Pharmacological Mechanisms and Adjuvant Properties of Licorice Glycyrrhiza in Treating Gastric Cancer
Abstract
1. Introduction
2. Botanical Description
3. Chemical Composition of Licorice
3.1. Saponins
3.2. Flavonoids
3.3. Polysaccharides
3.4. Phenolic Compounds
3.5. Volatile Compounds
3.6. Others
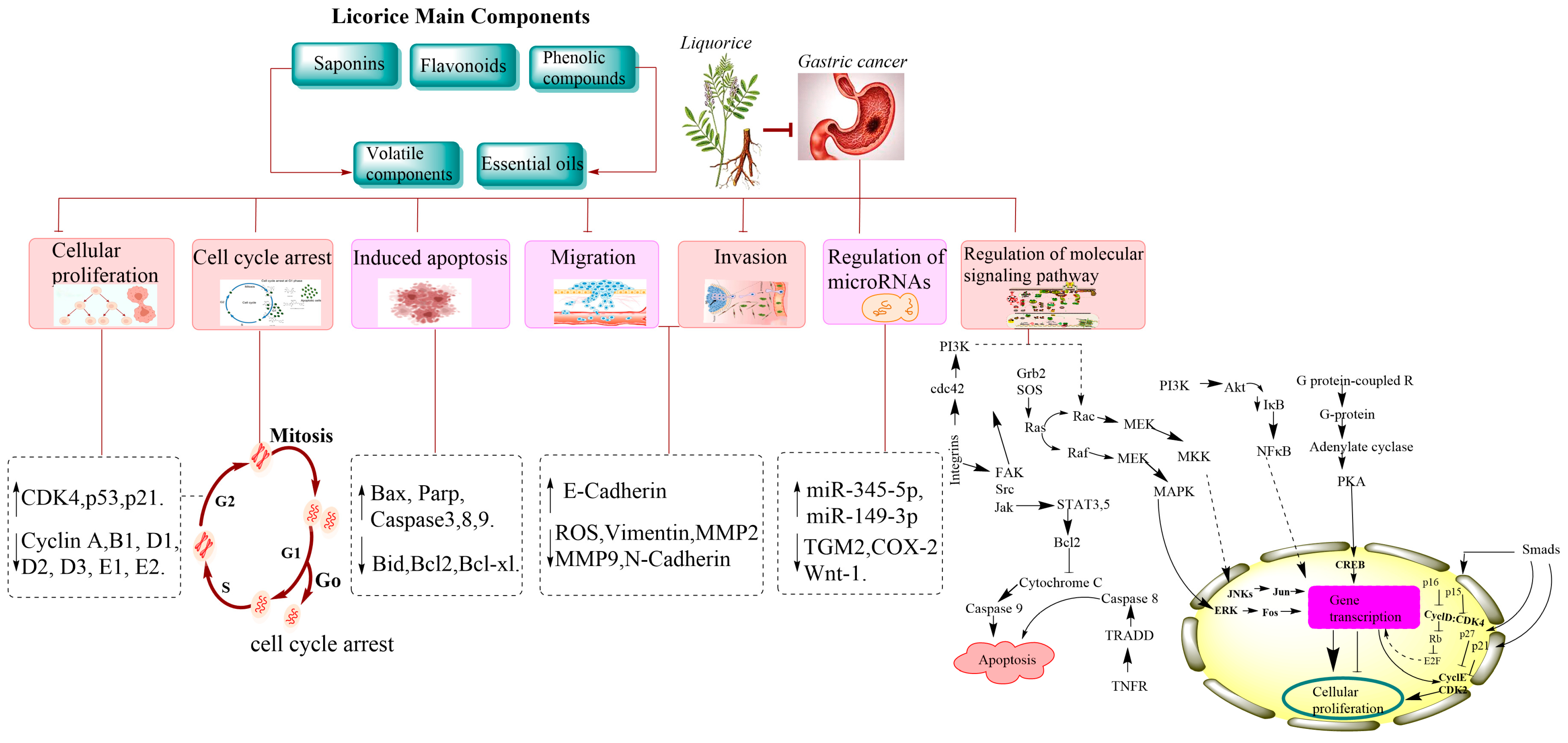
4. The Pharmacological Mechanism of Licorice in Gastric Cancer
4.1. Suppression of Cellular Proliferation in Gastric Cancer
4.2. Apoptosis Induction in Gastric Cancer Cells
4.3. Inhibition of Cellular Invasion and Metastasis in Gastric Cancer
4.4. Regulation of microRNAs
4.5. Regulation of Molecular Signaling Pathways
4.6. Immunoregulatory Functions
5. Pharmacological Mechanisms of Licorice in Conjunction with Other Drugs against Gastric Cancer
5.1. Combined with Chemotherapy Medications against Gastric Cancer
5.2. Combined with Other Compounds or Drugs against Gastric Cancer
| Active Components of Licorice | Experimental Model | Mechanism of Action | Signaling Pathways Involved | Journal Citation |
|---|---|---|---|---|
| Licoricidin (LCD) | In vitro: Human gastric cancer cell line of MGC-803 In vivo: Male nude mice 5 weeks old, 20 ± 2 g four groups (n = 6) Administration: Dosage; 10 mg/kg of LCD, 20 mg/kg of LCD, 20 mg/kg of 5-FU Route; Subcutaneous | Inhibited cellular proliferation, cellular migration, and invasion, induced apoptosis and cell cycle arrest at G0/G1 phase. Inhibited tumor growth. | Isoprenyl carboxyl methyltransferase (ICMT)/RAS pathway | [40] |
| Glycyrrhizic acid (GA) | Human gastric cancer cell line of MGC-803, BGC-823, and SGC-7901. | Inhibited cellular proliferation, promoted cell cycle arrest at G1/S-phase by ↓ cyclin D1, D2, D3, E1, and E2. Induced apoptosis by ↑ levels of Bax, cleaved PARP, and procaspase-3, -8, -9. | PI3K/AKT pathway | [41] |
| 18β-glycyrrhetinic acid (GRA) | In vitro: Human gastric cancer cell line of MKN-1, and BGC-823 In vivo: Male transgenic mice 6-week-old, two groups (n = 40) Administration: Dosage; distilled water containing 0.05% GRA Route; Oral | Inhibited cellular proliferation, induced cell cycle arrest, and apoptosis. Inhibited tumor growth | miR-149-3p-Wnt-1 signaling | [50] |
| Liquiritin (LIQ) + Cisplatin (DDP) | Human gastric cancer cell line of SGC7901/DDP In vivo: male BALB/c-nu mice 5-week-old, 15–18 g four groups (n = 10) Administration: Dosage; 15 mg/kg of LIQ, 3 mg/kg of DDP Route; Intraperitoneal injection | LIQ relatively inhibited the proliferation and migration of DDP-resistant gastric cancer cells. DDP+LIQ promoted cell cycle arrest at G0/G1 by ↓ cyclin D1, cyclin A, and ↑ CDK4 and p53 and p21. DDP+LIQ induced apoptosis and autophagy. Inhibited tumor growth of xenograft mice. | [60] | |
| Licoflavone A (LA) | In vitro: Human gastric cancer cell line of SGC-7901, MKN-45, MGC-803and VEGF-stimulated MKN-45 cells. In vivo: Male BALB/c-nude mice 4–6-week-old, 18 ± 2 g Administration: Dosage; 50 mg/kg of LA Route; Oral | Suppressed cellular proliferation. Induced apoptosis and cell cycle arrest at G1 phase, Inhibited the migration, invasion, and EMT of VEGF-stimulated MKN-45 cells. Inhibited tumor growth. | PI3K/AKT and MEK/ERK signaling pathways. | [63] |
| Isoliquiritigenin (ISL) | In vitro: Human gastric cancer cell line of MKN28 | Inhibited cellular proliferation, migration, and invasion. Promoted apoptosis and autophagy | PI3K/AKT/mTOR | [44] |
| 18β-glycyrrhetinic acid (18β-GA) | In vitro: Human gastric cancer cell line of SGC-7901 | Inhibited cellular proliferation, migration, and invasion. ↓ ROS formation, and expression of MMP-2 and 9, PKC-α, ERK, and vimentin. | ROS/PKC-α/ERK pathway | [64] |
| Quercetin (QC) | In vivo: Human gastric cancer cell line of EBV (+) SNU719, EBV (−) MKN74 Female NOD/SCID mice five weeks old, two groups (n = 15) Administration: Dosage; 30 mg/kg of QC Route; Oral | Inhibited tumor growth of the xenograft mice. Suppressed EBV viral proteins expression; (EBNA-1 and LMP-2) Promoted p53-dependent apoptosis by increasing the expression of caspase-3, -9, and Parp. | [42] | |
| Licochalcone A (LCA) + 5-fluorouracil (5-FU) | In vitro: Human gastric cancer cell line of SGC7901 and MKN-45 | LCA suppressed cellular proliferation, induced apoptosis, and cell cycle arrest at G2/M transition. LCA+5-FU enhanced the anticancer effects. | [65] | |
| Liquiritin (LIQ) + TRAIL | In vitro: Human gastric cancer cell line of AGS and SNU-216. In vivo: Male BALB/c-nu mice 5 weeks old,15–18 g Administration: Dosage; 20 mg/kg of LIQ, 100 mg/mouse of TRAIL Route; Intraperitoneal | Suppressed cellular proliferation, and migration. Induced apoptosis both in vitro and in vivo, enhanced activation of ROS and JNK. Inhibited tumor growth in vivo. | [62] | |
| Licochalcone A | In vitro: Human gastric cancer cell line of AGS, MKN-28, and MKN-45. | Inhibited cellular proliferation. Promoted cell cycle arrest at the G2/M transition by ↓ levels of cyclin A, B, and MDM2 and ↑ Rb expression. Induced apoptosis by regulating PARP, caspase-3, Bcl-2 and Bax expressions. | [43] | |
| Glycyrrhetinic acid (GA) 11-deoxy glycyrrhetinic acid (11-DOGA) | In vitro: Human gastric cancer cell line of BGC823 and SGC7901. In vivo: Nude Mice Administration: Dosage; 0, 10, 20, and 30 mg/kg of GA, 0, 10, 20, and 30 mg/kg of 11-DOGA Route; Subcutaneous injection | Suppressed cellular proliferation. Promoted cell cycle arrest in G2 Phase by ↑ p21 expression and ↓ cdc2 and cyclin B1. Induced apoptosis by ↓ Bid expression and activated PARP cleavage. Inhibited tumor growth in vivo. | Bid-mediated mitochondrial pathway. | [66] |
| Licochalcone A | In vitro: Human gastric cancer cell line of BGC. In vivo: SPF KM mice, 6–8 weeks, 13–15 g, two groups (n = 10). Administration: Dosage; 200 and 400 μM of LA Route; Intratumoral injection | Inhibited cell proliferation, and induced apoptosis. Inhibited tumor growth in vivo. | PI3K/AKT and ROS-mediated MAPK signaling pathway | [67] |
6. Toxicology Studies
7. Discussion
8. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med. Sci. Monit. 2019, 25, 3537–3541. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Maconi, G.; Manes, G.; Porro, G.B. Role of symptoms in diagnosis and outcome of gastric cancer. World J. Gastroenterol. 2008, 14, 1149–1155. [Google Scholar] [CrossRef]
- Cuzzuol, B.R.; Vieira, E.S.; Araújo, G.R.L.; Apolonio, J.S.; de Carvalho, L.S.; da Silva Junior, R.T.; de Brito, B.B.; de Melo, F.F. Gastric cancer: A brief review, from risk factors to treatment. Arch. Gastroenterol. Res. 2020, 1, 34–39. [Google Scholar]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belén, L.H.; Kaur, R.; Kregiel, D.; Uprety, Y.; Beyatli, A.; Yeskaliyeva, B.; Kırkın, C.; et al. Glycyrrhiza Genus: Enlightening Phytochemical Components for Pharmacological and Health-Promoting Abilities. Oxidative Med. Cell. Longev. 2021, 2021, 7571132. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Hayashi, H.; Sudo, H. Economic importance of licorice. Plant Biotechnol. 2009, 26, 101–104. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Han, S.; Zhang, L.; Bai, H.; Bao, F.; Zeng, Y.; Wang, J.; Du, H.; Liu, Y.; et al. Constituents Isolated from the Leaves of Glycyrrhiza uralansis and Their Anti-Inflammatory Activities on LPS-Induced RAW264.7 Cells. Molecules 2019, 24, 1923. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Son, D.H.; Chung, T.H.; Lee, Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef]
- Li, F.; Liu, B.; Li, T.; Wu, Q.; Xu, Z.; Gu, Y.; Li, W.; Wang, P.; Ma, T.; Lei, H. Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae radix et Rhizoma and Its Solubilization Characteristics. Molecules 2020, 25, 3904. [Google Scholar] [CrossRef]
- Bethapudi, B.; Murugan, S.K.; Nithyanantham, M.; Singh, V.K.; Agarwal, A.; Mundkinajeddu, D. Chapter 24—Gut health benefits of licorice and its flavonoids as dietary supplements. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Bagchi, D., Ohia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 377–417. [Google Scholar]
- Pan, L.C.; Zhu, Y.M.; Zhu, Z.Y.; Xue, W.; Liu, C.Y.; Sun, H.Q. Chemical structure and effects of antioxidation and against α-glucosidase of natural polysaccharide from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2020, 155, 560–571. [Google Scholar] [CrossRef]
- Ain, N.U.; Wu, S.; Li, X.; Li, D.; Zhang, Z. Isolation, Characterization, Pharmacology and Biopolymer Applications of Licorice Polysaccharides: Review. Materials 2022, 15, 3654. [Google Scholar] [CrossRef]
- Ayeka, P.A.; Bian, Y.; Mwitari, P.G.; Chu, X.; Zhang, Y.; Uzayisenga, R.; Otachi, E.O. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement. Altern. Med. 2016, 16, 206. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, X.; Hao, Y.; Zhang, X.; Zhu, Z. Chemical structure and inhibition on α-glucosidase of polysaccharide with alkaline-extracted from glycyrrhiza inflata residue. Int. J. Biol. Macromol. 2020, 147, 1125–1135. [Google Scholar] [CrossRef]
- Hong, Y.K.; Wu, H.T.; Ma, T.; Liu, W.J.; He, X.J. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int. J. Biol. Macromol. 2009, 45, 61–64. [Google Scholar] [CrossRef]
- Huan, C.; Xu, Y.; Zhang, W.; Ni, B.; Gao, S. Glycyrrhiza Polysaccharide Inhibits Pseudorabies Virus Infection by Interfering with Virus Attachment and Internalization. Viruses 2022, 14, 1772. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Rafi, M.M.; Vastano, B.C.; Zhu, N.; Ho, C.T.; Ghai, G.; Rosen, R.T.; Gallo, M.A.; DiPaola, R.S. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J. Agric. Food Chem. 2002, 50, 677–684. [Google Scholar] [CrossRef]
- Tao, W.W.; Duan, J.A.; Yang, N.Y.; Tang, Y.P.; Liu, M.Z.; Qian, Y.F. Antithrombotic phenolic compounds from Glycyrrhiza uralensis. Fitoterapia 2012, 83, 422–425. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Kim, J.H.; Park, S.J.; Chang, J.S.; Rho, M.C.; Bae, K.H.; Park, K.H.; Lee, W.S. Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg. Med. Chem. Lett. 2010, 20, 971–974. [Google Scholar] [CrossRef]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Azelmat, J.; Grenier, D.; Gafne, R.S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. [Google Scholar] [CrossRef]
- He, M.; Yang, Z.Y.; Guan, W.N.; Vicente Gonçalves, C.M.; Nie, J.; Wu, H. GC-MS Analysis and Volatile Profile Comparison for the Characteristic Smell from Liang-wai Gan Cao (Glycyrrhiza uralensis) and Honey-Roasting Products. J. Chromatogr. Sci. 2016, 54, 879–887. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Rúa, J.; Del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Abd-Allah, A.R.; Mansour, A.M.; El-Arabey, A.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2015, 29, 165–172. [Google Scholar]
- El-Sayedel, S.M.; Mansour, A.M.; Abdul-Hameed, M.S. Thymol and Carvacrol Prevent Doxorubicin-Induced Cardiotoxicity by Abrogation of Oxidative Stress, Inflammation, and Apoptosis in Rats. J. Biochem. Mol. Toxicol. 2016, 30, 37–44. [Google Scholar] [CrossRef]
- Azizi, Z.; Choopani, S.; Salimi, M.; Majlessi, N.; Naghdi, N. Protein Kinase C Involvement in Neuroprotective Effects of Thymol and Carvacrol Against Toxicity Induced by Amyloid-β in Rat Hippocampal Neurons. Basic Clin. Neurosci. 2022, 13, 295–304. [Google Scholar] [CrossRef]
- Gholijani, N.; Amirghofran, Z. Effects of thymol and carvacrol on T-helper cell subset cytokines and their main transcription factors in ovalbumin-immunized mice. J. Immunotoxicol. 2016, 13, 729–737. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Ma, H.; Wu, F.; Bai, Y.; Wang, T.; Ma, S.; Guo, L.; Liu, G.; Leng, G.; Kong, Y.; Zhang, Y. Licoricidin combats gastric cancer by targeting the ICMT/Ras pathway in vitro and in vivo. Front. Pharmacol. 2022, 13, 972825. [Google Scholar] [CrossRef]
- Wang, H.; Ge, X.; Qu, H.; Wang, N.; Zhou, J.; Xu, W.; Xie, J.; Zhou, Y.; Shi, L.; Qin, Z.; et al. Glycyrrhizic Acid Inhibits Proliferation of Gastric Cancer Cells by Inducing Cell Cycle Arrest and Apoptosis. Cancer Manag. Res. 2020, 12, 2853–2861. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, S.; Shin, Y.S.; Cho, M.; Kang, H.; Cho, H. Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma. Molecules 2016, 21, 1286. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Hao, M.; Yang, X.Y.; Ba, Q.; Li, M.; Ni, S.J.; Wang, L.S.; Du, X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011, 302, 69–75. [Google Scholar] [CrossRef]
- Zhang, X.R.; Wang, S.Y.; Sun, W.; Wei, C. Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2018, 18, 3429–3436. [Google Scholar] [CrossRef]
- Wittekind, C.; Neid, M.J.O. Cancer invasion and metastasis. Oncology 2005, 69 (Suppl. S1), 14–16. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.; Yang, Y.; Chen, Y.; Bu, Y.; Ye, M.; Mao, X.; Ma, T.; Yu, L.; Nan, Y. LINC00514 promotes gastric cancer cell growth and EMT progression via miR-204-3p/KRAS. Aging 2021, 13, 12007–12015. [Google Scholar] [CrossRef]
- Huang, L.; Xu, A.M.; Liu, W. Transglutaminase 2 in cancer. Am. J. Cancer Res. 2015, 5, 2756–2776. [Google Scholar]
- Li, X.; Ma, X.L.; Nan, Y.; Du, Y.H.; Yang, Y.; Lu, D.D.; Zhang, J.F.; Chen, Y.; Zhang, L.; Niu, Y.; et al. 18β-glycyrrhetinic acid inhibits proliferation of gastric cancer cells through regulating the miR-345-5p/TGM2 signaling pathway. World J. Gastroenterol. 2023, 29, 3622–3644. [Google Scholar] [CrossRef]
- Cao, D.; Jia, Z.; You, L.; Wu, Y.; Hou, Z.; Suo, Y.; Zhang, H.; Wen, S.; Tsukamoto, T.; Oshima, M.; et al. 18β-glycyrrhetinic acid suppresses gastric cancer by activation of miR-149-3p-Wnt-1 signaling. Oncotarget 2016, 7, 71960–71973. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Wang, F.; Zhou, Y.; Xia, G.; Xu, W. ICMT contributes to hepatocellular carcinoma growth, survival, migration and chemoresistance via multiple oncogenic pathways. Biochem. Biophys. Res. Commun. 2019, 518, 584–589. [Google Scholar] [CrossRef]
- Kole, C.; Charalampakis, N.; Tsakatikas, S.; Kouris, N.I.; Papaxoinis, G.; Karamouzis, M.V.; Koumarianou, A.; Schizas, D. Immunotherapy for gastric cancer: A 2021 update. Immunotherapy 2022, 14, 41–64. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.; Wei, K.; Zhang, T.; Che, Y.; Nguyễn, A.D.; Pandita, S.; Wan, X.; Cui, X.; Zhou, B.; et al. Structure of a new glycyrrhiza polysaccharide and its immunomodulatory activity. Front. Immunol. 2022, 13, 1007186. [Google Scholar] [CrossRef]
- Richard, S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediat. Inflamm. 2021, 2021, 6699560. [Google Scholar] [CrossRef]
- Bordbar, N.; Karimi, M.H.; Amirghofran, Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell Immunol. 2012, 280, 44–49. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc. Biol. 2012, 91, 967–976. [Google Scholar] [CrossRef]
- Ayeka, P.A.; Bian, Y.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Devarajan, N.; Manjunathan, R.; Ganesan, S.K. Tumor hypoxia: The major culprit behind cisplatin resistance in cancer patients. Crit. Rev. Oncol./Hematol. 2021, 162, 103327. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Sun, J.; Lv, H.; Wang, Y.; Ni, Y.; Chen, S.; Hu, C.; Wang, L.; Chen, W.; et al. Cisplatin resistance in gastric cancer cells is involved with GPR30-mediated epithelial-mesenchymal transition. J. Cell Mol. Med. 2020, 24, 3625–3633. [Google Scholar] [CrossRef]
- Wei, F.; Jiang, X.; Gao, H.Y.; Gao, S.H. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 2017, 51, 1383–1394. [Google Scholar] [CrossRef]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer. Cancers 2023, 15, 2752. [Google Scholar] [CrossRef]
- Xie, R.; Gao, C.C.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Zhang, J.L.; Yan, W.; Ma, T.H. Combining TRAIL and liquiritin exerts synergistic effects against human gastric cancer cells and xenograft in nude mice through potentiating apoptosis and ROS generation. Biomed. Pharmacother. 2017, 93, 948–960. [Google Scholar] [CrossRef]
- Hongxia, G.; Xiaojie, J.; Guangxian, L.; Min, Z.; Shiwei, N.; Wangjie, C.; Han, Z.; Yuanding, Z.; Chenghao, L.; Yaling, L.; et al. Licoflavone A Suppresses Gastric Cancer Growth and Metastasis by Blocking the VEGFR-2 Signaling Pathway. J. Oncol. 2022, 2022, 5497991. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chen, X.; Zhang, J.; Wang, J. 18β-glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J. Nat. Med. 2018, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tian, L.; Wang, L.; Li, W.; Xu, Q.; Xiao, X. Antitumor effects and the underlying mechanism of licochalcone A combined with 5-fluorouracil in gastric cancer cells. Oncol. Lett. 2017, 13, 1695–1701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.; Zhong, W.; Li, J.; Zhang, B.; Song, G.; Hu, T. Involvement of BID translocation in glycyrrhetinic acid and 11-deoxy glycyrrhetinic acid-induced attenuation of gastric cancer growth. Nutr. Cancer 2014, 66, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Yuan, X.; Yu, L.; Gao, C.; Sun, X.; Wang, D.; Zheng, Q. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 10336. [Google Scholar] [CrossRef]
- Lüde, S.; Vecchio, S.; Sinno-Tellier, S.; Dopter, A.; Mustonen, H.; Vucinic, S.; Jonsson, B.; Müller, D.; Veras Gimenez Fruchtengarten, L.; Hruby, K.; et al. Adverse effects of plant food supplements and plants consumed as food: Results from the poisons centres-based PlantLIBRA study. Phytother. Res. 2016, 30, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H.J.P.R. Toxicological effects of Glycyrrhiza glabra (licorice): A review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; May, B.H.; Zhou, I.W.; Zhang, A.L.; Xue, C.C. Integrative Medicine for Relief of Nausea and Vomiting in the Treatment of Colorectal Cancer Using Oxaliplatin-Based Chemotherapy: A Systematic Review and Meta-Analysis. Phytother. Res. 2016, 30, 741–753. [Google Scholar] [CrossRef]
- Wu, T.H.; Chiu, T.Y.; Tsai, J.S.; Chen, C.Y.; Chen, L.C.; Yang, L.L. Effectiveness of Taiwanese traditional herbal diet for pain management in terminal cancer patients. Asia Pac. J. Clin. Nutr. 2008, 17, 17–22. [Google Scholar]
- Najafi, S.; Koujan, S.E.; Manifar, S.; Kharazifard, M.J.; Kidi, S.; Hajheidary, S. Preventive Effect of Glycyrrhiza Glabra Extract on Oral Mucositis in Patients Under Head and Neck Radiotherapy: A Randomized Clinical Trial. J. Dent. 2017, 14, 267–274. [Google Scholar]
- Pakravan, F.; Salehabad, N.H.; Karimi, F.; Isfahani, M.N. Comparative Study of the Effect of Licorice Muco-adhesive Film on Radiotherapy Induced Oral Mucositis, A Randomized Controlled Clinical Trial. Gulf. J. Oncolog. 2021, 1, 42–47. [Google Scholar] [PubMed]
- Racková, L.; Jancinová, V.; Petríková, M.; Drábiková, K.; Nosál, R.; Stefek, M.; Kostálová, D.; Prónayová, N.; Kovácová, M. Mechanism of anti-inflammatory action of liquorice extract and glycyrrhizin. Nat. Prod. Res. 2007, 21, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Oh, S.M.; Kwon, H.S.; Oh, Y.S.; Lim, S.S.; Shin, H.K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem. Biophys. Res. Commun. 2006, 345, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, Y.; Liu, Z.; Li, Q.; Jin, H. Effects of Buzhong Yiqi Decoction Combined with THP Bladder Perfusion on Postoperative Efficacy in Patients with Bladder Cancer. Evid. Based Complement. Altern. Med. 2021, 2021, 3685213. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Copur, M.S.; Rose, M.; Wadler, S.; Stephenson, J.; O’Rourke, M.; Brenckman, W.; Tilton, R.; Liu, S.H.; Jiang, Z.; et al. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin. Color Cancer 2011, 10, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Cheon, C.; Kang, S.; Ko, Y.; Kim, M.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Sipjeondaebo-tang in patients with breast cancer with fatigue: A protocol for a pilot, randomised, double-blind, placebo-controlled, cross-over trial. BMJ Open 2018, 8, e021242. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T.; et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase II study (JORTC KMP-02). J. Gynecol. Oncol. 2017, 28, e44. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, Y.C.; Chen, H.Y.; Chiang, Y.F.; Ali, M.; Shieh, T.M.; Hsia, S.M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites 2022, 12, 535. [Google Scholar] [CrossRef]
- Lee, C.H.; Tsai, H.Y.; Chen, C.L.; Chen, J.L.; Lu, C.C.; Fang, Y.P.; Wu, D.C.; Huang, Y.B.; Lin, M.W. Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation. Biomedicines 2022, 10, 1350. [Google Scholar] [CrossRef]
- Zhu, X.; Cong, J.; Lin, Z.; Sun, J.; Yang, B.; Li, A. Inhibition of HMGB1 Overcomes Resistance to Radiation and Chemotherapy in Nasopharyngeal Carcinoma. OncoTargets Ther. 2020, 13, 4189–4199. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Zhang, L.; Zhang, J.; Chen, X.; Yang, M.; Chen, T.; Hong, J. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice. J. Radiat. Res. 2017, 58, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.; Nakahashi, Y.; Hachimine, D.; Seki, T.; Okazaki, K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007, 31, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xing, Y.; Li, M.; Zhang, Z.; Wang, J.; Ri, M.; Jin, C.; Xu, G.; Piao, L.; Jin, H.; et al. Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, R.; Yang, R.; Xiao, Y.; Yan, J.; Zheng, C.; Xiao, W.; Huang, C.; Wang, Y. Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-Cyclin D1 complex and increasing CD8(+) T cell infiltration. Cancer Cell Int. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.W.; Jiang, X.M.; Xu, Y.L.; Huang, M.Y.; Chen, Y.C.; Yu, W.B.; Su, M.X.; Ye, Z.H.; Chen, X.; Wang, Y.; et al. Licochalcone A inhibits interferon-gamma-induced programmed death-ligand 1 in lung cancer cells. Phytomedicine 2021, 80, 153394. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.T.; de la Cruz, J.; Hwang, S.G.; Hong, H. Tumorigenic effects of endocrine-disrupting chemicals are alleviated by licorice (Glycyrrhiza glabra) root extract through suppression of AhR expression in mammalian cells. Asian Pac. J. Cancer Prev. 2014, 15, 4809–4813. [Google Scholar] [CrossRef]
| Compound | Chemical Formula | Chemical Structure | Category |
|---|---|---|---|
| Glycyrrhizin | C42H62O16 | 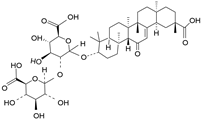 | Triterpene saponin |
| Glycyrrhetinic acid | C30H46O4 | 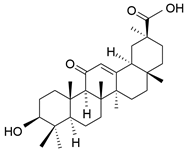 | Triterpene saponin |
| 18β-glycyrrhetyl-3-O-sulfate | C30H46O7S |  | Triterpene saponin |
| Liquiritin | C21H22O4 |  | Triterpene saponin |
| Licochalcone A | C21H22O4 | 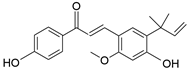 | Flavonoid |
| Glabridin | C20H20O4 | 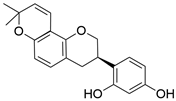 | Flavonoid |
| Isoliquiritigenin | C15H12O4 | 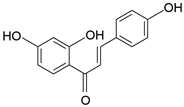 | Flavonoid |
| Liquiritin apioside | C26H30O13 | 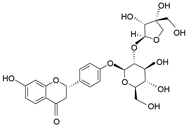 | Flavonoid |
| Liquiritigenin | C15H12O4 |  | Flavonoid |
| Isoliquiritin | C21H22O9 | 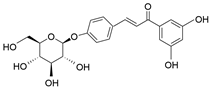 | Flavonoid |
| Licoriphenone | C21H24O6 | 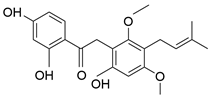 | Phenol |
| Kanzonol R | C22H26O5 | 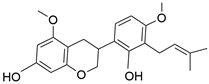 | Phenol |
| Compounds | Cell Lines | Dosage | Cytotoxic Outcome/IC50 (μM) |
|---|---|---|---|
| Licochalcone A | GES-1 AGS MKN-28 MKN-45 | 0, 10, 25, 50 and 100 µM, 48 h | 92.7 41.1 42.0 83.7 |
| Licoricidin (LCD) | MCG-803 | 1.5625, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μM, 24 h | 10.41 |
| Glycyrrhizic acid | MGC-803 BGC-823 SGC-7901 | 0, 0.5, 1, 2, 3, 4 mg/mL, 48 h | ≈2 mg/mL |
| Licoflavone A (LA) | GES-1 SGC-7901 MKN-45 MGC-803 | 0, 6.25, 12.5, 25, 50, and 100 μM, 24 h | 180.30 78.08 43.26 124.50 |
| 18β-glycyrrhetinic acid (18β-GA) | SGC-7901 cells | 0, 20, 40, 60, 80, 100 and 120 μM, 24 h | cytotoxicity was observed at a concentration > 80 μM |
| Liquiritin (LIQ) | GES-1 AGS SNU-216 | 0, 25, 50, 100, 150 and 200 uM, 24 h | cytotoxicity > 150 μM 185.73 198.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tibenda, J.J.; Du, Y.; Huang, S.; Chen, G.; Ning, N.; Liu, W.; Ye, M.; Nan, Y.; Yuan, L. Pharmacological Mechanisms and Adjuvant Properties of Licorice Glycyrrhiza in Treating Gastric Cancer. Molecules 2023, 28, 6966. https://doi.org/10.3390/molecules28196966
Tibenda JJ, Du Y, Huang S, Chen G, Ning N, Liu W, Ye M, Nan Y, Yuan L. Pharmacological Mechanisms and Adjuvant Properties of Licorice Glycyrrhiza in Treating Gastric Cancer. Molecules. 2023; 28(19):6966. https://doi.org/10.3390/molecules28196966
Chicago/Turabian StyleTibenda, Joanna Japhet, Yuhua Du, Shicong Huang, Guoqing Chen, Na Ning, Wenjing Liu, Mengyi Ye, Yi Nan, and Ling Yuan. 2023. "Pharmacological Mechanisms and Adjuvant Properties of Licorice Glycyrrhiza in Treating Gastric Cancer" Molecules 28, no. 19: 6966. https://doi.org/10.3390/molecules28196966
APA StyleTibenda, J. J., Du, Y., Huang, S., Chen, G., Ning, N., Liu, W., Ye, M., Nan, Y., & Yuan, L. (2023). Pharmacological Mechanisms and Adjuvant Properties of Licorice Glycyrrhiza in Treating Gastric Cancer. Molecules, 28(19), 6966. https://doi.org/10.3390/molecules28196966






