Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy
Abstract
1. Introduction
2. Results
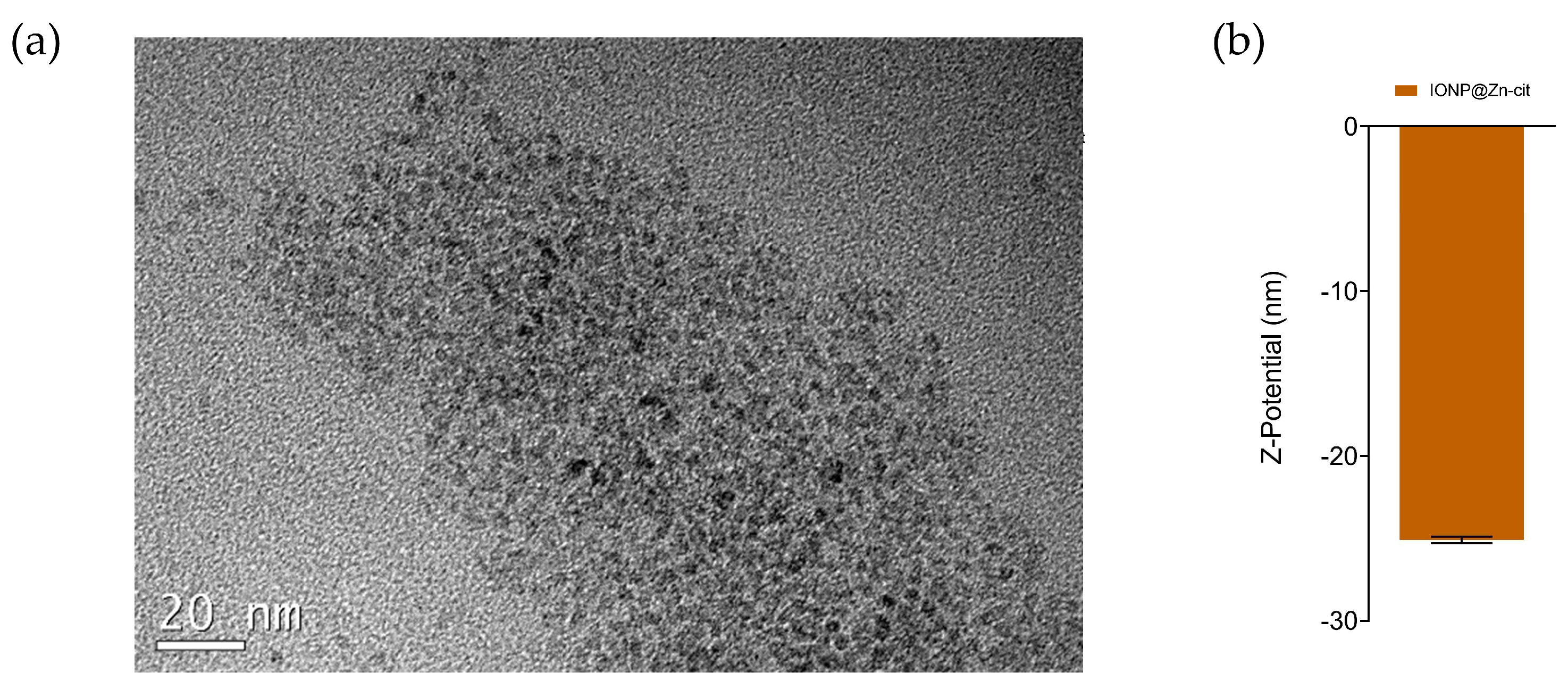
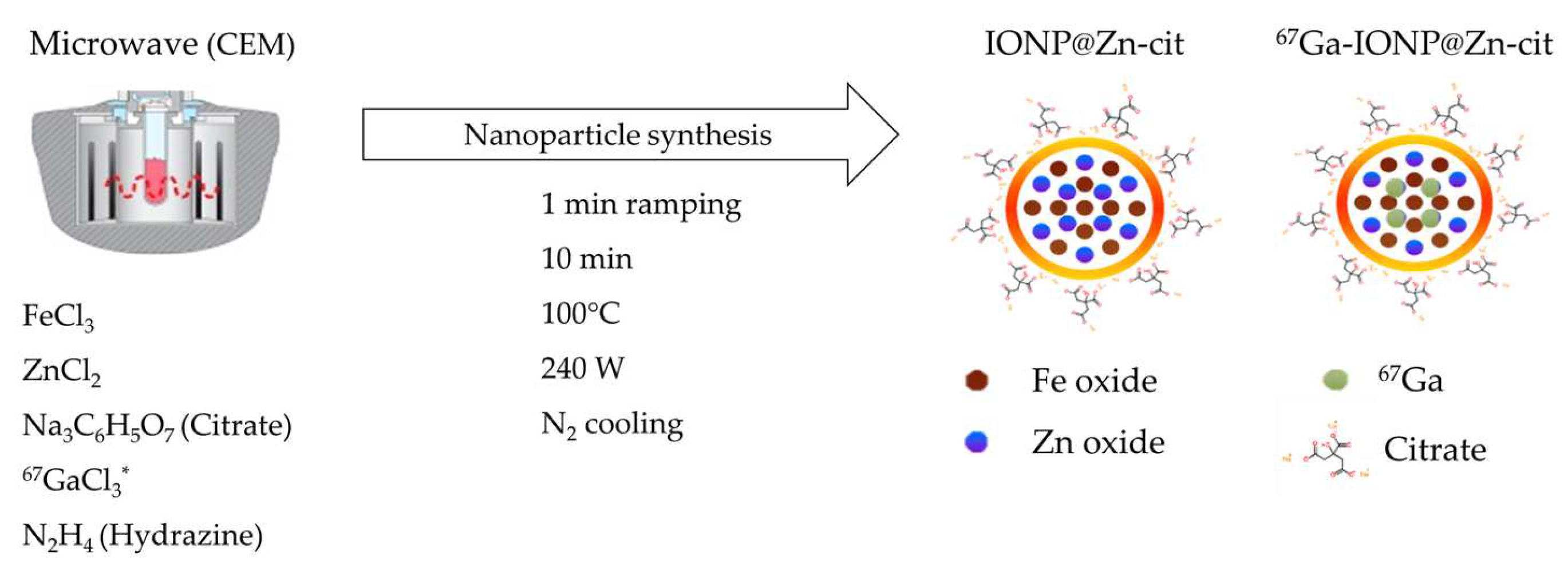
2.1. Nanoparticle Synthesis and Characterization
2.2. IONP@Zn-cit Cytotoxicity
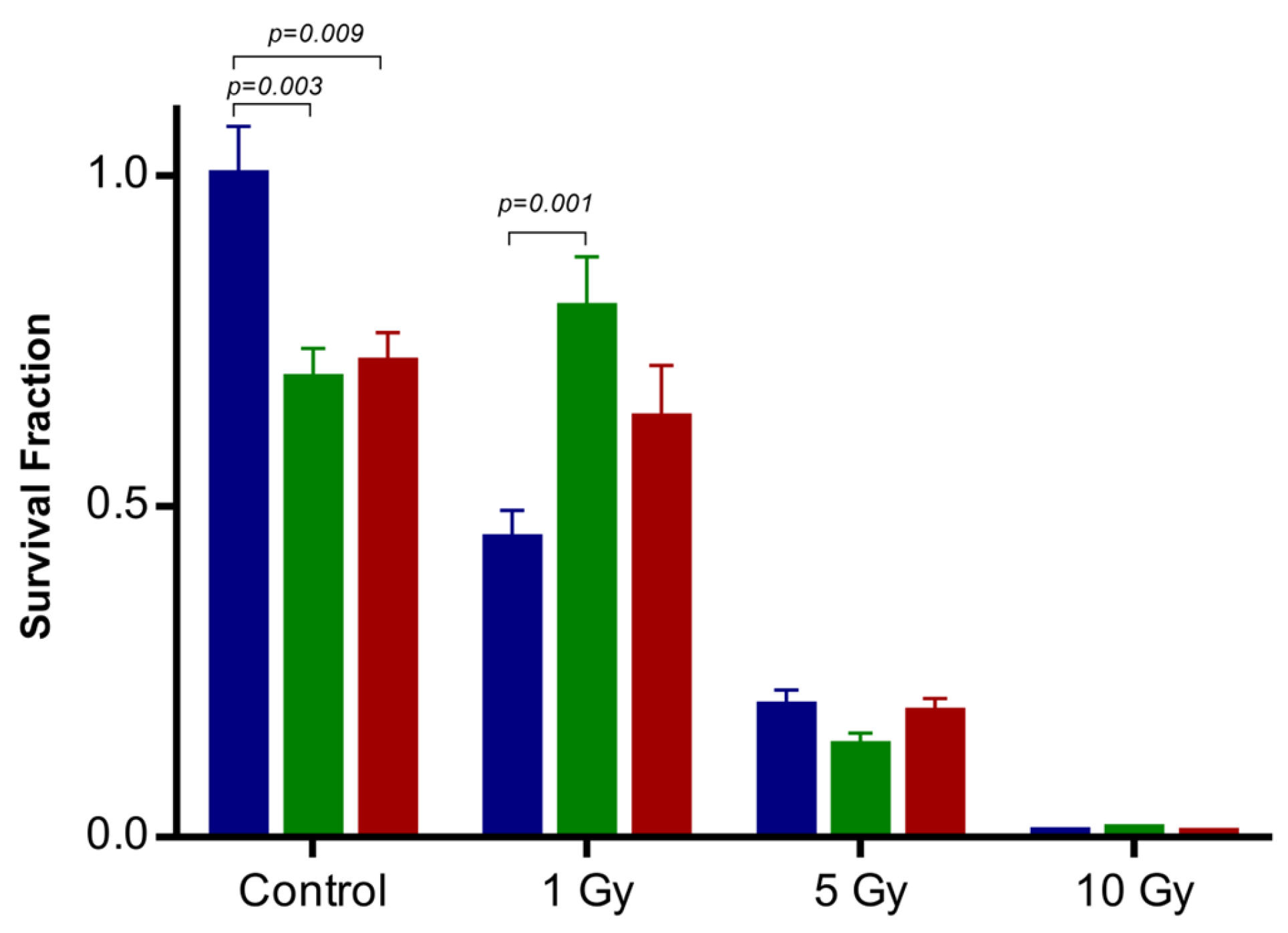
2.3. Influence of IONP@Zn-cit on X-ray Radiation-Induced Clonogenic Cell Death
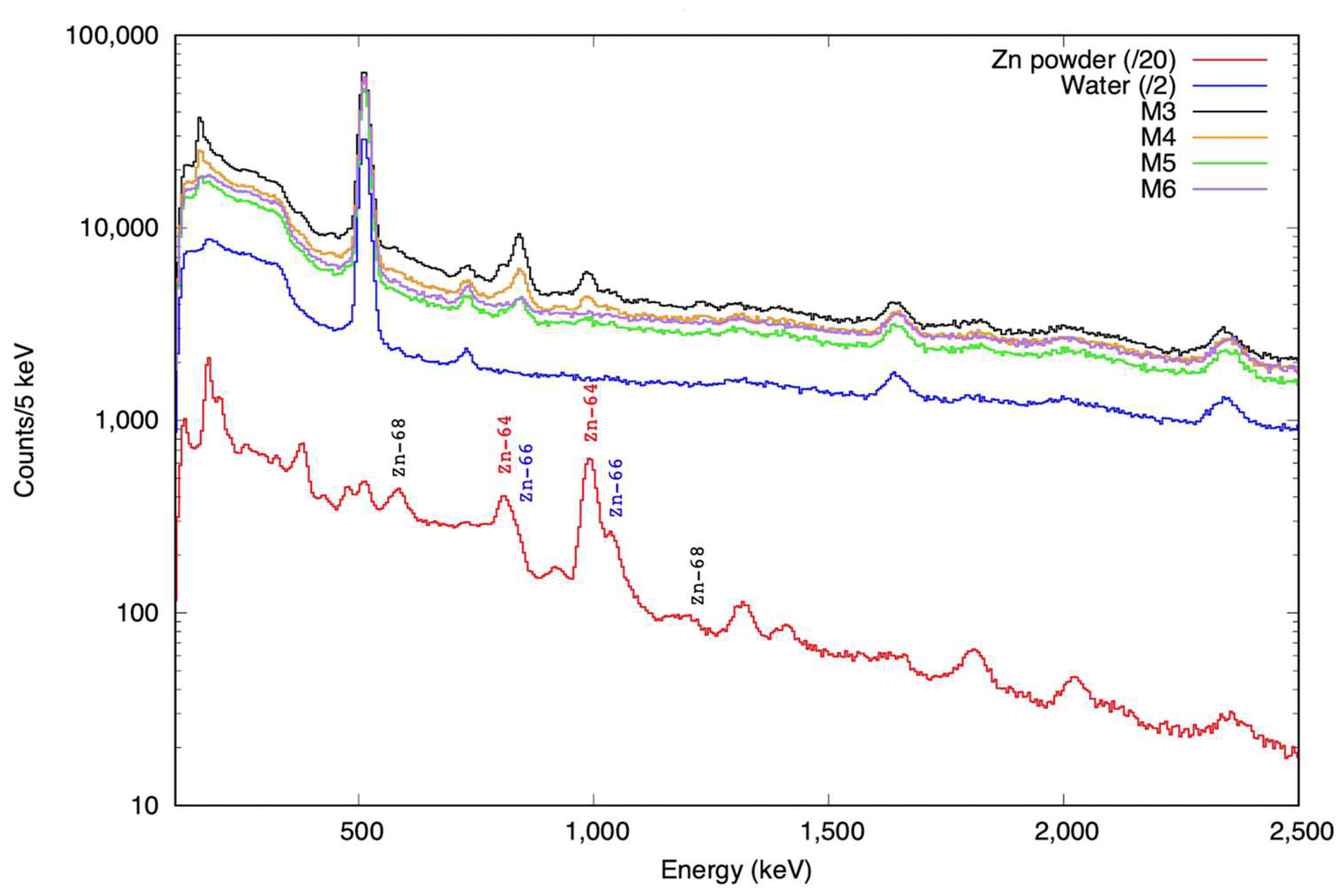
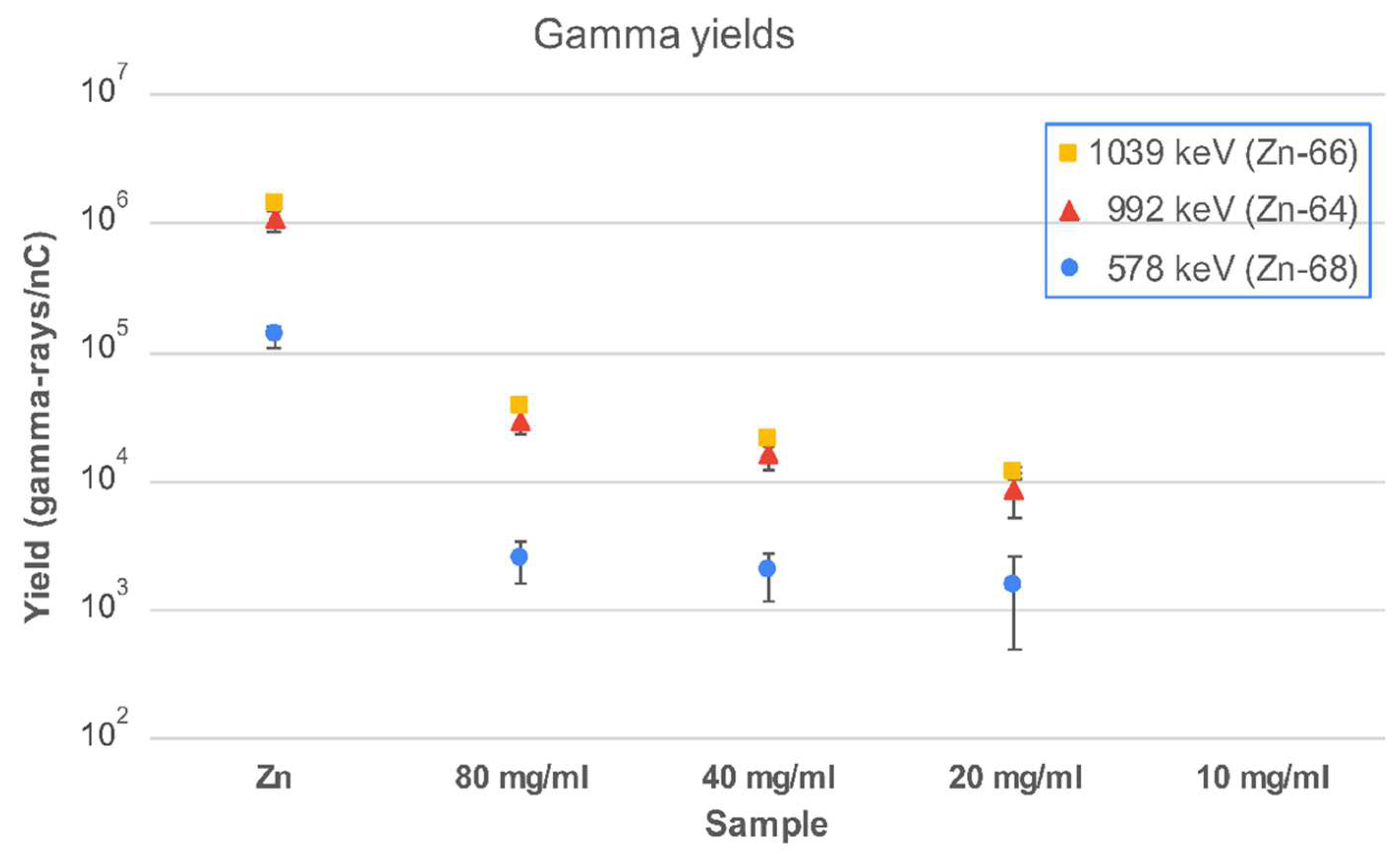
2.4. Proton Irradiation of IONP@Zn-cit
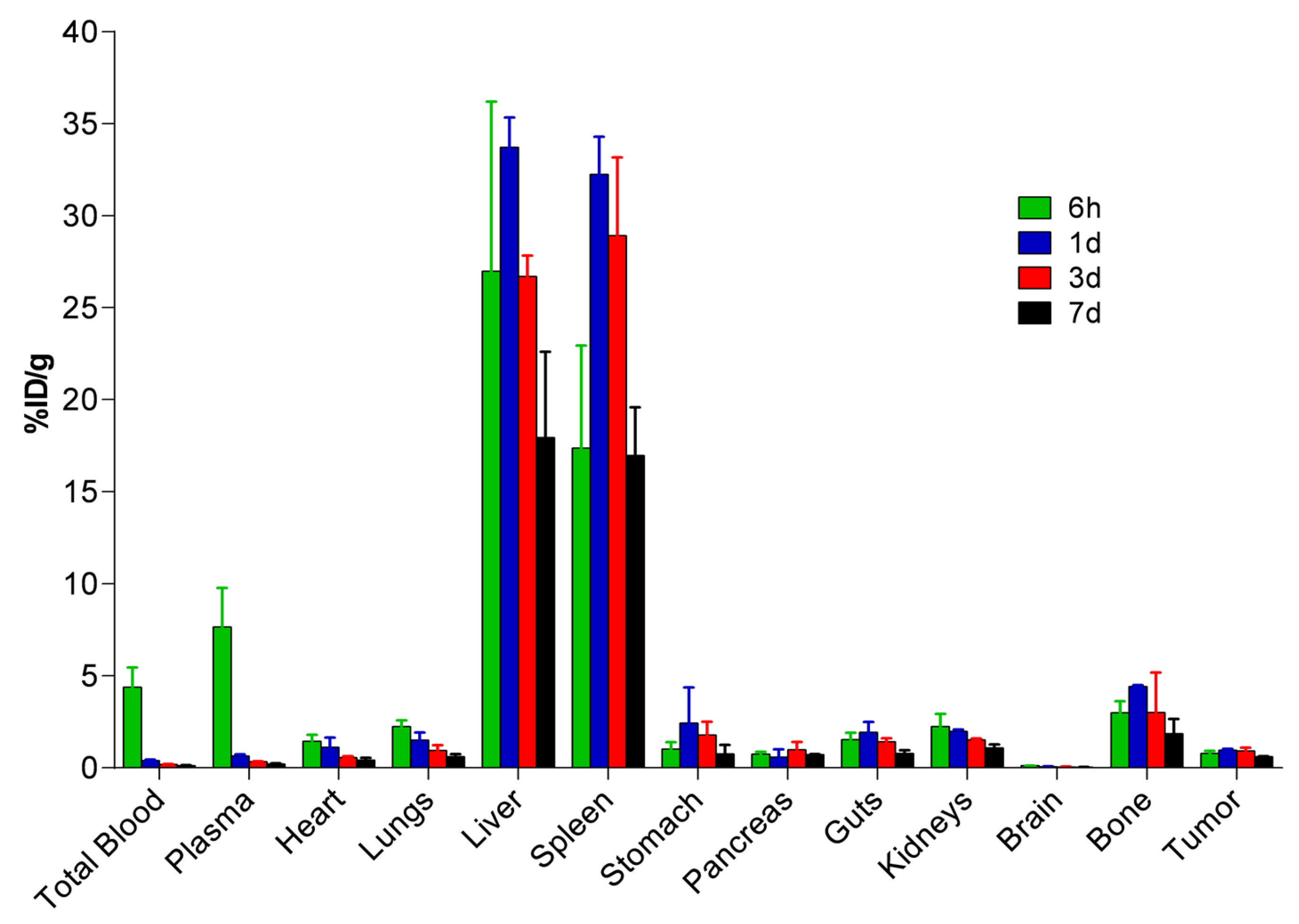
2.5. Biodistribution and Pharmacokinetic Studies of 67Ga-IONP@Zn-cit
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Synthesis
4.1.1. IONP@Zn-cit
4.1.2. Radiolabeled 67Ga-IONP@Zn-cit
4.2. Nanoparticle Characterization
4.2.1. Hydrodynamic Size and Zeta Potential Measurements
4.2.2. TEM Images
4.3. In Vitro Studies
4.3.1. IONP@Zn-cit Colloidal Stability in Cell Culture Medium
4.3.2. Cell Culture
4.3.3. Cytotoxicity Assay
4.3.4. Colony Formation Assay
4.4. Irradiations
4.4.1. X-ray Irradiation
4.4.2. Proton Irradiation
4.5. In Vivo Studies
4.5.1. Animal Model
4.5.2. Biodistribution Study
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McNamara, A.; Willers, H.; Paganetti, H. Modelling Variable Proton Relative Biological Effectiveness for Treatment Planning. BJR 2020, 93, 20190334. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.R. Radiological Use of Fast Protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Parodi, K. On- and off-Line Monitoring of Ion Beam Treatment. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2016, 809, 113–119. [Google Scholar] [CrossRef]
- Knopf, A.-C.; Lomax, A. In Vivo Proton Range Verification: A Review. Phys. Med. Biol. 2013, 58, R131–R160. [Google Scholar] [CrossRef] [PubMed]
- Parodi, K.; Paganetti, H.; Shih, H.A.; Michaud, S.; Loeffler, J.S.; DeLaney, T.F.; Liebsch, N.J.; Munzenrider, J.E.; Fischman, A.J.; Knopf, A.; et al. Patient Study of In Vivo Verification of Beam Delivery and Range, Using Positron Emission Tomography and Computed Tomography Imaging After Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Parodi, K.; Polf, J.C. In Vivo Range Verification in Particle Therapy. Med. Phys. 2018, 45, e1036–e1050. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Pausch, G.; Barczyk, S.; Priegnitz, M.; Keitz, I.; Thiele, J.; Smeets, J.; Stappen, F.V.; Bombelli, L.; Fiorini, C.; et al. First Clinical Application of a Prompt Gamma Based in Vivo Proton Range Verification System. Radiother. Oncol. 2016, 118, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Carlier, B.; Heymans, S.V.; Nooijens, S.; Toumia, Y.; Ingram, M.; Paradossi, G.; D’Agostino, E.; Himmelreich, U.; D’hooge, J.; Van Den Abeele, K.; et al. Proton Range Verification with Ultrasound Imaging Using Injectable Radiation Sensitive Nanodroplets: A Feasibility Study. Phys. Med. Biol. 2020, 65, 065013. [Google Scholar] [CrossRef]
- Schauer, J.; Wieser, H.-P.; Huang, Y.; Ruser, H.; Lascaud, J.; Würl, M.; Chmyrov, A.; Vidal, M.; Herault, J.; Ntziachristos, V.; et al. Proton Beam Range Verification by Means of Ionoacoustic Measurements at Clinically Relevant Doses Using a Correlation-Based Evaluation. Front. Oncol. 2022, 12, 925542. [Google Scholar] [CrossRef]
- Paganetti, H.; El Fakhri, G. Monitoring Proton Therapy with PET. BJR 2015, 88, 20150173. [Google Scholar] [CrossRef]
- España, S.; Zhu, X.; Daartz, J.; El Fakhri, G.; Bortfeld, T.; Paganetti, H. The reliability of proton-nuclear interaction cross-section data to predict proton-induced PET images in proton therapy. Phys. Med. Biol. 2011, 56, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.M.; Herraiz, J.L.; Udías, J.M.; Cal-González, J.; Corzo, P.M.G.; España, S.; Herranz, E.; Pérez-Liva, M.; Picado, E.; Vicente, E.; et al. Experimental Validation of Gallium Production and Isotope-Dependent Positron Range Correction in PET. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2016, 814, 110–116. [Google Scholar] [CrossRef][Green Version]
- Cho, J.; Campbell, P.; Wang, M.; Alqathami, M.; Mawlawi, O.; Kerr, M.; Cho, S.H. Feasibility of Hydrogel Fiducial Markers for in Vivo Proton Range Verification Using PET. Phys. Med. Biol. 2016, 61, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Backer, C.M.; Baumer, C.; Bley, A.; Costa, P.F.; Gerhardt, M.; Herrmann, K.; Kauer, S.; Kroninger, K.; Nitsch, C.; Siregar, H.M.; et al. Proton Beam Range Verification with Secondary Radiation from Titanium Implants. In Proceedings of the 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Manchester, UK, 26 October–2 November 2019; IEEE: Manchester, UK, 2019; pp. 1–2. [Google Scholar] [CrossRef]
- España, S.; Sánchez-Parcerisa, D.; Ibáñez, P.; Sánchez-Tembleque, V.; Udías, J.M.; Onecha, V.V.; Gutierrez-Uzquiza, A.; Bäcker, C.M.; Bäumer, C.; Herrmann, K.; et al. Direct Proton Range Verification Using Oxygen-18 Enriched Water as a Contrast Agent. Radiat. Phys. Chem. 2021, 182, 109385. [Google Scholar] [CrossRef]
- Espinosa Rodriguez, A.; Onecha, V.V.; Sánchez-Tembleque, V.; Gutiérrez-Neira, C.; García-Díez, M.; Ibáñez, P.; España, S.; Sánchez-Parcerisa, D.; Udías, J.M.; Fraile, L.M. Can Iodine Be Used as a Contrast Agent for Protontherapy Range Verification? Measurement of the 127I(p,n)127mXe (Reaction) Cross Section in the 4.5–10 MeV Energy Range. Radiat. Phys. Chem. 2021, 185, 109485. [Google Scholar] [CrossRef]
- España, S.; Sánchez-Parcerisa, D.; Bragado, P.; Gutiérrez-Uzquiza, Á.; Porras, A.; Gutiérrez-Neira, C.; Espinosa, A.; Onecha, V.V.; Ibáñez, P.; Sánchez-Tembleque, V.; et al. In Vivo Production of Fluorine-18 in a Chicken Egg Tumor Model of Breast Cancer for Proton Therapy Range Verification. Sci. Rep. 2022, 12, 7075. [Google Scholar] [CrossRef] [PubMed]
- Galanakou, P.; Leventouri, T.; Muhammad, W. Non-Radioactive Elements for Prompt Gamma Enhancement in Proton Therapy. Radiat. Phys. Chem. 2022, 196, 110132. [Google Scholar] [CrossRef]
- Stichelbaut, F.; Jongen, Y. Verification of the proton beam position in the patient by the detection of prompt gamma-rays emission. In Proceedings of the 39th Meeting of the Particle Therapy Co-Operative Group, San Francisco, CA, USA, 25 October 2003; Volume 16. [Google Scholar]
- Hueso-González, F.; Rabe, M.; Ruggieri, T.A.; Bortfeld, T.; Verburg, J.M. A Full-Scale Clinical Prototype for Proton Range Verification Using Prompt Gamma-Ray Spectroscopy. Phys. Med. Biol. 2018, 63, 185019. [Google Scholar] [CrossRef]
- Parodi, K. Vision 20/20: Positron Emission Tomography in Radiation Therapy Planning, Delivery, and Monitoring: PET in RT Planning, Delivery, and Monitoring. Med. Phys. 2015, 42, 7153–7168. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 Years of Biofunctionalization and Surface Chemistry of Inorganic Nanoparticles for Nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef]
- Choi, S.-J.; Choy, J.-H. Biokinetics of Zinc Oxide Nanoparticles: Toxicokinetics, Biological Fates, and Protein Interaction. Int. J. Nanomedicine 2014, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.J.; Scherzad, A.; Moratin, H.; Gehrke, T.E.; Killisperger, J.; Hagen, R.; Wohlleben, G.; Polat, B.; Dembski, S.; Kleinsasser, N.; et al. The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing is Associated with Oxidative Stress In Vitro. Materials 2019, 12, 4062. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of Nanomaterials for Medical Imaging and Therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Molecular Imaging with 68Ga Radio-Nanomaterials: Shedding Light on Nanoparticles. Appl. Sci. 2018, 8, 1098. [Google Scholar] [CrossRef]
- Alphandéry, E. Biodistribution and Targeting Properties of Iron Oxide Nanoparticles for Treatments of Cancer and Iron Anemia Disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Schettino, G.; Royle, G.; Barry, M.; Pankhurst, Q.A.; Tillement, O.; Russell, B.; Ricketts, K. Radiobiological Implications of Nanoparticles Following Radiation Treatment. Part. Part. Syst. Charact. 2020, 37, 1900411. [Google Scholar] [CrossRef] [PubMed]
- Paro, A.D.; Shanmugam, I.; van de Ven, A.L. Nanoparticle-Mediated X-ray Radiation Enhancement for Cancer Therapy. In Cancer Nanotechnol; Zeineldin, R., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1530, pp. 391–401. ISBN 978-1-4939-6644-8. [Google Scholar]
- Penninckx, S.; Hespeels, F.; Smeets, J.; Colaux, J.L.; Lucas, S.; Heuskin, A.-C. Metallic Nanoparticles: A Useful Prompt Gamma Emitter for Range Monitoring in Proton Therapy? Radiation 2021, 1, 305–316. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Synthesis of 68Ga Core-Doped Iron Oxide Nanoparticles for Dual Positron Emission Tomography/(T1)Magnetic Resonance Imaging. J. Vis. Exp. 2018, 141, e58269. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Pellico, J.; Lechuga-Vieco, A.V.; Almarza, E.; Hidalgo, A.; Mesa-Nuñez, C.; Fernández-Barahona, I.; Quintana, J.A.; Bueren, J.; Enríquez, J.A.; Ruiz-Cabello, J.; et al. In vivo imaging of lung inflammation with neutrophil-specific 68Ga nano-radiotracer. Sci. Rep. 2017, 7, 13242. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Saiz-Alía, M.; del Rosario, G.; Caja, S.; Montoya, M.; Fernández de Manuel, L.; Morales, M.P.; Gutiérrez, L.; Galiana, B.; et al. Fast Synthesis and Bioconjugation of 68 Ga Core-Doped Extremely Small Iron Oxide Nanoparticles for PET/MR Imaging: Chelator-Free68 Ga-Iron Oxide Nanoparticles. Contrast Media Mol. Imaging 2016, 11, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Manickam, V.; Dhakshinamoorthy, V.; Perumal, E. Iron Oxide Nanoparticles Induces Cell Cycle-Dependent Neuronal Apoptosis in Mice. J. Mol. Neurosci. 2018, 64, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of Long-Term Exposure to Zinc Oxide Nanoparticles on Development, Zinc Metabolism and Biodistribution of Minerals (Zn, Fe, Cu, Mn) in Mice. PLoS ONE 2016, 11, e0164434. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In Vivo Delivery, Pharmacokinetics, Biodistribution and Toxicity of Iron Oxide Nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cubero, A.; Borge, M.J.G.; Gordillo, N.; Gutiérrez, P.C.; Olivares, J.; Pérez Casero, R.; Ynsa, M.D. Current status and future developments of the ion beam facility at the centre of micro-analysis of materials in Madrid. Eur. Phys. J. Plus 2021, 136, 175. [Google Scholar] [CrossRef]
- Vedia, V.; Carmona-Gallardo, M.; Fraile, L.M.; Mach, H.; Udías, J.M. Performance evaluation of novel LaBr3(Ce) scintillator geometries for fast-timing applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2017, 857, 98–105. [Google Scholar] [CrossRef]
- Rosman, K.J.R. A Survey of the Isotopic and Elemental Abundance of Zinc. Geochim. Cosmochim. Acta 1972, 36, 801–819. [Google Scholar] [CrossRef]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef]
- Rosário, F.; Bessa, M.J.; Brandão, F.; Costa, C.; Lopes, C.B.; Estrada, A.C.; Tavares, D.S.; Teixeira, J.P.; Reis, A.T. Unravelling the Potential Cytotoxic Effects of Metal Oxide Nanoparticles and Metal(Loid) Mixtures on A549 Human Cell Line. Nanomaterials 2020, 10, 447. [Google Scholar] [CrossRef]
- Bastos, V.; Ferreira-de-Oliveira, J.M.P.; Carrola, J.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. Coating Independent Cytotoxicity of Citrate- and PEG-Coated Silver Nanoparticles on a Human Hepatoma Cell Line. J. Environ. Sci. Int. 2017, 51, 191–201. [Google Scholar] [CrossRef]
- Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J.-F. The Effects of Aggregation and Protein Corona on the Cellular Internalization of Iron Oxide Nanoparticles. Biomaterials 2011, 32, 9353–9363. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Li, W.; Bai, H.; Gao, Y.; Ma, J.; Liu, W.; Xi, G. Dextran Coated Fe3O4 Nanoparticles as a Near-Infrared Laser-Driven Photothermal Agent for Efficient Ablation of Cancer Cells in Vitro and in Vivo. Mater. Sci. Eng. C 2018, 90, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Líbalová, H.; Costa, P.M.; Olsson, M.; Farcal, L.; Ortelli, S.; Blosi, M.; Topinka, J.; Costa, A.L.; Fadeel, B. Toxicity of Surface-Modified Copper Oxide Nanoparticles in a Mouse Macrophage Cell Line: Interplay of Particles, Surface Coating and Particle Dissolution. Chemosphere 2018, 196, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.S.; López, J.L.; Lacerda, S.M.S.N.; Procópio, M.S.; Figueiredo, A.F.A.; Martins, E.M.N.; Guimarães, P.P.G.; Ladeira, L.O.; Kitten, G.T.; Dias, F.F.; et al. Biotechnological Approach to Induce Human Fibroblast Apoptosis Using Superparamagnetic Iron Oxide Nanoparticles. J. Inorg. Biochem. 2020, 206, 111017. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.; Mozar, F.S.; Chowdhury, E.H. Citrate Association Dramatically Reduces Diameter with Concomitant Increase in Uptake of Drug-Loaded Carbonate Apatite Particles in Breast Cancer Cells. J. Nanosci. Nanotechnol. 2019, 19, 6881–6892. [Google Scholar] [CrossRef] [PubMed]
- Lévy, M.; Lagarde, F.; Maraloiu, V.-A.; Blanchin, M.-G.; Gendron, F.; Wilhelm, C.; Gazeau, F. Degradability of Superparamagnetic Nanoparticles in a Model of Intracellular Environment: Follow-up of Magnetic, Structural and Chemical Properties. Nanotechnology 2010, 21, 395103. [Google Scholar] [CrossRef] [PubMed]
- Plan Sangnier, A.; Van de Walle, A.B.; Curcio, A.; Le Borgne, R.; Motte, L.; Lalatonne, Y.; Wilhelm, C. Impact of Magnetic Nanoparticle Surface Coating on Their Long-Term Intracellular Biodegradation in Stem Cells. Nanoscale 2019, 11, 16488–16498. [Google Scholar] [CrossRef] [PubMed]
- Mekseriwattana, W.; Srisuk, S.; Kriangsaksri, R.; Niamsiri, N.; Prapainop, K. The Impact of Serum Proteins and Surface Chemistry on Magnetic Nanoparticle Colloidal Stability and Cellular Uptake in Breast Cancer Cells. AAPS PharmSciTech 2019, 20, 55. [Google Scholar] [CrossRef]
- de Lucas, A.G.; Schuhmacher, A.J.; Oteo, M.; Romero, E.; Cámara, J.A.; de Martino, A.; Arroyo, A.G.; Morcillo, M.Á.; Squatrito, M.; Martinez-Torrecuadrada, J.L.; et al. Targeting MT1-MMP as an ImmunoPET-Based Strategy for Imaging Gliomas. PLoS ONE 2016, 11, e0158634. [Google Scholar] [CrossRef]
- Chambrelant, I.; Eber, J.; Antoni, D.; Burckel, H.; Noël, G.; Auvergne, R. Proton Therapy and Gliomas: A Systematic Review. Radiation 2021, 1, 218–233. [Google Scholar] [CrossRef]
- Horie, M.; Shimizu, K.; Tabei, Y. Validation of Metallothionein, Interleukin-8, and Heme Oxygenase-1 as Markers for the Evaluation of Cytotoxicity Caused by Metal Oxide Nanoparticles. Toxicol. Mech. Methods 2018, 28, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Generalov, R.; Kuan, W.B.; Chen, W.; Kristensen, S.; Juzenas, P. Radiosensitizing Effect of Zinc Oxide and Silica Nanocomposites on Cancer Cells. Colloids Surf. B 2015, 129, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, M.; Janko, C.; Unterweger, H.; Friedrich, R.P.; Friedrich, B.; Band, J.; Cebulla, N.; Alexiou, C.; Dudziak, D.; Lee, G.; et al. Functionalization of T Lymphocytes With Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles For Magnetically Controlled Immune Therapy. Int. J. Nanomedicine 2019, 14, 8421–8432. [Google Scholar] [CrossRef] [PubMed]
- Uzar, N.K.; Abudayyak, M.; Akcay, N.; Algun, G.; Özhan, G. Zinc Oxide Nanoparticles Induced Cyto- and Genotoxicity in Kidney Epithelial Cells. Toxicol. Mech. Methods 2015, 25, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.; Wingett, D.G.; Rasmussen, J.W.; Layne, J.; Johnson, L.; Tenne, D.A.; Zhang, J.; Hanna, C.B.; Punnoose, A. Improving the Selective Cancer Killing Ability of ZnO Nanoparticles Using Fe Doping. Nanotoxicology 2012, 6, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of Iron and Hydrogen Peroxide: The Fenton Reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive Cytotoxicity Studies of Superparamagnetic Iron Oxide Nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Xia, Z.; Teng, L.; Chen, Y.; Meng, J.; Li, C. Zinc Oxide Nanoparticles Promotes Ferroptosis to Repress Cancer Cell Survival and Inhibits Invasion and Migration by Targeting MiR-27a-3p/YAP Axis in Renal Cell Carcinoma. Arab. J. Chem. 2022, 15, 103753. [Google Scholar] [CrossRef]
- Palmer, L.D.; Jordan, A.T.; Maloney, K.N.; Farrow, M.A.; Gutierrez, D.B.; Gant-Branum, R.; Burns, W.J.; Romer, C.E.; Tsui, T.; Allen, J.L.; et al. Zinc Intoxication Induces Ferroptosis in A549 Human Lung Cells. Metallomics 2019, 11, 982–993. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chi, J.-T. Unexpected Zinc Dependency of Ferroptosis: What Is in a Name? Oncotarget 2021, 12, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and Methodologies for Characterizing Radiobiological Impact of High-Z Nanoparticles. Theranostics 2016, 6, 1651–1671. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Broche, L.M.; Ezdoglian, A.; Li, D.; Yuecel, R.; James Ross, P.; Cheyne, L.; Wilson, H.M.; Lurie, D.J.; Dawson, D.K. Fast Field-Cycling Magnetic Resonance Detection of Intracellular Ultra-Small Iron Oxide Particles in Vitro: Proof-of-Concept. J. Magn. Reson. 2020, 313, 106722. [Google Scholar] [CrossRef] [PubMed]
- Heuskin, A.; Gallez, B.; Feron, O.; Martinive, P.; Michiels, C.; Lucas, S. Metallic Nanoparticles Irradiated by Low-energy Protons for Radiation Therapy: Are There Significant Physical Effects to Enhance the Dose Delivery? Med. Phys. 2017, 44, 4299–4312. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.; Nedaei, H.A.; Mozdarani, H.; Mahmoudzadeh, A.; Salimi, M. Enhanced Cytotoxic and Genotoxic Effects of Gadolinium-Doped ZnO Nanoparticles on Irradiated Lung Cancer Cells at Megavoltage Radiation Energies. Mater. Sci. Eng. 2019, 103, 109739. [Google Scholar] [CrossRef] [PubMed]
- Burger, N.; Biswas, A.; Barzan, D.; Kirchner, A.; Hosser, H.; Hausmann, M.; Hildenbrand, G.; Herskind, C.; Wenz, F.; Veldwijk, M.R. A Method for the Efficient Cellular Uptake and Retention of Small Modified Gold Nanoparticles for the Radiosensitization of Cells. Nanomedicine: NBM. 2014, 10, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Kempson, I. Mechanisms of Nanoparticle Radiosensitization. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1656. [Google Scholar] [CrossRef] [PubMed]
- Deylam, M.; Alizadeh, E.; Sarikhani, M.; Hejazy, M.; Firouzamandi, M. Zinc Oxide Nanoparticles Promote the Aging Process in a Size-Dependent Manner. J. Mater. Sci. Mater. Med. 2021, 32, 128. [Google Scholar] [CrossRef]
- Matsui, T.; Nuryadi, E.; Komatsu, S.; Hirota, Y.; Shibata, A.; Oike, T.; Nakano, T. Robustness of Clonogenic Assays as a Biomarker for Cancer Cell Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 4148. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Jin, X.; He, P.; Zheng, X.; Dai, Z.; Ye, F.; Zhao, T.; Chen, W.; Li, Q. The Dependence of Radiation Enhancement Effect on the Concentration of Gold Nanoparticles Exposed to Low- and High-LET Radiations. Phys. Med. 2015, 31, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Lou, Z.; Chen, F.; Chang, S.; Miao, Y.; Zhou, Z.; Hu, X.; Feng, J.; Ding, Q.; et al. Radiosensitivity Enhancement of Fe3O4@Ag Nanoparticles on Human Glioblastoma Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 975–984. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Jin, X.; Liu, Y.; Zhang, P.; Li, P.; Shen, Z.; Wu, A.; Chen, W.; Li, Q. Ultra-Small Gadolinium Oxide Nanocrystal Sensitization of Non-Small-Cell Lung Cancer Cells toward X-ray Irradiation by Promoting Cytostatic Autophagy. Int. J. Nanomedicine 2019, 14, 2415–2431. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale Energy Deposition by X-ray Absorbing Nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Kim, E.-H. Gold Nanoparticles as Dose-Enhancement Agent for Kilovoltage X-ray Therapy of Melanoma. Int. J. Radiat. Biol. 2017, 93, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Rajaee, A.; Wang, S.; Zhao, L.; Wang, D.; Liu, Y.; Wang, J.; Ying, K. Multifunction Bismuth Gadolinium Oxide Nanoparticles as Radiosensitizer in Radiation Therapy and Imaging. Phys. Med. Biol. 2019, 64, 195007. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, J. Cu, Fe, Mn, and Zn Chelates Offer a Medicinal Chemistry Approach to Overcoming Radiation Injury. CMC 2002, 9, 639–662. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Ge, M.H.; Tian, H.; Mao, L.; Li, D.Y.; Lin, J.Q.; Hu, H.S.; Huang, S.C.; Zhang, C.J.; Mei, X.F. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci. Ther. 2021, 27, 1023–1040. [Google Scholar] [CrossRef]
- Brero, F.; Calzolari, P.; Albino, M.; Antoccia, A.; Arosio, P.; Berardinelli, F.; Bettega, D.; Ciocca, M.; Facoetti, A.; Gallo, S.; et al. Proton Therapy, Magnetic Nanoparticles and Hyperthermia as Combined Treatment for Pancreatic BxPC3 Tumor Cells. Nanomaterials 2023, 13, 791. [Google Scholar] [CrossRef]
- Mikuš, P.; Melník, M.; Forgácsová, A.; Krajčiová, D.; Havránek, E. Gallium Compounds in Nuclear Medicine and Oncology. Main Group Met. Chem. 2014, 37, 53–65. [Google Scholar] [CrossRef]
- Jødal, L.; Afzelius, P.; Alstrup, A.K.O.; Jensen, S.B. Radiotracers for Bone Marrow Infection Imaging. Molecules 2021, 26, 3159. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, C. Nuclear Medicine Imaging With an Emphasis on Spinal Infections. In Interventional Spine; Elsevier: Amsterdam, The Netherlands, 2008; pp. 89–93. ISBN 978-0-7216-2872-1. [Google Scholar]
- Boros, E.; Bowen, A.M.; Josephson, L.; Vasdev, N.; Holland, J.P. Chelate-Free Metal Ion Binding and Heat-Induced Radiolabeling of Iron Oxide Nanoparticles. Chem. Sci. 2015, 6, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood–Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A.; Guiberteau, M.J. 1—Radioactivity, Radionuclides, and Radiopharmaceuticals. In Essentials of Nuclear Medicine and Molecular Imaging, 7th ed.; Mettler, F.A., Guiberteau, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18. ISBN 978032348319. [Google Scholar] [CrossRef]
- Slavotinek, A.; McMillan, T.J.; Steel, C.M. Measurement of Radiation Survival Using the MTT Assay. Eur. J. Cancer 1994, 30, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Brix, N.; Samaga, D.; Belka, C.; Zitzelsberger, H.; Lauber, K. Analysis of Clonogenic Growth in Vitro. Nat. Protoc. 2021, 16, 4963–4991. [Google Scholar] [CrossRef]
- Flint, D.B.; Ruff, C.E.; Bright, S.J.; Yepes, P.; Wang, Q.; Manandhar, M.; Kacem, M.B.; Turner, B.X.; Martinus, D.K.J.; Shaitelman, S.F.; et al. An empirical model of proton RBE based on the linear correlation between X-ray and proton radiosensitivity. Med. Phys. 2022, 49, 6221–6236. [Google Scholar] [CrossRef]
- Akbasak, A.; Toevs, C.C.; Laske, D.W. Reconstituted Basement Membrane (Matrigel) Enhances the Growth of Human Glioma Cell Lines in Nude Mice. J. Neuro-Oncol. 1996, 27, 23–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]

| NP Component | IC50 (μg/mL) | 95% Confidence Intervals |
|---|---|---|
| Fe | 64 | 61 to 67 |
| Zn | 100 | 96 to 104 |
| Fe + Zn | 164 | 157 to 171 |
| [IONP@Zn-cit] (µg Zn/mL) | α (Gy−1) | β (Gy−2) | SF2Gy | D50% (Gy) | D10% (Gy) | MID |
|---|---|---|---|---|---|---|
| 0 | 0.527 ± 0.286 | −0.005 ± 0.028 | 0.355 | 1.33 | 4.55 | 2.62 |
| 1 | 0.206 ± 0.162 | 0.019 ± 0.016 | 0.613 | 2.69 | 6.82 | 3.11 |
| 10 | 0.056 ± 0.010 | 0.039 ± 0.001 | 0.766 | 3.57 | 7.03 | 2.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-Moragues, M.; Fernández-Barahona, I.; Santacruz, R.; Oteo, M.; Luján-Rodríguez, V.M.; Muñoz-Hernando, M.; Magro, N.; Lagares, J.I.; Romero, E.; España, S.; et al. Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy. Molecules 2023, 28, 6874. https://doi.org/10.3390/molecules28196874
Ibáñez-Moragues M, Fernández-Barahona I, Santacruz R, Oteo M, Luján-Rodríguez VM, Muñoz-Hernando M, Magro N, Lagares JI, Romero E, España S, et al. Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy. Molecules. 2023; 28(19):6874. https://doi.org/10.3390/molecules28196874
Chicago/Turabian StyleIbáñez-Moragues, Marta, Irene Fernández-Barahona, Rocío Santacruz, Marta Oteo, Víctor M. Luján-Rodríguez, María Muñoz-Hernando, Natalia Magro, Juan I. Lagares, Eduardo Romero, Samuel España, and et al. 2023. "Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy" Molecules 28, no. 19: 6874. https://doi.org/10.3390/molecules28196874
APA StyleIbáñez-Moragues, M., Fernández-Barahona, I., Santacruz, R., Oteo, M., Luján-Rodríguez, V. M., Muñoz-Hernando, M., Magro, N., Lagares, J. I., Romero, E., España, S., Espinosa-Rodríguez, A., García-Díez, M., Martínez-Nouvilas, V., Sánchez-Tembleque, V., Udías, J. M., Valladolid-Onecha, V., Martín-Rey, M. Á., Almeida-Cordon, E. I., Viñals i Onsès, S., ... Morcillo, M. Á. (2023). Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy. Molecules, 28(19), 6874. https://doi.org/10.3390/molecules28196874