The History of Animal and Plant Sulfite Oxidase—A Personal View
Abstract
1. Introduction
2. The History of SOX
2.1. How Everything Began
2.2. Early Biochemistry of SOX: Cows, Dogs, Chicken, and Rats (the 1950s, 1960s, and 1970s)
- pH optimum 8.5;
- Km 2 × 10−5 M (sulfite);
- Km 3–9 × 10−7 M (cytochrome c);
- cyt c, oxygen, ferricyanide were reduced by the enzyme;
- p-chloromercuribenzoate inhibited the enzyme;
- absorption spectrum typical for cyt b5;
- formation of peroxide during catalysis;
- most activity found in the microsomal fraction;
- high amounts in liver, kidney, heart (rat);
- relatively low amounts in brain, lung and spleen (rat);
- no flavin found.
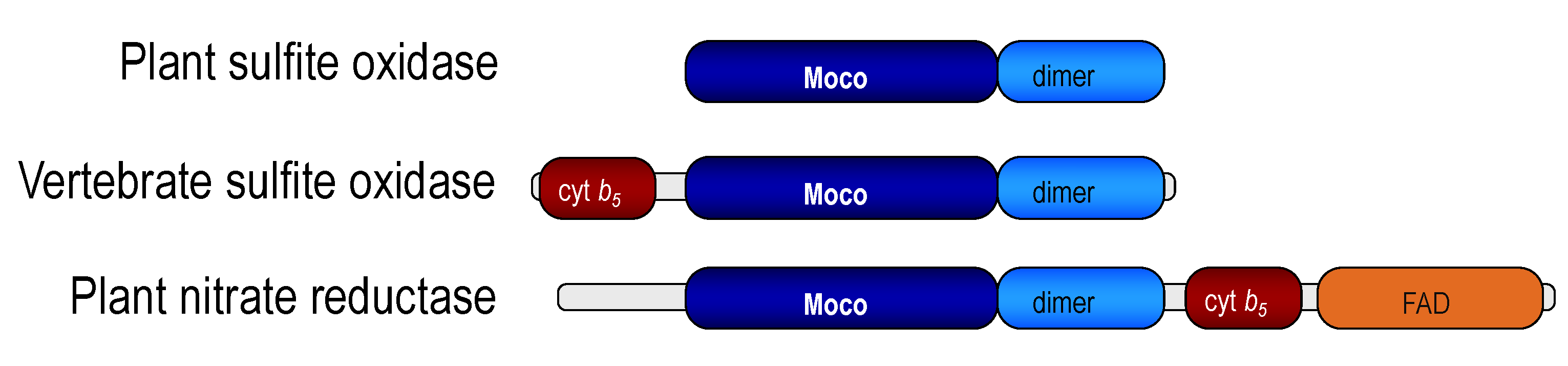
2.3. Amino Acid Sequences, Gene Cloning, and First Structure (the 1980s and 1990s)
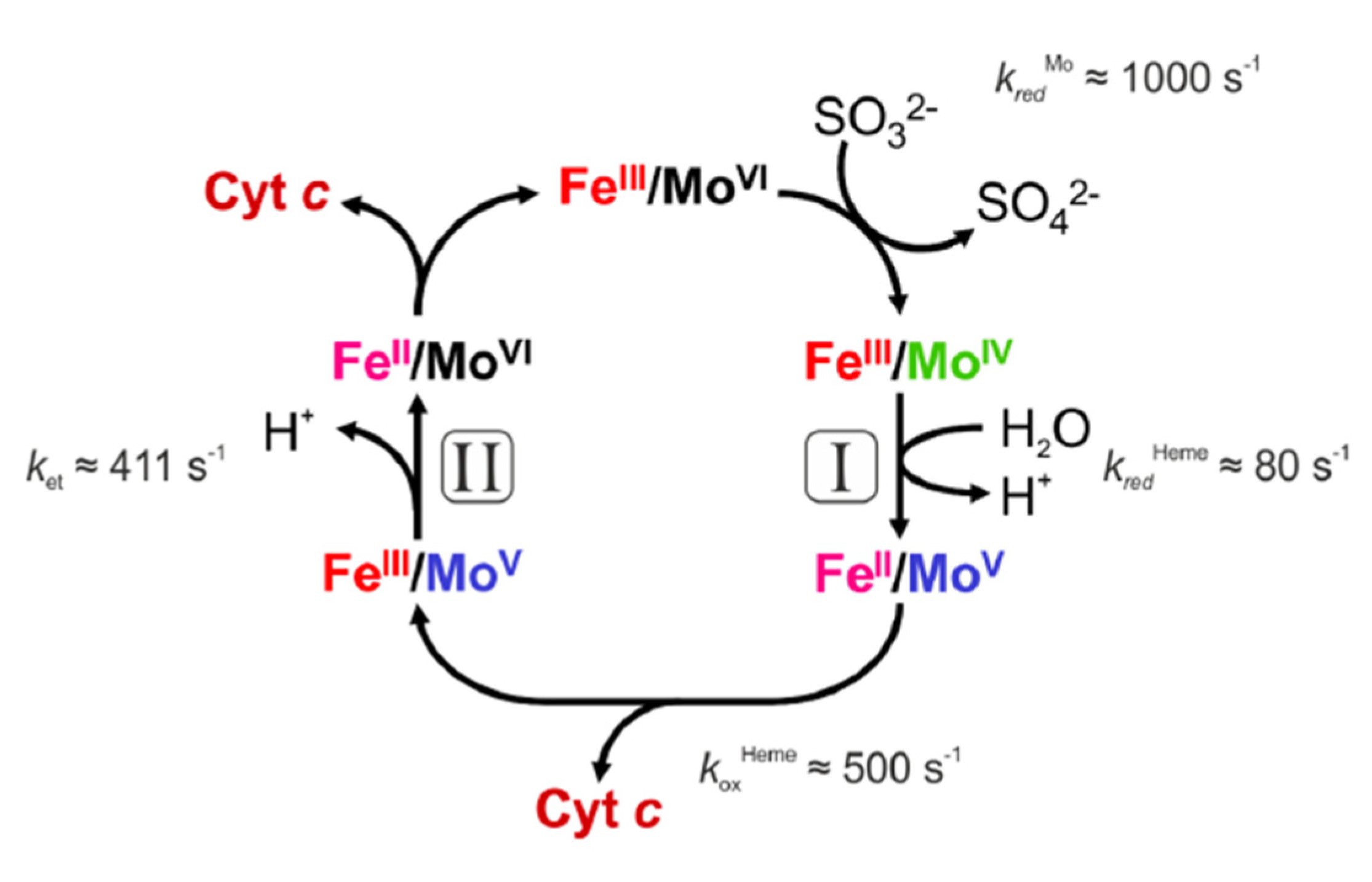
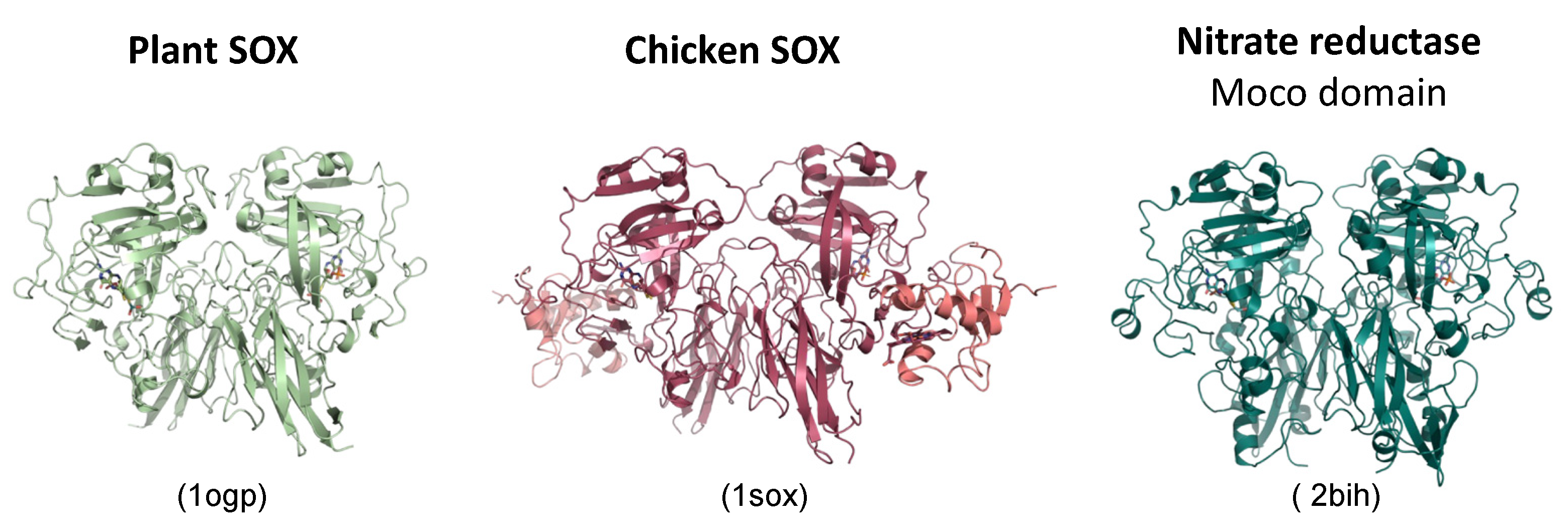
2.4. The Beauty of the SOX Structure and Unanswered Questions
2.5. The Multiple Roles of SOX in Metabolism
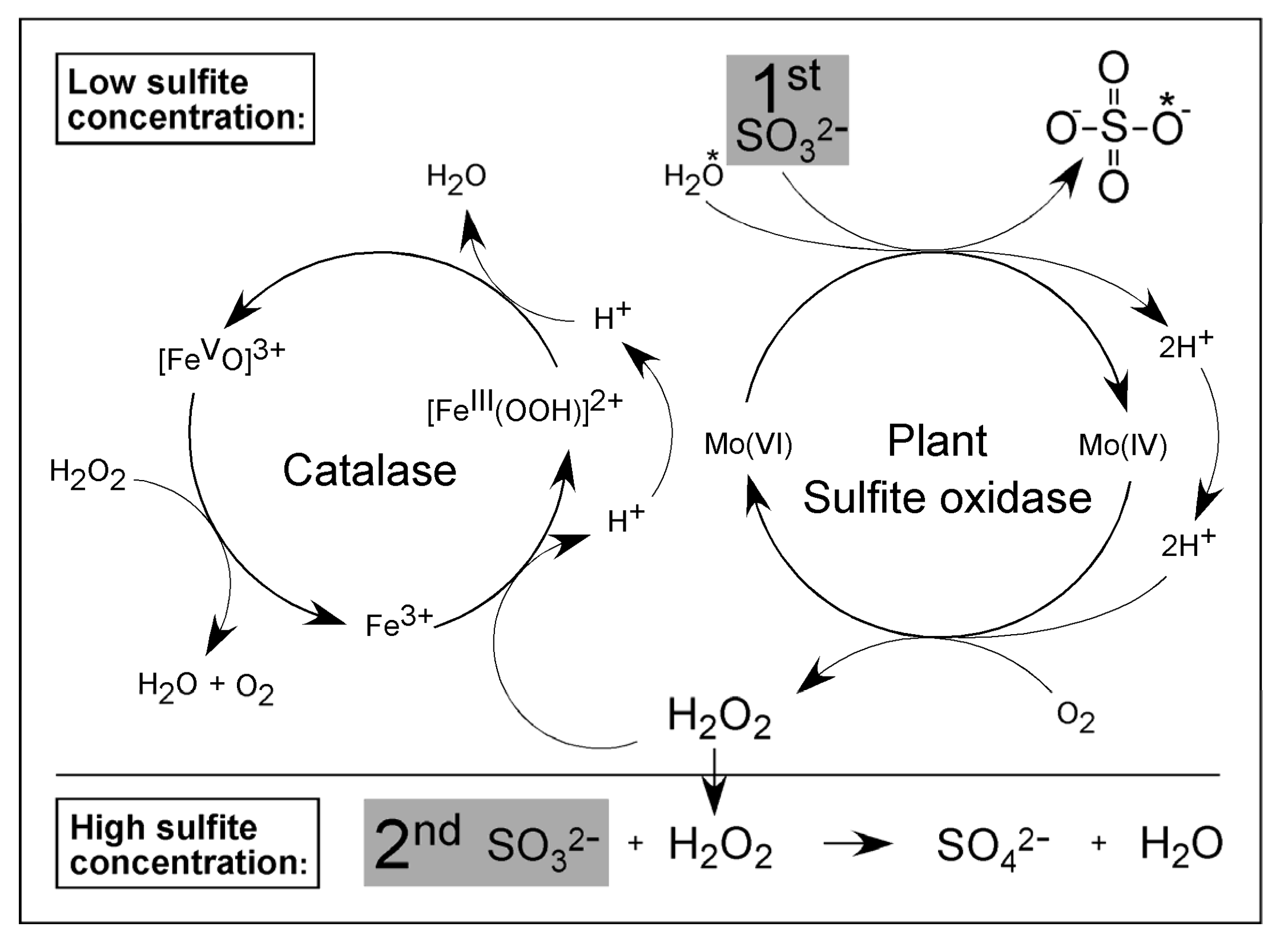
3. Plant SOX Is Different—A Personal View by Ralf Mendel (Years 2001 Onward)
4. Human SOX-Deficiency and Therapy—A Personal View by Günter Schwarz (Years 2002 Onward)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, M.C.; Preiner, M.; Xavier, J.C.; Zimorski, V.; Martin, W.F. The last universal common ancestor between ancient Earth chemistry and the onset of genetics. PLoS Genet. 2018, 14, e1007518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rump, S.; Gladyshev, V.N. Comparative Genomics and Evolution of Molybdenum Utilization. Coord. Chem. Rev. 2011, 255, 1206–1217. [Google Scholar] [CrossRef]
- Hille, R.; Nishino, T.; Bittner, F. Molybdenum enzymes in higher organisms. Coord. Chem. Rev. 2011, 255, 1179–1205. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. Xanthine Oxidase—A Personal History. Molecules 2023, 28, 1921. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T. XDH and XO Research and Drug Discovery—Personal History. Molecules 2023, 28, 4440. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.J.G. The History of Desulfovibrio gigas Aldehyde Oxidoreductase—A Personal View. Molecules 2023, 28, 4229. [Google Scholar] [CrossRef] [PubMed]
- Clement, B.; Struwe, M.A. The History of mARC. Molecules 2023, 28, 4713. [Google Scholar] [CrossRef]
- Leimkuhler, S. Metal-Containing Formate Dehydrogenases, a Personal View. Molecules 2023, 28, 5338. [Google Scholar] [CrossRef]
- Bortels, H. Molybdän als Katalysator bei der biologischen Stickstoffbindung. Arch. Mikrobiol. 1930, 1, 333–342. [Google Scholar] [CrossRef]
- Ter Meulen, H. Distribution of molybdenum. Nature 1932, 130, 966. [Google Scholar] [CrossRef]
- Renzo, E.C.D.; Kaleita, E.; Heytler, P.; Oleson, J.J.; Hutchings, B.L.; Williams, J.H. The Nature of the Xanthine Oxidase Factor. J. Am. Chem. Soc. 1953, 75, 753. [Google Scholar] [CrossRef]
- Mahler, H.R.; Mackler, B.; Green, D.E. Studies on metallo-flavoproteins. III. Aldehydeoxidase: A molybdoflavoprotein. J. Biol. Chem. 1954, 210, 65–480. [Google Scholar]
- Nicholas, D.J.; Nason, A.; McElroy, W.D. Molybdenum and nitrate reductase. I. Effect of molybdenum deficiency on the Neurospora enzyme. J. Biol. Chem. 1954, 207, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Medes, G.; Floyd, N. Metabolism of sulphur. Cysteic acid. Biochem. J. 1942, 36, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Heimberg, M.; Fridovich, I.; Handler, P. The enzymatic oxidation of sulfite. J. Biol. Chem. 1953, 204, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I.; Handler, P. The initial step in enzymatic sulfite oxidation. J. Biol. Chem. 1956, 223, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.V.; Fridovich, I.; Handler, P. Hepatic aldehyde oxidase. I. Purification and properties. J. Biol. Chem. 1962, 237, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.V.; Handler, P. Hepatic Aldehyde Oxidase. 3. The Substrate-Binding Site. J. Biol. Chem. 1964, 239, 2027–2035. [Google Scholar] [CrossRef]
- Rajagopalan, K.V.; Handler, P.; Palmer, G.; Beinert, H. Studies of aldehyde oxidase by electron paramagnetic resonance spectroscopy. I. Spectra at equilibrium states. J. Biol. Chem. 1968, 243, 3784–3796. [Google Scholar] [CrossRef]
- Mudd, S.H.; Irreverre, F.; Laster, L. Sulfite oxidase deficiency in man: Demonstration of the enzymatic defect. Science 1967, 156, 1599–1602. [Google Scholar] [CrossRef]
- Cohen, H.J.; Fridovich, I. Hepatic sulfite oxidase. Purification and properties. J. Biol. Chem. 1971, 246, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.L.; Rajagopalan, K.V. Purification and properties of sulfite oxidase from chicken liver. Presence of molybdenum in sulfite oxidase from diverse sources. J. Biol. Chem. 1972, 247, 6566–6573. [Google Scholar] [CrossRef]
- Johnson, J.L.; Cohen, H.J.; Rajagopalan, K.V. Molecular basis of the biological function of molybdenum. Molybdenum- free sulfite oxidase from livers of tungsten-treated rats. J. Biol. Chem. 1974, 249, 5046–5055. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Rajagopalan, K.V. Human sulfite oxidase deficiency. Characterization of the molecular defect in a multicomponent system. J. Clin. Investig. 1976, 58, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Jones, H.P.; Rajagopalan, K.V. In vitro reconstitution of demolybdosulfite oxidase by a molybdenum cofactor from rat liver and other sources. J. Biol. Chem. 1977, 252, 4994–5003. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.E.; Abroms, I.F.; Johnson, J.L.; Carney, M.; Mandell, R.; Robb, R.M.; Cloherty, J.P.; Rajagopalan, K.V. Sulfite oxidase deficiency. Biochemical and clinical investigations of a hereditary metabolic disorder in sulfur metabolism. N. Engl. J. Med. 1977, 297, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. The History of the Molybdenum Cofactor—A Personal View. Molecules 2022, 27, 4934. [Google Scholar] [CrossRef] [PubMed]
- Kresge, N.; Simoni, R.D.; Hill, R.L. The Discovery and Characterization of Molybdopterin: The Work of K. V. Rajagopalan. J. Biol. Chem. 2009, 284, e10. [Google Scholar] [CrossRef]
- Macleod, R.M.; Farkas, W.; Fridovich, I.; Handler, P. Purification and properties of hepatic sulfite oxidase. J. Biol. Chem. 1961, 236, 1841–1846. [Google Scholar] [CrossRef]
- Cohen, H.J.; Fridovich, I.; Rajagopalan, K.V. Hepatic sulfite oxidase. A functional role for molybdenum. J. Biol. Chem. 1971, 246, 374–382. [Google Scholar] [CrossRef]
- Lyric, R.M.; Suzuki, I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: Cytochrome c oxidoreductase. Can. J. Biochem. 1970, 48, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Betcher-Lange, S.; Kessler, D.L.; Rajagopalan, K.V. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J. Biol. Chem. 1972, 247, 7759–7766. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, E.; Yoder, R.E.; Frank, N.R. Distribution of 35S in the blood and its excretion in urine dogs exposed to 35SO2. Arch. Environ. Health 1971, 22, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Drew, R.T.; Johnson, J.L.; Rajagopalan, K.V. Molecular basis of the biological function of molybdenum: The relationship between sulfite oxidase and the acute toxicity of bisulfite and SO2. Proc. Natl. Acad. Sci. USA 1973, 70, 3655–3659. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Rajagopalan, K.V. Tryptic cleavage of rat liver sulfite oxidase. Isolation and characterization of molybdenum and heme domains. J. Biol. Chem. 1977, 252, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Southerland, W.M.; Winge, D.R.; Rajagopalan, K.V. The domains of rat liver sulfite oxidase. Proteolytic separation and characterization. J. Biol. Chem. 1978, 253, 8747–8752. [Google Scholar] [CrossRef] [PubMed]
- Neame, P.J.; Barber, M.J. Conserved domains in molybdenum hydroxylases—The amino acid sequence of chicken hepatic sulfite oxidase. J. Biol. Chem. 1989, 264, 20894–20901. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.J.; Neame, P.J. A conserved cysteine in molybdenum oxotransferases. J. Biol. Chem. 1990, 265, 20912–20915. [Google Scholar] [CrossRef]
- Cramer, S.P.; Solomonson, L.P.; Adams, M.W.W.; Mortenson, L.E. Molybdenum sites of Escherichia coli and Chlorella vulgaris nitrate reductase: A comparison by EXAFS. J. Am. Chem. Soc. 1984, 106, 1467–1471. [Google Scholar] [CrossRef]
- George, G.N.; Kipke, C.A.; Prince, R.C.; Sunde, R.A.; Enemark, J.H.; Cramer, S.P. Structure of the active site of sulfite oxidase. X-ray absoprtion spectroscopy of the Mo(IV), Mo(V), and Mo(VI) oxidation states. Biochemistry 1989, 28, 5075–5080. [Google Scholar] [CrossRef]
- Garrett, R.M.; Rajagopalan, K.V. Molecular cloning of rat liver sulfite oxidase. Expression of a eukaryotic Mo-pterin-containing enzyme in Escherichia coli. J. Biol. Chem. 1994, 269, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.M.; Bellissimo, D.B.; Rajagopalan, K.V. Molecular cloning of human liver sulfite oxidase. Biochim. Biophys. Acta 1995, 1262, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.M.; Rajagopalan, K.V. Site-directed mutagenesis of recombinant sulfite oxidase. J. Biol. Chem. 1996, 271, 7387–7391. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.M.; Johnson, J.L.; Graf, T.N.; Feigenbaum, A.; Rajagopalan, K.V. Human sulfite oxidase R160Q: Identification of the mutation in a sulfite oxidase-deficient patient and expression and characterization of the mutant enzyme. Proc. Natl. Acad. Sci. USA 1998, 95, 6394–6398. [Google Scholar] [CrossRef] [PubMed]
- Kisker, C.; Schindelin, H.; Pacheco, A.; Wehbi, W.A.; Garrett, R.M.; Rajagopalan, K.V.; Enemark, J.H.; Rees, D.C. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell 1997, 91, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.; Mukund, S.; Kletzin, A.; Adams, M.W.; Rees, D.C. Structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase. Science 1995, 267, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Romao, M.J.; Archer, M.; Moura, I.; Moura, J.J.; LeGall, J.; Engh, R.; Schneider, M.; Hof, P.; Huber, R. Crystal structure of the xanthine oxidase-related aldehyde oxido- reductase from D. gigas. Science 1995, 270, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.S.; Hille, R. The kinetic behavior of chicken liver sulfite oxidase. Biochemistry 1999, 38, 6668–6677. [Google Scholar] [CrossRef]
- Feng, C.; Kedia, R.V.; Hazzard, J.T.; Hurley, J.K.; Tollin, G.; Enemark, J.H. Effect of solution viscosity on intramolecular electron transfer in sulfite oxidase. Biochemistry 2002, 41, 5816–5821. [Google Scholar] [CrossRef]
- Johnson-Winters, K.; Tollin, G.; Enemark, J.H. Elucidating the catalytic mechanism of sulfite oxidizing enzymes using structural, spectroscopic, and kinetic analyses. Biochemistry 2010, 49, 7242–7254. [Google Scholar] [CrossRef]
- Kaczmarek, A.T.; Bahlmann, N.; Thaqi, B.; May, P.; Schwarz, G. Machine learning-based identification and characterization of 15 novel pathogenic SUOX missense mutations. Mol. Genet. Metab. 2021, 134, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.M.; Schwarz, G. Cofactor-dependent maturation of mammalian sulfite oxidase links two mitochondrial import pathways. J. Cell Sci. 2012, 125, 4876–4885. [Google Scholar] [CrossRef] [PubMed]
- Eh, M.; Kaczmarek, A.T.; Schwarz, G.; Bender, D. Molecular mechanism of intramolecular electron transfer in dimeric sulfite oxidase. J. Biol. Chem. 2022, 298, 101668. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.C.; Stagliano, C.; Liu, A. Structural insights into the half-of-sites reactivity in homodimeric and homotetrameric metalloenzymes. Curr. Opin. Chem. Biol. 2023, 75, 102332. [Google Scholar] [CrossRef] [PubMed]
- Kohl, J.B.; Mellis, A.T.; Schwarz, G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 2019, 176, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; Kopriva, S. Sulfation pathways in plants. Chem. Biol. Interact. 2016, 259, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mellis, A.T.; Misko, A.L.; Arjune, S.; Liang, Y.; Erdelyi, K.; Ditroi, T.; Kaczmarek, A.T.; Nagy, P.; Schwarz, G. The role of glutamate oxaloacetate transaminases in sulfite biosynthesis and H2S metabolism. Redox Biol. 2021, 38, 101800. [Google Scholar] [CrossRef] [PubMed]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di Meo, I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef]
- Friederich, M.W.; Elias, A.F.; Kuster, A.; Laugwitz, L.; Larson, A.A.; Landry, A.P.; Ellwood-Digel, L.; Mirsky, D.M.; Dimmock, D.; Haven, J.; et al. Pathogenic variants in SQOR encoding sulfide:quinone oxidoreductase are a potentially treatable cause of Leigh disease. J. Inherit. Metab. Dis. 2020, 43, 1024–1036. [Google Scholar] [CrossRef]
- Bender, D.; Kaczmarek, A.T.; Santamaria-Araujo, J.A.; Stueve, B.; Waltz, S.; Bartsch, D.; Kurian, L.; Cirak, S.; Schwarz, G. Impaired mitochondrial maturation of sulfite oxidase in a patient with severe sulfite oxidase deficiency. Hum. Mol. Genet. 2019, 28, 2885–2899. [Google Scholar] [CrossRef]
- Kaczmarek, A.T.; Bender, D.; Gehling, T.; Kohl, J.B.; Daimaguler, H.S.; Santamaria-Araujo, J.A.; Liebau, M.C.; Koy, A.; Cirak, S.; Schwarz, G. A defect in molybdenum cofactor binding causes an attenuated form of sulfite oxidase deficiency. J. Inherit. Metab. Dis 2022, 45, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Zuckerbraun, B.S.; Shiva, S.; Ifedigbo, E.; Mathier, M.A.; Mollen, K.P.; Rao, J.; Bauer, P.M.; Choi, J.J.; Curtis, E.; Choi, A.M.; et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation 2010, 121, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Krizowski, S.; Fischer-Schrader, K.; Niks, D.; Tejero, J.; Sparacino-Watkins, C.; Wang, L.; Ragireddy, V.; Frizzell, S.; Kelley, E.E.; et al. Sulfite Oxidase Catalyzes Single-Electron Transfer at Molybdenum Domain to Reduce Nitrite to Nitric Oxide. Antioxid. Redox Signal. 2015, 23, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R.; Müller, A.J. A common genetic determinant of xanthine dehydrogenase and nitrate reductase in Nicotiana tabacum. Biochem. Physiol. Pflanz. 1976, 170, 538–541. [Google Scholar] [CrossRef]
- Mendel, R.R.; Müller, A. Nitrate reductase-deficient mutant cell lines of Nicotiana tabacum. Further biochemical characterization. Mol. Gen. Genet. 1979, 177, 145–153. [Google Scholar] [CrossRef]
- Fischer, K.; Barbier, G.G.; Hecht, H.-J.; Mendel, R.R.; Campbell, W.H.; Schwarz, G. Crystal Structure of the Yeast Nitrate Reductase Molybdenum Domain Provides Insight into Eukaryotic Nitrate Assimilation. Plant Cell 2005, 17, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Hesberg, C.; Hansch, R.; Mendel, R.R.; Bittner, F. Tandem orientation of duplicated xanthine dehydrogenase genes from Arabidopsis thaliana: Differential gene expression and enzyme activities. J. Biol. Chem. 2004, 279, 13547–13554. [Google Scholar] [CrossRef]
- Rennenberg, H. The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 121–153. [Google Scholar] [CrossRef]
- Thomas, M.D.; Hendricks, R.H.; Hill, G.R. Some Chemical Reactions of Sulphur Dioxide after Absorption by Alfalfa and Sugar Beets. Plant Physiol. 1944, 19, 212–226. [Google Scholar] [CrossRef]
- Leustek, T.; Saito, K. Sulfate transport and assimilation in plants. Plant Physiol. 1999, 120, 637–644. [Google Scholar] [CrossRef]
- Pfanz, H.; Oppmnn, B. The possible role of apoplastic peroxidases in detoxifying the air pollutant sulfur dioxide. In Biochemical, Molecular and Physiological Aspects of Plant Peroxidases; Lobarzewski, J., Penel, H.G.C., Gaspar, T., Eds.; University of Geneva: Geneva, Switzerland, 1991; pp. 401–417. [Google Scholar]
- Eilers, T.; Schwarz, G.; Brinkmann, H.; Witt, C.; Richter, T.; Nieder, J.; Koch, B.; Hille, R.; Hansch, R.; Mendel, R.R. Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. A new player in plant sulfur metabolism. J. Biol. Chem. 2001, 276, 46989–46994. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.T.; Lee, M.S.; Flynn, C.R.; Trelease, R.N. Diverse amino acid residues function within the type 1 peroxisomal targeting signal. Implications for the role of accessory residues upstream of the type 1 peroxisomal targeting signal. Plant Physiol. 1997, 115, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Luniak, N.; Witt, C.; Wustefeld, Y.; Wachter, A.; Mendel, R.R.; Hansch, R. Peroxisomal localization of sulfite oxidase separates it from chloroplast-based sulfur assimilation. Plant Cell Physiol. 2004, 45, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Truglio, J.J.; Theis, K.; Leimkuhler, S.; Rappa, R.; Rajagopalan, K.V.; Kisker, C. Crystal Structures of the Active and Alloxanthine-Inhibited Forms of Xanthine Dehydrogenase from Rhodobacter capsulatus. Structure 2002, 10, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Schrader, N.; Fischer, K.; Theis, K.; Mendel, R.R.; Schwarz, G.; Kisker, C. The crystal structure of plant sulfite oxidase provides insights into sulfite oxidation in plants and animals. Structure 2003, 11, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Havemeyer, A.; Bittner, F.; Wollers, S.; Mendel, R.; Kunze, T.; Clement, B. Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. J. Biol. Chem. 2006, 281, 34796–34802. [Google Scholar] [CrossRef] [PubMed]
- Hemann, C.; Hood, B.L.; Fulton, M.; Hansch, R.; Schwarz, G.; Mendel, R.R.; Kirk, M.L.; Hille, R. Spectroscopic and kinetic studies of Arabidopsis thaliana sulfite oxidase: Nature of the redox-active orbital and electronic structure contributions to catalysis. J. Am. Chem. Soc. 2005, 127, 16567–16577. [Google Scholar] [CrossRef] [PubMed]
- Astashkin, A.V.; Hood, B.L.; Feng, C.; Hille, R.; Mendel, R.R.; Raitsimring, A.M.; Enemark, J.H. Structures of the Mo(V) forms of sulfite oxidase from Arabidopsis thaliana by pulsed EPR spectroscopy. Biochemistry 2005, 44, 13274–13281. [Google Scholar] [CrossRef]
- Byrne, R.S.; Hansch, R.; Mendel, R.R.; Hille, R. Oxidative half-reaction of Arabidopsis thaliana sulfite oxidase: Generation of superoxide by a peroxisomal enzyme. J. Biol. Chem. 2009, 284, 35479–35484. [Google Scholar] [CrossRef]
- Hansch, R.; Lang, C.; Riebeseel, E.; Lindigkeit, R.; Gessler, A.; Rennenberg, H.; Mendel, R.R. Plant sulfite oxidase as novel producer of H2O2: Combination of enzyme catalysis with a subsequent non-enzymatic reaction step. J. Biol. Chem. 2006, 281, 6884–6888. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Roper, J.; Arjune, S.; Krizowski, S.; Trifunovic, A.; Schwarz, G. Oxygen reactivity of mammalian sulfite oxidase provides a concept for the treatment of sulfite oxidase deficiency. Biochem. J. 2015, 469, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Theisen, S.; Hansch, R.; Kothe, L.; Leist, U.; Galensa, R. A fast and sensitive HPLC method for sulfite analysis in food based on a plant sulfite oxidase biosensor. Biosens. Bioelectron. 2010, 26, 175–181. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Schwarz, G. Metal insertion into the molybdenum cofactor: Product-substrate channelling demonstrates the functional origin of domain fusion in gephyrin. Biochem. J. 2013, 450, 149–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peiser, G.; Shang, F.Y. Biochemical and physiological effects of SO2 on nonphotosynthetic processes in plants. In Sulfur Dioxide and Vegetation; Winner, W.E., Mooney, H.A., Goldstein, R.A., Eds.; Stanford University Press: Stanford, CA, USA, 1985; pp. 148–161. [Google Scholar]
- Heber, U.; Hueve, K. Action of SO2 on plants and metabolic detoxification of SO2. Int. Rev. Cytol. 1998, 177, 255–286. [Google Scholar]
- Lang, C.; Popko, J.; Wirtz, M.; Hell, R.; Herschbach, C.; Kreuzwieser, J.; Rennenberg, H.; Mendel, R.R.; Hansch, R. Sulphite oxidase as key enzyme for protecting plants against sulphur dioxide. Plant Cell Environ. 2007, 30, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, D.; Randewig, D.; Schliesky, S.; Brautigam, A.; Weber, A.P.M.; Geffers, R.; Herschbach, C.; Rennenberg, H.; Mendel, R.R.; Hansch, R. Impact of SO2 on Arabidopsis thaliana transcriptome in wildtype and sulfite oxidase knockout plants analyzed by RNA deep sequencing. New Phytol. 2012, 196, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Baillie, C.K.; Meinen, R.; Kaufholdt, D.; Hänsch, S.; Schmidt, N.; Zwerschke, D.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Bloem, E.; et al. Apoplastic peroxidases enable an additional sulphite detoxification strategy and act as first line of defence upon exposure to sulphur containing gas. Environ. Exp. Bot. 2019, 157, 140–150. [Google Scholar] [CrossRef]
- Baillie, C.-K.; Kaufholdt, D.; Meinen, R.; Hu, B.; Rennenberg, H.; Hänsch, R.; Bloem, E. Surviving volcanic environments–Interaction of soil mineral content and plant element composition. Front. Environ. Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- Weber, J.-N.; Kaufholdt, D.; Minner-Meinen, R.; Bloem, E.; Shahid, A.; Rennenberg, H.; Hänsch, R. Impact of wildfires on SO2 detoxification mechanisms in leaves of oak and beech trees. Environ. Pollut. 2020, 272, 116389. [Google Scholar] [CrossRef]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157C, 3–32. [Google Scholar] [CrossRef]
- Olney, J.W.; Misra, C.H.; de Gubareff, T. Cysteine-S-sulfate: Brain damaging metabolite in sulfite oxidase deficiency. J. Neuropathol. Exp. Neurol. 1975, 34, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Waud, W.R.; Rajagopalan, K.V.; Duran, M.; Beemer, F.A.; Wadman, S.K. Inborn errors of molybdenum metabolism: Combined deficiencies of sulfite oxidase and xanthine dehydrogenase in a patient lacking the molybdenum cofactor. Proc. Natl. Acad. Sci. USA 1980, 77, 3715–3719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Rajagopalan, K.V. Structural and metabolic relationship between the molybdenum cofactor and urothione. Proc. Natl. Acad. Sci. USA 1982, 79, 6856–6860. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Wuebbens, M.M.; Mandell, R.; Shih, V.E. Molybdenum cofactor biosynthesis in humans. Identification of two complementation groups of cofactor-deficient patients and preliminary characterization of a diffusible molybdopterin precursor. J. Clin. Investig. 1989, 83, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Wuebbens, M.M.; Rajagopalan, K.V. The structure of a molybdopterin precursor. Characterization of a stable, oxidized derivative. J. Biol. Chem. 1989, 264, 13440–13447. [Google Scholar] [CrossRef]
- Schwarz, G.; Santamaria-Araujo, J.A.; Wolf, S.; Lee, H.J.; Adham, I.M.; Grone, H.J.; Schwegler, H.; Sass, J.O.; Otte, T.; Hanzelmann, P.; et al. Rescue of lethal molybdenum cofactor deficiency by a biosynthetic precursor from Escherichia coli. Hum. Mol. Gen. 2004, 13, 1249–1255. [Google Scholar] [CrossRef]
- Reiss, J.; Cohen, N.; Dorche, C.; Mandel, H.; Mendel, R.R.; Stallmeyer, B.; Zabot, M.T.; Dierks, T. Mutations in a polycistronic nuclear gene associated with molybdenum cofactor deficiency. Nat. Genet. 1998, 20, 51–53. [Google Scholar] [CrossRef]
- Stallmeyer, B.; Drugeon, G.; Reiss, J.; Haenni, A.L.; Mendel, R.R. Human molybdopterin synthase gene: Identification of a bicistronic transcript with overlapping reading frames. Am. J. Hum. Genet. 1999, 64, 698–705. [Google Scholar] [CrossRef]
- Stallmeyer, B.; Schwarz, G.; Schulze, J.; Nerlich, A.; Reiss, J.; Kirsch, J.; Mendel, R.R. The neurotransmitter receptor-anchoring protein gephyrin reconstitutes molybdenum cofactor biosynthesis in bacteria, plants, and mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, G.; Schrader, N.; Mendel, R.R.; Hecht, H.J.; Schindelin, H. Crystal structures of human gephyrin and plant Cnx1 G domains: Comparative analysis and functional implications. J. Mol. Biol. 2001, 312, 405–418. [Google Scholar] [CrossRef]
- Kim, E.Y.; Schrader, N.; Smolinsky, B.; Bedet, C.; Vannier, C.; Schwarz, G.; Schindelin, H. Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J. 2006, 25, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Schrader, N.; Kim, E.Y.; Winking, J.; Paulukat, J.; Schindelin, H.; Schwarz, G. Biochemical characterization of the high affinity binding between the glycine receptor and gephyrin. J. Biol. Chem. 2004, 279, 18733–18741. [Google Scholar] [CrossRef] [PubMed]
- Herweg, J.; Schwarz, G. Splice-specific glycine receptor binding, folding, and phosphorylation of the scaffolding protein gephyrin. J. Biol. Chem. 2012, 287, 12645–12656. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Djemie, T.; Grunewald, N.; Suls, A.; Kress, V.; Hetsch, F.; Craiu, D.; Zemel, M.; Gormley, P.; Lal, D.; et al. Simultaneous impairment of neuronal and metabolic function of mutated gephyrin in a patient with epileptic encephalopathy. EMBO Mol. Med. 2015, 7, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Lal, D.; Catarino, C.B.; Arjune, S.; Belaidi, A.A.; Trucks, H.; Vollmar, C.; Surges, R.; Kunz, W.S.; Motameny, S.; et al. Exonic microdeletions of the gephyrin gene impair GABAergic synaptic inhibition in patients with idiopathic generalized epilepsy. Neurobiol. Dis. 2014, 67, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Araujo, J.A.; Fischer, B.; Otte, T.; Nimtz, M.; Mendel, R.R.; Wray, V.; Schwarz, G. The tetrahydropyranopterin structure of the sulfur-free and metal-free molybdenum cofactor precursor. J. Biol. Chem. 2004, 279, 15994–15999. [Google Scholar] [CrossRef]
- Lee, H.-J.; Adham, I.M.; Schwarz, G.; Kneussel, M.; Sass, J.-O.; Engel, W.; Reiss, J. Molybdenum cofactor-deficient mice resemble the phenotype of human patients. Hum. Mol. Gen. 2002, 11, 3309–3317. [Google Scholar] [CrossRef]
- Veldman, A.; Santamaria-Araujo, J.A.; Sollazzo, S.; Pitt, J.; Gianello, R.; Yaplito-Lee, J.; Wong, F.; Ramsden, C.A.; Reiss, J.; Cook, I.; et al. Successful treatment of molybdenum cofactor deficiency type A with cPMP. Pediatrics 2010, 125, e1249–e1254. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Van Spronsen, F.J.; Belaidi, A.A.; Bowhay, S.; Christodoulou, J.; Derks, T.G.; Hennermann, J.B.; Jameson, E.; Konig, K.; McGregor, T.L.; et al. Efficacy and safety of cyclic pyranopterin monophosphate substitution in severe molybdenum cofactor deficiency type A: A prospective cohort study. Lancet 2015, 386, 1955–1963. [Google Scholar] [CrossRef]
- Johannes, L.; Fu, C.Y.; Schwarz, G. Molybdenum Cofactor Deficiency in Humans. Molecules 2022, 27, 6896. [Google Scholar] [CrossRef]
- Del Rizzo, M.; Burlina, A.P.; Sass, J.O.; Beermann, F.; Zanco, C.; Cazzorla, C.; Bordugo, A.; Giordano, L.; Manara, R.; Burlina, A.B. Metabolic stroke in a late-onset form of isolated sulfite oxidase deficiency. Mol. Genet. Metab. 2013, 108, 263–266. [Google Scholar] [CrossRef]
- Warnhoff, K.; Ruvkun, G. Molybdenum cofactor transfer from bacteria to nematode mediates sulfite detoxification. Nat. Chem. Biol. 2019, 15, 480–488. [Google Scholar] [CrossRef]
- Warnhoff, K.; Hercher, T.W.; Mendel, R.R.; Ruvkun, G. Protein-bound molybdenum cofactor is bioavailable and rescues molybdenum cofactor-deficient C. elegans. Genes Dev. 2021, 35, 212–217. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendel, R.R.; Schwarz, G. The History of Animal and Plant Sulfite Oxidase—A Personal View. Molecules 2023, 28, 6998. https://doi.org/10.3390/molecules28196998
Mendel RR, Schwarz G. The History of Animal and Plant Sulfite Oxidase—A Personal View. Molecules. 2023; 28(19):6998. https://doi.org/10.3390/molecules28196998
Chicago/Turabian StyleMendel, Ralf R., and Günter Schwarz. 2023. "The History of Animal and Plant Sulfite Oxidase—A Personal View" Molecules 28, no. 19: 6998. https://doi.org/10.3390/molecules28196998
APA StyleMendel, R. R., & Schwarz, G. (2023). The History of Animal and Plant Sulfite Oxidase—A Personal View. Molecules, 28(19), 6998. https://doi.org/10.3390/molecules28196998





