Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals
Abstract
:1. Introduction
2. Structural Features and Chemistry of Schiff Bases
- (1)
- Imine Functional Group: The defining structural feature of Schiff bases is the presence of an imine (-C=N-) functional group. This double bond between carbon and nitrogen is formed through the condensation reaction between a primary amine and an aldehyde or ketone. The imine group imparts unique chemical and physical properties to Schiff bases, making them versatile compounds [30].
- (2)
- Chelation: Schiff bases can act as ligands, forming coordination complexes with metal ions. The imine nitrogen atom can donate its lone pair of electrons to form a coordination bond with a metal ion, forming Schiff base metal complexes. This chelation capability enables the use of Schiff bases in various fields such as catalysis, materials science, and coordination chemistry [31].
- (3)
- Tautomeric Forms: Schiff bases can exist in different tautomeric forms, primarily the imine and enamine tautomers. The imine form has a double bond between the carbon and nitrogen atoms, while the enamine form has a carbon–carbon double bond adjacent to the imine nitrogen. The equilibrium between these tautomeric forms can influence the reactivity and properties of Schiff bases [32].
- (4)
- Acid-Base Properties: Schiff bases can act as both acids and bases. The imine nitrogen can participate in protonation reactions, making the Schiff base compound acidic. On the other hand, the nitrogen lone pair can accept protons, making Schiff bases basic. Schiff bases’ acidic and basic characteristics contribute to their reactivity and coordination ability [33].
- (5)
- Reactivity: Schiff bases exhibit rich reactivity due to the presence of the imine group. They can undergo chemical transformations such as hydrolysis, reduction, oxidation, and nucleophilic addition reactions. The reactivity of Schiff bases makes them valuable intermediates for the synthesis of diverse organic compounds and coordination complexes [34].
- (6)
- Biological Activity: Schiff bases have shown a range of biological activities and applications. They have been studied for their antimicrobial and antifungal activity, such as Schiff base derivatives of quinoline, anticancer by interfering with cancer cell growth and inducing apoptosis, and antioxidant properties in neutralizing harmful free radicals in the body. Schiff base complexes with transition metal ions have been widely applied in various biological areas, such as metal ion detection, catalysis, and medicinal chemistry [35,36].
- (1)
- Absorption and Emission Ability: Schiff bases typically have broad absorption and emission spectra, meaning that they can absorb light at a specific wavelength and emit light at a longer wavelength [37]. The absorption spectra cover a wide range of wavelengths, allowing them to absorb light in the visible or near-ultraviolet region. Such optical behavior of Schiff bases is highly dependent on their molecular structure and electronic environment. By modifying the structure of Schiff bases, their fluorescence properties can be tuned, leading to the development of fluorescent probes and sensors [38].
- (2)
- Solvent and pH Sensitivity: The photophysical properties of Schiff bases can be influenced by the surrounding medium’s polarity and acidity/basicity. Solvent polarity or pH changes can alter Schiff bases’ absorption and emission wavelengths, quantum yields, and fluorescence lifetimes. This sensitivity to the environment makes them useful for sensing applications [39].
- (3)
- Nonlinear Optical Properties: Schiff bases with extended π-conjugation can possess nonlinear optical properties. They exhibit efficient nonlinear optical responses such as second harmonic generation and third-order frequency mixing, making them potential candidates for applications in nonlinear optics, photonic devices, and optical data storage [40,41].
3. Schiff Bases as Fluorescent Chemosensors for Hazardous Ions
- (1)
- Selective Binding: Schiff bases can be designed to exhibit high selectivity and affinity for specific hazardous cations. The imine moiety in Schiff bases can act as a binding site for these cations, facilitating coordination through lone pair–electron interactions. By incorporating specific functional groups or structural modifications, Schiff bases can be tuned to selectively recognize and bind specific hazardous cations, even in the presence of other metal ions [44].
- (2)
- Fluorescence Quenching Mechanism: Schiff bases with inherent fluorescence properties can undergo fluorescence quenching upon binding to hazardous cations. The coordination between the Schiff base’s cation and the imine group disrupts the fluorescent properties. This quenching effect can arise due to energy transfer, electron transfer, or other quenching mechanisms associated with coordination with the hazardous cation [45].
- (3)
- Fluorescence Recovery: The fluorescence quenching of the Schiff base upon cation binding provides the basis for sensing hazardous cations. The fluorescence quenching occurs when the Schiff base is exposed to a hazardous cation sample. The presence and concentration of the cation can be determined by monitoring the fluorescence recovery upon chelation or displacement of the hazardous cation [46].
- (4)
- Sensitivity and LOD: Schiff bases can exhibit high sensitivity towards hazardous cations, allowing for their detection at low concentrations. The extent of fluorescence quenching or recovery correlates with the concentration of the hazardous cation in the sample, which allows for quantitative detection within a specific range. The detection limits can be optimized by modifying the Schiff base structure and carefully selecting the fluorescent properties [47].
- (5)
- Sensor Design: The design of chemosensors for hazardous cations is critical to achieving high selectivity and sensitivity. Factors such as the choice of Schiff base structure, the nature of the coordinating groups, and the optimal fluorophore functionality are considered to enhance the sensing properties. This design approach ensures minimal interference from other metal ions and improved analytical performance [48].
- (6)
- Real-Time Monitoring: The reversible binding of hazardous cations to Schiff bases enables real-time monitoring of cation concentrations. This is particularly valuable for continuous monitoring or in-field measurements of hazardous cations in environmental, industrial, or biological settings [49].
- (1)
- Chelation-Induced Fluorescence Modulation (CHEF): Schiff base chemosensors can exhibit chelation-induced fluorescence modulation. The formation of a chelate complex between the Schiff base and the analyte induces conformational changes, altering the molecular environment around the fluorophore. These changes can affect the fluorophore’s excited state and result in fluorescence intensity, emission wavelength, or fluorescence lifetime changes [50].
- (2)
- Protonation/Deprotonation: Schiff bases can act as pH-responsive chemosensors. Changes in pH alter the protonation/deprotonation state of the Schiff base, leading to changes in the electronic environment and fluorescence properties. Protonation or deprotonation can induce changes in the chromophore’s electronic structure, such as charge redistribution or intramolecular charge transfer (ICT), resulting in fluorescence changes that can be measured [51].
- (3)
- Photoinduced Electron Transfer (PET): Schiff base chemosensors can exhibit fluorescence changes due to photoinduced electron transfer processes. This mechanism involves electron transfer between the analyte and the excited state of the Schiff base. The analyte’s electron-withdrawing or electron-donating properties can influence fluorescence, leading to fluorescence quenching or enhancement [52].
- (4)
- Excited-State Intramolecular Proton Transfer (ESIPT): Some Schiff bases undergo ESIPT processes, where upon excitation, a proton migrates intramolecularly from the hydroxyl or amino group of the Schiff base to an adjacent electron-rich group. This results in a significant change in the fluorescence emission wavelength. ESIPT can lead to dual-emission and ratiometric sensing capabilities, making Schiff bases valuable for fluorescence-based pH sensors [53].
- (5)
- Aggregation-Induced Emission (AIE): AIE in Schiff base compounds refers to the phenomenon where these compounds exhibit enhanced fluorescence intensity upon aggregation or confinement. When Schiff base compounds undergo aggregation or are confined within specific environments, their molecular motions become restricted, and non-radiative energy pathways that typically cause fluorescence quenching are blocked. As a result, the fluorescence emission of Schiff base compounds with AIE characteristics is significantly increased, leading to bright fluorescence signals. This unique behavior has attracted significant attention and has been extensively explored for various applications [54,55,56].
- (6)
- Förster Resonance Energy Transfer (FRET): Schiff base chemosensors can utilize FRET as a sensing mechanism. FRET occurs when the excited-state energy of the Schiff base donor fluorophore is transferred to an acceptor fluorophore upon analyte binding. The analyte-induced proximity between the donor and acceptor leads to a decrease or increase in fluorescence intensity, depending on the specific design and energy transfer efficiency [57].
4. Modifications and Variations in Functional Groups
4.1. Schiff Bases as Chemosensors for Hg2+
4.2. Schiff Bases as Chemosensors for Cu2+
4.3. Schiff Bases as Chemosensors for Fe2+ and Fe3+
4.4. Schiff Bases as Chemosensors for Zn2+
4.5. Schiff Bases as Chemosensors for Other Ions
5. Integration of Nanomaterials or Auxiliary Receptors
- Enhanced Stability: Immobilizing the functional nanomaterials ensures their stability and prevents their aggregation or leaching out. It helps maintain the nanomaterials’ structural integrity and fluorescence properties, leading to reliable and long-lasting sensor performance [116].
- Improved Sensitivity: Immobilization can enhance the sensitivity of fluorescence chemosensors by providing a controlled environment for the nanomaterials. It minimizes background interference, reduces signal noise, and increases the signal-to-noise ratio, enabling the detection of trace amounts of target analytes with higher accuracy and lower LOD values [117].
- Specificity and Selectivity: Functional nanomaterials can be modified with ligands or receptors to interact with specific analytes selectively. Immobilization allows for precisely positioning these recognition elements, promoting selective analyte binding and minimizing nonspecific interactions. This ensures high specificity and selectivity of the chemosensor towards the target analyte [118].
- Easy Handling and Integration: Immobilization facilitates the handling and integration of functional nanomaterials into different sensor formats or platforms. It enables their incorporation into various devices such as lab-on-a-chip systems, wearable sensors, or surface-based arrays, enabling practical and convenient application in real-world settings [119].
- Reusability: Immobilization can enable the regeneration and reuse of functional nanomaterials in sensing applications. For example, suppose the nanomaterials are immobilized on a solid support. In that case, they can be easily separated, regenerated, and reused after each sensing cycle, reducing the cost of sensor fabrication and operation [120].
5.1. Nanoparticle Materials-Based Chemosensors
5.1.1. Nanoparticles-Based Chemosensors for Hg2+
5.1.2. Nanoparticles-Based Chemosensors for Cu2+
5.1.3. Nanoparticles-Based Chemosensors for Other Ions
5.2. Nanoporous Materials-Based Chemosensors
5.2.1. Nanoporous-Based Chemosensors for Hg2+
5.2.2. Nanoporous-Based Chemosensors for Cu2+
5.2.3. Nanoporous-Based Chemosensors for Fe3+
5.2.4. Nanoporous-Based Chemosensors for Other Ions
5.3. Metal Nanoclusters-Based Chemosensors
6. Concluding Remarks
- Spacer length optimization: Researchers can find ways to adjust the length and flexibility of the spacer connecting the chelating unit and the reporter moiety. This modification helps Schiff base compounds achieve the optimum distance and orientation for efficient cation binding and signal transduction.
- Conformational control: Sensor design should consider incorporating conformational switches within the Schiff base structure. These switches can be responsive to the presence of the target cation, leading to conformational changes that amplify the signal output, thereby improving sensitivity.
- Signal amplification strategies: Introduce amplification mechanisms, such as signal amplification tags, to enhance the signal response generated by the Schiff base structure upon cation binding.
- Analyte-binding pocket modification: Rational design of the binding pocket to optimize interactions with the target cation. This can involve introducing specific binding sites, modifying the cavity size, or incorporating steric hindrance to exclude interfering cations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Soufeena, P.P.; Nibila, T.A.; Aravindakshan, K. Coumarin based yellow emissive AIEE active probe: A colorimetric sensor for Cu2+ and fluorescent sensor for picric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117201. [Google Scholar] [CrossRef]
- Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, İ. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics 2023, 15, 779. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.; Gahlyan, P.; Kumar, R.; Pani, B. Recent developments on the colorimetric and fluorometric detection of 3d block metal ions using Schiff base probes. J. Mol. Struct. 2023, 1289, 135859. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Duan, H. Schiff base fluorescence probes for Cu2+ based on imidazole and benzimidazole. Sens. Actuators B Chem. 2015, 214, 138–143. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorganica Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Güngör, Ö.; Gürkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- El-wakiel, N.; El-keiy, M.; Gaber, M. Synthesis, spectral, antitumor, antioxidant and antimicrobial studies on Cu(II), Ni(II) and Co(II) complexes of 4-[(1H-Benzoimidazol-2-ylimino)-methyl]-benzene-1,3-diol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 147, 117–123. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef]
- Pandiarajan, S.; Hajarabeevi, N.; Shaikh, R.R.; Raghunathan, S.; MubarakAli, D. Synthesis and Characterization of Novel Schiff Bases Derived from 2-Butyl-4-chloro Imidazole for the Enhanced Antimicrobial Property. Appl. Biochem. Biotechnol. 2023, 195, 253–263. [Google Scholar] [CrossRef]
- Asraf, M.; Asraf, M. Synthesis and Characterization of Schiff Base Complexes of Cu(II), Co(II) and Cd(II) Derived from Ethylenediamine and Benzaldehyde Derivatives. Asian J. Appl. Chem. Res. 2020, 6, 34–46. [Google Scholar]
- Jadhav, S.; Kapadnis, K. Synthesis and characterization of schiff base derived from vanillin with various amine and formation of co(ii), cu(ii) and ni(ii) metal complexes with derived schiff base. World J. Pharm. Res. 2017, 6, 8. [Google Scholar]
- Nan, X.; Huyan, Y.; Li, H.; Sun, S.; Xu, Y. Reaction-based fluorescent probes for Hg2+, Cu2+ and Fe3+/Fe2+. Coord. Chem. Rev. 2021, 426, 213580. [Google Scholar] [CrossRef]
- Ahmed, F.; Xiong, H. Recent developments in 1,2,3-triazole-based chemosensors. Dye. Pigment. 2021, 185, 108905. [Google Scholar] [CrossRef]
- Alharbi, K.H. A Review on Organic Colorimetric and Fluorescent Chemosensors for the Detection of Zn(II) Ions. Crit. Rev. Anal. Chem. 2022, 2022, 2033611. [Google Scholar] [CrossRef]
- Banik, D.; Manna, S.K.; Maiti, A.; Mahapatra, A.K. Recent Advancements in Colorimetric and Fluorescent pH Chemosensors: From Design Principles to Applications. Crit. Rev. Anal. Chem. 2021, 2021, 2023002. [Google Scholar] [CrossRef]
- Kowser, Z.; Rayhan, U.; Akther, T.; Redshaw, C.; Yamato, T. A brief review on novel pyrene based fluorometric and colorimetric chemosensors for the detection of Cu2+. Mater. Chem. Front. 2021, 5, 2173–2200. [Google Scholar] [CrossRef]
- Roy, A.; Nandi, M.; Roy, P. Dual chemosensors for metal ions: A comprehensive review. TrAC Trends Anal. Chem. 2021, 138, 116204. [Google Scholar] [CrossRef]
- Saha, S.; Alam, R. Recent developments in the creation of a single molecular sensing tool for ternary iron (III), chromium (III), aluminium (III) ionic species: A review. Luminescence 2022, 38, 1026–2046. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Inbaraj, V. A Review on Schiff Base Fluorescent Chemosensors for Cell Imaging Applications. J. Fluoresc. 2020, 30, 1203–1223. [Google Scholar] [CrossRef]
- Mansha, A.; Asad, S.; Asim, S.; Bibi, S.; Urrehman, S.; Shahzad, A. Review of recent advancements in fluorescent chemosensor for ion detection via coumarin derivatives. Chem. Pap. 2022, 76, 3303–3349. [Google Scholar] [CrossRef]
- Pooja; Pandey, H.; Aggarwal, S.; Vats, M.; Rawat, V.; Pathak, S.R. Coumarin-based Chemosensors for Metal Ions Detection. Asian J. Org. Chem. 2022, 11, 23–75. [Google Scholar] [CrossRef]
- Choudhury, N.; De, P. Recent progress in pendant rhodamine-based polymeric sensors for the detection of copper, mercury and iron ions. J. Macromol. Sci. Part A 2021, 58, 835–848. [Google Scholar] [CrossRef]
- Sarkar, S.; Chatterjee, A.; Biswas, K. A Recent Update on Rhodamine Dye Based Sensor Molecules: A Review. Crit. Rev. Anal. Chem. 2023, 2023, 2169598. [Google Scholar] [CrossRef]
- Muhammad, M.; Khan, S.; Shehzadi, S.A.; Gul, Z.; Al-Saidi, H.M.; Waheed Kamran, A.; Alhumaydhi, F.A. Recent advances in colorimetric and fluorescent chemosensors based on thiourea derivatives for metallic cations: A review. Dye Pigment. 2022, 205, 110477. [Google Scholar] [CrossRef]
- Trevino, K.M.; Wagner, C.R.; Tamura, E.K.; Garcia, J.; Louie, A.Y. Small molecule sensors for the colorimetric detection of Copper(II): A review of the literature from 2010 to 2022. Dye Pigment. 2023, 214, 110881. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Wang, S.; Song, Q.; Guo, H.; Li, Z. Recent progress in macrocyclic chemosensors for lead, cadmium and mercury heavy metal ions. Dye Pigment. 2023, 216, 111380. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases-Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Dubey, M.; Biswas, A.; Pandey, D.S. Detection of copper (II) and aluminium (III) by a new bis-benzimidazole Schiff base in aqueous media via distinct routes. RSC Adv. 2015, 5, 88612–88624. [Google Scholar] [CrossRef]
- Matamoros, E.; Cintas, P.; Palacios, J.C. Tautomerism and stereodynamics in Schiff bases from gossypol and hemigossypol with N-aminoheterocycles. Org. Biomol. Chem. 2019, 17, 6229–6250. [Google Scholar] [CrossRef]
- Kumar, R.; Mani, G. Exhibition of the Brønsted acid–base character of a Schiff base in palladium(ii) complex formation: Lithium complexation, fluxional properties and catalysis of Suzuki reactions in water. Dalton Trans. 2015, 44, 6896–6908. [Google Scholar] [CrossRef]
- Hylland, K.; Gerz, I.; Wragg, D.; Øien, S.; Tilset, M. The Reactivity of Multidentate Schiff base ligands derived from bi- and terphenyl polyamines towards M(II) (M = Ni, Cu, Zn, Cd) and M(III) (M = Co, Y, Lu). Eur. J. Inorg. Chem. 2021, 2021, 1869–1889. [Google Scholar] [CrossRef]
- Yapar, G.; Demir, N.; Kiraz, A.; Özkat, G.Y.; Yıldız, M. Synthesis, Biological Activities, Antioxidant Properties, and Molecular Docking Studies of Novel Bis-Schiff Base Podands as Responsive Chemosensors for Anions. J. Mol. Struct. 2022, 1266, 133530. [Google Scholar] [CrossRef]
- Tsacheva, I.; Todorova, Z.; Momekova, D.; Momekov, G.; Koseva, N. Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates. Pharmaceuticals 2023, 16, 938. [Google Scholar] [CrossRef]
- Afrin, A.; Jayaraj, A.; Gayathri, M.S.; Chinna, A.S.P. An overview of Schiff base-based fluorescent turn-on probes: A potential candidate for tracking live cell imaging of biologically active metal ions. Sens. Diagn. 2023, 2, 988–1076. [Google Scholar] [CrossRef]
- Tang, H.-H.; Zhang, L.; Zeng, L.-L.; Fang, X.-M.; Lin, L.-R.; Zhang, H. A pair of Schiff base enantiomers studied by absorption, fluorescence, electronic and vibrational circular dichroism spectroscopies and density functional theory calculation. RSC Adv. 2015, 5, 36813–36819. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Yazdani, M.; Sharghi, H. Effects of solvent and substituent on the electronic absorption spectra of some substituted Schiff bases: A chemometrics study. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2012, 91, 198–205. [Google Scholar] [CrossRef]
- Derkowska-Zielinska, B.; Barwiolek, M.; Cassagne, C.; Boudebs, G. Nonlinear optical study of Schiff bases using Z-scan technique. Opt. Laser Technol. 2020, 124, 105968. [Google Scholar] [CrossRef]
- Maza, S.; Kijatkin, C.; Bouhidel, Z.; Pillet, S.; Schaniel, D.; Imlau, M.; Guillot, B.; Cherouana, A.; Bendeif, E.-E. Synthesis, structural investigation and NLO properties of three 1,2,4-triazole Schiff bases. J. Mol. Struct. 2020, 1219, 128492. [Google Scholar] [CrossRef]
- M Basheer, S.; Rasin, P.; Manakkadan, V.; Namboothiri, V.P.V.; Sreekanth, A. Recent Advancements in Schiff Base Fluorescence Chemosensors for the Detection of Heavy Metal Ions. In Schiff Base in Organic, Inorganic and Physical Chemistry; IntechOpen: London, UK, 2023. [Google Scholar]
- İnal, E.K. A Fluorescent Chemosensor Based on Schiff Base for the Determination of Zn2+, Cd2+ and Hg2+. J. Fluoresc. 2020, 30, 891–900. [Google Scholar] [CrossRef]
- Chethanakumar; Budri, M.; Gudasi, K.B.; Vadavi, R.S.; Bhat, S.S. Luminescent Pyrene-based Schiff base Receptor for Hazardous Mercury(II) Detection Demonstrated by Cell Imaging and Test Strip. J. Fluoresc. 2023, 33, 539–551. [Google Scholar] [CrossRef]
- Aroua, L.M.; Ali, R.; Albadri, A.E.A.E.; Messaoudi, S.; Alminderej, F.M.; Saleh, S.M. A New, Extremely Sensitive, Turn-Off Optical Sensor Utilizing Schiff Base for Fast Detection of Cu(II). Biosensors 2023, 13, 359. [Google Scholar] [CrossRef]
- Sadia, M.; Khan, J.; Naz, R.; Zahoor, M.; Khan, E.; Ullah, R.; Bari, A. Schiff-Based Fluorescent-ON Sensor L Synthesis and Its Application for Selective Determination of Cerium in Aqueous Media. J. Sens. 2020, 2020, 2192584. [Google Scholar] [CrossRef]
- Pang, Y.; Meng, D.; Liu, J.; Duan, S.; Fan, J.; Gao, L.; Long, X. Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Al(3+) Ions. Molecules 2023, 28, 3090. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Attinà, A.; Di Bella, S. A Zinc(II) Schiff Base Complex as Fluorescent Chemosensor for the Selective and Sensitive Detection of Copper(II) in Aqueous Solution. Sensors 2023, 23, 3925. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, J.; Park, S.; Jung, J.; Kim, D. A Schiff Base Fluorescence Enhancement Probe for Fe(III) and Its Sensing Applications in Cancer Cells. Sensors 2019, 19, 2500. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Muthusamy, S.; Zhu, D.; Xue, Z.; Yu, Q.; Chiyumba, C.N.; Mack, J.; Nyokong, T.; Wang, S.; Zhao, L. A simple quinoline-thiophene Schiff base turn-off chemosensor for Hg2+ detection: Spectroscopy, sensing properties and applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120338. [Google Scholar] [CrossRef]
- Horak, E.; Kassal, P.; Hranjec, M.; Steinberg, I.M. Benzimidazole functionalised Schiff bases: Novel pH sensitive fluorescence turn-on chromoionophores for ion-selective optodes. Sens. Actuators B Chem. 2018, 258, 415–423. [Google Scholar] [CrossRef]
- Rout, K.; Manna, A.K.; Sahu, M.; Mondal, J.; Singh, S.K.; Patra, G.K. Triazole-based novel bis Schiff base colorimetric and fluorescent turn-on dual chemosensor for Cu2+ and Pb2+: Application to living cell imaging and molecular logic gates. RSC Adv. 2019, 9, 25919–25931. [Google Scholar] [CrossRef]
- Chaihan, K.; Semakul, N.; Promarak, V.; Bui, T.-T.; Kungwan, N.; Goubard, F. Tunable far-red fluorescence utilizing π-extension and substitution on the excited state intramolecular proton transfer (ESIPT) of naphthalene-based Schiff bases: A combined experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2022, 431, 114047. [Google Scholar] [CrossRef]
- Harathi, J.; Thenmozhi, K. AIE-active Schiff base compounds as fluorescent probes for the highly sensitive and selective detection of Fe3+ ions. Mater. Chem. Front. 2020, 4, 1471–1482. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Gurusamy, S.; Rajakumar, K.; Sathish, V.; Thanasekaran, P.; Mathavan, A. Aggregation induced emission (AIE), selective fluoride ion sensing and lysozyme interaction properties of Julolidinesulphonyl derived Schiff base. J. Photochem. Photobiol. A Chem. 2022, 427, 113822. [Google Scholar] [CrossRef]
- Patel, D.A.; Ashok Kumar, S.K.; Sahoo, S.K. Aggregation-induced emission active salicylaldehyde hydrazone with multipurpose sensing applications. J. Photochem. Photobiol. A Chem. 2023, 437, 114465. [Google Scholar] [CrossRef]
- Sathish, V.; Ramdass, A.; Thanasekaran, P.; Lu, K.-L.; Rajagopal, S. Aggregation-induced phosphorescence enhancement (AIPE) based on transition metal complexes—An overview. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 25–44. [Google Scholar] [CrossRef]
- Li, A.; Liu, Y.-H.; Yuan, L.-Z.; Ma, Z.-Y.; Zhao, C.-L.; Xie, C.-Z.; Bao, W.-G. Association of structural modifications with bioactivity in three new copper(II) complexes of Schiff base ligands derived from 5-chlorosalicyladehyde and amino acids. J. Inorg. Biochem. 2015, 146, 52–60. [Google Scholar] [CrossRef]
- Sansul, S.; Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Kariuki, B.M.; Hashim, H.; Ahmed, A. Pendant Modification of Poly(methyl methacrylate) to Enhance Its Stability against Photoirradiation. Polymers 2023, 15, 2989. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Kopchuk, D.S.; Platonov, V.A.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. Mechanosynthesis of Diaminobiphenyls-Based Schiff’s Bases as Simple Probes for the Naked-Eye Detection of Cyanide Ion. Chemistry 2023, 5, 66. [Google Scholar] [CrossRef]
- Mohd Izham, N.Z.; Yusoff, H.M.; Ul Haq Bhat, I.; Endo, T.; Fukumura, H.; Kwon, E.; Yoshida, S.I.; Asari, A.; Osman, U.M.; Mohd Yusof, M.S. Data on synthesis and characterization of new p-nitro stilbene Schiff bases derivatives as an electrochemical DNA potential spacer. Data Brief 2020, 30, 105568. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Zhou, Z.-Y.; Zhuo, S.-P.; Shan, G.-G.; Xing, L.-B.; Wang, H.-N.; Su, Z.-M. Rational design of a highly sensitive and selective “turn-on” fluorescent sensor for PO43− detection. Dalton Trans. 2015, 44, 20830–20833. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.-J.; Sun, X.-J.; Liu, T.-T.; Xing, Z.-Y. A dual-functional chemosensor for fluorescent on-off and ratiometric detection of Cu2+ and Hg2+ and its application in cell imaging. Dye Pigment. 2019, 170, 107642. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhang, Y.-M.; Kan, X.-T.; Yang, Q.-Y.; Li, Y.-J.; Wei, T.-B.; Yao, H.; Lin, Q. Tripodal aroyl hydrazone based AIE fluorescent sensor for relay detection Hg2+ and Br− in living cells. Dye Pigment. 2021, 191, 109389. [Google Scholar] [CrossRef]
- Bawa, R.; Deswal, N.; Negi, S.; Dalela, M.; Kumar, A.; Kumar, R. Pyranopyrazole based Schiff base for rapid colorimetric detection of arginine in aqueous and real samples. RSC Adv. 2022, 12, 11942–11952. [Google Scholar] [CrossRef]
- Yang, G.; Meng, X.; Fang, S.; Duan, H.; Wang, L.; Wang, Z. A highly selective colorimetric fluorescent probe for detection of Hg2+ and its application on test strips. RSC Adv. 2019, 9, 8529–8536. [Google Scholar] [CrossRef]
- Tekuri, V.; Sahoo, S.K.; Trivedi, D.R. Hg2+ induced hydrolysis of thiazole amine based Schiff base: Colorimetric and fluorogenic chemodosimeter for Hg2+ ions in an aqueous medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 19–26. [Google Scholar] [CrossRef]
- Cui, C.; Gao, X.; Jia, X.; Jiao, Y.; Duan, C. A rhodamine B-based turn on fluorescent probe for selective recognition of mercury(II) ions. Inorganica Chim. Acta 2021, 520, 120285. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, L.; Qin, D.; Zhou, J.; Duan, H. Two Novel Fluorescent Probes as Systematic Sensors for Multiple Metal Ions: Focus on Detection of Hg2+. ACS Omega 2020, 5, 24285–24295. [Google Scholar] [CrossRef]
- Vanjare, B.D.; Mahajan, P.G.; Ryoo, H.-I.; Dige, N.C.; Choi, N.G.; Han, Y.; Kim, S.J.; Kim, C.-H.; Lee, K.H. Novel rhodamine based chemosensor for detection of Hg2+: Nanomolar detection, real water sample analysis, and intracellular cell imaging. Sens. Actuators B 2021, 330, 129308. [Google Scholar] [CrossRef]
- Dewangan, S.; Barik, T.; Halder, B.; Mishra, A.; Dhiman, R.; Sasamori, T.; Chatterjee, S. Rhodamine tethered 1,1′-unsymmetrical ferrocene functionalization: Metal sensing, cell imaging and logic gate properties. J. Organomet. Chem. 2021, 948, 121922. [Google Scholar] [CrossRef]
- Christopher Leslee, D.B.; Karuppannan, S.; Kothottil, M.M. Carbazole-hydrazinobenzothiazole a selective turn-on fluorescent sensor for Hg2+ions—Its protein binding and electrochemical application studies. J. Photochem. Photobiol. A Chem. 2021, 415, 113303. [Google Scholar] [CrossRef]
- Singh, R.; Das, G. Fluorogenic detection of Hg2+ and Ag+ ions via two mechanistically discrete signal genres: A paradigm of differentially responsive metal ion sensing. Sens. Actuators B: Chem. 2018, 258, 478–483. [Google Scholar] [CrossRef]
- Yin, J.; Bing, Q.; Wang, L.; Wang, G. Ultrasensitive and highly selective detection of Cu2+ ions based on a new carbazole-Schiff. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 495–501. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Wu, W.-N.; Yu, Y.-P.; Zhao, X.-L.; Xu, Z.-H.; Xu, Z.-Q.; Fan, Y.-C. Aggregation-induced ratiometric emission active monocarbazone: Ratiometric fluorescent probe for Cu2+ in either solution or aggregation states. J. Lumin. 2018, 204, 289–295. [Google Scholar] [CrossRef]
- Yang, M.; Ma, L.; Li, J.; Kang, L. Fluorescent probe for Cu2+ and the secondary application of the resultant complex to detect cysteine. RSC Adv. 2019, 9, 16812–16818. [Google Scholar] [CrossRef]
- Ni, H.; Wang, Q.; Jin, L.; Wang, W.; Dai, L.; Zhao, C. High selectivity and reversibility/reusability red emitting fluorescent probe for copper ions detection and imaging in living cells. J. Lumin. 2019, 206, 125–131. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, C. Selective monitoring of Cu(II) with a fluorescence–on naphthalene–based probe in aqueous solution. Inorg. Chem. Commun. 2019, 107, 107467. [Google Scholar] [CrossRef]
- Venkatesan, V.; Selva Kumar, R.; Ashok Kumar, S.K.; Sahoo, S.K. Dual optical properties of new schiff base based on bisthiophene for sensing of Cu2+ in protic media. J. Mol. Struct. 2019, 1198, 126906. [Google Scholar] [CrossRef]
- Slassi, S.; Aarjane, M.; El-Ghayoury, A.; Amine, A. A highly turn-on fluorescent CHEF-type chemosensor for selective detection of Cu2+ in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 348–353. [Google Scholar] [CrossRef]
- Fang, H.; Huang, P.-C.; Wu, F.-Y. A novel jointly colorimetric and fluorescent sensor for Cu2+ recognition and its complex for sensing S2− by a Cu2+ displacement approach in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 568–575. [Google Scholar] [CrossRef]
- Arjunan, S.; Gopalan, S.; Selvaraj, S.; Thangaraj, S.; Krishna, K.; Nanjan, B.; Raju, N.; Palathurai Subramaniam, M. A selective Fluorescence Chemosensor: Pyrene motif Schiff base derivative for detection of Cu2+ ions in living cells. J. Photochem. Photobiol. A Chem. 2018, 364, 424–432. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.; Pu, S. A highly selective fluorescent probe for Cu2+ based on a diarylethene with a benzo[1,2,5]oxadiazol-4-ylamine Schiff base unit. J. Photochem. Photobiol. A Chem. 2018, 364, 208–218. [Google Scholar] [CrossRef]
- Bing, Q.; Wang, L.; Li, D.; Wang, G. A new high selective and sensitive turn-on fluorescent and ratiometric absorption chemosensor for Cu2+ based on benzimidazole in aqueous solution and its application in live cell. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 202, 305–313. [Google Scholar] [CrossRef]
- Nouri Moghadam, F.; Amirnasr, M.; Meghdadi, S.; Eskandari, K.; Buchholz, A.; Plass, W. A new fluorene derived Schiff-base as a dual selective fluorescent probe for Cu2+ and CN−. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 6–15. [Google Scholar] [CrossRef]
- Kouser, R.; Zehra, S.; Khan, R.A.; Alsalme, A.; Arjmand, F.; Tabassum, S. “Turn–on” benzophenone based fluorescence and colorimetric sensor for the selective detection of Fe2+ in aqueous media: Validation of sensing mechanism by spectroscopic and computational studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119156. [Google Scholar] [CrossRef]
- Hou, L.; Liu, T.; Gong, Y.; Li, J.; Deng, C.; Zhang, C.; Wang, Y.; Shuang, S.; Liang, W. A turn-on Schiff base fluorescent probe for the exogenous and endogenous Fe3+ ion sensing and bioimaging of living cells. New J. Chem. 2020, 44, 19642–19649. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, T.; Xiao, D.; Yuan, F.; Li, T.; Wang, E.; Liu, H.; Niu, Q. A dual-responsive colorimetric and fluorescent chemosensor based on diketopyrrolopyrrole derivative for naked-eye detection of Fe3+ and its practical application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 594–600. [Google Scholar] [CrossRef]
- Tamil Selvan, G.; Varadaraju, C.; Tamil Selvan, R.; Enoch, I.V.M.V.; Mosae Selvakumar, P. On/Off Fluorescent Chemosensor for Selective Detection of Divalent Iron and Copper Ions: Molecular Logic Operation and Protein Binding. ACS Omega 2018, 3, 7985–7992. [Google Scholar] [CrossRef]
- Zuo, Z.; Song, X.; Guo, D.; Guo, Z.; Niu, Q. A dual responsive colorimetric/fluorescent turn-on sensor for highly selective, sensitive and fast detection of Fe3+ ions and its applications. J. Photochem. Photobiol. A Chem. 2019, 382, 111876. [Google Scholar] [CrossRef]
- Guo, Z.; Niu, Q.; Li, T. Highly sensitive oligothiophene-phenylamine-based dual-functional fluorescence “turn-on” sensor for rapid and simultaneous detection of Al3+ and Fe3+ in environment and food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 76–84. [Google Scholar] [CrossRef]
- Senthil Murugan, A.; Kiruthika, M.; Abel Noelson, E.R.; Yogapandi, P.; Kumar, G.G.; Annaraj, J. Fluorescent sensor for in-vivo bio-imaging, precise tracking of Fe3+ ions in Zebrafish embryos and visual measuring of Cu2+ ions in pico-molar level. Arab. J. Chem. 2021, 14, 102910. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, X.; Li, Z.; Zhou, Q.; Lei, M.; Hu, S.; Wu, X.; Li, C.; Xu, Z.; Wang, Y. A pyrrole-containing hydrazone and its Cu2+ complex: An easily accessible optical chemosensor system for the successive detection of Zn2+/Cu2+ and pyrophosphate. Anal. Methods 2018, 10, 5790–5796. [Google Scholar] [CrossRef]
- He, X.; Ding, F.; Sun, X.; Zheng, Y.; Xu, W.; Ye, L.; Chen, H.; Shen, J. Reversible Chemosensor for Bioimaging and Biosensing of Zn(II) and hpH in Cells, Larval Zebrafish, and Plants with Dual-Channel Fluorescence Signals. Inorg. Chem. 2021, 60, 5563–5572. [Google Scholar] [CrossRef]
- Wu, Q.; Feng, L.; Chao, J.B.; Wang, Y.; Shuang, S. Ratiometric sensing of Zn2+ with a new benzothiazole-based fluorescent sensor and living cell imaging. Analyst 2021, 146, 4348–4356. [Google Scholar] [CrossRef]
- Malthus, S.J.; Cameron, S.A.; Brooker, S. Improved Access to 1,8-Diformyl-carbazoles Leads to Metal-Free Carbazole-Based [2 + 2] Schiff Base Macrocycles with Strong Turn-On Fluorescence Sensing of Zinc(II) Ions. Inorg. Chem. 2018, 57, 2480–2488. [Google Scholar] [CrossRef]
- Upadhyay, Y.; Anand, T.; Babu, L.T.; Paira, P.; Crisponi, G.; Sk, A.K.; Kumar, R.; Sahoo, S.K. Three-in-one type fluorescent sensor based on a pyrene pyridoxal cascade for the selective detection of Zn(ii), hydrogen phosphate and cysteine. Dalton Trans. 2018, 47, 742–749. [Google Scholar] [CrossRef]
- Yun, J.Y.; Chae, J.B.; Kim, M.; Lim, M.H.; Kim, C. A multiple target chemosensor for the sequential fluorescence detection of Zn2+ and S2− and the colorimetric detection of Fe3+/2+ in aqueous media and living cells. Photochem. Photobiol. Sci. 2019, 18, 166–176. [Google Scholar] [CrossRef]
- Wu, W.-N.; Mao, P.-D.; Wang, Y.; Zhao, X.-L.; Xu, Z.-Q.; Xu, Z.-H.; Xue, Y. Quinoline containing acetyl hydrazone: An easily accessible switch-on optical chemosensor for Zn2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 324–331. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, H. A multi-responsive AIE-active tetraphenylethylene-functioned salicylaldehyde-based schiff base for reversible mechanofluorochromism and Zn2+ and CO32− detection. Org. Electron. 2019, 73, 55–61. [Google Scholar] [CrossRef]
- Liu, L.-m.; Yang, Z.-y. A rhodamine and chromone based “turn-on” fluorescent probe (RC1) for Zn(II) in aqueous solutions and its application. J. Photochem. Photobiol. A Chem. 2018, 364, 558–563. [Google Scholar] [CrossRef]
- Liu, C.; Qin, J.-C.; Xue, J.; Tian, L.-M.; Li, T.-R.; Yang, Z.-Y. Development of a novel fluorescent probe for Zn2+/Cu2+/S2− in different solutions based on benzocoumarin derivative. J. Photochem. Photobiol. A Chem. 2019, 385, 112091. [Google Scholar] [CrossRef]
- Rani, B.K.; John, S.A. A highly selective turn-on fluorescent chemosensor for detecting zinc ions in living cells using symmetrical pyrene system. J. Photochem. Photobiol. A Chem. 2021, 418, 113372. [Google Scholar] [CrossRef]
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Zhu, D.; Tang, M.; Xue, Z.; Wang, S.; Zhao, L. A naphthol hydrazone Schiff base bearing benzothiadiazole unit for fluorescent detection of Fe3+ in PC3 cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122242. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Huang, Z.; Ma, Q.; Liang, Y.; Xue, Z.; Feng, L.; Zhao, L. A pyridine modified naphthol hydrazone Schiff base chemosensor for Al3+ via intramolecular charge transfer process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122961. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Zhang, Y.; Zhu, D.; Xue, Z.; Yuan, R.; Wang, S.; Zhao, L. Turn-off detection of Cr(III) with chelation enhanced fluorescence quenching effect by a naphthyl hydrazone Shiff base chemosensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121599. [Google Scholar] [CrossRef]
- Hwang, S.M.; Yun, D.; Lee, H.; Kim, M.; Lim, M.H.; Kim, K.-T.; Kim, C. Relay detection of Zn2+ and S2− by a quinoline-based fluorescent chemosensor in aqueous media and zebrafish. Dye Pigment. 2019, 165, 264–272. [Google Scholar] [CrossRef]
- Li, D.; Liu, A.; Xing, Y.; Li, Z.; Luo, Y.; Zhao, S.; Dong, L.; Xie, T.; Guo, K.; Li, J. A smart chemosensor with different response mechanisms to multi-analytes: Chromogenic and fluorogenic recognition of Cu2+, Fe3+, and Zn2+. Dye Pigment. 2023, 213, 111180. [Google Scholar] [CrossRef]
- Kang, J.H.; Han, J.; Lee, H.; Lim, M.H.; Kim, K.-T.; Kim, C. A water-soluble fluorescence chemosensor for the sequential detection of Zn2+ and pyrophosphate in living cells and zebrafish. Dye Pigment. 2018, 152, 131–138. [Google Scholar] [CrossRef]
- Velmathi, S.; Reena, V.; Suganya, S.; Anandan, S. Pyrrole Based Schiff Bases as Colorimetric and Fluorescent Chemosensors for Fluoride and Hydroxide Anions. J. Fluoresc. 2012, 22, 155–162. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Velmathi, S. Colorimetric chemosensor for multi-signaling detection of metal ions using pyrrole based Schiff bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 428–435. [Google Scholar] [CrossRef]
- Maity, D.; Kumar Mandal, S.; Guha, B.; Roy, P. A salicylaldehyde based dual chemosensor for zinc and arsenate ion detection: Biological application. Inorganica Chim. Acta 2021, 519, 120258. [Google Scholar] [CrossRef]
- Minhaz, A.; Khan, N.; Jamila, N.; Javed, F.; Imran, M.; Shujah, S.; Noor Khan, S.; Atlas, A.; Shah, M.R. Schiff base stabilized silver nanoparticles as potential sensor for Hg(II) detection, and anticancer and antibacterial agent. Arab. J. Chem. 2020, 13, 8898–8908. [Google Scholar] [CrossRef]
- Elisabete, O.; Nunes-Miranda, J.; Santos, H. From colorimetric chemosensors to metal nanoparticles using two tyrosine Schiff-base ligands for Cu2+ detection. Inorganica Chim. Acta 2012, 380, 22–30. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, X.; Wang, M.; Liu, Q.; Shao, Y.; Li, H.; Liang, C.; Chen, X.; Wang, H. A Schiff-Base Modified Pt Nano-Catalyst for Highly Efficient Synthesis of Aromatic Azo Compounds. Catalysts 2019, 9, 339. [Google Scholar] [CrossRef]
- Zhu, T.; Gou, Q.; Yang, Y.; Zhang, Y.; Chen, M. Bis-Schiff base functionalized Fe3O4 nanoparticles for the sensitive fluorescence sensation of copper ions in aqueous medium. J. Mol. Struct. 2022, 1264, 133258. [Google Scholar] [CrossRef]
- Singh, G.; Sushma; Singh, A.; Satija, P.; Shilpy; Mohit; Priyanka; Singh, J.; Khosla, A. Schiff base derived bis-organosilanes: Immobilization on silica nanosphere and Cu2+ and Fe3+ dual ion sensing. Inorganica Chim. Acta 2020, 514, 120028. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Sui, D.; Wang, Y. Highly selective fluorescence chemosensors based on functionalized SBA-15 for detection of Ag+ in aqueous media. Sens. Actuators B Chem. 2017, 242, 1043–1049. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Chan, W.; Wang, Y.; You, Q.; Liu, C.; Zheng, J.; Li, J.; Yang, S.; Yang, R. Selective Tracking of Lysosomal Cu2+ Ions Using Simultaneous Target- and Location-Activated Fluorescent Nanoprobes. Anal. Chem. 2015, 87, 584–591. [Google Scholar] [CrossRef]
- Singh, N.; Mulrooney, R.C.; Kaur, N.; Callan, J.F. A nanoparticle based chromogenic chemosensor for the simultaneous detection of multiple analytes. Chem. Commun. 2008, 40, 4900–4902. [Google Scholar] [CrossRef]
- Suneetha, G.; Ayodhya, D.; Sunitha Manjari, P. Schiff base stabilized gold nanoparticles: Synthesis, characterization, catalytic reduction of nitroaromatic compounds, fluorometric sensing, and biological activities. Results Chem. 2023, 5, 100688. [Google Scholar] [CrossRef]
- Khashei Siuki, H.; Ghamari Kargar, P.; Bagherzade, G. New Acetamidine Cu(II) Schiff base complex supported on magnetic nanoparticles pectin for the synthesis of triazoles using click chemistry. Sci. Rep. 2022, 12, 3771. [Google Scholar] [CrossRef]
- Omar, M.S.; Sanif, M.; Ali, N.; Hamid, M.; Taha, H.; Mahadi, A.H.; Usman, A.J.S. Synthesis of Schiff Base Encapsulated ZnS Nanoparticles: Characterization and Antibacterial Screening. Int. J. Technol. 2020, 11, 1309–1318. [Google Scholar] [CrossRef]
- Kakanejadifard, A.; Khojasteh, V.; Zabardasti, A.; Azarbani, F. New Azo-Schiff Base Ligand Capped Silver and Cadmium Sulfide Nanoparticles Preparation, Characterization, Antibacterial and Antifungal Activities. J. Org. Chem. Res. 2018, 4, 210–226. [Google Scholar] [CrossRef]
- Adwin Jose, P.; Sankarganesh, M.; Dhaveethu Raja, J.; Senthilkumar, G.S.; Nandini Asha, R.; Raja, S.J.; Sheela, C.D. Bio-inspired nickel nanoparticles of pyrimidine-Schiff base: In vitro anticancer, BSA and DNA interactions, molecular docking and antioxidant studies. J. Biomol. Struct. Dyn. 2022, 40, 10715–10729. [Google Scholar] [CrossRef]
- Sahudin, M.A.; Su’ait, M.S.; Tan, L.L.; Abd Karim, N.H. Schiff base complex/TiO2 chemosensor for visual detection of food freshness level. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119129. [Google Scholar] [CrossRef]
- Lee, I.; Kim, S.; Kim, S.-n.; Jang, Y.; Jang, J. Highly Fluorescent Amidine/Schiff Base Dual-Modified Polyacrylonitrile Nanoparticles for Selective and Sensitive Detection of Copper Ions in Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 17151–17156. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, L.; Song, X.; Zhao, L. Developing an Electrochemically Reversible Switch for Modulating the Optical Signal of Gold Nanoparticles. Molecules 2023, 28, 6233. [Google Scholar] [CrossRef]
- Amourizi, F.; Dashtian, K.; Ghaedi, M.; Hosseinzadeh, B. An asymmetric Schiff base-functionalized gold nanoparticle-based colorimetric sensor for Hg2+ ion determination: Experimental and DFT studies. Anal. Methods 2021, 13, 2603–2611. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Shin, J.S.; Dige, N.C.; Vanjare, B.D.; Han, Y.; Choi, N.G.; Kim, S.J.; Seo, S.Y.; Lee, K.H. Chelation enhanced fluorescence of rhodamine based novel organic nanoparticles for selective detection of mercury ions in aqueous medium and intracellular cell imaging. J. Photochem. Photobiol. A Chem. 2020, 397, 112579. [Google Scholar] [CrossRef]
- Čonková, M.; Montes-García, V.; Konopka, M.; Ciesielski, A.; Samori, P.; Stefankiewicz, A.R. Schiff base capped gold nanoparticles for transition metal cation sensing in organic media. Chem. Commun. 2022, 58, 5773–5776. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Chen, X.; Zhang, X.; Zhou, H.; Du, H.; Wang, M.; Ma, Y.; Jin, X. Detection of Cu2+ and S2− with fluorescent polymer nanoparticles and bioimaging in HeLa cells. Anal. Bioanal. Chem. 2021, 413, 3945–3953. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Fabrication of Schiff base coordinated ZnS nanoparticles for enhanced photocatalytic degradation of chlorpyrifos pesticide and detection of heavy metal ions. J. Mater. 2019, 5, 446–454. [Google Scholar] [CrossRef]
- Srisukjaroen, R.; Wechakorn, K.; Teepoo, S. A smartphone based-paper test strip chemosensor coupled with gold nanoparticles for the Pb2+ detection in highly contaminated meat samples. Microchem. J. 2022, 179, 107438. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Abednatanzi, S.; Esquivel, D.; Krishnaraj, C.; Jena, H.S.; Wang, G.; Leus, K.; Van Deun, R.; Romero–Salguero, F.J.; Van Der Voort, P. Amine-containing (nano-) Periodic Mesoporous Organosilica and its application in catalysis, sorption and luminescence. Microporous Mesoporous Mater. 2020, 291, 109687. [Google Scholar] [CrossRef]
- Selvan, K.S.; Narayanan, S.S. Synthesis and characterization of carbon nanotubes/asymmetric novel tetradentate ligand forming complexes on PIGE modified electrode for simultaneous determination of Pb(II) and Hg(II) in sea water, Lake water and well water using anodic stripping voltammetry. J. Electroanal. Chem. 2018, 810, 176–184. [Google Scholar] [CrossRef]
- Parsaee, Z.; Karachi, N.; Razavi, R. Ultrasound assisted fabrication of a novel optode base on a triazine based Schiff base immobilized on TEOS for copper detection. Ultrason. Sonochemistry 2018, 47, 36–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Wu, G.; Wang, J.; Zhang, T. Quaternized salicylaldehyde Schiff base modified mesoporous silica for efficiently sensing Cu(II) ions and their removal from aqueous solution. Appl. Surf. Sci. 2020, 527, 146803. [Google Scholar] [CrossRef]
- Chen, G.; Lan, H.-H.; Cai, S.-L.; Sun, B.; Li, X.-L.; He, Z.-H.; Zheng, S.-R.; Fan, J.; Liu, Y.; Zhang, W.-G. Stable Hydrazone-Linked Covalent Organic Frameworks Containing O,N,O′-Chelating Sites for Fe(III) Detection in Water. ACS Appl. Mater. Interfaces 2019, 11, 12830–12837. [Google Scholar] [CrossRef]
- Gai, F.; Yin, L.; Fan, M.; Li, L.; Grahn, J.; Ao, Y.; Yang, X.; Wu, X.; Liu, Y.; Huo, Q. Novel Schiff base (DBDDP) selective detection of Fe (III): Dispersed in aqueous solution and encapsulated in silica cross-linked micellar nanoparticles in living cell. J. Colloid Interface Sci. 2018, 514, 357–363. [Google Scholar] [CrossRef]
- Mahmoud, N.F.; Fouad, O.A.; Ali, A.E.; Mohamed, G.G. Potentiometric Determination of the Al(III) Ion in Polluted Water and Pharmaceutical Samples by a Novel Mesoporous Copper Metal–Organic Framework-Modified Carbon Paste Electrode. Ind. Eng. Chem. Res. 2021, 60, 2374–2387. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, R.; Kaur, V.; Talwar, D. Reusable Schiff base functionalized silica as a multi-purpose nanoprobe for fluorogenic recognition, quantification and extraction of Zn2+ ions. Sens. Actuators B Chem. 2018, 254, 533–541. [Google Scholar] [CrossRef]
- Liu, X.; Fu, C.; Ren, X.; Liu, H.; Li, L.; Meng, X. Fluorescence switching method for cascade detection of salicylaldehyde and zinc(II) ion using protein protected gold nanoclusters. Biosens. Bioelectron. 2015, 74, 322–328. [Google Scholar] [CrossRef]
- Bothra, S.; Babu, L.T.; Paira, P.; Ashok Kumar, S.K.; Kumar, R.; Sahoo, S.K. A biomimetic approach to conjugate vitamin B6 cofactor with the lysozyme cocooned fluorescent AuNCs and its application in turn-on sensing of zinc(II) in environmental and biological samples. Anal. Bioanal. Chem. 2018, 410, 201–210. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Wang, S.; Schipper, D.; Jones, R.A. Construction of a Large High-Nuclearity Cd–Sm Schiff Base Cluster with Nanoscale Inner Cavity as Luminescent Probe for Metal Cations. Cryst. Growth Des. 2019, 19, 2149–2154. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Yang, X.; Shi, D.; Schipper, D.; Jones, R.A. Construction of Chiral “Triple-Decker” Nd(III) Nanocluster with High NIR Luminescence Sensitivity toward Co(II). Inorg. Chem. 2020, 59, 8652–8656. [Google Scholar] [CrossRef]
- Yang, H.; Lu, F.; Zhan, X.; Tian, M.; Yuan, Z.; Lu, C.J.T. A Eu3+-inspired fluorescent carbon nanodot probe for the sensitive visualization of anthrax biomarker by integrating EDTA chelation. Talanta 2020, 208, 120368. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, Q.; Yin, J.-H.; Xu, N.J.J.O.P.; Biology, P.B. Polyethyleneimine protected silver nanoclusters luminescence probe for sensitive detection of cobalt (II) in living cells. J. Photochem. Photobiol. B Biol. 2017, 173, 508–513. [Google Scholar] [CrossRef]
- Ren, Q.; Shen, X.; Sun, Y.; Fan, R.; Zhang, J.J.F.C. A highly sensitive competitive immunosensor based on branched polyethyleneimine functionalized reduced graphene oxide and gold nanoparticles modified electrode for detection of melamine. Food Chem. 2020, 304, 125397. [Google Scholar] [CrossRef]
- Dharmalingam, P.; Palani, G.; Apsari, R.; Kannan, K.; Lakkaboyana, S.K.; Venkateswarlu, K.; Kumar, V.; Ali, Y. Synthesis of metal oxides/sulfides-based nanocomposites and their environmental applications: A review. Mater. Today Sustain. 2022, 20, 100232. [Google Scholar] [CrossRef]
- Fan, Y.; Tao, T.; Wang, H.; Liu, Z.; Huang, W.; Cao, H. A Schiff base-functionalized graphene quantum dot nanocomposite for preferable picric acid sensing. Dye Pigment. 2021, 191, 109355. [Google Scholar] [CrossRef]
- Sun, J.-K.; Antonietti, M.; Yuan, J. Nanoporous ionic organic networks: From synthesis to materials applications. Chem. Soc. Rev. 2016, 45, 6627–6656. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Javadi, F.; Mohajer, F.; Anafcheh, M.; Badiei, A.; Ghasemi, J.B. A new Fe3+ colorimetric sensor: Nitrophenyl bispyrazole derivative synthesis using Fe3O4@SiO2@Si-Pr-NH-(CH2)2-NH2 and its DFT study. Mater. Chem. Phys. 2022, 275, 125285. [Google Scholar] [CrossRef]
- Pundir, M.; Prasher, P.; Vasić, K.; Leitgeb, M.; Kumar, A.; Prakash, R.; Knez, Ž.; Pandey, J.K.; Kumar, S. Enzyme modified CNTs for biosensing application: Opportunities and challenges. Colloid Interface Sci. Commun. 2021, 44, 100506. [Google Scholar] [CrossRef]
- Fach, M.; Radi, L.; Wich, P.R. Nanoparticle Assembly of Surface-Modified Proteins. J. Am. Chem. Soc. 2016, 138, 14820–14823. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks bearing free carboxylic acids: Preparation, modification, and applications. Coord. Chem. Rev. 2022, 450, 214237. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Deng, H.; Huang, K.; Xiu, L.; Sun, W.; Yao, Q.; Fang, X.; Huang, X.; Noreldeen, H.A.A.; Peng, H.; Xie, J.; et al. Bis-Schiff base linkage-triggered highly bright luminescence of gold nanoclusters in aqueous solution at the single-cluster level. Nat. Commun. 2022, 13, 3381. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Jiang, D.; Shi, D.; Zhang, L. Construction of NIR luminescent polynuclear lanthanide-based nanoclusters with sensing properties towards metal ions. Dalton Trans. 2018, 47, 13880–13886. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Wang, S.; Zhu, T.; Bo, L.; Zhang, L.; Chen, H.; Jiang, D.; Dong, X.; Huang, S. Anion dependent self-assembly of drum-like 30- and 32-metal Cd–Ln nanoclusters: Visible and NIR luminescent sensing of metal cations. J. Mater. Chem. C 2018, 6, 865–874. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering luminescent metal nanoclusters for sensing applications. Coord. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]

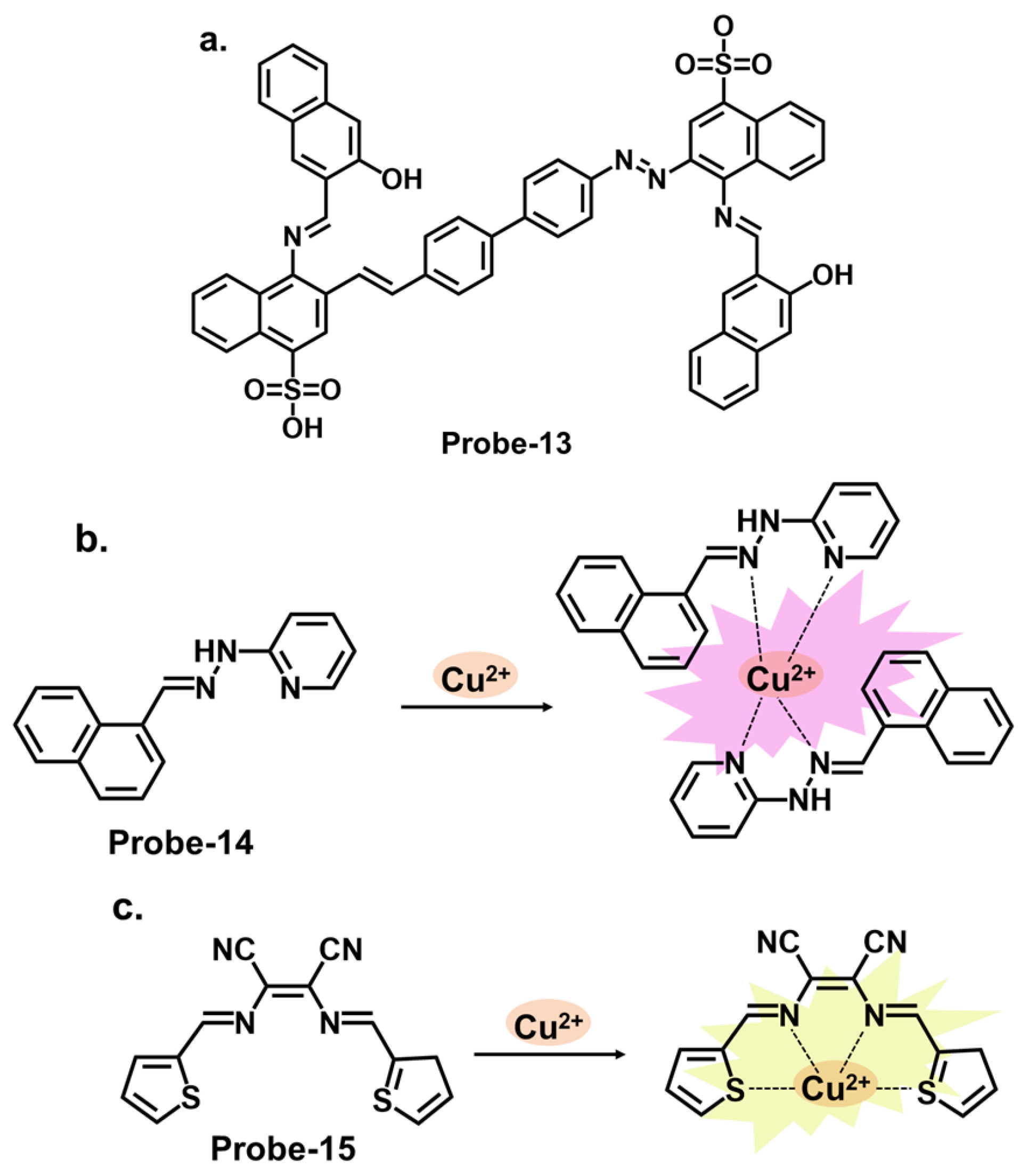
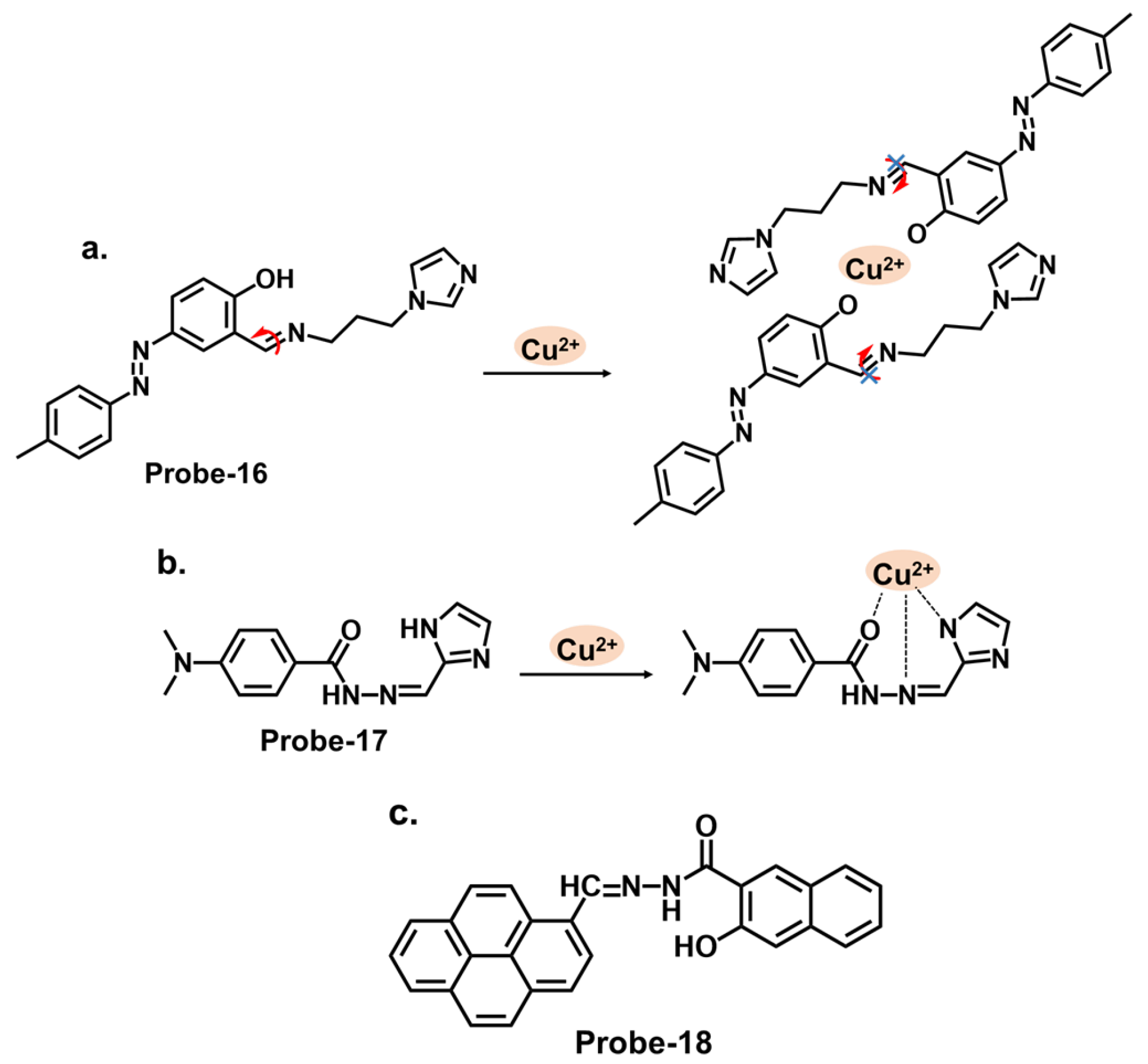
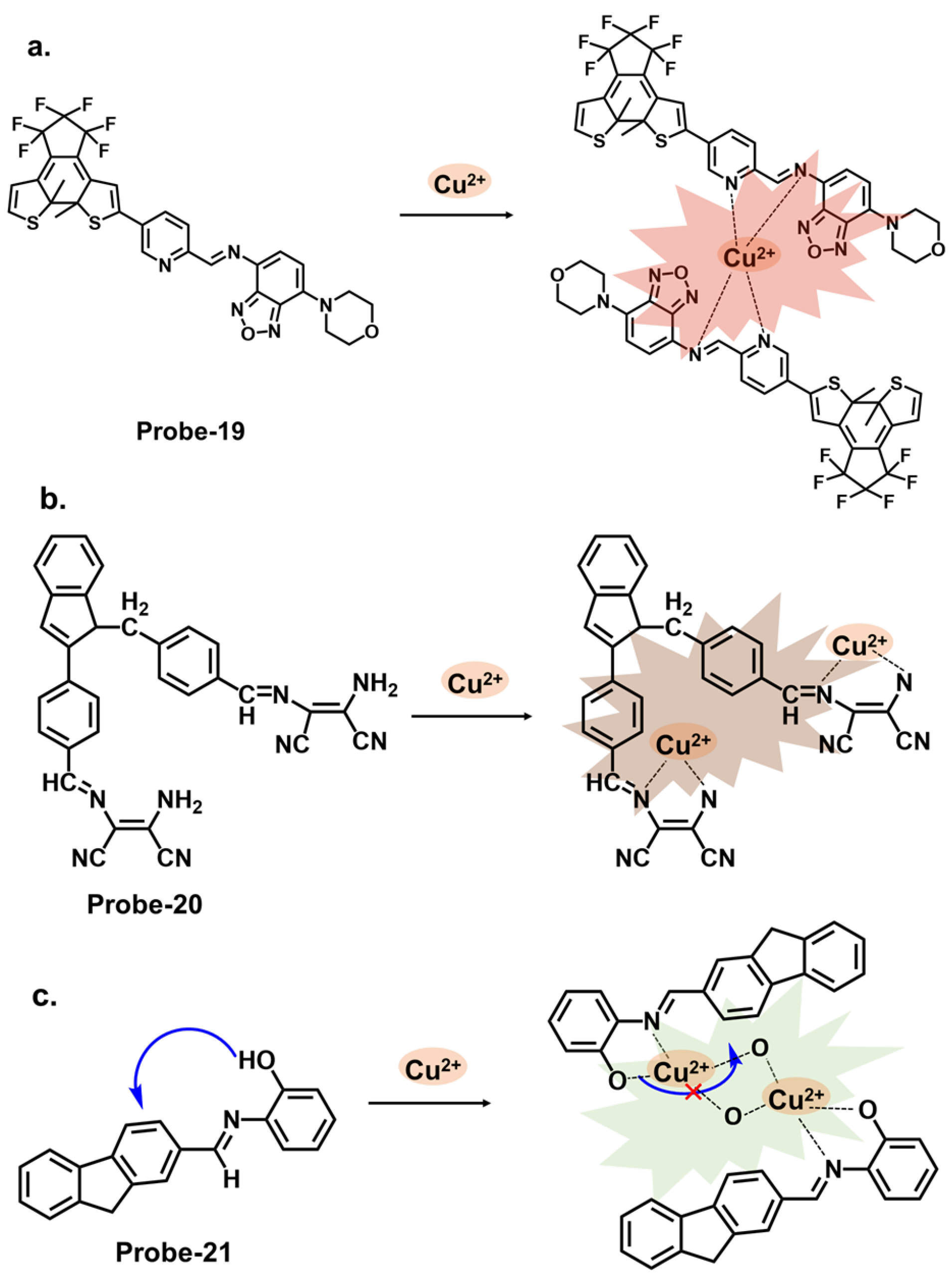
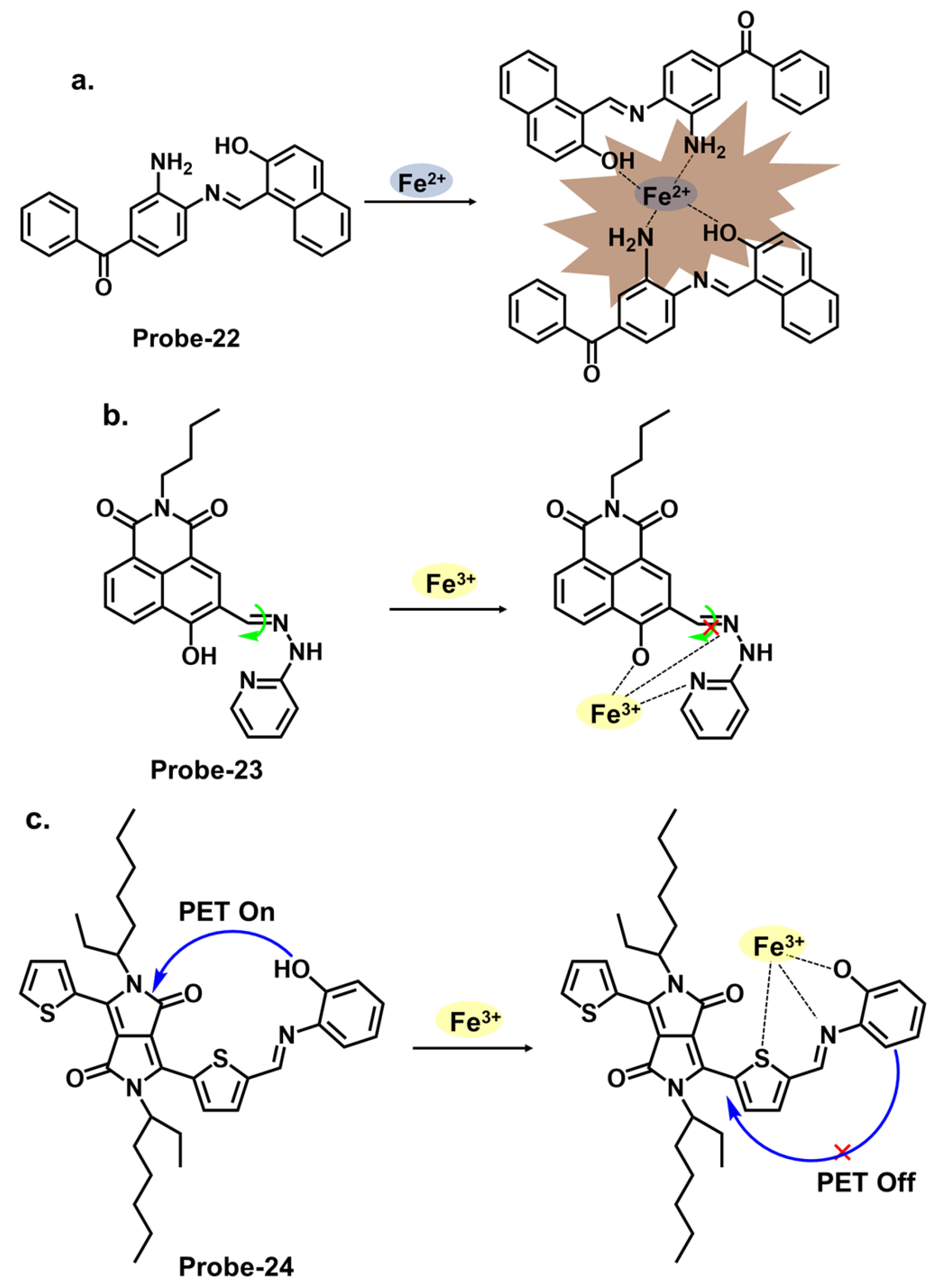
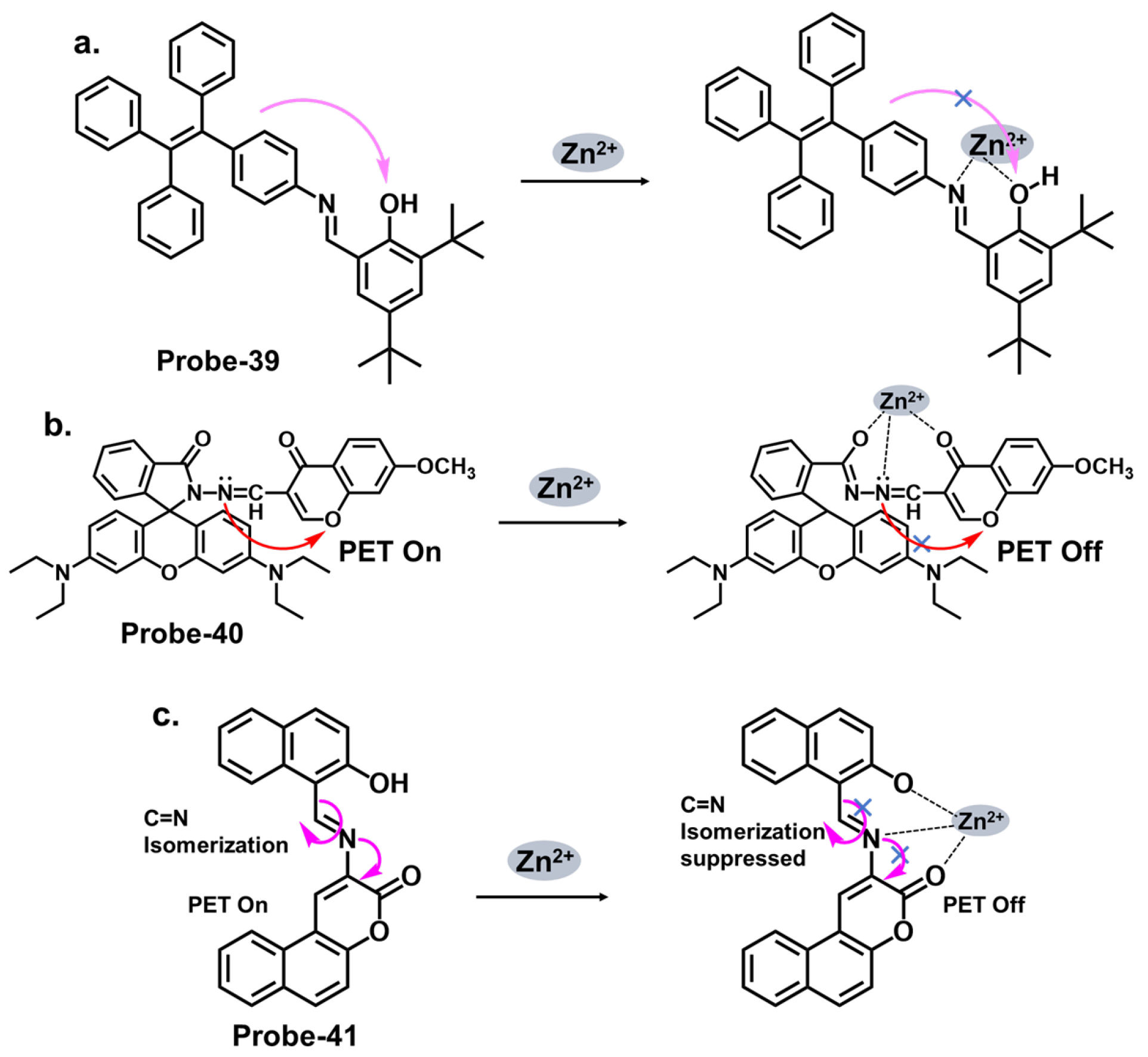
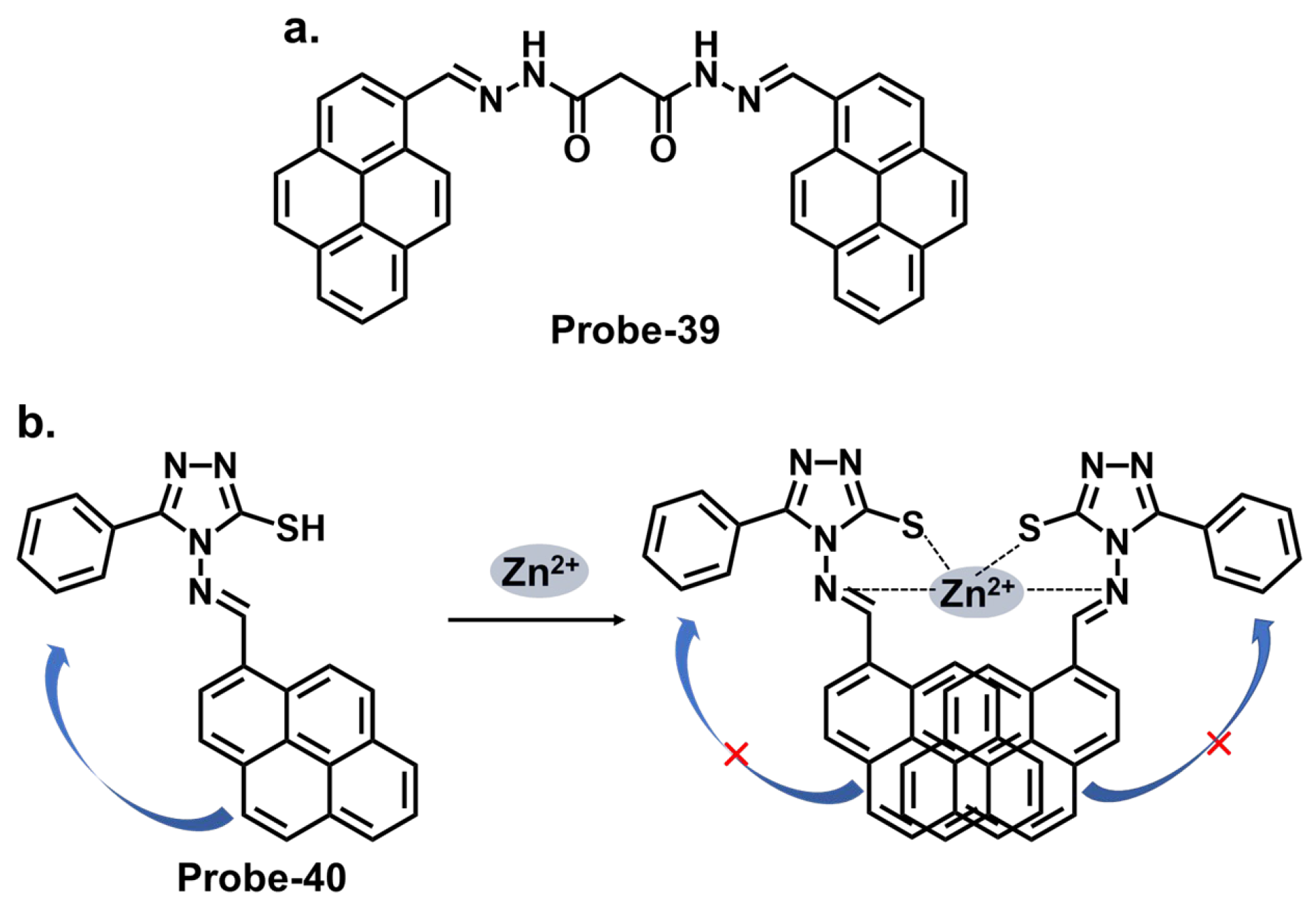
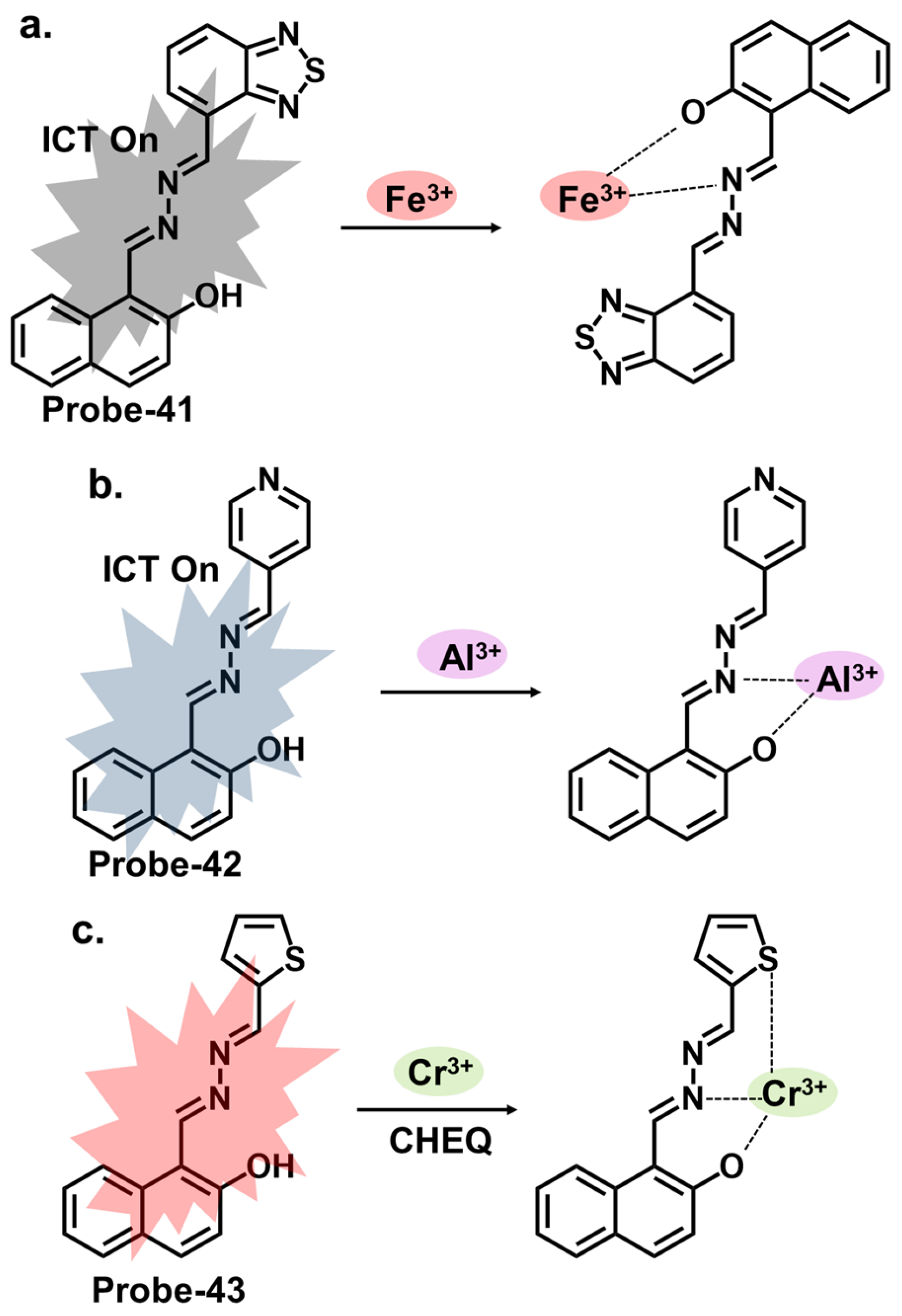
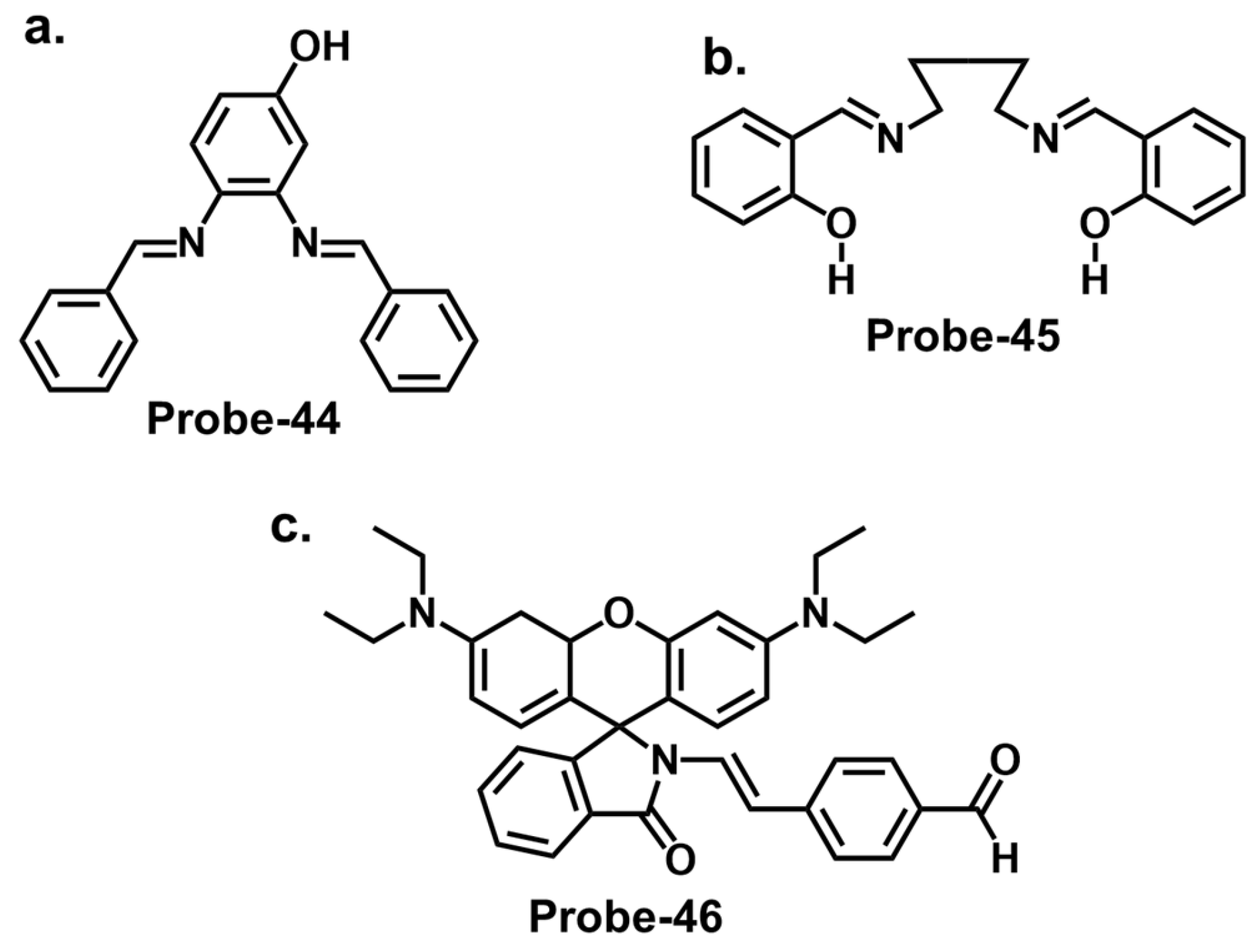
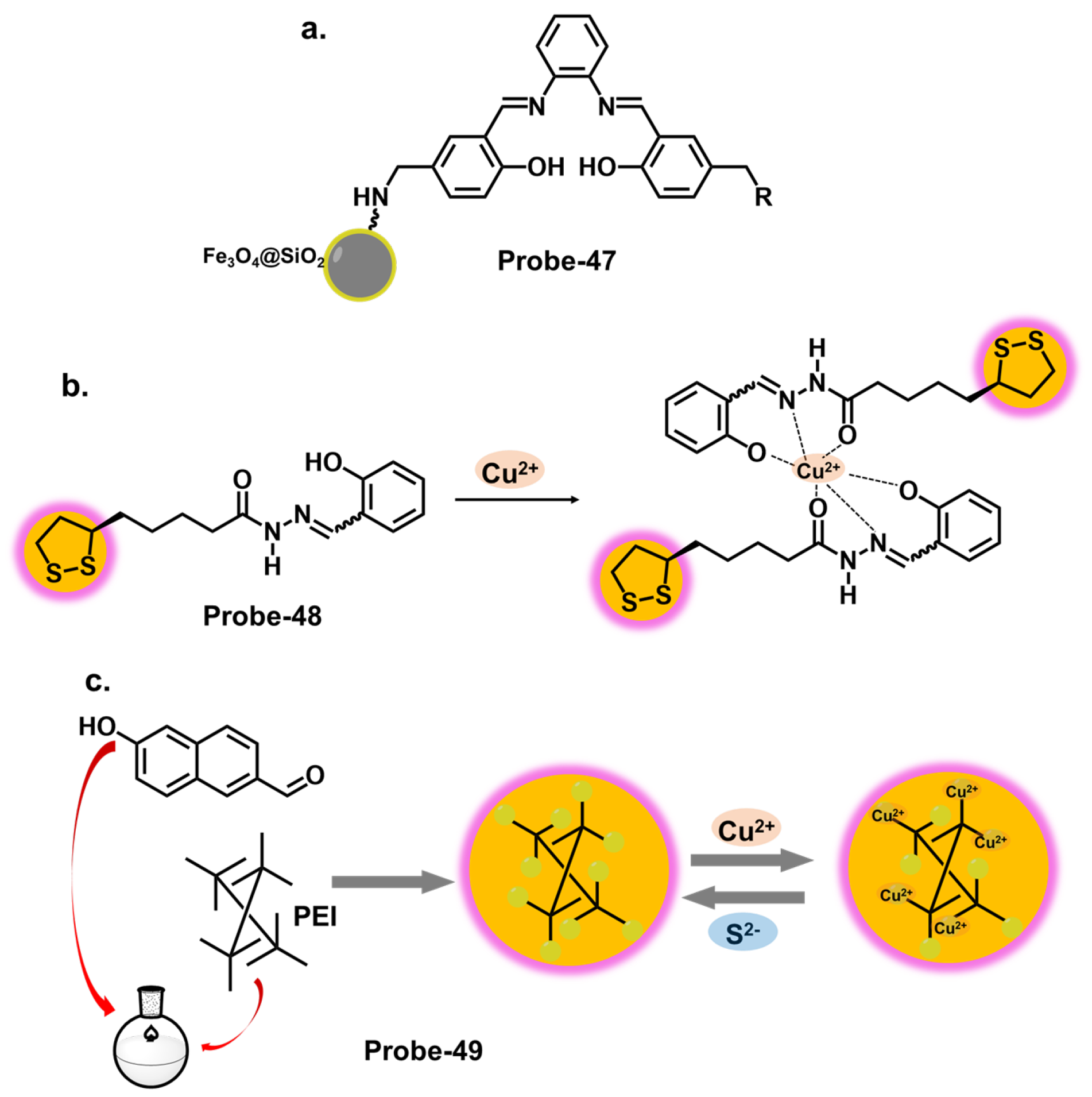
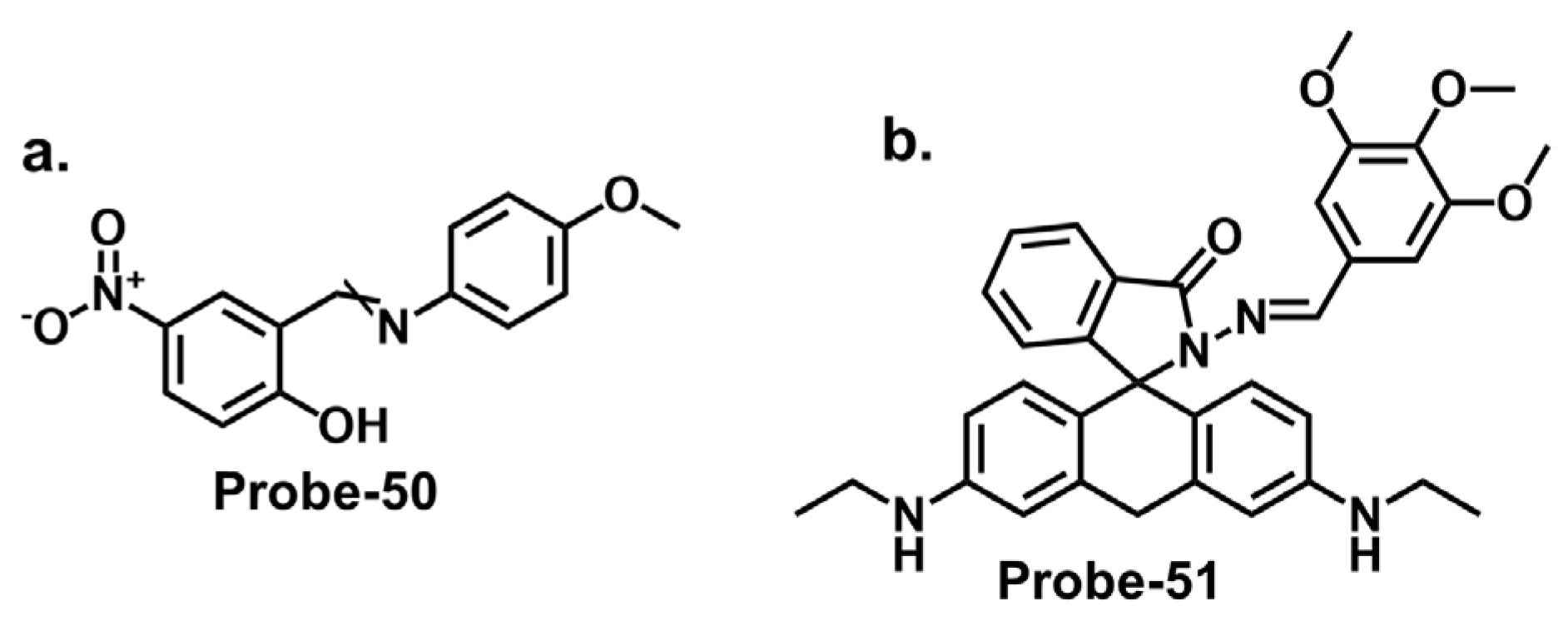
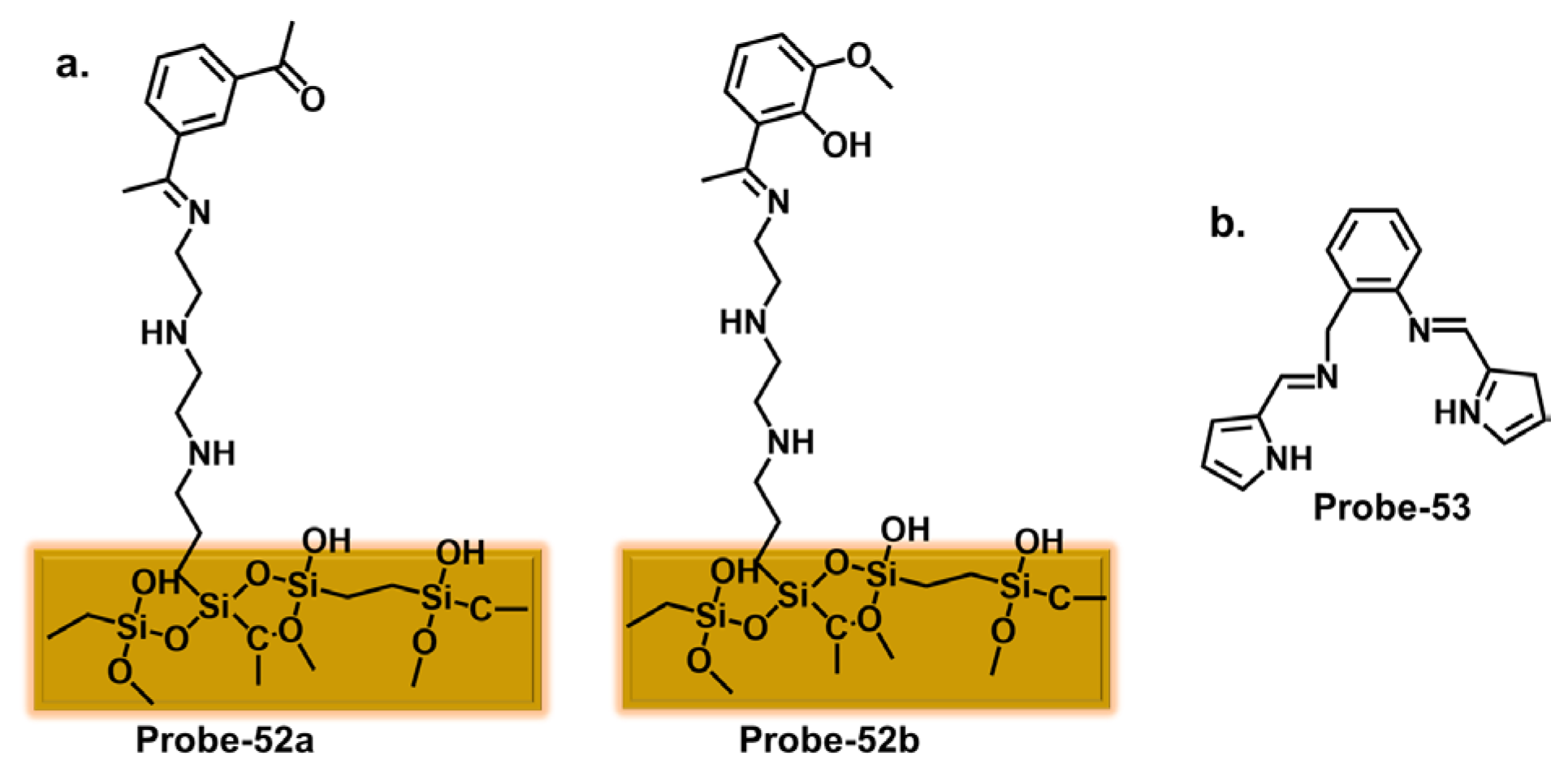
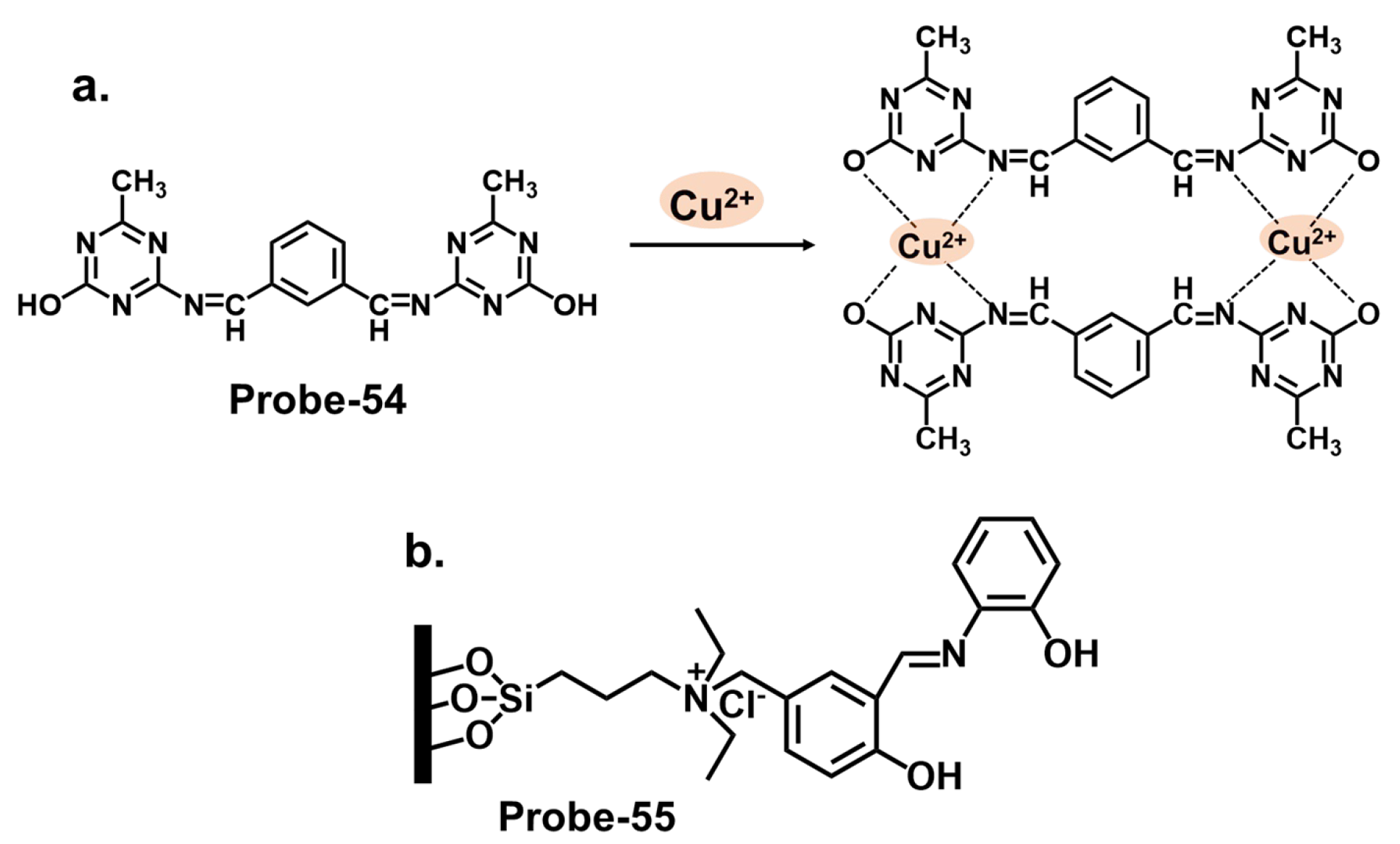
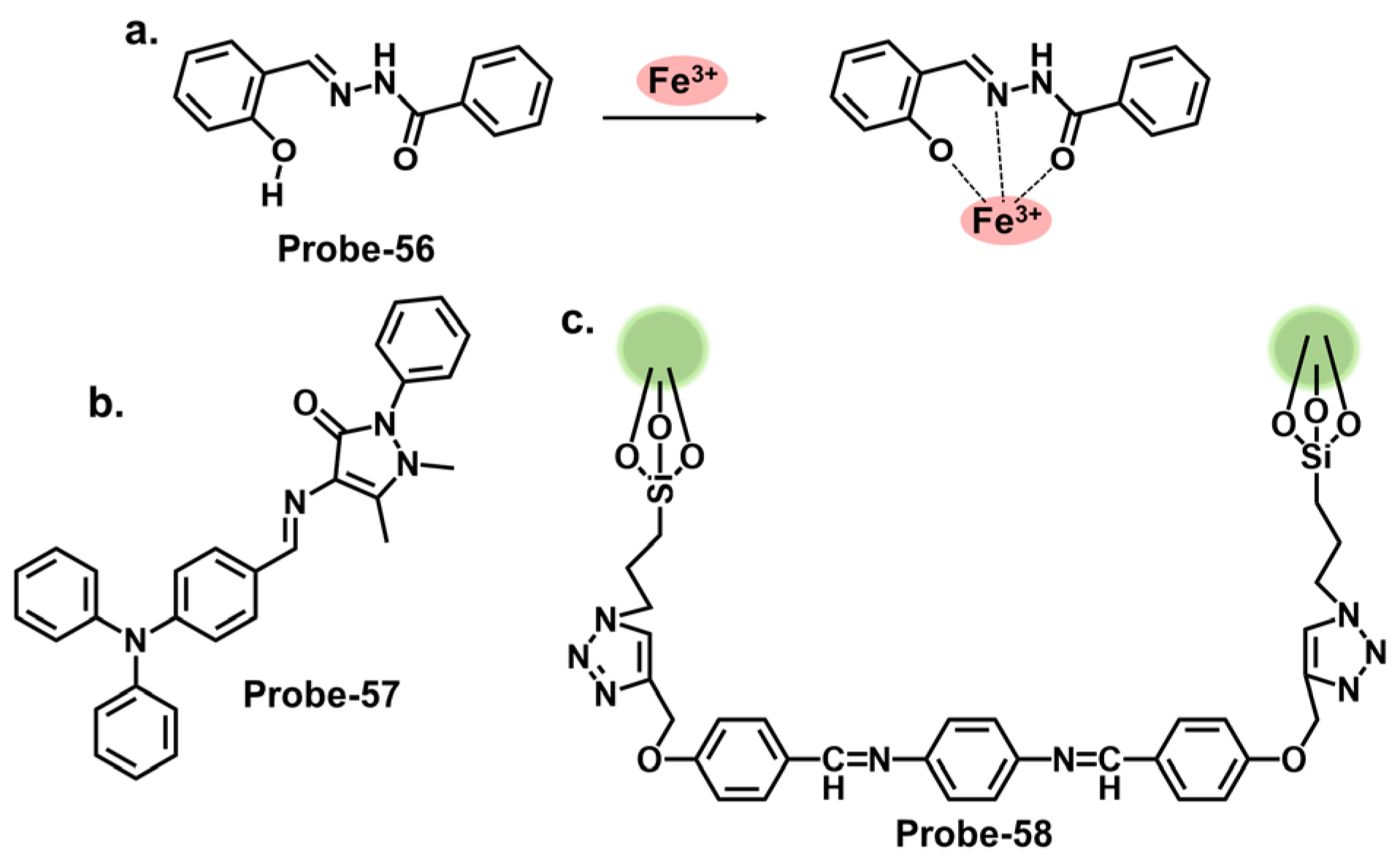
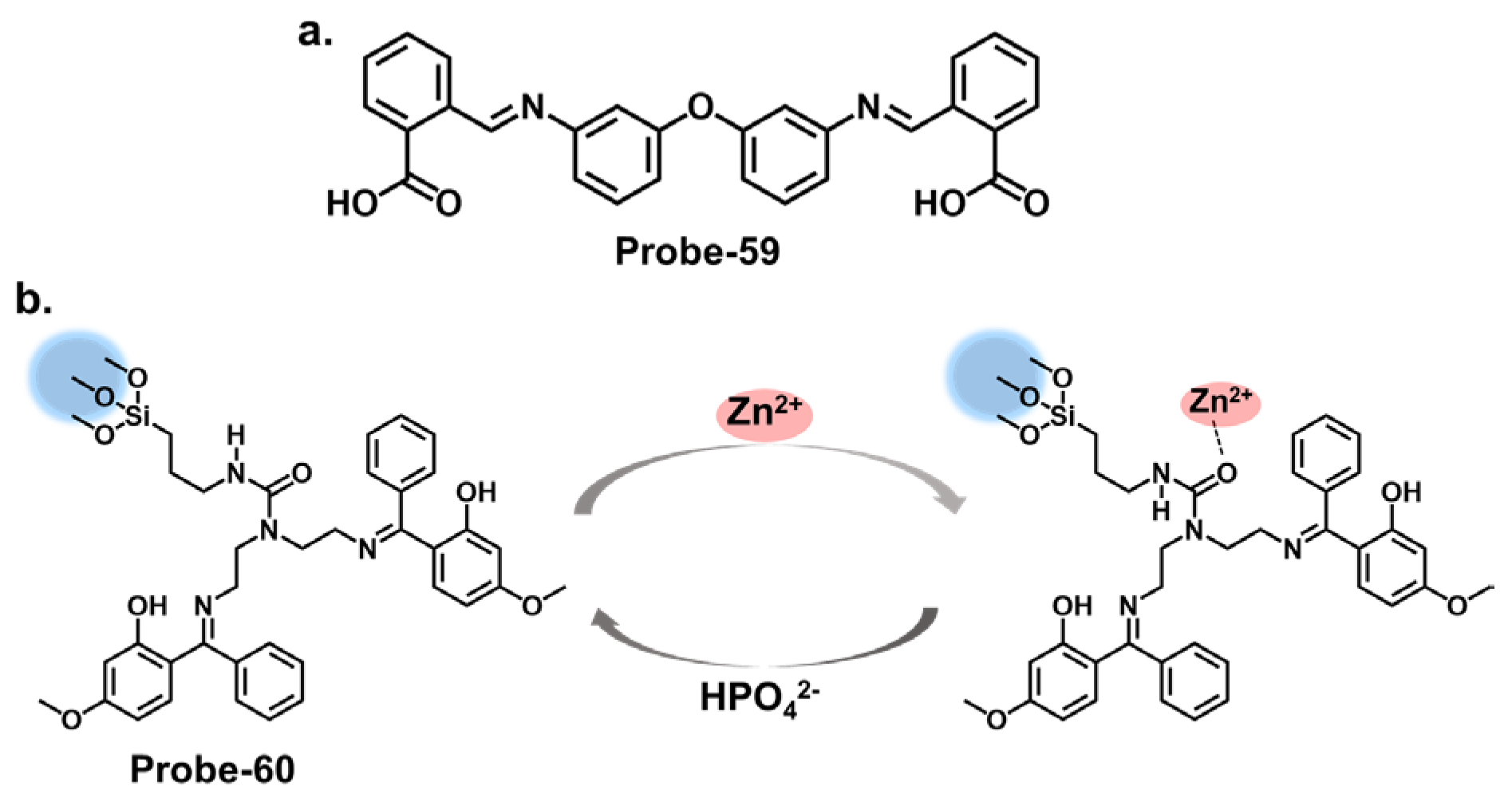
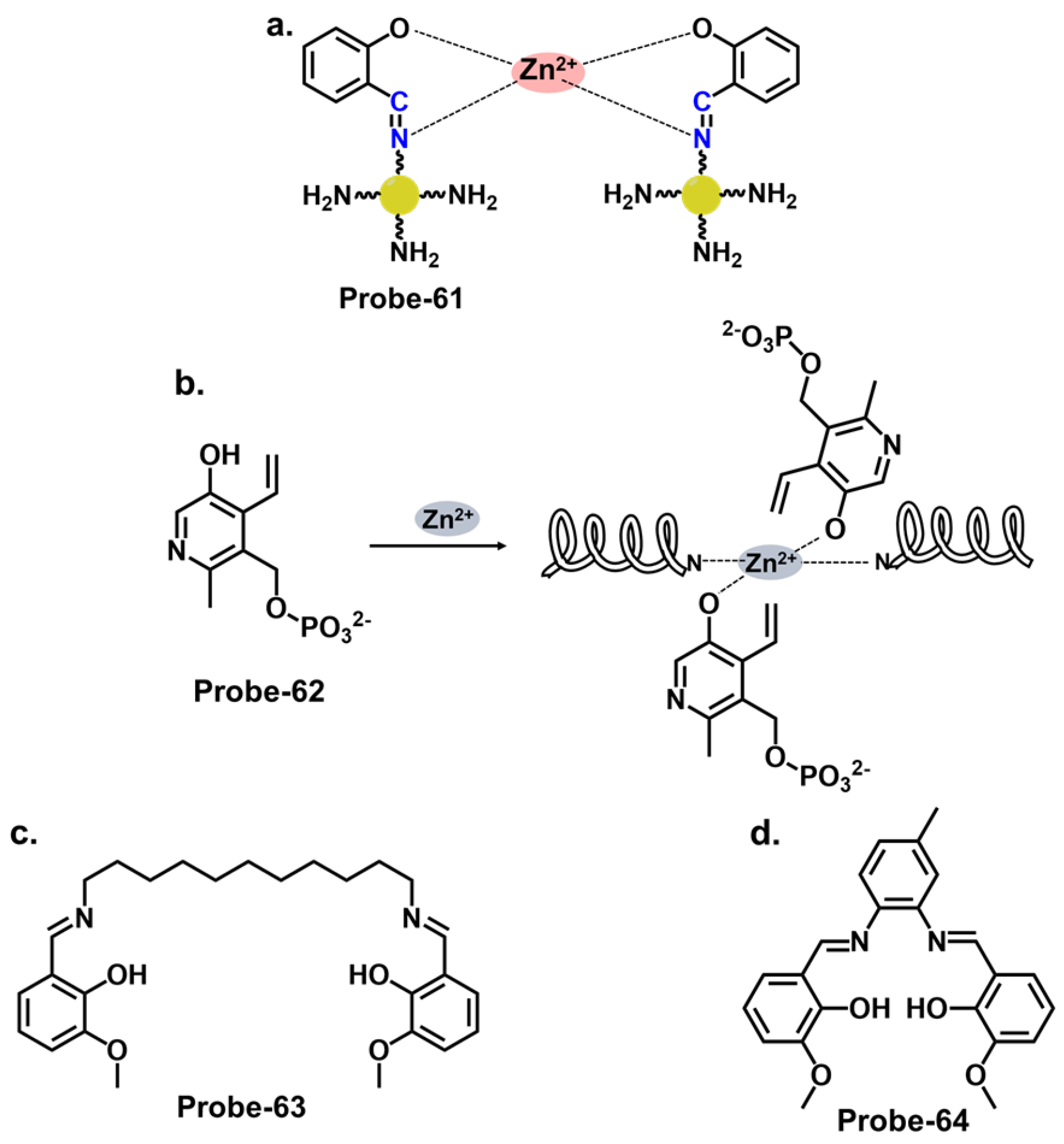
| Probe | Analyte | Mechanism | LOD (μM) | Applications | Ref. |
|---|---|---|---|---|---|
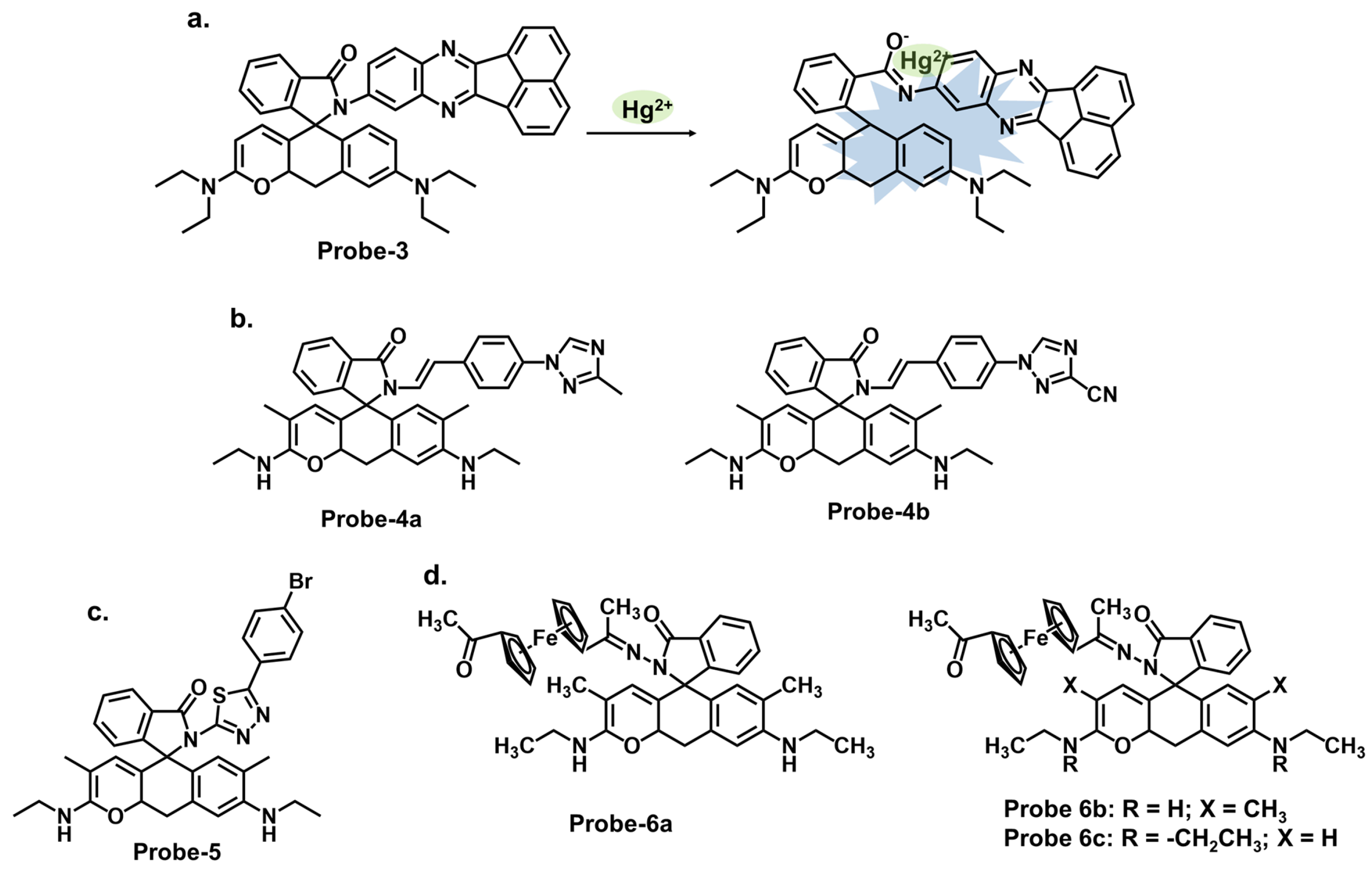
| 1 | Hg2+ | PET/C=N Isomerization/CHEF | 0.02 | Paper strips, Hg2+ recovery from water samples | [66] |
| 2a | Hg2+ | CHEF | 0.27 | Hg2+ recovery from water samples | [67] |
| 3 | Hg2+ | Metal coordination-spiro ring opening | 0.01 | HeLa cells, zebrafish | [68] |
| 4a, 4b | Hg2+ | PET | 0.01, 0.01 | MCF-7 cells | [69] |
| 5 | Hg2+ | Metal coordination-spiro ring opening | 0.03 | Cancer (MDA-MB-231) cell imaging | [70] |
| 6 | Hg2+ | CHEF | / | THP-1 cancer cells | [71] |
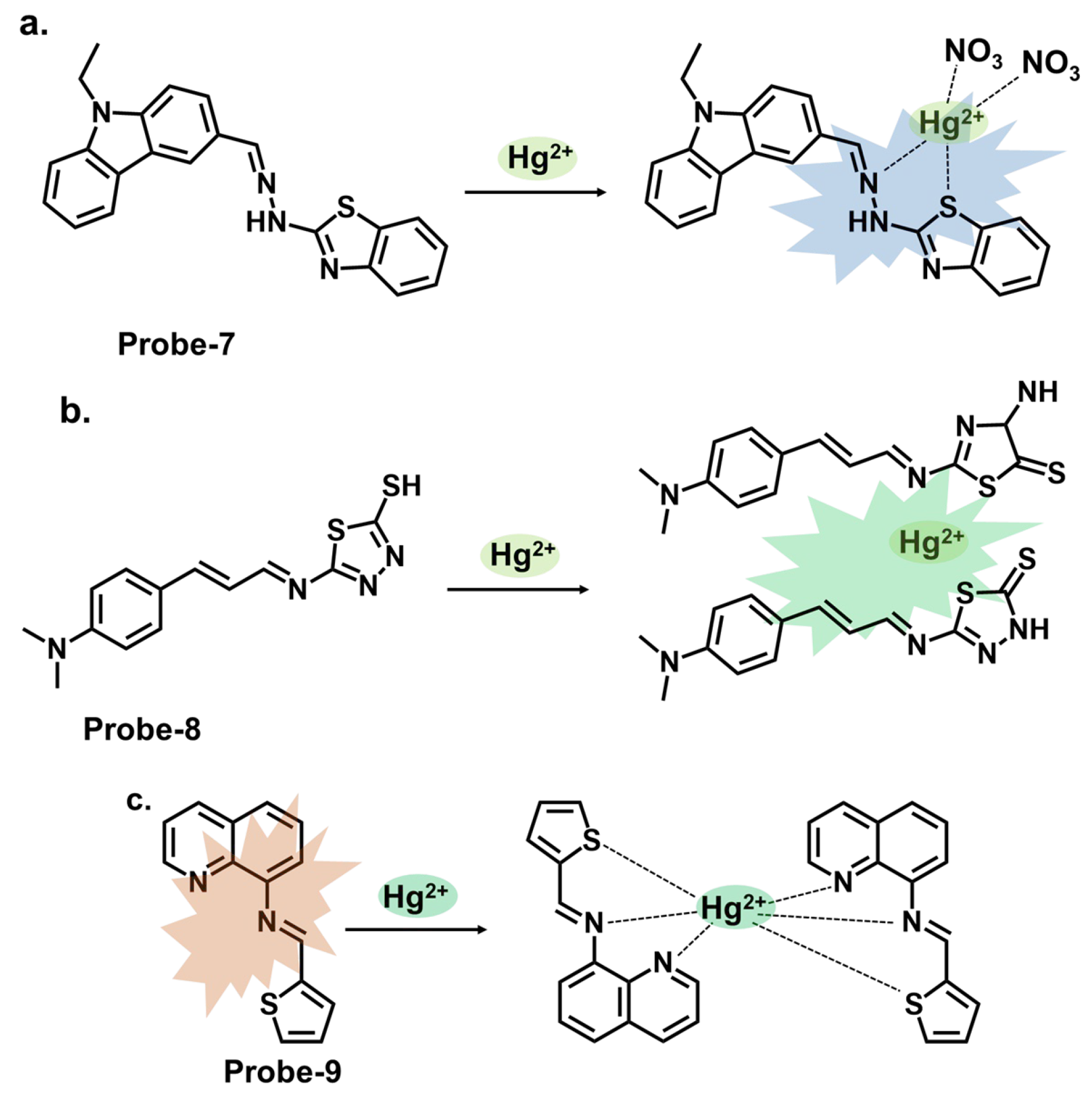
| 7 | Hg2+ | ICT | 0.14 | Bovine Serum Albumin, organelle targeting | [72] |
| 8 | Hg2+ | CHEF, aggregation-derived quenching | 2 | Hg2+ recovery from water samples | [73] |
| 9 | Hg2+ | CHEQ | 0.023 | HeLa cells, paper strips | [50] |
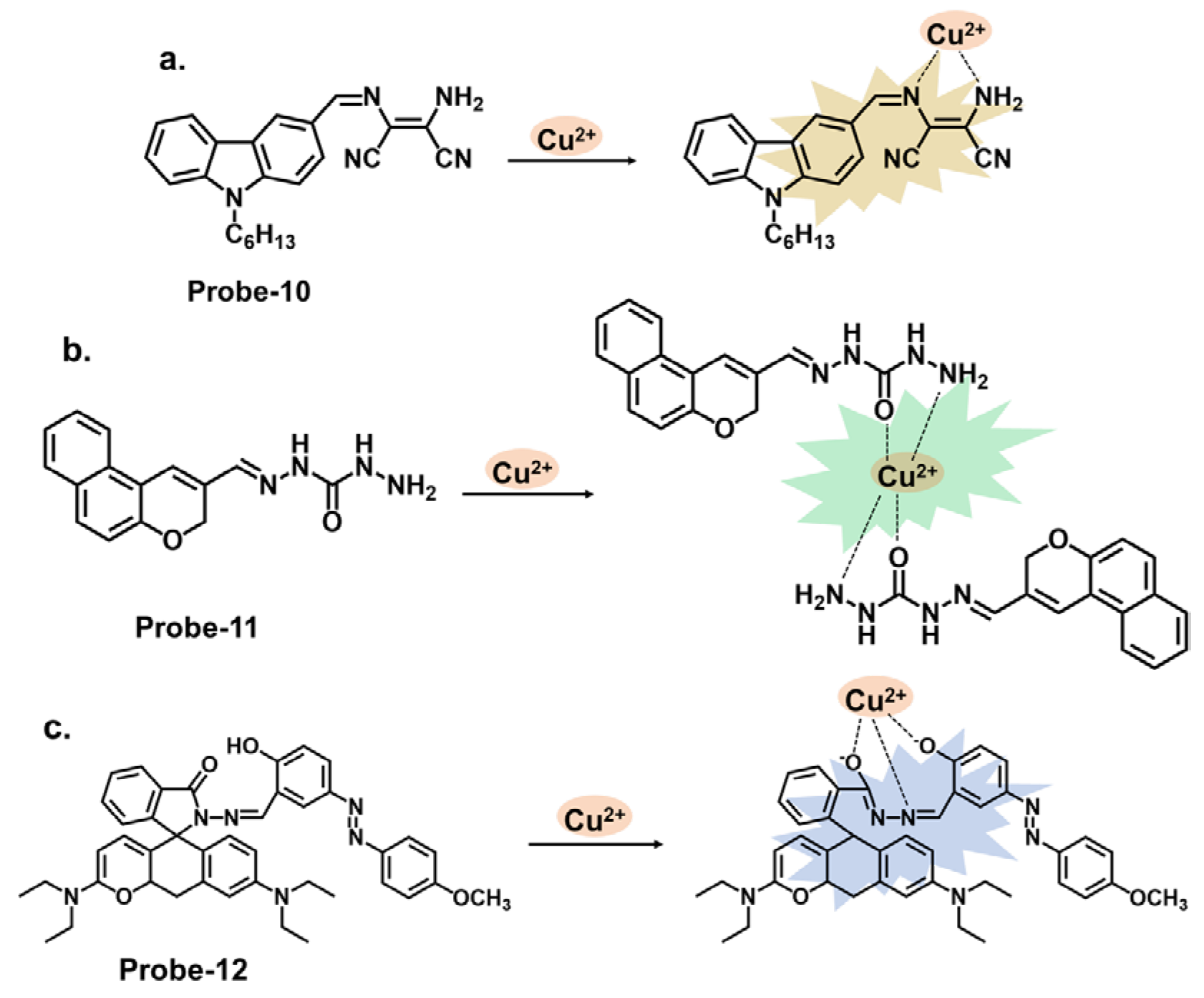
| 10 | Cu2+ | ICT | 0.027 | / | [74] |
| 11 | Cu2+ | ICT/Metal-induced assembly | 0.01 | HeLa cells | [75] |
| 12 | Cu2+ | C=N Isomerization/CHEF | 0.01 | HepG2 Cells, Kunming mouse | [76] |
| 13 | Cu2+ | C=N Isomerization/CHEF | 0.0001 | HepG2 cells | [77] |
| 14 | Cu2+ | PET | 0.003 | / | [78] |
| 15 | Cu2+ | CHEF | 0.014 | Cu2+ recovery from water samples | [79] |
| 16 | Cu2+ | C=N Isomerization/CHEF | 1.8 | / | [80] |
| 17 | Cu2+, S2− | PET | 0.015 | Paper strips, Cu2+ recovery from water and blood samples | [81] |
| 18 | Cu2+ | PET | 0.26 | RAW 264.7 cells | [82] |
| 19 | Cu2+ | C=N Isomerization/CHEF | 1.49 | Cu2+ recovery from water samples | [83] |
| 20 | Cu2+ | C=N Isomerization/PET | 0.49 | HepG2 cells | [84] |
| 21 | Cu2+ | C=N Isomerization/ICT/CHEF | 0.001 | Paper strips, wastewater samples | [85] |
| 22 | Fe2+ | C=N Isomerization/PET | 0.036 | Fe2+ detection in water samples | [86] |
| 23 | Fe3+ | C=N Isomerization | 0.038 | Cancer cells | [87] |
| 24 | Fe3+ | PET/CHEF | 0.014 | Fe3+ detection in water samples | [88] |
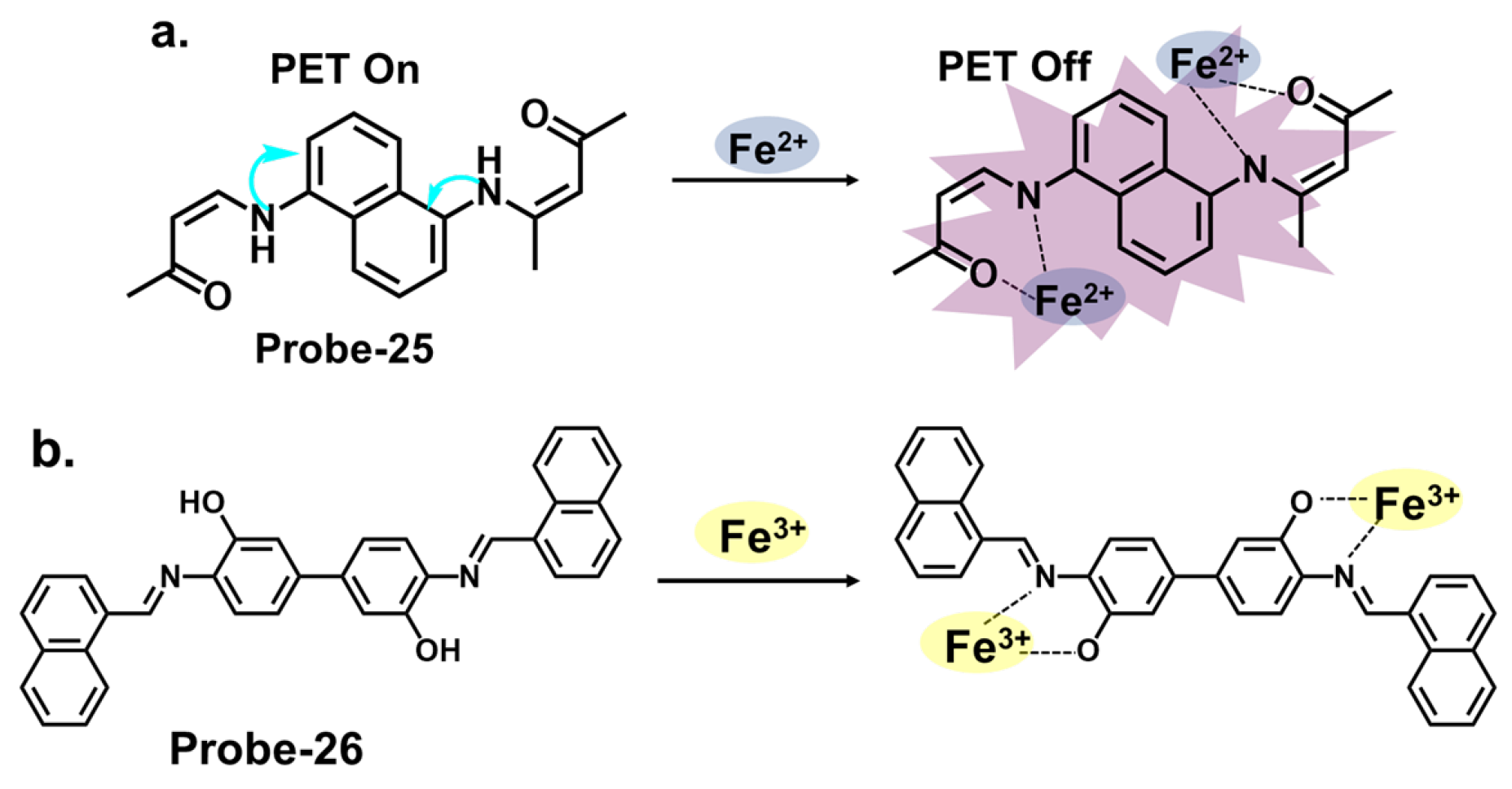
| 25 | Fe2+, Cu2+ | PET (Fe2+)/MLCT (Cu2+) | 0.5 (Fe2+) | Bovine Serum Albumin | [89] |
| 26 | Fe3+ | C=N Isomerization/PET/LMCT | 0.178 | Food samples, Fe3+ recovery from water samples | [90] |
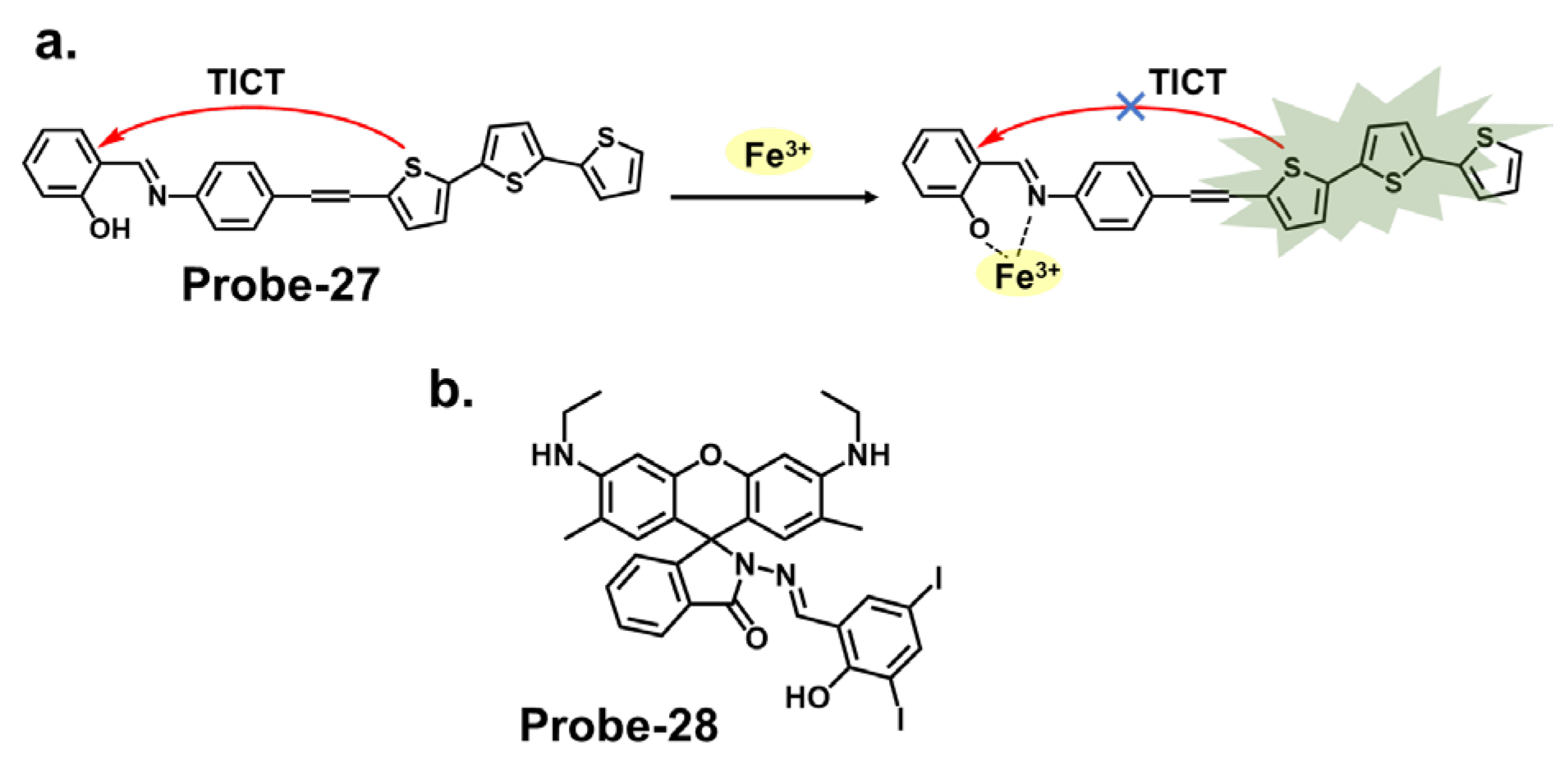
| 27 | Fe3+, Al3+ | TICT | 0.172, 0.177 | Real water and food samples | [91] |
| 28 | Fe3+, Cu2+ | ICT | 0.003, 0.002 | Zebrafish | [92] |
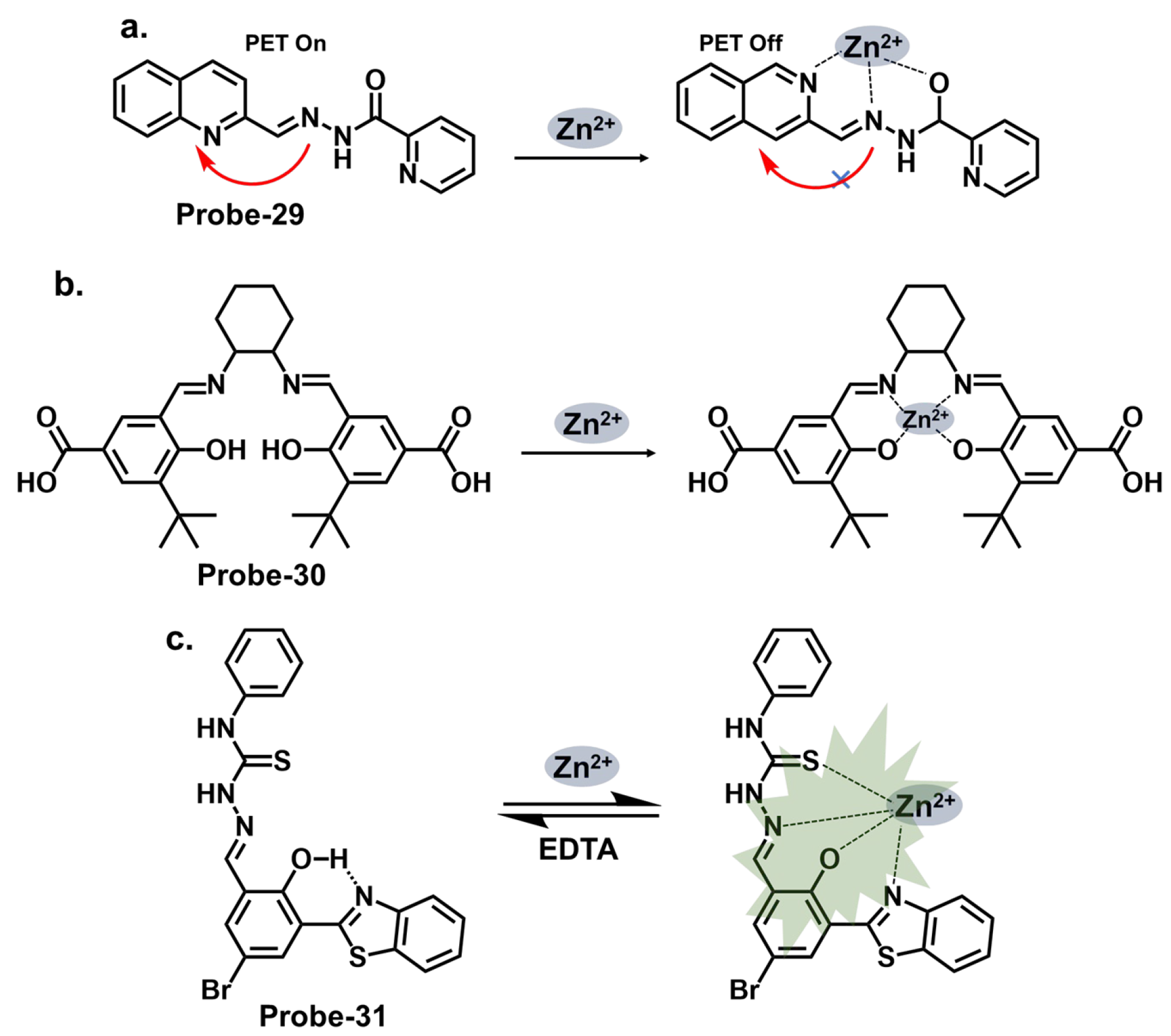
| 29 | Zn2+ and Cu2+ | CHEF | 0.18 | U251 Glioma Cells | [93] |
| 30 | Zn2+ and pH | PET | 0.056 | Zebrafish, live cells | [94] |
| 31 | Zn2+ | ESIPT/CHEF | 0.037 | HeLa cells, SH-SY5Y neuroblastoma cells | [95] |
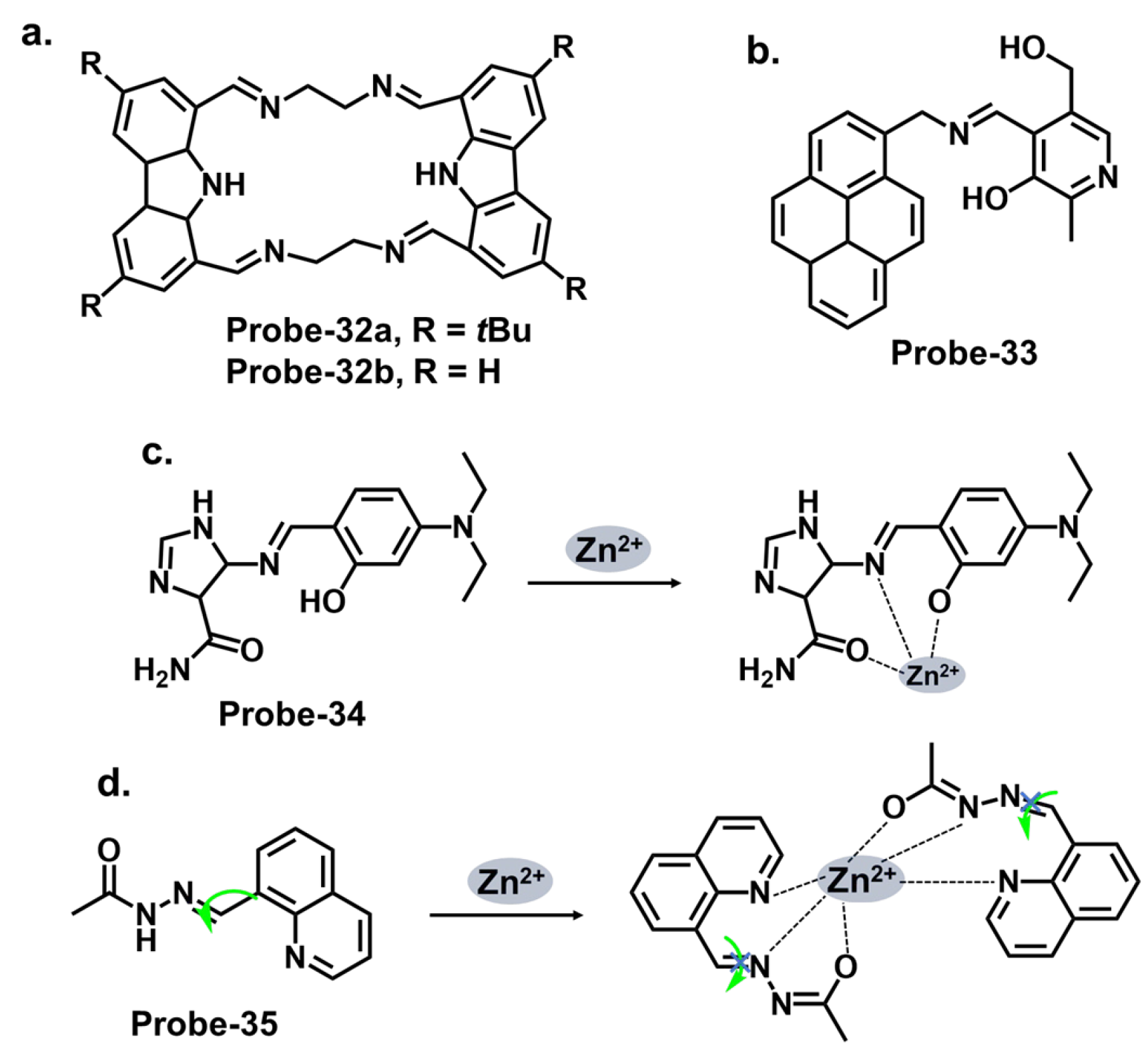
| 32 | Zn2+ | PET/CHEF | / | / | [96] |
| 33 | Zn2+, hydrogen phosphate and cysteine | PET | 2.3, 0.21 and 0.16 | HeLa cells | [97] |
| 34 | Zn2+, S2−, Fe3+/2+ | PET/CHEF | 1.59, 8.03, 0.73, 1.11 | Fe3+ tracking in water samples | [98] |
| 35 | Zn2+ | C=N isomerism/CHEF | 0.089 | HeLa cells | [99] |
| 36 | Zn2+ | PET/ESIPT | 0.080 | / | [100] |
| 37 | Zn2+ | PET | 0.34 | Paper strips | [101] |
| 38 | Zn2+, Cu2+, S2− | C=N isomerism/ICT | 3.6 (Zn2+), 0.47 (Cu2+) | / | [102] |
| 39 | Zn2+ | PET | 0.005 | HeLa cells | [103] |
| 40 | Zn2+ | PET | 0.0007 | B16F10 cell lines 773 and zebrafish | [104] |
| 41 | Fe3+ | ICT/CHEQ | 0.036 | PC3 cells | [105] |
| 42 | Al3+ | ICT | 0.164 | HeLa cells, Fe3+ recovery from water samples | [106] |
| 43 | Cr3+ | CHEQ | 0.041 | PC3 cells | [107] |
| Probe | Analyte | Nanomaterial | LOD (μM) | Applications | Ref. |
|---|---|---|---|---|---|
| 44 | Hg2+ | Gold | 0.0006 | Hg2+ recovery from water samples | [130] |
| 45 | Hg2+ | Silver | 0.01 | Anticancer, and antibacterial uses | [114] |
| 46 | Hg2+ | Organic | 0.008 | Hg2+ recovery from water samples and living A375 cells | [131] |
| 47 | Cu2+ | Silica/black iron oxide | 0.005 | Cu2+ recovery from water samples | [117] |
| 48 | Cu2+, Ni2+, Fe2+ | Gold | 0.001–0.011 | Ni2+ tracking in organic waste | [132] |
| 49 | Cu2+ | Polymer | 0.243 | HeLa cells | [133] |
| 50 | Fe3+, Cr2+, Cd2+ | Zinc Sulfide | 10.24, 31.48, 64.56 | / | [134] |
| 51 | Pb2+ | (3-Aminopropyl) triethoxysilane/Gold | 0.0001 | Paper strips | [135] |
| 52 | Hg2+ | Mesoporous organosilica | / | / | [136] |
| 53 | Hg2+, Pb2+ | Graphite | 0.0003, 0.001 | Hg2+and Pb2+ tracking in water samples | [137] |
| 54 | Cu2+ | Glass slides | 0.015 | / | [138] |
| 55 | Cu2+ | Mesoporous silica | 0.37 | Cu2+ extraction from aqueous solutions | [139] |
| 56 | Fe3+ | COFs | / | / | [140] |
| 57 | Fe3+ | Silica cross-linked micellar nanoparticles | 0.21 | HeLa cells | [141] |
| 58 | Fe3+, Cu2+ | Silica | 0.44, 0.87 | / | [118] |
| 59 | Al3+ | Copper MOFs/Carbon | 0.033 | Al3+ tracking in pharmaceutical and water samples | [142] |
| 60 | Zn2+ | Nanosilica | 0.17 | / | [143] |
| 61 | Zn2+ | Gold nanoclusters | 0.029 | Zn2+ recovery from fetal bovine serum | [144] |
| 62 | Zn2+ | Gold nanoclusters | 0.039 | Zn2+ recovery from water samples, plasma, urine, and beet root extract; HeLa cells imaging. | [145] |
| 63 | Fe2+, Zn2+ | Cd–Ln clusters | / | / | [146] |
| 64 | Co2+ | Lanthanide nanocluster | 0.97 | / | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musikavanhu, B.; Liang, Y.; Xue, Z.; Feng, L.; Zhao, L. Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals. Molecules 2023, 28, 6960. https://doi.org/10.3390/molecules28196960
Musikavanhu B, Liang Y, Xue Z, Feng L, Zhao L. Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals. Molecules. 2023; 28(19):6960. https://doi.org/10.3390/molecules28196960
Chicago/Turabian StyleMusikavanhu, Brian, Yongdi Liang, Zhaoli Xue, Lei Feng, and Long Zhao. 2023. "Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals" Molecules 28, no. 19: 6960. https://doi.org/10.3390/molecules28196960
APA StyleMusikavanhu, B., Liang, Y., Xue, Z., Feng, L., & Zhao, L. (2023). Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals. Molecules, 28(19), 6960. https://doi.org/10.3390/molecules28196960