centrum Nauki) under project no. 2020/37/N/ST5/02257.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds MHyCdCl3 and MHyCuCl3 are available from the authors.
References
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Xu, X.; Zhang, L. The Opto-Electronic Functional Devices Based on Three-Dimensional Lead Halide Perovskites. Appl. Sci. 2021, 11, 1453. [Google Scholar] [CrossRef]
- Fu, A.; Yang, P. Lower Threshold for Nanowire Lasers. Nat. Mater. 2015, 14, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid Organic-Inorganic Perovskites: Low-Cost Semiconductors with Intriguing Charge-Transport Properties. Nat. Rev. Mater. 2016, 1, 15007. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Bithell, E.G.; Batsanov, A.S.; Barton, P.T.; Saines, P.J.; Jain, P.; Howard, C.J.; Carpenter, M.A.; Cheetham, A.K. Ferroelasticity in a Metal-Organic Framework Perovskite; towards a New Class of Multiferroics. Acta Mater. 2013, 61, 4928–4938. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, J.B.; Xia, B.; Wang, Q.L.; Tong, Y.Z.; Ma, Y.; Bu, X.H. Ferroelastic Phase Transition and Switchable Dielectric Constant in Heterometallic Niccolite Formate Frameworks. Inorg. Chem. 2018, 57, 537–540. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Shivaramaiah, R.; Navrotsky, A. Thermochemistry of Multiferroic Organic-Inorganic Hybrid Perovskites [(CH3)2NH2][M(HCOO)3] (M = Mn, Co, Ni, and Zn). J. Am. Chem. Soc. 2015, 137, 10351–10356. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, K.; Gao, S.; Kobayashi, H. Formate-Based Magnetic Metal-Organic Frameworks Templated by Protonated Amines. Adv. Mater. 2010, 22, 1526–1533. [Google Scholar] [CrossRef]
- Britvin, S.N.; Spiridonova, D.V.; Siidra, O.I.; Lotnyk, A.; Kienle, L.; Krivovichev, S.V.; Depmeier, W. Synthesis, Structure and Properties of Hydrazinium Germanate Pharmacosiderite, (N2H5)3Ge7O15(OH)·2.5H2O. Microporous Mesoporous Mater. 2010, 131, 282–288. [Google Scholar] [CrossRef]
- Zhao, T.M.; Chen, S.; Shang, R.; Wang, B.W.; Wang, Z.M.; Gao, S. Perovskite-Like Polar Lanthanide Formate Frameworks of [NH2NH3][Ln(HCOO)4] (Ln=Tb-Lu and Y): Synthesis, Structures, Magnetism, and Anisotropic Thermal Expansion. Inorg. Chem. 2016, 55, 10075–10082. [Google Scholar] [CrossRef]
- Mączka, M.; Sobczak, S.; Kryś, M.; Leite, F.F.; Paraguassu, W.; Katrusiak, A. Mechanism of Pressure-Induced Phase Transitions and Structure-Property Relations in Methylhydrazinium Manganese Hypophosphite Perovskites. J. Phys. Chem. C 2021, 125, 10121–10129. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Vasconcelos, D.L.M.; Giriunas, L.; Freire, P.T.C.; Bertmer, M.; Banys, J.; Simenas, M. NMR and Raman Scattering Studies of Temperature-and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Ptak, M.; Paraguassu, W.; Da Silva, T.A.; Sieradzki, A.; Pikul, A. Phase Transitions and Coexistence of Magnetic and Electric Orders in the Methylhydrazinium Metal Formate Frameworks. Chem. Mater. 2017, 29, 2264–2275. [Google Scholar] [CrossRef]
- Mączka, M.; Gagor, A.; Zareba, J.K.; Stefanska, D.; Drozd, M.; Balciunas, S.; Šimenas, M.; Banys, J.; Sieradzki, A. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zaręba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Oku, T. Crystal Structures of Perovskite Halide Compounds Used for Solar Cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Capitaine, A.; Sciacca, B. Monocrystalline Methylammonium Lead Halide Perovskite Materials for Photovoltaics. Adv. Mater. 2021, 33, 2102588. [Google Scholar] [CrossRef]
- Rosales, B.A.; Hanrahan, M.P.; Boote, B.W.; Rossini, A.J.; Smith, E.A.; Vela, J. Lead Halide Perovskites: Challenges and Opportunities in Advanced Synthesis and Spectroscopy. ACS Energy Lett. 2017, 2, 906–914. [Google Scholar] [CrossRef]
- Ünlü, F.; Jung, E.; Haddad, J.; Kulkarni, A.; Öz, S.; Choi, H.; Fischer, T.; Chakraborty, S.; Kirchartz, T.; Mathur, S. Understanding the Interplay of Stability and Efficiency in A-Site Engineered Lead Halide Perovskites. APL Mater. 2020, 8, 070901. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Raval, P.; Kennard, R.M.; Vasileiadou, E.S.; Dahlman, C.J.; Spanopoulos, I.; Chabinyc, M.L.; Kanatzidis, M.; Manjunatha Reddy, G.N. Understanding Instability in Formamidinium Lead Halide Perovskites: Kinetics of Transformative Reactions at Grain and Subgrain Boundaries. ACS Energy Lett. 2022, 7, 1534–1543. [Google Scholar] [CrossRef]
- Petrosova, H.R.; Kucheriv, O.I.; Shova, S.; Gural’skiy, I.A. Aziridinium Cation Templating 3D Lead Halide Hybrid Perovskites. Chem. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ferrandin, S.; Slawin, A.M.Z.; Harrison, W.T.A. Syntheses and Crystal Structures of a New Family of Hybrid Perovskites: C5H14N2·ABr3·0.5H2O (A = K, Rb, Cs). Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 1243–1248. [Google Scholar] [CrossRef]
- Paton, L.A.; Harrison, W.T.A. Structural Diversity in Non-Layered Hybrid Perovskites of the RMCl3 Family. Angew. Chemie Int. Ed. 2010, 49, 7684–7687. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Tang, Y.Y.; Li, P.F.; Shi, P.P.; Liao, W.Q.; Fu, D.W.; Ye, H.Y.; Zhang, Y.; Xiong, R.G. Precise Molecular Design of High-Tc 3D Organic-Inorganic Perovskite Ferroelectric: [MeHdabco]RbI3 (MeHdabco = N-Methyl-1,4-Diazoniabicyclo[2.2.2]Octane). J. Am. Chem. Soc. 2017, 139, 10897–10902. [Google Scholar] [CrossRef]
- Chen, X.-G.; Gao, J.-X.; Hua, X.-N.; Liao, W.-Q. Three-Dimensional Organic-Inorganic Hybrid Sodium Halide Perovskite: C4H12N2·NaI3 and a Hydrogen-Bonded Supramolecular Three-Dimensional Network in 3C4H12N2·NaI4·3I·H2O. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 728–733. [Google Scholar] [CrossRef]
- Mączka, M.; Sobczak, S.; Ratajczyk, P.; Leite, F.F.; Paraguassu, W.; Dybała, F.; Herman, A.P.; Kudrawiec, R.; Katrusiak, A. Pressure-Driven Phase Transition in Two-Dimensional Perovskite MHy2PbBr4. Chem. Mater. 2022, 34, 7867–7877. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Mączka, M. Methylhydrazinium Lead Iodide—One Dimensional Chain Phase with Excitonic Absorption and Large Energy Band Gap. J. Mol. Struct. 2022, 1249, 131660. [Google Scholar] [CrossRef]
- Zienkiewicz, J.A.; Ptak, M.; Drozdowski, D.; Fedoruk, K.; Stefanski, M.; Pikul, A. Hybrid Organic-Inorganic Crystals of [Methylhydrazinium]MIICl3. J. Phys. Chem. C 2022, 3, 15809–15818. [Google Scholar] [CrossRef]
- Ciupa-Litwa, A.; Ptak, M.; Kucharska, E.; Hanuza, J.; Mączka, M. Vibrational Properties and DFT Calculations of Perovskite-Type Methylhydrazinium Manganese Hypophosphite. Molecules 2020, 25, 5215. [Google Scholar] [CrossRef] [PubMed]
- 3Sreenivasa Kumar, N.R.; Nethaji, M.; Patil, K.C. Synthesis, Characterization and Structure of (N2H5)3MnX5 (X = Cl and Br). J. Coord. Chem. 1991, 24, 333–337. [Google Scholar] [CrossRef]
- Goedken, V.L.; Vallarino, L.M.; Quagliano, J.V. Cationic Ligands. Coordination of the 1,1,1-Trimethylhydrazinium Cation to Nickel(II). Inorg. Chem. 1971, 10, 2682–2685. [Google Scholar] [CrossRef]
- Burns, P.; Hawthorne, F. Static and Dynamic Jahn-Teller Effects in Cu2+ Oxysalt Minerals. Can. Mineral. 1996, 34, 1089–1105. [Google Scholar]
- Momma, K.; Izumi, F. IUCr VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Kubiak, M.; Głowiak, T.; Kozłowski, H. Structure of 2-Amino-4,5-Dihydro-3H+-1,3-Thiazolium Trichlorocadmate(II), C3H7N2S+ CdCl3−. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1983, 39, 1637–1639. [Google Scholar] [CrossRef]
- Mączka, M.; Zienkiewicz, J.A.; Ptak, M. Comparative Studies of Phonon Properties of Three-Dimensional Hybrid Organic-Inorganic Perovskites Comprising Methylhydrazinium, Methylammonium, and Formamidinium Cations. J. Phys. Chem. C 2022, 2022, 4056. [Google Scholar] [CrossRef]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X=I, Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef]
- Mączka, M.; Stefańska, D.; Ptak, M.; Gągor, A.; Pikul, A.; Sieradzki, A. Cadmium and Manganese Hypophosphite Perovskites Templated by Formamidinium Cations: Dielectric, Optical and Magnetic Properties. Dalt. Trans. 2021. [Google Scholar] [CrossRef]
- Mączka, M.; Stefańska, D.; Zaręba, J.K.; Nyk, M.; Sieradzki, A. Temperature-Dependent Luminescence and Second-Harmonic Generation of Perovskite-Type Manganese and Cadmium Dicyanamide Frameworks Templated by Tetrapropylammonium Cations. J. Alloys Compd. 2020, 821, 153464. [Google Scholar] [CrossRef]
- Bourwina, M.; Msalmi, R.; Walha, S.; Turnbull, M.M.; Roisnel, T.; Costantino, F.; Mosconi, E.; Naïli, H. A New Lead-Free 1D Hybrid Copper Perovskite and Its Structural, Thermal, Vibrational, Optical and Magnetic Characterization. J. Mater. Chem. C 2021, 9, 5970–5976. [Google Scholar] [CrossRef]
- Valiente, R.; de Lucas, M.C.M.; Rodriguez, F. Polarized Charge Transfer Spectroscopy of Cu2+ in Doped One-Dimensional [N(CH3)4]CdCl3 and [N(CH3)4]CdBr3 Crystals. J. Phys. Condens. Matter 1994, 6, 4527–4540. [Google Scholar] [CrossRef]
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Grätzel, M.; Mhaisalkar, S.; Soci, C.; et al. Lead-Free MA2CuClxBr4-x Hybrid Perovskites. Inorg. Chem. 2016, 55, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.; Lin, Y.; Mao, W.L.; Karunadasa, H.I. Pressure-Induced Conductivity and Yellow-to-Black Piezochromism in a Layered Cu–Cl Hybrid Perovskite. J. Am. Chem. Soc. 2015, 137, 1673–1678. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ptak, M.; Sieradzki, A.; Šimėnas, M.; Maczka, M. Molecular Spectroscopy of Hybrid Organic-Inorganic Perovskites and Related Compounds. Coord. Chem. Rev. 2021, 448, 214180. [Google Scholar] [CrossRef]
- Gómez-Aguirre, L.C.; Pato-Doldán, B.; Stroppa, A.; Yáñez-Vilar, S.; Bayarjargal, L.; Winkler, B.; Castro-García, S.; Mira, J.; Sánchez-Andújar, M.; Señarís-Rodríguez, M.A. Room-Temperature Polar Order in [NH4][Cd(HCOO)3]—A Hybrid Inorganic-Organic Compound with a Unique Perovskite Architecture. Inorg. Chem. 2015, 54, 2109–2116. [Google Scholar] [CrossRef]
- Szymborska-Małek, K.; Trzebiatowska-Gusowska, M.; Maczka, M.; Gagor, A. Temperature-Dependent IR and Raman Studies of Metal-Organic Frameworks [(CH3)2NH2][M(HCOO)3], M = Mg and Cd. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2016, 159, 35–41. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).