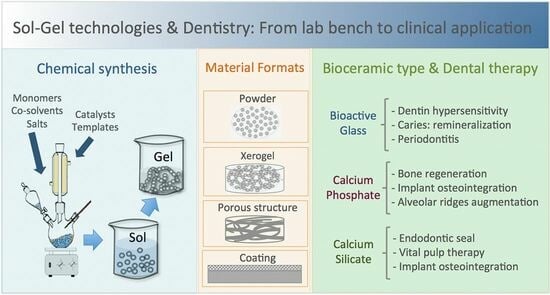
Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics
Abstract
:1. Introduction
2. Commercial Bioceramics Currently in Use
2.1. Bioactive Glasses
2.2. Calcium Phosphates
2.3. Calcium Silicates
3. Current Research on Sol–Gel Bioceramics for Application in Dentistry
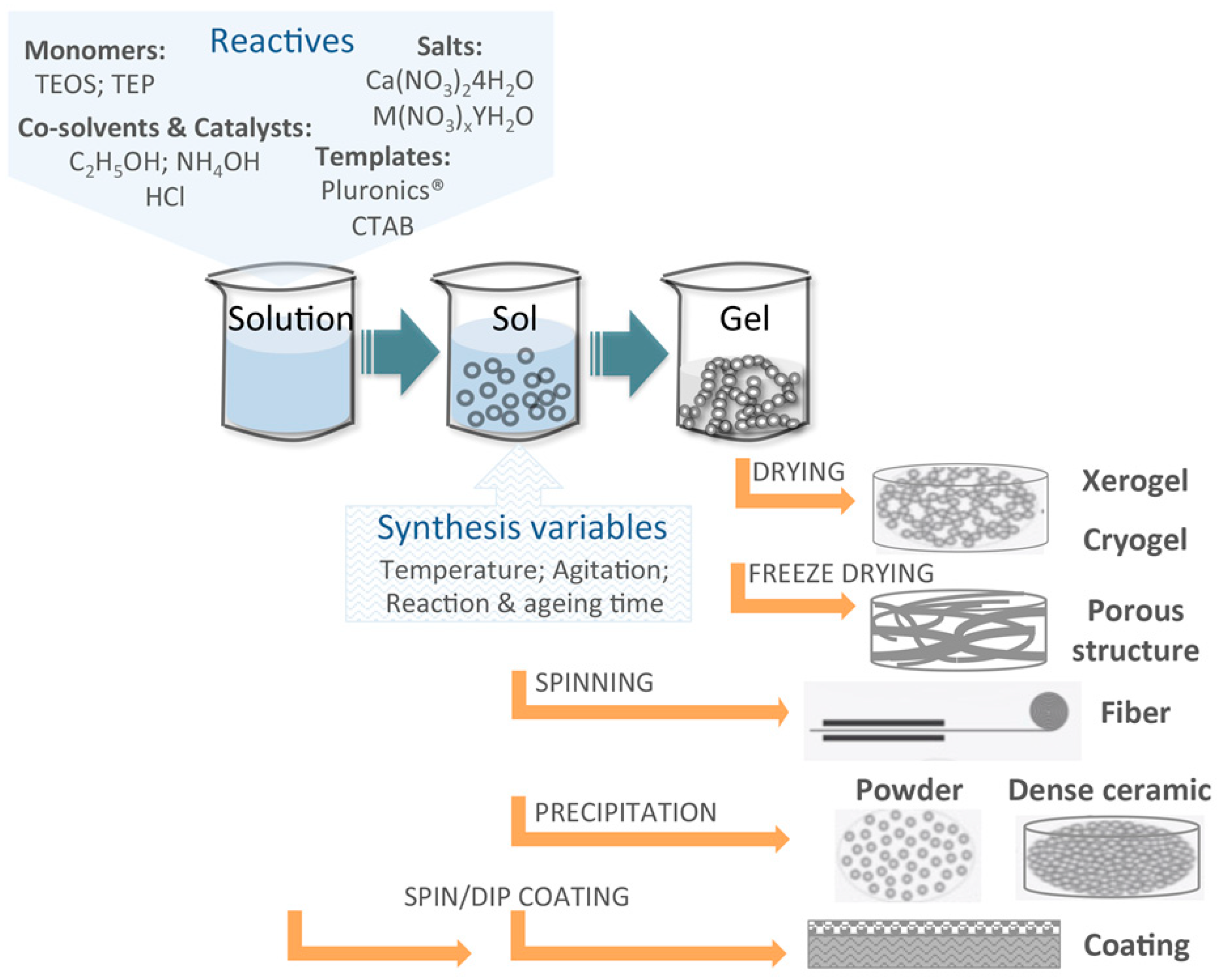
3.1. Basics of the Sol–Gel Synthesis Technique
3.2. Progress in Bioactive Glasses Research
3.2.1. Compositions and Chemical Routes
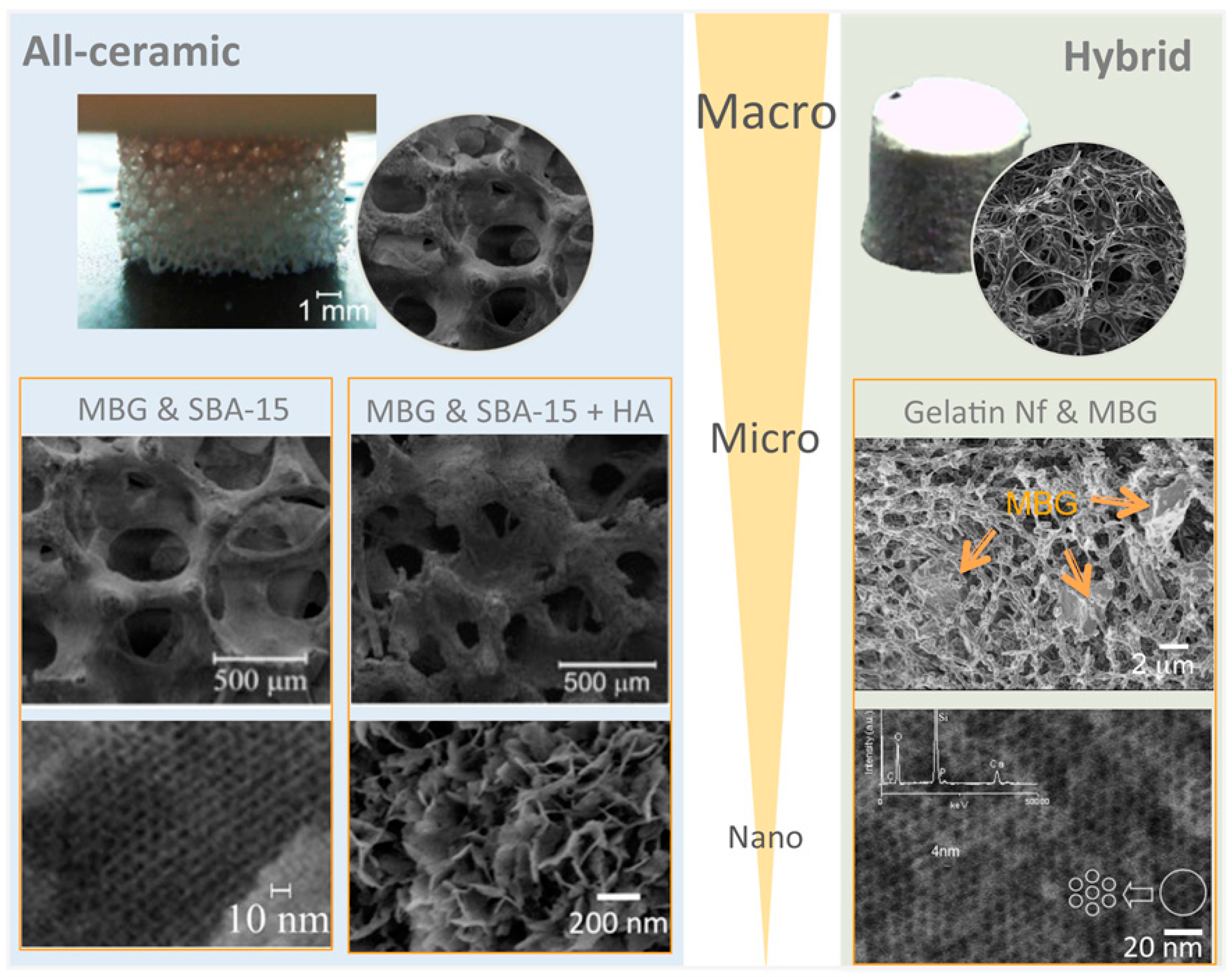
3.2.2. Processing and Final Formatting of Materials
3.2.3. Therapeutic and Clinical Uses
3.3. Progress in Calcium Phosphate Bioceramics Research
3.3.1. Compositions and Chemical Routes
3.3.2. Processing and Final Formatting of Materials
3.3.3. Therapeutic and Clinical Uses
3.4. Progress in Calcium Silicate Cements Research
3.4.1. Compositions and Synthesis Routes
3.4.2. Final Processing of Materials
3.4.3. Therapeutic and Clinical Uses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ICMS | Materials Science Institute of Seville |
| NCDs | Noncommunicable diseases |
| sol | Solution |
| BG | Bioactive glass |
| CaP | Calcium phosphate |
| CaSi | Calcium silicate |
| ERMI | Endosseous ridge maintenance implant |
| GICs | Glass-ionomer cements |
| MCP | Monocalcium phosphate |
| DCPA | Dicalcium phosphate |
| TCP | Tricalcium phosphate |
| HA | Hydroxyapatite |
| BECs | Bioactive endodontic cement |
| MTA | Mineral trioxide aggregate |
| GMTA | Gray ProRoot MTA |
| WMTA | White ProRoot MTA |
| TEOS | Tetraethyl orthosilicate |
| TEP | Triethyl phosphate |
| CaN | Calcium nitrate tetrahydrate |
| SBF | Simulated body fluid |
| HCA | Hydroxy carbonate apatite |
| MBG | Mesoporous bioactive glass |
| DDA | Dodecylamine |
| CTAB | Hexadecyltrimethyl ammonium bromide |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene oxide) |
| PPO | Poly(propylene oxide) |
| P/L | Powder to liquid ratio |
| TMOS | Tetramethylorthosilicate |
| hPDLCs | Human periodontal ligament-derived cells |
| SP | Silica particles |
| SBA-15 | Santa Barbara Amorphous-15 |
| GTR | Guided tissue regeneration |
| TIPS | Thermally induced phase separation |
| GS | Gentamicin sulphate |
| Nf | Nanofibres |
| PRP | Platelet-rich plasma |
| MBN | Mesoporous bioactive glass nanoparticles |
| WSLs | White spot lesions |
| ALP | Alkaline phosphatase |
| YAG | Yttrium aluminum garnet |
| SA | Stearic acid |
| PVP | Poly(vinyl pyrrolidone) |
| SEM | Scanning electron microscopy |
| Si-HA | Silicon-substituted hydroxyapatite |
| MT | Methionine |
| C3S | Tri-calcium silicate |
| C2S | Di-calcium silicate |
| CSH | Calcium silicate hydrate |
| BT | Barium titanate |
| BC | Biocellulose |
| hDPSCs | Human dental pulp stem cells |
Appendix A
| Bioceramic | Properties | References |
|---|---|---|
| BG 1 | Highly versatile for formulating variations in chemical composition; Bioactive; Biocompatible; Osteointegrative; Osteogenic and osteoinductive; Biodegradable; Bactericide | [28,35,189,192] |
| CaP 2 | Biomimetic to natural human hard tissues composition; Biocompatible; Osteoconductive; Bioresorbable | [88,89,227,232] |
| CaSi 3 | Hydraulic; Biocompatible; Bioactive; Osteoconductive; Osteoinductive | [66,115,156,258] |
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Seymour, R.A. Is gum disease killing your patient? Br. Dent. J. 2009, 206, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.C.; Fouad, A.F. The Impact of Endodontic Infections on the Pathogenesis of Cardiovascular Disease(s): A Systematic Review with Meta-analysis Using GRADE. J. Endod. 2018, 44, 1361–1366.e3. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Jimenez-Moreno, E.; Calvo-Monroy, C.; Ríos-Santos, J.V.; Velasco-Ortega, E.; Sánchez-Domínguez, B.; Castellanos-Cosano, L.; Llamas-Carreras, J.M. Hypertension and dental periapical condition. J. Endod. 2010, 36, 1800–1804. [Google Scholar] [CrossRef]
- Cabanillas-Balsera, D.; Martín-González, J.; Montero-Miralles, P.; Sánchez-Domínguez, B.; Jiménez-Sánchez, M.C.; Segura-Egea, J.J. Association between diabetes and nonretention of root filled teeth: A systematic review and meta-analysis. Int. Endod. J. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Velioğlu, E.M.; Aydındoğan, S.; Hakkı, S.S. Metabolic Syndrome and Periodontal Disease. Curr. Oral Health Rep. 2023, 10, 43–51. [Google Scholar] [CrossRef]
- Palmeira, E.; de Liz Pérez-Losada, F.; Díaz-Flores-García, V.; Segura-Sampedro, J.J.; Segura-Egea, J.J.; López-López, J. Prevalence of oral infections in chronic kidney disease patients: A cross-sectional study. Oral Dis. 2023. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamanashi, H.; Kitamura, M.; Miyata, J.; Nonaka, F.; Nakamichi, S.; Saito, T.; Nagata, Y.; Maeda, T. Association between periodontitis and chronic kidney disease by functional atherosclerosis status among older Japanese individuals: A cross-sectional study. J. Clin. Periodontol. 2023, 50, 430–439. [Google Scholar] [CrossRef]
- Segura-Sampedro, J.J.; Jiménez-Giménez, C.; Jane-Salas, E.; Cabanillas-Balsera, D.; Martín-González, J.; Segura-Egea, J.J.; López-López, J. Periapical and endodontic status of patients with inflammatory bowel disease: Age- and sex-matched case–control study. Int. Endod. J. 2022, 55, 748–757. [Google Scholar] [CrossRef]
- Madsen, G.R.; Bertl, K.; Pandis, N.; Stavropoulos, A.; Burisch, J. The Impact of Periodontitis on Inflammatory Bowel Disease Activity. Inflamm. Bowel Dis. 2023, 29, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Leech, M.T.; Bartold, P.M. The association between rheumatoid arthritis and periodontitis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 189–201. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.Y.; Kang, J.H. Associations between oral health status and risk of fractures in elder adults. Sci. Rep. 2023, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Borrell, L.N.; Papapanou, P.N.; Elkind, M.S.V.; Scarmeas, N.; Wright, C.B. Periodontitis is associated with cognitive impairment among older adults: Analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Han, Y.W. Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontology 2000 2022, 89, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Min, M.; Sun, C.; Zhang, Y.; Liang, M.; Sun, Y. Periodontal disease and susceptibility to breast cancer: A meta-analysis of observational studies. J. Clin. Periodontol. 2018, 45, 1025–1033. [Google Scholar] [CrossRef]
- Nwizu, N.N.; Marshall, J.R.; Moysich, K.; Genco, R.J.; Hovey, K.M.; Mai, X.; LaMonte, M.J.; Freudenheim, J.L.; Wactawski-Wende, J. Periodontal disease and incident cancer risk among postmenopausal women: Results from the women’s health initiative observational cohort. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1255–1265. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Bush, R.B.; Paju, S. In Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann. Periodontol./Am. Acad. Periodontol. 2003, 8, 54–69. [Google Scholar] [CrossRef]
- Costa, C.A.; Vilela, A.C.S.; Oliveira, S.A.; Gomes, T.D.; Andrade, A.A.C.; Leles, C.R.; Costa, N.L. Poor oral health status and adverse COVID-19 outcomes: A preliminary study in hospitalized patients. J. Periodontol. 2022, 93, 1889–1901. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Marí-Beffa, M.; Segura-Egea, J.J.; Díaz-Cuenca, A. Regenerative Endodontic Procedures: A Perspective from Stem Cell Niche Biology. J. Endod. 2017, 43, 52–62. [Google Scholar] [CrossRef]
- Borrego-González, S.; Dalby, M.J.; Díaz-Cuenca, A. Nanofibrous gelatin-based biomaterial with improved biomimicry using d-periodic self-assembled atelocollagen. Biomimetics 2021, 6, 20. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium phosphates and human beings. J. Chem. Educ. 2006, 83, 713–719. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. MTA HP Repair stimulates in vitro an homogeneous calcium phosphate phase coating deposition. J. Clin. Exp. Dent. 2019, 11, e322–e326. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, M.D.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. Higher hydration performance and bioactive response of the new endodontic bioactive cement MTA HP repair compared with ProRoot MTA white and NeoMTA plus. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cuenca, A.; Rabadjieva, D.; Sezanova, K.; Gergulova, R.; Ilieva, R.; Tepavitcharova, S. Biocompatible calcium phosphate-based ceramics and composites. Mater. Today Proc. 2022, 61, 1217–1225. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. Off. J. Soc. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Hench, L.L.; Wheeler, D.L.; Greenspan, D.C. Molecular Control of Bioactivity in Sol-Gel Glasses. J. Sol-Gel Sci. Technol. 1998, 13, 245–250. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Ramiro-Gutiérrez, M.L.; Will, J.; Boccaccini, A.R.; Díaz-Cuenca, A. Reticulated bioactive scaffolds with improved textural properties for bone tissue engineering: Nanostructured surfaces and porosity. J. Biomed. Mater. Res.-Part A 2014, 102, 2982–2992. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Borrego-González, S.; Romero-Sánchez, L.B.; Blázquez, J.; Díaz-Cuenca, A. Nanostructured hybrid device mimicking bone extracellular matrix as local and sustained antibiotic delivery system. Microporous Mesoporous Mater. 2018, 256, 165–176. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Histochemical responses at a biomaterial’s interface. J. Biomed. Mater. Res. 1974, 8, 49–64. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Sly, J.J.; Hill, R.G.; Mauro, J.C. Trends and perspectives on the commercialization of bioactive glasses. Acta Biomater. 2023, 160, 14–31. [Google Scholar] [CrossRef]
- Stanley, H.R.; Hall, M.B.; Colaizzi, F.; Clark, A.E. Residual alveolar ridge maintenance with a new endosseous implant material. J. Prosthet. Dent. 1987, 58, 607–613. [Google Scholar] [CrossRef]
- Wilson, J.; Clark, A.E.; Hall, M.; Hench, L.L. Tissue response to Bioglass endosseous ridge maintenance implants. J. Oral Implantol. 1993, 19, 295–302. [Google Scholar]
- Stanley, H.R.; Hall, M.B.; Clark, A.E.; King Iii, C.J.; Hench, L.L.; Berte, J.J. Using 45S5 Bioglass Cones as Endosseous Ridge Maintenance Implants to Prevent Alveolar Ridge Resorption: A 5-Year Evaluation. Int. J. Oral Maxillofac. Implant. 1997, 12, 95–105. [Google Scholar]
- Carinci, F.; Palmieri, A.; Martinelli, M.; Perrotti, V.; Piattelli, A.; Brunelli, G.; Arlotti, M.; Pezzetti, F. Genetic portrait of osteoblast-like cells cultured on PerioGlas. J. Oral Implantol. 2007, 33, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Abati, S.; Celestino, S.; Chiapasco, M.; Bosshardt, D.; Lang, N.P. Oral implants placed in bone defects treated with Bio-Oss®, Ostim®-Paste or PerioGlas: An experimental study in the rabbit tibiae. Clin. Oral Implant. Res. 2008, 19, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, A.; Nohlert, E.; Tegelberg, Å. Endodontic surgery with and without inserts of bioactive glass PerioGlas®—A clinical and radiographic follow-up. Oral Maxillofac. Surg. 2009, 13, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cancian, D.C.J.; Hochuli-Vieira, E.; Marcantonio, R.A.C.; Marcantonio, E., Jr. Use of BioGran and Calcitite in Bone Defects: Histologic Study in Monkeys (Cebus apella). Int. J. Oral Maxillofac. Implant. 1999, 14, 859–864. [Google Scholar]
- Trisi, P.; Rebaudi, A.; Calvari, F.; Lazzara, R.J. Sinus graft with biogran, autogenous bone, and PRP: A report of three cases with histology and micro-CT. Int. J. Periodontics Restor. Dent. 2006, 26, 113–125. [Google Scholar]
- Pereira, R.D.S.; Menezes, J.D.; Bonardi, J.P.; Griza, G.L.; Okamoto, R.; Hochuli-Vieira, E. Histomorphometric and immunohistochemical assessment of RUNX2 and VEGF of Biogran™ and autogenous bone graft in human maxillary sinus bone augmentation: A prospective and randomized study. Clin. Implant Dent. Relat. Res. 2017, 19, 867–875. [Google Scholar] [CrossRef]
- Frigério, P.B.; Gomes-Ferreira, P.H.S.; de Souza Batista, F.R.; Moura, J.; Júnior, I.R.G.; Botticelli, D.; Lisboa-Filho, P.N.; Okamoto, R. Effect of topical PTH 1-34 functionalized to biogran® in the process of alveolar repair in rats submitted to orchiectomy. Materials 2022, 15, 207. [Google Scholar] [CrossRef]
- Wotiz, A.W. Novabone may stimulate new bone growth as well as BMP-2. Adv. Mater. Process. 2005, 163, 60–61. [Google Scholar]
- Qiu, Z.; Yang, H.; Wu, J.; Wei, L.; Li, J. Ionic dissolution products of NovaBone® promote osteoblastic proliferation via influences on the cell cycle. J. Int. Med. Res. 2009, 37, 737–745. [Google Scholar] [CrossRef]
- Malik, R.; Gupta, A.; Bansal, P.; Sharma, R.; Sharma, S. Evaluation of Alveolar Ridge Height Gained by Vertical Ridge Augmentation Using Titanium Mesh and Novabone Putty in Posterior Mandible. J. Maxillofac. Oral Surg. 2020, 19, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Du, M.Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (NovaMin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar]
- Greenspan, D.C. NovaMin® and tooth sensitivity-An overview. J. Clin. Dent. 2010, 21, 61–65. [Google Scholar] [PubMed]
- LaTorre, G.; Greenspan, D.C. The role of ionic release from NovaMin® (calcium sodium phosphosilicate) in tubule occlusion: An exploratory in vitro study using radio-labeled isotopes. J. Clin. Dent. 2010, 21, 72–76. [Google Scholar] [PubMed]
- Earl, J.S.; Leary, R.K.; Muller, K.H.; Langford, R.M.; Greenspan, D.C. Physical and chemical characterization of dentin surface following treatment with NovaMin® technology. J. Clin. Dent. 2011, 22, 62–67. [Google Scholar]
- Layer, T.M. Development of a fluoridated, daily-use toothpaste containing novamin® technology for the treatment of dentin hypersensitivity. J. Clin. Dent. 2011, 22, 59–61. [Google Scholar]
- Tiwari, A.; Jain, R.K. Comparative Evaluation of White Spot Lesion incidence between NovaMin, Probiotic, And Fluoride containing Dentifrices during Orthodontic treatment Using Laser Fluorescence—A Prospective Randomized Controlled Clinical Trial. Clin. Investig. Orthod. 2023, 82, 75–82. [Google Scholar] [CrossRef]
- Tai, B.J.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Zhong, J.; Clark, A.E.; Du, M.Q. Anti-gingivitis effect of a dentifrice containing bioactive glass (NovaMin®) particulate. J. Clin. Periodontol. 2006, 33, 86–91. [Google Scholar] [CrossRef]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A novel fluoride containing bioactive glass paste is capable of re-mineralizing early caries lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef]
- Bakry, A.S.; Al-Harbi, N.; Al-Hadeethi, Y.; Abbassy, M.A.; Katturi, N.K.; Xin, B.; Roqan, I.S.; Mohammed, H.; Hill, R. Invitro evaluation of new treatment for dentin hypersensitivity using BioMin F and BioMin C. J. Non-Cryst. Solids 2023, 602, 122072. [Google Scholar] [CrossRef]
- Pintado-Palomino, K.; Peitl Filho, O.; Zanotto, E.D.; Tirapelli, C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J. Dent. 2015, 43, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Pintado-Palomino, K.; Tirapelli, C. The effect of home-use and in-office bleaching treatments combined with experimental desensitizing agents on enamel and dentin. Eur. J. Dent. 2015, 9, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. in vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Characterization of fluoride releasing restorative dental materials. Dent. Mater. J. 2018, 37, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive glass-based endodontic sealer as a promising root canal filling material without semisolid core materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Hanada, K.; Morotomi, T.; Washio, A.; Yada, N.; Matsuo, K.; Teshima, H.; Yokota, K.; Kitamura, C. In vitro and in vivo effects of a novel bioactive glass-based cement used as a direct pulp capping agent. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 161–168. [Google Scholar] [CrossRef]
- Ting, H.K.; Page, S.J.; Poologasundarampillai, G.; Chen, S.; Yu, B.; Hanna, J.V.; Jones, J.R. Phosphate content affects structure and bioactivity of sol-gel silicate bioactive glasses. Int. J. Appl. Glass Sci. 2017, 8, 372–382. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, H.E.; Lee, J.E.; Park, I.S.; Kim, H.G.; Kwon, J.; Kim, D.S. Effects of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef]
- Makanjuola, J.; Deb, S. Chemically Activated Glass-Ionomer Cements as Bioactive Materials in Dentistry: A Review. Prosthesis 2023, 5, 327–345. [Google Scholar] [CrossRef]
- Six, N.; Lasfargues, J.J.; Goldberg, M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J. Dent. 2000, 28, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Marczuk-Kolada, G.; Waszkiel, D.; Luczaj-Cepowicz, E.; Kierklo, A.; Pawińska, M.; Mystkowska, J. The effect of glass ionomer cement Fuji IX on the hard tissues of teeth treated by sparing methods (ART and CMCR). Adv. Med. Sci. 2006, 51 (Suppl. 1), 138–141. [Google Scholar]
- Munhoz, T.; Karpukhina, N.; Hill, R.G.; Law, R.V.; De Almeida, L.H. Setting of commercial glass ionomer cement Fuji IX by 27Al and 19F MAS-NMR. J. Dent. 2010, 38, 325–330. [Google Scholar] [CrossRef]
- Kukreja, R.; Singla, S.; Bhadoria, N.; Pawar, P.; Gupta, K.; Khandelwal, D.; Dewani, N. An In Vitro Study to Compare the Release of Fluoride from Glass Ionomer Cement (Fuji IX) and Zirconomer. Int. J. Clin. Pediatr. Dent. 2022, 15, 35–57. [Google Scholar] [PubMed]
- Hosoya, Y.; García-Godoy, F. Bonding mechanism of Ketac-Molar Aplicap and Fuji IX GP to enamel and dentin. Am. J. Dent. 1998, 11, 235–239. [Google Scholar]
- Peez, R.; Frank, S. The physical-mechanical performance of the new Ketac™ Molar Easymix compared to commercially available glass ionomer restoratives. J. Dent. 2006, 34, 582–587. [Google Scholar] [CrossRef]
- Zainuddin, N.; Karpukhina, N.; Law, R.V.; Hill, R.G. Characterisation of a remineralising Glass Carbomer® ionomer cement by MAS-NMR Spectroscopy. Dent. Mater. 2012, 28, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Olegário, I.C.; Malagrana, A.P.V.F.P.; Kim, S.S.H.; Hesse, D.; Tedesco, T.K.; Calvo, A.F.B.; Camargo, L.B.; Raggio, D.P. Mechanical properties of high-viscosity glass ionomer cement and nanoparticle glass carbomer. J. Nanomater. 2015, 16, 37. [Google Scholar] [CrossRef]
- Hasan, A.M.H.R.; Sidhu, S.K.; Nicholson, J.W. Fluoride release and uptake in enhanced bioactivity glass ionomer cement (“glass carbomer™”) compared with conventional and resin-modified glass ionomer cements. J. Appl. Oral Sci. 2019, 27. [Google Scholar] [CrossRef]
- Mahmoud, R.; Ibrahim, A.; Elzoghby, A.; Shaalan, O. Clinical evaluation of Carbomer compared with high viscosity glass ionomer in restoration of root caries in geriatric patients: A randomized controlled trial. J. Int. Oral Health 2022, 14, 118–127. [Google Scholar]
- Dhummarungrong, S.; Moore, B.K.; Avery, D.R. Properties related to strength and resistance to abrasion of VariGlass VLC, Fuji II L.C. Ketac-Silver, and Z-100 composite resin. ASDC J. Dent. Child. 1994, 61, 17–20. [Google Scholar]
- Mallow, P.K.; Durward, C.S.; Klaipo, M. Restoration of permanent teeth in young rural children in Cambodia using the atraumatic restorative treatment (ART) technique and Fuji II glass ionomer cement. Int. J. Paediatr. Dent. 1998, 8, 35–40. [Google Scholar] [CrossRef]
- Ghashami, M.; Nouri, F.; Heidari, S.; Mohammadpour, M.; Mirzadeh, M.; Asgari, N. Comparative evaluation of net setting time and radiopacity in Fuji II (GC-Japan) restorative glass ionomer and Iranian glass ionomer. Dent. Res. J. 2022, 19, 109. [Google Scholar]
- Croll, T.P.; Cavanaugh, R.R. Vitremer cement for Class I restoration of permanent teeth. Pract. Periodontics Aesthetic Dent. PPAD 1994, 6, 25–32; quiz 33. [Google Scholar]
- Pereira, A.C.; Basting, R.T.; Pinelli, C.; Meneghim, M.D.C.; Werner, C.W. Retention and caries prevention of Vitremer and Ketac-Bond used as occlusal sealants. Am. J. Dent. 1999, 12, 62–64. [Google Scholar] [PubMed]
- Donly, K.J.; Liu, J.A. Dentin and enamel demineralization inhibition at restoration margins of Vitremer, Z 100 and Cention N. Am. J. Dent. 2018, 31, 166–168. [Google Scholar] [PubMed]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic material for bone, periodontal, and dental tissue regeneration: Where are we now, and where are we heading next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Braly, T.E., Jr. The use of Synthograft in periodontal bony defects. J. Oral Implantol. 1983, 10, 611–618. [Google Scholar] [PubMed]
- Hoexter, D.L. The use of tricalcium (Synthograft). Part I: Its use in extensive periodontal defects. J. Oral Implantol. 1983, 10, 599–610. [Google Scholar]
- Louise, F.; Fourel, J.; Roig, R. Bioceramics in periodontal bone surgery. Clinical trial of Synthograft. J. Parodontol. 1985, 4, 277–285. [Google Scholar] [PubMed]
- Shen, T.C. Tricalcium phosphate (Synthograft) in a vital homogeneous tooth transplant. Gen. Dent. 1985, 33, 518–519. [Google Scholar]
- Rothstein, S.S.; Paris, D.; Sage, B. Use of durapatite for the rehabilitation of resorbed alveolar ridges. J. Am. Dent. Assoc. (1939) 1984, 109, 571–574. [Google Scholar] [CrossRef]
- Greenstein, G.; Jaffin, R.A.; Hilsen, K.L.; Berman, C.L. Repair of anterior gingival deformity with durapatite. A case report. J. Periodontol. 1985, 56, 200–203. [Google Scholar] [CrossRef]
- Koslen, R.H. Two-stage maxillary ridge augmentation using durapatite. J. Oral Implantol. 1987, 13, 428–441. [Google Scholar]
- Yukna, R.A.; Mayer, E.T.; Amos, S.M. 5-year evaluation of durapatite ceramic alloplastic implants in periodontal osseous defects. J. Periodontol. 1989, 60, 544–551. [Google Scholar] [CrossRef]
- Yukna, R.A.; Cassingham, R.J.; Caudill, R.F.; Evans, G.H.; Miller, S.; Mayer, E.T.; Simon, J.F. Six month evaluation of Calcitite (hydroxyapatite ceramic) in periodontal osseous defects. Int. J. Periodontics Restor. Dent. 1986, 6, 34–45. [Google Scholar]
- Shepard, W.K.; Bohat, O.; Joseph, C.E.; LoPiccolo, P.; Bernick, S. Human clinical and histological responses to a Calcitite implant in intraosseous lesions. Int. J. Periodontics Restor. Dent. 1986, 6, 46–63. [Google Scholar]
- Kwon, H.J. Ridge augmentation with hydroxylapatite (Alveograf). CDS Rev. 1984, 77, 29–33. [Google Scholar]
- Zijderveld, S.A.; Zerbo, I.R.; Van Den Bergh, J.P.A.; Schulten, E.A.J.M.; Ten Bruggenkate, C.M. Maxillary sinus floor augmentation using a β-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int. J. Oral Maxillofac. Implant. 2005, 20, 432–440. [Google Scholar]
- Bokan, I.; Bill, J.S.; Schlagenhauf, U. Primary flap closure combined with Emdogain® alone or Emdogain® and Cerasorb® in the treatment of intra-bony defects. J. Clin. Periodontol. 2006, 33, 885–893. [Google Scholar] [CrossRef]
- Plenk, H., Jr.; Chyplyk, L.; Lederer, J. Unique bone substitution of TCP-granulates (Cerasorb®) during degradation in human sinus floor elevations. Eur. Cells Mater. 2007, 14 (Suppl. 1), 24. [Google Scholar]
- Ogose, A.; Kondo, N.; Umezu, H.; Hotta, T.; Kawashima, H.; Tokunaga, K.; Ito, T.; Kudo, N.; Hoshino, M.; Gu, W.; et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion®) in human bones. Biomaterials 2006, 27, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Licina, P.; Coughlan, M.; Johnston, E.; Pearcy, M. Comparison of Silicate-Substituted Calcium Phosphate (Actifuse) with Recombinant Human Bone Morphogenetic Protein-2 (Infuse) in Posterolateral Instrumented Lumbar Fusion. Glob. Spine J. 2015, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Spongostan™ leads to increased regeneration of a rat calvarial critical size defect compared to nanobone® and actifuse. Materials 2021, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Koduru, S.; Aghanashini, S.; Nadiger, S.; Apoorva, S.; Bhat, D.; Puvvalla, B. A clinical and radiographic evaluation of the efficacy of nanohydroxyapatite (Sybograf™) versus bioactive calcium phosphosilicate putty (Novabone®) in the treatment of human periodontal infrabony defects: A randomized clinical trial. Contemp. Clin. Dent. 2019, 10, 16–23. [Google Scholar]
- Gerike, W.; Bienengräber, V.; Henkel, K.O.; Bayerlein, T.; Proff, P.; Gedrange, T.; Gerber, T. The manufacture of synthetic non-sintered and degradable bone grafting substitutes. Folia Morphol. 2006, 65, 54–55. [Google Scholar]
- Götz, W.; Gerber, T.; Michel, B.; Lossdörfer, S.; Henkel, K.O.; Heinemann, F. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone®) osteogenesis: A study on biopsies from human jaws. Clin. Oral Implant. Res. 2008, 19, 1016–1026. [Google Scholar] [CrossRef]
- Meier, J.; Wolf, E.; Bienengräber, V. Application of the synthetic nanostructured bone grafting material NanoBone in sinus floor elevation. Implantologie 2008, 16, 301–314. [Google Scholar]
- Jasrasaria, N.; Tikku, A.P.; Bharti, R. Analysis of porosity, sealer dissolution and apical extrusion of endodontic sealers: A micro computed tomography study. J. Oral Biol. Craniofacial Res. 2023, 13, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Lee, S.J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef]
- Camilleri, J.; Pitt Ford, T.R. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int. Endod. J. 2006, 39, 747–754. [Google Scholar] [CrossRef]
- Dammaschke, T.; Gerth, H.U.V.; Züchner, H.; Schäfer, E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent. Mater. 2005, 21, 731–738. [Google Scholar] [CrossRef]
- Song, J.S.; Mante, F.K.; Romanow, W.J.; Kim, S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 809–815. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Camilleri, J. Investigation of Biodentine as dentine replacement material. J. Dent. 2013, 41, 600–610. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W.X. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef]
- Martens, L.; Rajasekharan, S.; Cauwels, R. Pulp management after traumatic injuries with a tricalcium silicate-based cement (Biodentine™): A report of two cases, up to 48 months follow-up. Eur. Arch. Paediatr. Dent. 2015, 16, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bani, M.; Aktaş, N.; Çinar, Ç.; Odabaş, M.E. The clinical and radiographic success of primary molar pulpotomy using biodentine" and mineral trioxide aggregate: A 24-month randomized clinical trial. Pediatr. Dent. 2017, 39, 284–288. [Google Scholar] [PubMed]
- Patel, S.; Vincer, L. Case report: Single visit indirect pulp cap using biodentine. Dent. Update 2017, 44, 141–145. [Google Scholar] [CrossRef]
- Caruso, S.; Dinoi, T.; Marzo, G.; Campanella, V.; Giuca, M.R.; Gatto, R.; Pasini, M. Clinical and radiographic evaluation of biodentine versus calcium hydroxide in primary teeth pulpotomies: A retrospective study. BMC Oral Health 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.; Noueiri, B.E. Biodentine™ Pulpotomy in Stage I Primary Molars: A 12-month Follow-up. Int. J. Clin. Pediatr. Dent. 2022, 15, 660–666. [Google Scholar] [PubMed]
- Kim, M.; Yang, W.; Kim, H.; Ko, H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and endocem MTA cements. J. Endod. 2014, 40, 1649–1653. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Carvalho, N.K.; Guberman, M.R.D.C.L.; Prado, M.; Senna, P.M.; Souza, E.M.; De-Deus, G. Push-out Bond Strength of Fast-setting Mineral Trioxide Aggregate and Pozzolan-based Cements: ENDOCEM MTA and ENDOCEM Zr. J. Endod. 2017, 43, 801–804. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Collado-González, M.; López-García, S.; García-Bernal, D.; Moraleda, J.M.; Lozano, A.; Forner, L.; Murcia, L.; Oñate-Sánchez, R.E. Evaluation of changes in ion release and biological properties of NeoMTA-Plus and Endocem-MTA exposed to an acidic environment. Int. Endod. J. 2019, 52, 1196–1209. [Google Scholar] [CrossRef]
- Sharma, V.; Nawal, R.R.; Augustine, J.; Urs, A.B.; Talwar, S. Evaluation of Endosequence Root Repair Material and Endocem MTA as direct pulp capping agents: An in vivo study. Aust. Endod. J. 2022, 48, 251–257. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Reis, C.; Brandão, C.; Fidel, S.; Fidel, R.A.S. The Ability of Portland Cement, MTA, and MTA Bio to Prevent Through-and-Through Fluid Movement in Repaired Furcal Perforations. J. Endod. 2007, 33, 1374–1377. [Google Scholar] [CrossRef]
- De-Deus, G.; Audi, C.; Murad, C.; Fidel, S.; Fidel, R. Similar expression of through-and-through fluid movement along orthograde apical plugs of MTA Bio™ and white Portland cement. Int. Endod. J. 2008, 41, 1047–1053. [Google Scholar] [CrossRef]
- Adl, A.; Shojaee, N.S.; Pourhatami, N. Evaluation of the dislodgement resistance of a new pozzolan-based cement (endoseal MTA) compared to proroot MTA and biodentine in the presence and absence of blood. Scanning 2019, 2019, 3863069. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.T.; Leong, X.F.; Tew, I.M.; Kumolosasi, E.; Wong, L. In Vitro Evaluation of the Antibacterial Activity of EndoSeal MTA, iRoot SP, and AH Plus against Planktonic Bacteria. Materials 2022, 15, 2012. [Google Scholar] [CrossRef]
- Vitti, R.P.; Prati, C.; Silva, E.J.N.L.; Sinhoreti, M.A.C.; Zanchi, C.H.; De Souza E Silva, M.G.; Ogliari, F.A.; Piva, E.; Gandolfi, M.G. Physical properties of MTA fillapex sealer. J. Endod. 2013, 39, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Yavari, H.; Ghasemi, N.; Divband, B.; Rezaei, Y.; Jabbari, G.; Payahoo, S. The effect of photodynamic therapy and polymer solution containing nano-particles of Ag /ZnO on push-out bond strength of the sealers AH-Plus and MTA Fillapex. J. Clin. Exp. Dent. 2017, 9, e1109–e1114. [Google Scholar] [CrossRef]
- Saraiva, J.A.; Da Fonseca, T.S.; Da Silva, G.F.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Cerri, P.S. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed. Mater. 2018, 13, 035002. [Google Scholar] [CrossRef]
- Delfino, M.M.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Cerri, P.S. Immunoinflammatory response and bioactive potential of GuttaFlow bioseal and MTA Fillapex in the rat subcutaneous tissue. Sci. Rep. 2020, 10, 7173. [Google Scholar] [CrossRef]
- Elsayed, M.A.; Hassanien, E.E.; Elgendy, A.A.E. Ageing of TotalFill BC Sealer and MTA Fillapex in Simulated Body Fluid. Eur. Endod. J. 2021, 6, 183–188. [Google Scholar]
- Kumar, A.; Kour, S.; Kaul, S.; Malik, A.; Dhani, R.; Kaul, R. Cytotoxicity evaluation of Bio-C, CeraSeal, MTA-Fillapex, and AH Plus root canal sealers by microscopic and 3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. J. Conserv. Dent. 2023, 26, 73–78. [Google Scholar]
- Camilleri, J.; Laurent, P.; About, I. Hydration of biodentine, theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014, 40, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.Z.; Rabi, T. TheraCal LC: From Biochemical and Bioactive Properties to Clinical Applications. Int. J. Dent. 2018, 2018, 3484653. [Google Scholar] [CrossRef] [PubMed]
- Alazrag, M.A.; Abu-Seida, A.M.; El-Batouty, K.M.; El Ashry, S.H. Marginal adaptation, solubility and biocompatibility of TheraCal LC compared with MTA-angelus and biodentine as a furcation perforation repair material. BMC Oral Health 2020, 20, 298. [Google Scholar] [CrossRef]
- Bala Anusha, D.; Prathima, G.; Sanguida, A.; Nandakumar, S.; Kavitha, M. Role of TheraCal LC in pediatric dentistry: A narrative review. J. Int. Oral Health 2022, 14, 111–117. [Google Scholar]
- Kayad, M.; Koura, A.; El-Nozahy, A. A comparative histological study of the effect of TheraCal LC and biodentine on direct pulp capping in rabbits: An experimental study. Clin. Oral Investig. 2023, 27, 1013–1022. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeek, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef]
- Zhang, H.; Pappen, F.G.; Haapasalo, M. Dentin Enhances the Antibacterial Effect of Mineral Trioxide Aggregate and Bioaggregate. J. Endod. 2009, 35, 221–224. [Google Scholar] [CrossRef]
- Tuna, E.B.; Dinçol, M.E.; Gençay, K.; Aktören, O. Fracture resistance of immature teeth filled with BioAggregate, mineral trioxide aggregate and calcium hydroxide. Dent. Traumatol. 2011, 27, 174–178. [Google Scholar] [CrossRef]
- Kum, K.Y.; Kim, E.C.; Yoo, Y.J.; Zhu, Q.; Safavi, K.; Bae, K.S.; Chang, S.W. Trace metal contents of three tricalcium silicate materials: MTA Angelus, Micro Mega MTA and Bioaggregate. Int. Endod. J. 2014, 47, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Bayram, E.; Bayram, H.M. Fracture resistance of immature teeth filled with mineral trioxide aggregate, bioaggregate, and biodentine. Eur. J. Dent. 2016, 10, 220–224. [Google Scholar] [CrossRef]
- Eram, A.; Zuber, M.; Keni, L.G.; Kalburgi, S.; Naik, R.; Bhandary, S.; Amin, S.; Badruddin, I.A. Finite element analysis of immature teeth filled with MTA, Biodentine and Bioaggregate. Comput. Methods Programs Biomed. 2020, 190, 105356. [Google Scholar] [CrossRef] [PubMed]
- Siboni, F.; Taddei, P.; Prati, C.; Gandolfi, M.G. Properties of NeoMTA plus and MTA plus cements for endodontics. Int. Endod. J. 2017, 50, e83–e94. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef]
- Lozano-Guillén, A.; López-García, S.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Lozano, A.; Llena, C.; Forner, L. Comparative cytocompatibility of the new calcium silicate-based cement NeoPutty versus NeoMTA Plus and MTA on human dental pulp cells: An in vitro study. Clin. Oral Investig. 2022, 26, 7219–7228. [Google Scholar] [CrossRef]
- Sharma, A.; Thomas, M.; Shetty, N.; Srikant, N. Evaluation of indirect pulp capping using pozzolan-based cement (ENDOCEM-Zr®) and mineral trioxide aggregate—A randomized controlled trial. J. Conserv. Dent. 2020, 23, 152–157. [Google Scholar]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Tomás-Catalá, C.J.; Santos, J.M.; Llena, C.; Lozano, A.; Murcia, L.; Forner, L. Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int. Endod. J. 2020, 53, 1216–1228. [Google Scholar] [CrossRef]
- Mann, A.; Zeng, Y.; Kirkpatrick, T.; van der Hoeven, R.; Silva, R.; Letra, A.; Chaves de Souza, L. Evaluation of the Physicochemical and Biological Properties of EndoSequence BC Sealer HiFlow. J. Endod. 2022, 48, 123–131. [Google Scholar] [CrossRef]
- Ersahan, S.; Aydin, C. Dislocation resistance of iRoot SP, a calcium silicate-based sealer, from radicular dentine. J. Endod. 2010, 36, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Forghani, M.; Gharechahi, M.; Karimpour, S. In vitro evaluation of tooth discolouration induced by mineral trioxide aggregate Fillapex and iRoot SP endodontic sealers. Aust. Endod. J. 2016, 42, 99–103. [Google Scholar] [CrossRef]
- Atav Ates, A.; Dumani, A.; Yoldas, O.; Unal, I. Post-obturation pain following the use of carrier-based system with AH Plus or iRoot SP sealers: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 3053–3061. [Google Scholar] [CrossRef]
- Bhor, S.; Rao, A.S.; Shah, U.; Mathur, M.; Reda, R.; Pagnoni, F.; Testarelli, L.; Luke, A.M.; Pawar, A.M. Comparative Evaluation of the Sealing Ability of a BioCeramic Sealer (iRoot SP) with AH Plus Sealer with Root Canal Dentin Using Three Different Techniques of Sealer Application: A Combined Dye Extraction and Scanning Electron Microscope Study. J. Compos. Sci. 2023, 7, 106. [Google Scholar] [CrossRef]
- Guneser, M.B.; Akman, M.; Kolcu, İ.B.; Eldeniz, A.U. Fracture resistance of roots obturated with a novel calcium silicate-based endodontic sealer (BioRoot RCS). J. Adhes. Sci. Technol. 2016, 30, 2420–2428. [Google Scholar] [CrossRef]
- Jeanneau, C.; Giraud, T.; Laurent, P.; About, I. BioRoot RCS Extracts Modulate the Early Mechanisms of Periodontal Inflammation and Regeneration. J. Endod. 2019, 45, 1016–1023. [Google Scholar] [CrossRef]
- Bianco, E.; Calvelli, C.; Citterio, C.L.; Pellegatta, A.; Venino, P.M.; Maddalone, M. Evaluation with micro-CT of the canal seal made with two different bioceramic cements: Guttaflow bioseal and bioroot RCS. J. Contemp. Dent. Pract. 2020, 21, 359–366. [Google Scholar] [PubMed]
- Alsubait, S.; Alhathlool, N.; Alqedairi, A.; Alfawaz, H. A micro-computed tomographic evaluation of retreatability of BioRoot RCS in comparison with AH Plus. Aust. Endod. J. 2022, 47, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. Physicochemical parameters-hydration performance relationship of the new endodontic cement MTA Repair HP. J. Clin. Exp. Dent. 2019, 11, e739–e744. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. A microstructure insight of MTA repair HP of rapid setting capacity and bioactive response. Materials 2020, 13, 1641. [Google Scholar] [CrossRef]
- Graham, T. On the properties of silicic acid and other analogous colloidal substances. J. Chem. Soc. 1864, 17, 318–327. [Google Scholar] [CrossRef]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol-gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef]
- Valdés-Sánchez, L.; Borrego-González, S.; Montero-Sánchez, A.; Massalini, S.; de la Cerda, B.; Díaz-Cuenca, A.; Díaz-Corrales, F.J. Mesoporous Silica-Based Nanoparticles as Non-Viral Gene Delivery Platform for Treating Retinitis Pigmentosa. J. Clin. Med. 2022, 11, 2170. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Cejudo-Guillén, M.; Ramiro-Gutiérrez, M.L.; Labrador-Garrido, A.; Díaz-Cuenca, A.; Pozo, D. Nanoporous silica microparticle interaction with toll-like receptor agonists in macrophages. Acta Biomater. 2012, 8, 4295–4303. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Balas, F.; Román, J.; Salinas, A.J.; Vallet-Regí, M. Influence of composition and surface characteristics on the in vitro bioactivity of SiO2-CaO-P2O5-MgO sol-gel glasses. J. Biomed. Mater. Res. 1999, 47, 170–175. [Google Scholar] [CrossRef]
- Rámila, A.; Balas, F.; Vallet-Regí, M. Synthesis routes for bioactive sol-gel glasses: Alkoxides versus nitrates. Chem. Mater. 2002, 14, 542–548. [Google Scholar] [CrossRef]
- Szu, S.P.; Klein, L.C.; Greenblatt, M. Effect of precursors on the structure of phosphosilicate gels: 29Si and 31P MAS-NMR study. J. Non-Cryst. Solids 1992, 143, 21–30. [Google Scholar] [CrossRef]
- Vargas Machuca Bueno, O.M.; San-Miguel, M.A.; Bertran, C.A.; Zacarias da Silva, E.; Lopes, J.H. Unveiling the mechanism of the triethyl phosphate hydrolysis reaction in the synthesis of the sol-gel-derived 58S bioactive glass. Mater. Today Chem. 2022, 24, 100929. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.; Drevet, R.; Lemelle, A.; Ben Jaber, N.; Tara, A.; El Btaouri, H.; Benhayoune, H. A new sol-gel synthesis of 45S5 bioactive glass using an organic acid as catalyst. Mater. Sci. Eng. C 2015, 47, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chitra, S.; Bargavi, P.; Balasubramaniam, M.; Chandran, R.R.; Balakumar, S. Impact of copper on in-vitro biomineralization, drug release efficacy and antimicrobial properties of bioactive glasses. Mater. Sci. Eng. C 2020, 109, 110598. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.G.; Manoharan, A.; Anbarasu, A. Preclinical evaluation of sol-gel synthesized modulated 45s5-bioglass based biodegradable bone graft intended for alveolar bone regeneration. J. Hard Tissue Biol. 2021, 30, 303–308. [Google Scholar] [CrossRef]
- Palza Cordero, H.; Castro Cid, R.; Diaz Dosque, M.; Cabello Ibacache, R.; Palma Fluxá, P. Li-doped bioglass® 45S5 for potential treatment of prevalent oral diseases. J. Dent. 2021, 105, 103575. [Google Scholar] [CrossRef]
- Esfahanizadeh, N.; Nourani, M.R.; Bahador, A.; Akhondi, N.; Montazeri, M. The Anti-biofilm Activity of Nanometric Zinc doped Bioactive Glass against Putative Periodontal Pathogens: An in vitro Study. Biomed. Glas. 2020, 4, 95–107. [Google Scholar] [CrossRef]
- Shankhwar, N.; Kothiyal, G.P.; Srinivasan, A. Influence of phosphate precursors on the structure, crystallization behaviour and bioactivity of sol-gel derived 45S5 bioglass. RSC Adv. 2015, 5, 100762–100768. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Victoria Ragel, C.; Salinas, A.J. Glasses with medical applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Zabidi, N.F.; Lai, J.C.H.; Noorani, T.Y. Effect of ammonia catalyst on the morphological structures of sol–gel-derived bioactive glass 58S for dental application. Oral Sci. Int. 2022, 19, 167–172. [Google Scholar] [CrossRef]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Firzok, H.; Zahid, S.; Asad, S.; Manzoor, F.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoridated and non-fluoridated bioactive glass ceramics-based dental adhesives: Compositional effect on re-mineralization around orthodontic brackets. J. Non-Cryst. Solids 2019, 521, 119469. [Google Scholar] [CrossRef]
- Gul, H.; Zahid, S.; Zahid, S.; Kaleem, M.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoride-doped bioactive glass powders: Structural and long-term fluoride release/pH analysis. J. Non-Cryst. Solids 2018, 498, 216–222. [Google Scholar] [CrossRef]
- Lins, C.E.C.; Oliveira, A.A.R.; Gonzalez, I.; Macedo, W.A.A.; Pereira, M.M. Structural analysis of fluorine-containing bioactive glass nanoparticles synthesized by sol–gel route assisted by ultrasound energy. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Jmal, N.; Bouaziz, J. Fluorapatite-glass-ceramics obtained by heat treatment of a gel synthesized by the sol-gel processing method. Mater. Lett. 2018, 215, 280–283. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Manafi, S.; Sharifianjazi, F. The effect of Ag2O and MgO dopants on the bioactivity, biocompatibility, and antibacterial properties of 58S bioactive glass synthesized by the sol-gel method. J. Non-Cryst. Solids 2023, 606, 122189. [Google Scholar] [CrossRef]
- Correia, B.L.; Gomes, A.T.P.C.; Noites, R.; Ferreira, J.M.F.; Duarte, A.S. New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials 2022, 12, 1577. [Google Scholar] [CrossRef]
- Dey, P.; Pal, S.K.; Sarkar, R. Effect of Alumina Addition on 45S5 Bioglass. Trans. Indian Ceram. Soc. 2014, 73, 105–109. [Google Scholar] [CrossRef]
- Prabhu, M.; Kavitha, K.; Sutha, S.; Manivasakan, P.; Rajendran, V.; Kulandaivelu, P.; Alameh, K. Bioactivity of zirconium-substituted nanobioactive glass particles. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 92–96. [Google Scholar] [CrossRef]
- Smith, S.; Elkashty, O.; Tamimi, F.; Tran, S.D.; Cerruti, M. Titanium-Containing Silicate-Based Sol-Gel Bioactive Glass: Development, Characterization, and Applications. Langmuir 2021, 37, 14243–14253. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Vitale-Brovarone, C.; Fiorilli, S. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics 2022, 14, 2636. [Google Scholar] [CrossRef]
- Huang, W.; Yang, J.; Feng, Q.; Shu, Y.; Liu, C.; Zeng, S.; Guan, H.; Ge, L.; Pathak, J.L.; Zeng, S. Mesoporous Bioactive Glass Nanoparticles Promote Odontogenesis and Neutralize Pathophysiological Acidic pH. Front. Mater. 2020, 7, 241. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Dong, Y. Effect of mesoporous bioactive glass on odontogenic differentiation of human dental pulp stem cells. PeerJ 2021, 9, e12421. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Qian, J.; Luo, L.; Zeng, J.; Sa, B.; Zhan, X.; Wang, J.; Sheng, L.; Zheng, Y. Effect of nitrogen on the structure evolution and biological properties of mesoporous bioactive glass nanospheres: Experiments and simulations. J. Non-Cryst. Solids 2022, 578, 121329. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, M.S.; Mahapatra, C.; Kim, H.W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS ONE 2016, 11, e0150727. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, T.H.; Kim, M.; Eltohamy, M.; Won, J.E.; Lee, E.J.; Kim, H.W. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale 2012, 4, 7475–7488. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Chen, W.; Luo, L.; Tong, W.; Wen, C.; Zhan, X.; Sa, B. Effect of B2O3 on the structural and in vitro biological assessment of mesoporous bioactive glass nanospheres. J. Am. Ceram. Soc. 2021, 104, 3058–3072. [Google Scholar] [CrossRef]
- Lee, J.H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef]
- Kung, J.C.; Wang, W.H.; Chiang, Y.C.; Yang-Wang, Y.T.; Wang, Y.C.; Chen, W.C.; Shih, C.J. The antibacterial and remineralization effect of silver-containing mesoporous bioactive glass sealing and er-yag laser on dentinal tubules treated in a streptococcus mutans cultivated environment. Pharmaceuticals 2021, 14, 1124. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Wang, Y.C.; Kung, J.C.; Shih, C.J. Antibacterial silver-containing mesoporous bioglass as a dentin remineralization agent in a microorganism-challenged environment. J. Dent. 2021, 106, 103563. [Google Scholar] [CrossRef]
- Romero-Sánchez, L.B.; Marí-Beffa, M.; Carrillo, P.; Medina, M.A.; Díaz-Cuenca, A. Copper-containing mesoporous bioactive glass promotes angiogenesis in an in vivo zebrafish model. Acta Biomater. 2018, 68, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.R.; West, N.X.; Su, B. Synthesis of nanobioglass and formation of apatite rods to occlude exposed dentine tubules and eliminate hypersensitivity. Acta Biomater. 2010, 6, 3740–3746. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Q.; Lin, C.; Li, X.; Chen, X.; Hu, Q. Facile synthesis and characterization of novel rapid-setting spherical sub-micron bioactive glasses cements and their biocompatibility in vitro. Mater. Sci. Eng. C 2017, 75, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.H.; Kim, H.W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin-Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Dutra, C.E.A.; Pereira, M.M.; Serakides, R.; Rezende, C.M.F. In vivo evaluation of bioactive glass foams associated with platelet-rich plasma in bone defects. J. Tissue Eng. Regen. Med. 2008, 2, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.A.; Zhu, X.; Morrow, B.R.; Thamma, U.; Kowal, T.J.; Moawad, H.M.; Falk, M.M.; Jain, H.; Huang, G.T.J. Potential of tailored amorphous multiporous calcium silicate glass for pulp capping regenerative endodontics—A preliminary assessment. J. Dent. 2021, 109, 103655. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li + ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, L.B.; Borrego-González, S.; Díaz-Cuenca, A. High surface area biopolymeric-ceramic scaffolds for hard tissue engineering. Biomed. Phys. Eng. Express 2017, 3, 035012. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of calcium precursor on the bioactivity and biocompatibility of sol-gel-derived glasses. J. Funct. Biomater. 2019, 10, 13. [Google Scholar] [CrossRef]
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1a and TNF-a pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef]
- Song, H.K.; Yoo, K.H.; Yoon, S.Y.; Na, H.S.; Chung, J.; Son, W.S.; Lee, S.M.; Kim, Y.I. In vitro effect of gallium-doped bioactive glass on enamel anti-demineralization and bond strength of orthodontic resins. Appl. Sci. 2019, 9, 4918. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Merzougui, M.; Mezahi, F.Z.; Dakhouche, A.; Kherifi, D.; Sahnoune, F. Improvement of the reactivity of triethyl phosphate and structural behavior of hydroxyapatite versus the synthesis conditions by sol–gel route. Chem. Pap. 2022, 76, 1045–1061. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, A.; Barui, A.; Basu, B. Epithelial cell functionality on electroconductive Fe/Sr co-doped biphasic calcium phosphate. J. Biomater. Appl. 2019, 33, 1035–1052. [Google Scholar] [CrossRef]
- Lett, J.A.; Sundareswari, M.; Ravichandran, K.; Latha, M.B.; Sagadevan, S.; Bin Johan, M.R. Tailoring the morphological features of sol–gel synthesized mesoporous hydroxyapatite using fatty acids as an organic modifier. RSC Adv. 2019, 9, 6228–6240. [Google Scholar] [CrossRef]
- Díaz, A.; López, T.; Manjarrez, J.; Basaldella, E.; Martínez-Blanes, J.M.; Odriozola, J.A. Growth of hydroxyapatite in a biocompatible mesoporous ordered silica. Acta Biomater. 2006, 2, 173–179. [Google Scholar] [CrossRef]
- Shu, C.; Wenjuan, Z.; Xu, G.; Wei, Z.; Wei, J.; Dongmei, W. Dissolution behavior and bioactivity study of glass ceramic scaffolds in the system of CaO-P2O5-Na2O-ZnO prepared by sol-gel technique. Mater. Sci. Eng. C 2010, 30, 105–111. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Feng, W.; Mu-Sen, L.; Yu-Peng, L.; Yong-Xin, Q. A simple sol-gel technique for preparing hydroxyapatite nanopowders. Mater. Lett. 2005, 59, 916–919. [Google Scholar] [CrossRef]
- Gan, L.; Pilliar, R. Calcium phosphate sol–gel-derived thin films on porous-surfaced implants for enhanced osteoconductivity. Part I: Synthesis and characterization. Biomaterials 2004, 25, 5303–5312. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E. Nanofiber generation of hydroxyapatite and fluor-hydroxyapatite bioceramics. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2006, 77, 323–328. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Yu, W.-X.; Hwang, P.-A.; Huang, S.-S.; Lin, H.-M.; Hsu, Y.-W.; Hsu, F.-Y. Fabrication and characterization of strontium-substituted hydroxyapatite-CaO-CaCO3 nanofibers with a mesoporous structure as drug delivery carriers. Pharmaceutics 2018, 10, 179. [Google Scholar] [CrossRef]
- Ramiro-Gutiérrez, M.L.; Santos-Ruiz, L.; Borrego-González, S.; Becerra, J.; Díaz-Cuenca, A. In vitro stimulation of MC3T3-E1cells and sustained drug delivery by a hierarchical nanostructured SiO2-CaO-P2O5 scaffold. Microporous Mesoporous Mater. 2016, 229, 31–43. [Google Scholar] [CrossRef]
- Besinis, A.; Van Noort, R.; Martin, N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent. Mater. 2012, 28, 1012–1023. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rebelo, A.H.S.; Lemos, A.F.; Rocha, J.H.G.; Ventura, J.M.G.; Ferreira, J.M.F. Suitability evaluation of sol-gel derived Si-substituted hydroxyapatite for dental and maxillofacial applications through in vitro osteoblasts response. Dent. Mater. 2008, 24, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, U.; Rajeswari, S. Influence of process parameters on the sol-gel synthesis of nano hydroxyapatite using various phosphorus precursors. J. Sol-Gel Sci. Technol. 2012, 63, 45–55. [Google Scholar] [CrossRef]
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55, 710–777. [Google Scholar] [CrossRef] [PubMed]
- Ylmén, R.; Jäglid, U.; Steenari, B.M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Liu, W.; Chang, J.; Yue, Z. Physicochemical properties and biocompatibility of tricalcium and dicalcium silicate composite cements after hydration. Int. J. Appl. Ceram. Technol. 2011, 8, 560–565. [Google Scholar] [CrossRef]
- Nettleship, I.; Slavick, K.G.; Kim, Y.J.; Kriven, W.M. Phase Transformations in Dicalcium Silicate: I, Fabrication and Phase Stability of Fine-Grained β Phase. J. Am. Ceram. Soc. 1992, 75, 2400–2406. [Google Scholar] [CrossRef]
- Hong, S.H.; Young, J.F. Hydration kinetics and phase stability of dicalcium silicate synthesized by the Pechini process. J. Am. Ceram. Soc. 1999, 82, 1681–1686. [Google Scholar] [CrossRef]
- Zhao, W.; Chang, J. Sol–gel synthesis and in vitro bioactivity of tricalcium silicate powders. Mater. Lett. 2004, 58, 2350–2353. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Zhai, W.; Wang, Z.; Chang, J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials 2005, 26, 6113–6121. [Google Scholar] [CrossRef]
- Gou, Z.; Chang, J. Synthesis and in vitro bioactivity of dicalcium silicate powders. J. Eur. Ceram. Soc. 2004, 24, 93–99. [Google Scholar] [CrossRef]
- Gou, Z.; Chang, J.; Zhai, W.; Wang, J. Study on the self-setting property and the in vitro bioactivity of β-Ca2SiO4. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 73, 244–251. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.-C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261. [Google Scholar] [CrossRef]
- Voicu, G.; Bădănoiu, A.; Andronescu, E.; Chifiruc, C.M. Synthesis, characterization and bioevaluation of partially stabilized cements for medical applications. Cent. Eur. J. Chem. 2013, 11, 1657–1667. [Google Scholar] [CrossRef]
- Voicu, G.; Popa, A.M.; Badanoiu, A.I.; Iordache, F. Influence of thermal treatment conditions on the properties of dental silicate cements. Molecules 2016, 21, 233. [Google Scholar] [CrossRef]
- Liu, W.C.; Hu, C.C.; Tseng, Y.Y.; Sakthivel, R.; Fan, K.S.; Wang, A.N.; Wang, Y.M.; Chung, R.J. Study on strontium doped tricalcium silicate synthesized through sol-gel process. Mater. Sci. Eng. C 2020, 108, 110431. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Hsu, T.-T.; Wang, K.; Shie, M.-Y. Preparation of the fast setting and degrading Ca–Si–Mg cement with both odontogenesis and angiogenesis differentiation of human periodontal ligament cells. Mater. Sci. Eng. C 2016, 60, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.-H.; Kim, Y.-I.; Yoon, S.-Y. Physicochemical and biological properties of mg-doped calcium silicate endodontic cement. Materials 2021, 14, 1843. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, M.; Gu, W.; Shen, Z.; Ma, X.; Lu, F.; Yang, X.; Zheng, Y.; Gou, Z. Zinc-/copper-substituted dicalcium silicate cement: Advanced biomaterials with enhanced osteogenesis and long-term antibacterial properties. J. Mater. Chem. B 2020, 8, 1060–1070. [Google Scholar] [CrossRef]
- Ding, S.-J.; Shie, M.-Y.; Wang, C.-Y. Novel fast-setting calcium silicate bone cements with high bioactivity and enhanced osteogenesis in vitro. J. Mater. Chem. 2009, 19, 1183–1190. [Google Scholar] [CrossRef]
- Xuereb, M.; Sorrentino, F.; Damidot, D.; Camilleri, J. Development of novel tricalcium silicate-based endodontic cements with sintered radiopacifier phase. Clin. Oral Investig. 2016, 20, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Zanfir, A.-V.; Nenu, N.; Voicu, G.; Badanoiu, A.-I.; Ghitulica, C.-D.; Iordache, F. Modified calcium silicophosphate cements with improved properties. Mater. Chem. Phys. 2019, 238, 121965. [Google Scholar] [CrossRef]
- Song, X.; Díaz-Cuenca, A. Sol–Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity. Materials 2022, 15, 6051. [Google Scholar] [CrossRef]
- Nishad, K.V.; Komath, M.; Unnikrishnan, G. Synthesis of strontium orthosilicate (Sr2SiO4) by sol-gel method for the use in endodontic cements to enhance bioactivity and radio-contrast. Mater. Res. Express 2019, 6, 105401. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Segura-Egea, J.J.; Díaz-Cuenca, A. Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules 2023, 28, 6967. https://doi.org/10.3390/molecules28196967
Song X, Segura-Egea JJ, Díaz-Cuenca A. Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules. 2023; 28(19):6967. https://doi.org/10.3390/molecules28196967
Chicago/Turabian StyleSong, Xiaozhe, Juan J. Segura-Egea, and Aránzazu Díaz-Cuenca. 2023. "Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics" Molecules 28, no. 19: 6967. https://doi.org/10.3390/molecules28196967
APA StyleSong, X., Segura-Egea, J. J., & Díaz-Cuenca, A. (2023). Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules, 28(19), 6967. https://doi.org/10.3390/molecules28196967