Influence of pH on Heat-Induced Changes in Skim Milk Containing Various Levels of Micellar Calcium Phosphate
Abstract
:1. Introduction
2. Results
2.1. Calcium Content of MCP-Adjusted Skim Milk
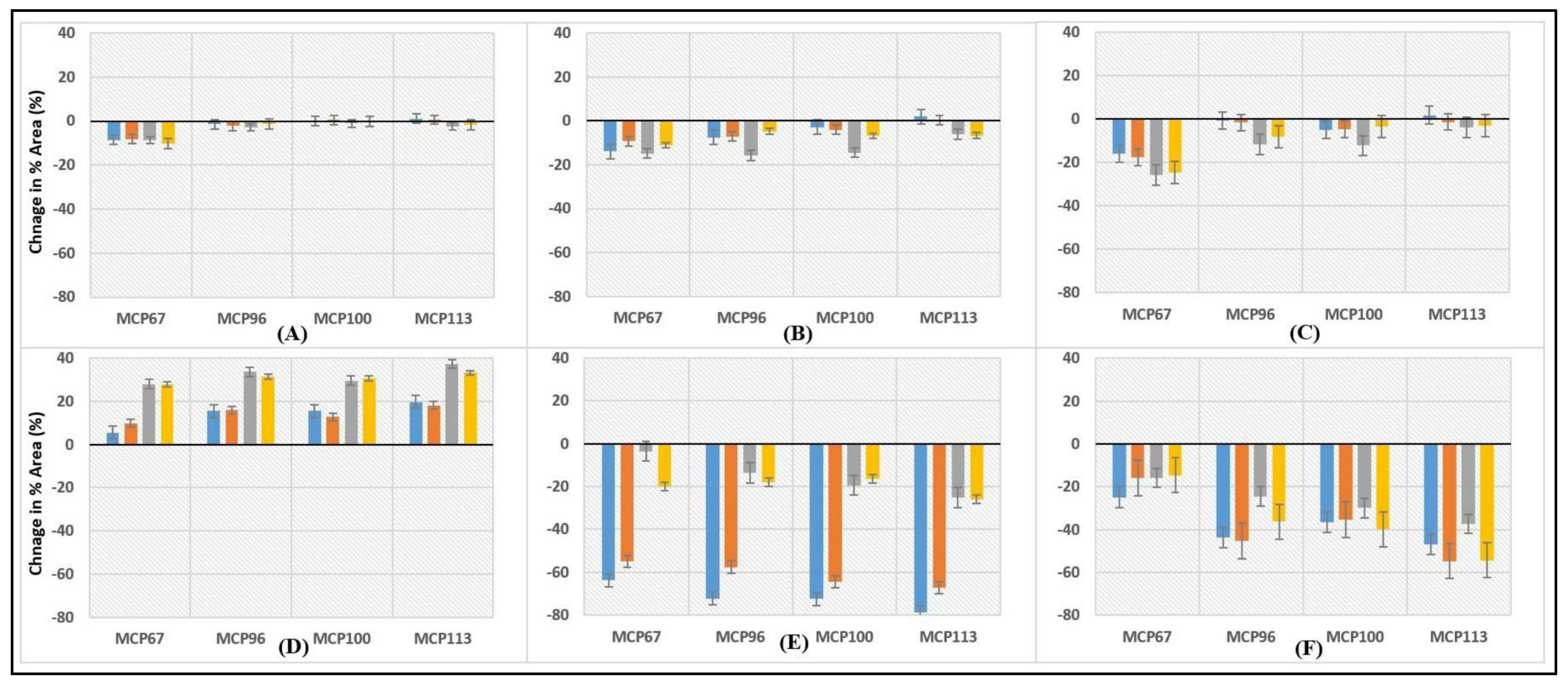
2.2. Physiochemical Changes in MCP-Adjusted Skim Milk Samples after Heat Treatment
2.3. Heat-Induced Changes in the Protein Distribution of MCP-Adjusted Skim Milk
2.4. FTIR Fingerprinting
2.4.1. Region I: Amide I (1700–1600 cm−1)
2.4.2. Region II (1200–900 cm−1)
3. Discussion
4. Materials and Methods
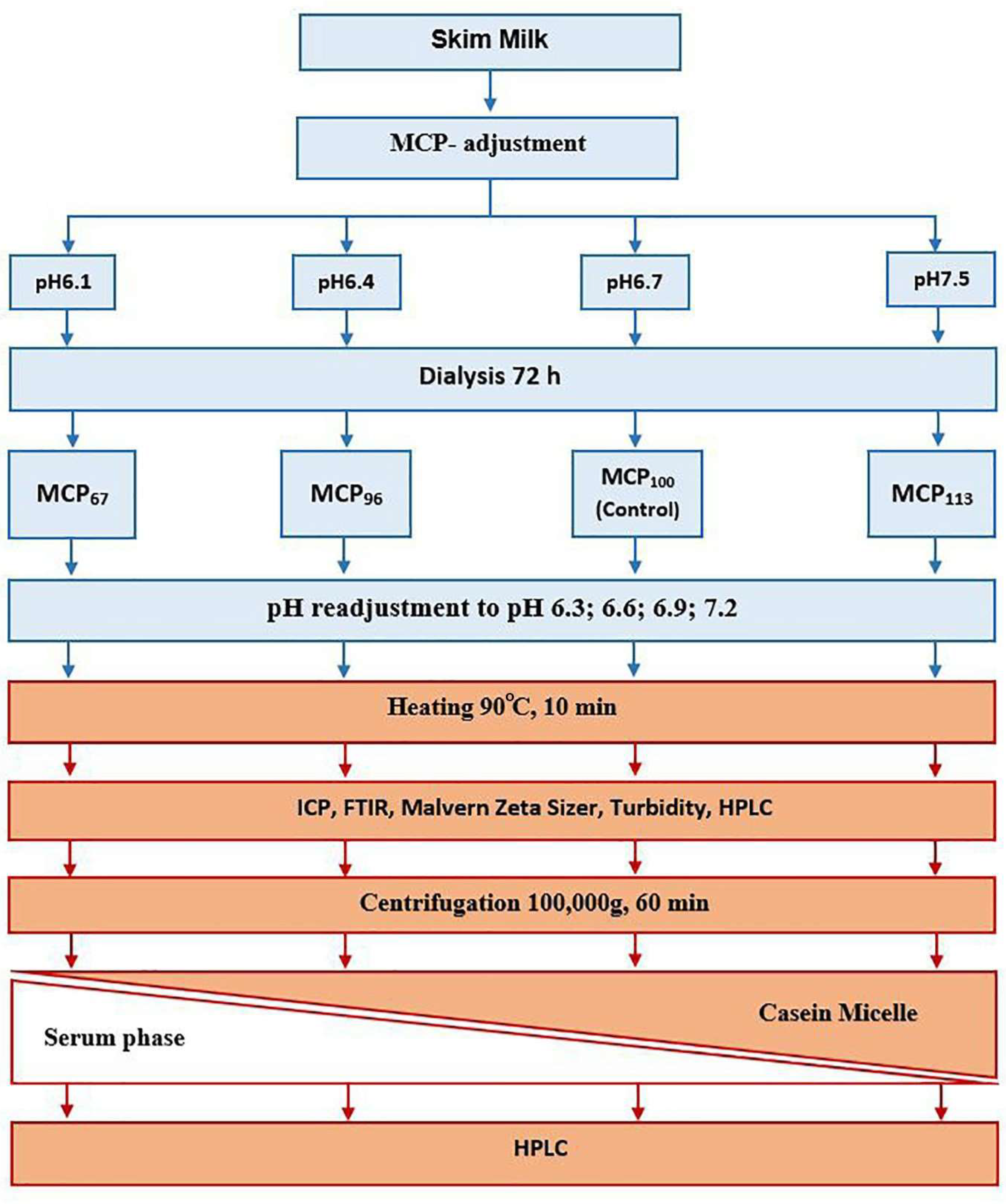
4.1. Sample Preparation
4.2. Sample Fractionation
4.3. Sample Analysis
4.3.1. Calcium Content
4.3.2. Turbidity
4.3.3. Particle Size Distribution
4.3.4. High-Performance Liquid Chromatography (HPLC)
4.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food. Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y. Effect of temperature and pH on salts equilibria and calcium phosphate in bovine milk. Int. Dairy J. 2020, 110, 104713. [Google Scholar] [CrossRef]
- Lewis, M.J.; Deeth, H.C. Heat Treatment of Milk. Milk Processing and Quality Management; Wiley: London, UK, 2009; pp. 168–204. [Google Scholar]
- Anema, S.G. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. Int. Dairy J. 2021, 122, 105–136. [Google Scholar] [CrossRef]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef]
- Fox, P.F.; Hoynes, M.C.T. Heat stability of milk: Influence of colloidal calcium phosphate and β-lactoglobulin. J. Dairy Res. 1975, 42, 427–435. [Google Scholar] [CrossRef]
- Singh, H.; Fox, P.F. Heat stability of milk: Influence of colloidal and soluble salts and protein modification on the pH-dependent dissociation of micellar κ-casein. J. Dairy Res. 1987, 54, 523–534. [Google Scholar] [CrossRef]
- Anema, S.G.; Li, Y. Further studies on the heat-induced, pH-dependent dissociation of casein from the micelles in reconstituted skim milk. LWT-Food. Sci. Technol. 2000, 33, 335–343. [Google Scholar] [CrossRef]
- Walstra, P.; Walstra, P.; Wouters, J.T.; Geurts, T.J. Heat Treatment. In Dairy Science and Technology, 2nd ed.; Barbosa-Cánovas, G.V., Davidson, P.M., Eds.; CRC press, Taylor and Francis Group: London, UK, 2006; pp. 236–239. [Google Scholar]
- Aydogdu, T.; O’Mahony, J.A.; Huppertz, T.; Magan, J.B.; McCarthy, N.A. Measuring pH of skim milk and milk permeate at ultra-high temperatures at laboratory and pilot scale. Int. Dairy J. 2023, 139, 105565. [Google Scholar] [CrossRef]
- Pyne, G.T.; McGann, T.C.A. The colloidal phosphate of milk: II. Influence of citrate. J. Dairy Res. 1960, 27, 9–17. [Google Scholar] [CrossRef]
- Anema, S.G.; Li, Y. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J. Dairy Res. 2003, 70, 73–83. [Google Scholar] [CrossRef]
- McSweeney, P.L.; Fox, P.F. Proteins: Basic aspects. In Advanced Dairy Chemistry, 4th ed.; Springer Science & Business Media: Cork, Ireland, 2013; p. 47. [Google Scholar]
- Grewal, M.K.; Vasiljevic, T.; Huppertz, T. Influence of calcium and magnesium on the secondary structure in solutions of individual caseins and binary casein mixtures. Int. Dairy J. 2020, 112, 104879. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Huang, S.; Ruan, X.; Zhou, Q.; Sun, W. Effects of spray drying and freeze drying on the properties of protein isolate from rice dreg protein. Food Bioprocess Technol. 2013, 6, 1759–1769. [Google Scholar] [CrossRef]
- Parris, N.; Purcell, J.M.; Ptashkin, S.M. Thermal denaturation of whey proteins in skim milk. J. Agric. Food. Chem. 1991, 39, 2167–2170. [Google Scholar] [CrossRef]
- Grewal, M.K.; Chandrapala, J.; Donkor, O.; Apostolopoulos, V.; Stojanovska, L.; Vasiljevic, T. Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. Int. Dairy J. 2017, 66, 99–107. [Google Scholar] [CrossRef]
- Pouliot, Y.; Boulet, M.; Paquin, P. Observations on the heat-induced salt balance changes in milk I. Effect of heating time between 4 and 90 C. J. Dairy Res. 1989, 56, 185–192. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Effect of temperature and pH on the interactions of whey proteins with casein micelles in skim milk. Food. Res. Int. 1996, 29, 49–55. [Google Scholar] [CrossRef]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Chemistry of the caseins. In Advanced Dairy Chemistry: Proteins, 2nd ed.; Elsevier: London, UK, 1992; pp. 63–110. [Google Scholar]
- Liu, X.C.; Jiang, Y.; Ahrné, L.M.; Skibsted, L.H. Temperature effects on calcium binding to caseins. Food. Res. Int. 2022, 154, 110981. [Google Scholar] [CrossRef]
- Grewal, M.K.; Huppertz, T.; Vasiljevic, T. FTIR fingerprinting of structural changes of milk proteins induced by heat treatment, deamidation and dephosphorylation. Food Hydrocoll. 2018, 80, 160–167. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Zhang, H.; Liu, C.; Jiao, W.; Chang, Z. Chaperone-like activity of β-casein. Int. J. Biochem. Cell Biol. 2005, 37, 1232–1240. [Google Scholar] [CrossRef]
- Liyanaarachchi, W.S.; Vasiljevic, T. Caseins and their interactions that modify heat aggregation of whey proteins in commercial dairy mixtures. Int. Dairy J. 2018, 83, 43–51. [Google Scholar] [CrossRef]
- Smits, P.; Van Brouwershaven, J.H. Heat-induced association of β-lactoglobulin and casein micelles. J. Dairy Res. 1980, 47, 313–325. [Google Scholar] [CrossRef]
- Huppertz, T.; Lambers, T.T. Influence of micellar calcium phosphate on in vitro gastric coagulation and digestion of milk proteins in infant formula model systems. Int. Dairy J. 2020, 107, 104717. [Google Scholar] [CrossRef]
- Jenness, R.; Koops, J. Preparation and properties of a salt solution which simulates milk ultrafiltrate. Neth. Milk Dairy J. 1962, 16, 153–164. [Google Scholar]
- Aprianita, A.; Vasiljevic, T.; Bannikova, A.; Kasapis, S. Physicochemical properties of flours and starches derived from traditional Indonesian tubers and roots. Food. Sci. Technol. 2014, 51, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
 ; pH 6.6
; pH 6.6  ; pH 6.9
; pH 6.9  ; and pH 7.2
; and pH 7.2  .
.
 ; pH 6.6
; pH 6.6  ; pH 6.9
; pH 6.9  ; and pH 7.2
; and pH 7.2  .
.
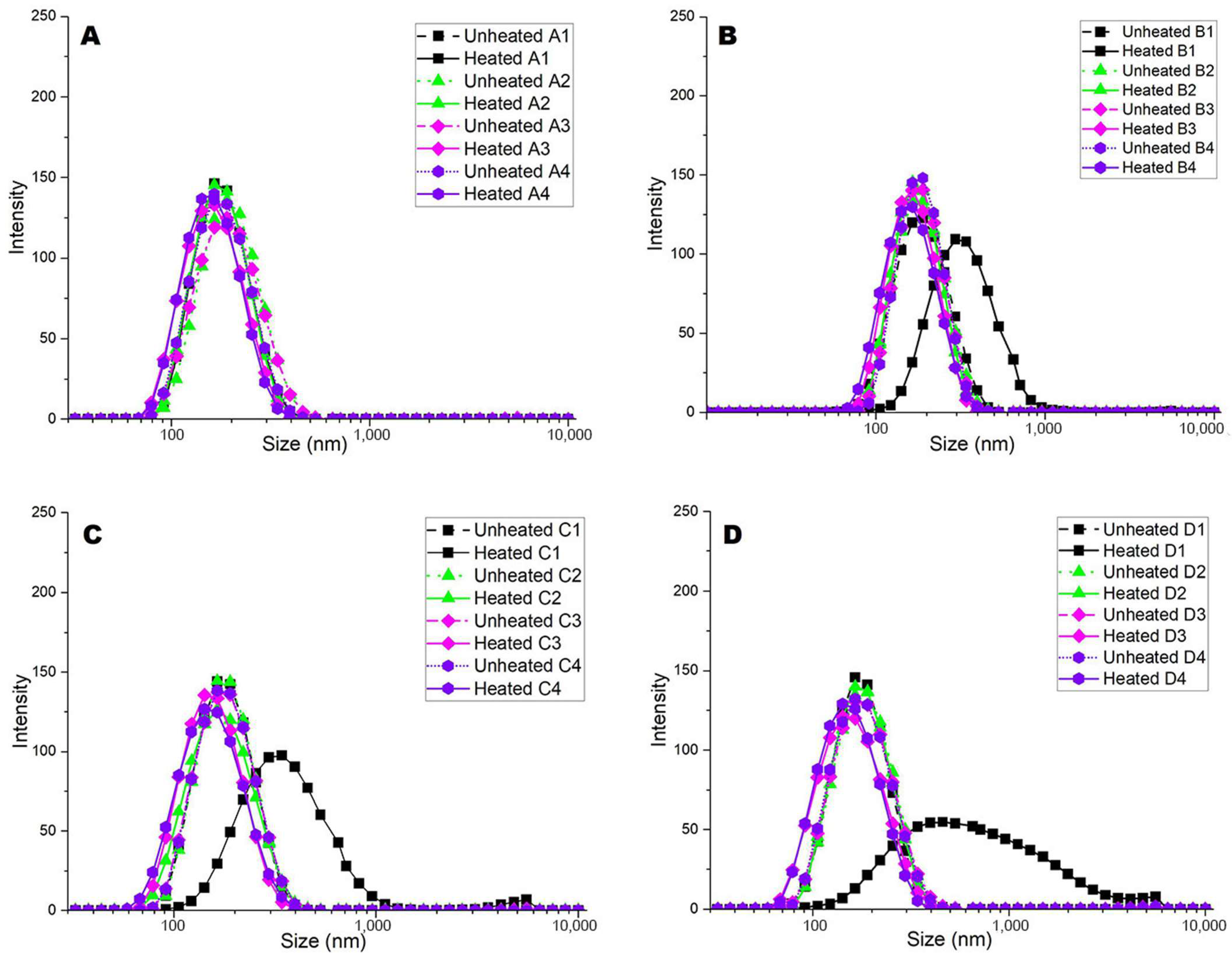
 Unheated 1;
Unheated 1;  Heated 1;
Heated 1;  Unheated 2;
Unheated 2;  Heated 2;
Heated 2;  Unheated 3;
Unheated 3;  Heated 3;
Heated 3;  Unheated 4;
Unheated 4;  Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
 Unheated 1;
Unheated 1;  Heated 1;
Heated 1;  Unheated 2;
Unheated 2;  Heated 2;
Heated 2;  Unheated 3;
Unheated 3;  Heated 3;
Heated 3;  Unheated 4;
Unheated 4;  Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.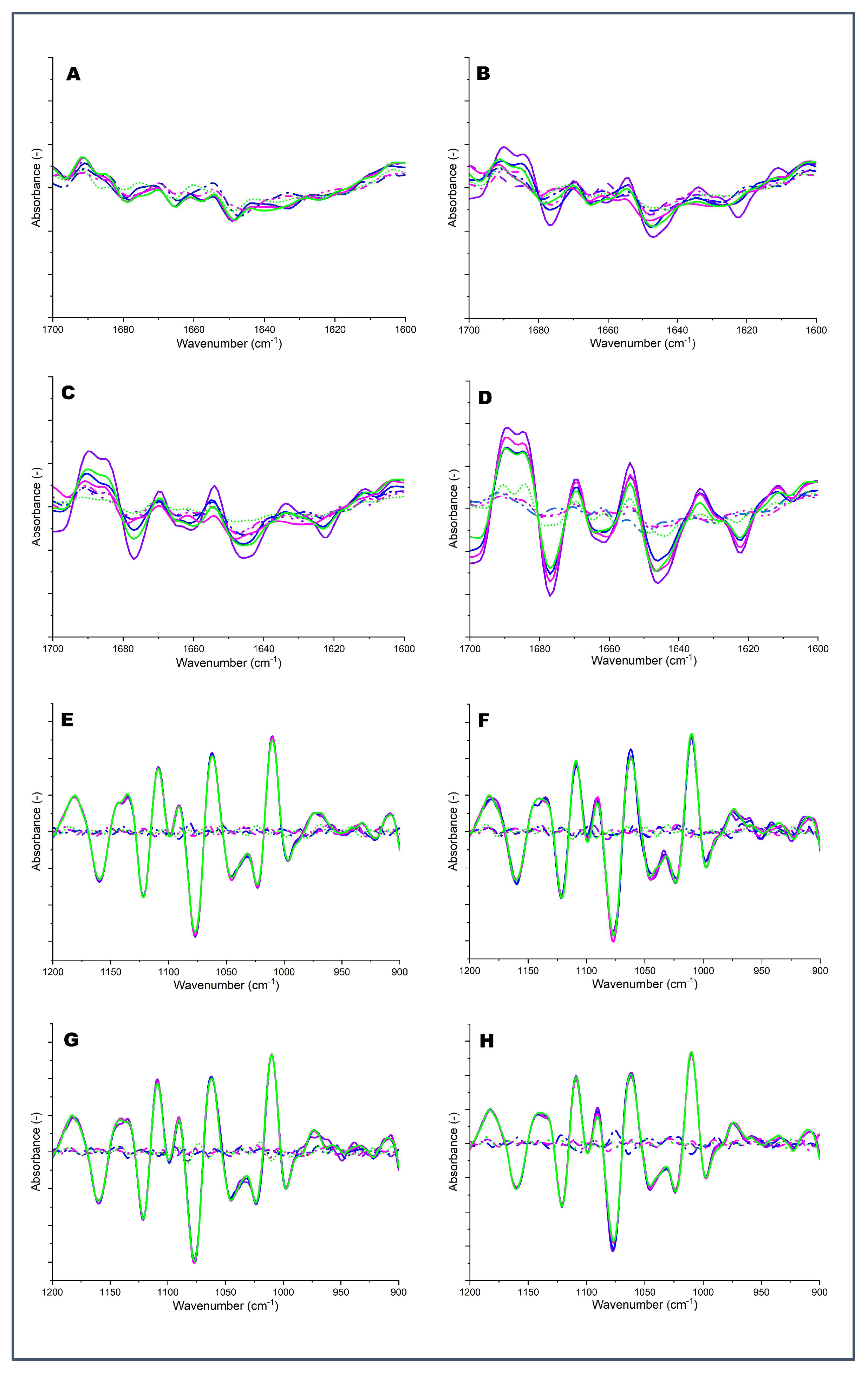
 Unheated 1;
Unheated 1;  Heated 1;
Heated 1;  Unheated 2;
Unheated 2;  Heated 2;
Heated 2;  Unheated 3;
Unheated 3;  Heated 3;
Heated 3;  Unheated 4;
Unheated 4;  Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
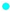 Unheated 1;
Unheated 1;  Heated 1;
Heated 1;  Unheated 2;
Unheated 2;  Heated 2;
Heated 2;  Unheated 3;
Unheated 3;  Heated 3;
Heated 3;  Unheated 4;
Unheated 4;  Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.
Heated 4. Numbers 1 to 4 represent 1: pH 6.3, 2: pH 6.6, 3: pH 6.9, and 4: pH 7.2.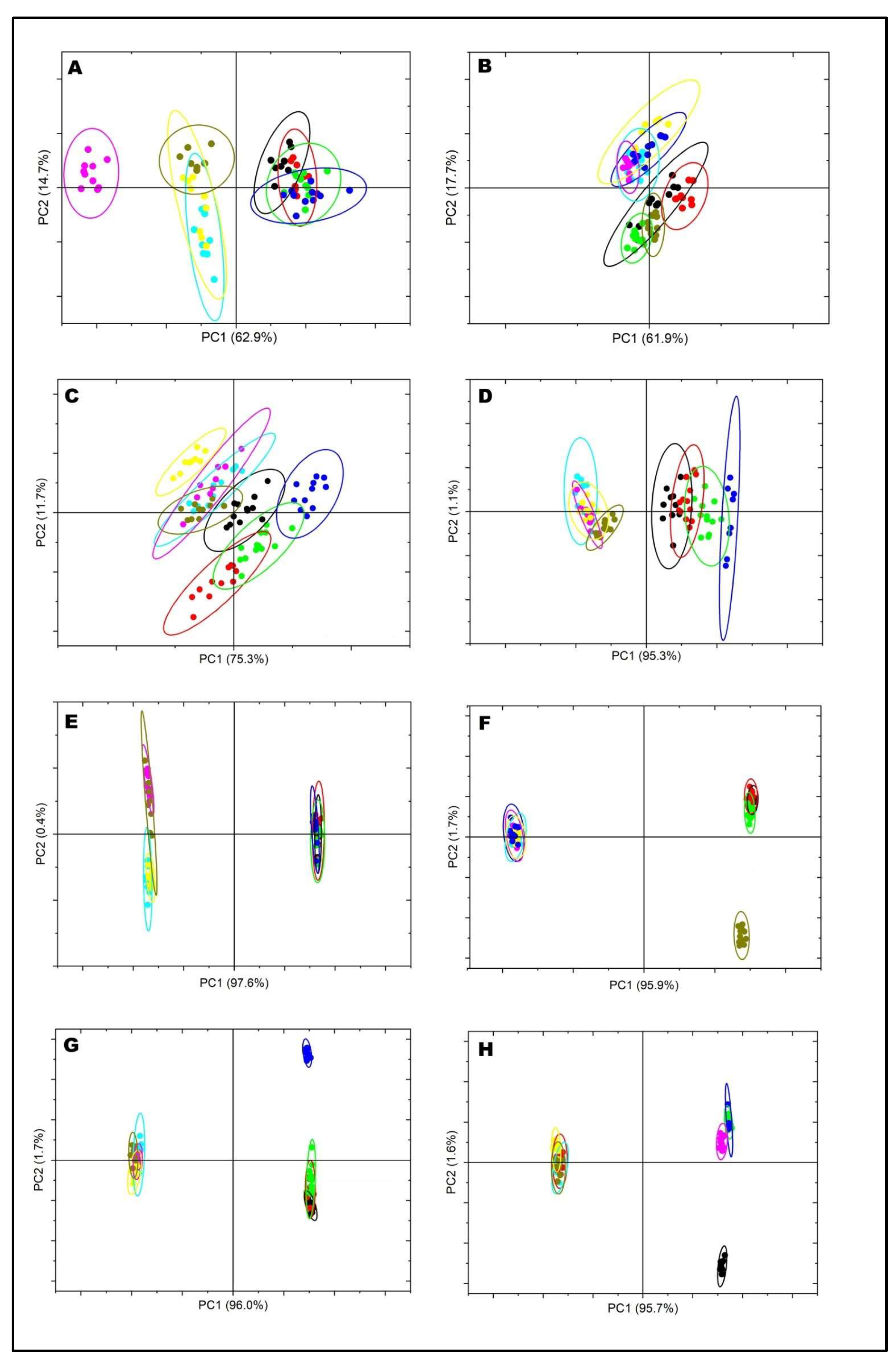
| MCP-Adjusted Samples | pH | Total Ca (mmoL L−1) | Particle Size Unheated (nm) | Particle Size Heated (nm) | Turbidity Unheated (cm−1) | Turbidity Heated (cm−1) |
|---|---|---|---|---|---|---|
| MCP67 | 6.3 | 21.76 ± 0.05 E | 160 ± 1 Ab | 207 ± 1 Da | 0.29 ± 0.01 Ab | 0.53 ± 0.03 Ca |
| 6.6 | 21.76 ± 0.11 E | 161 ± 2 Aa | 157 ± 1 Ea | 0.34 ± 0.01 Aa | 0.36 ± 0.02 Da | |
| 6.9 | 22.01 ± 0.05 E | 162 ± 1 Aa | 152 ±1 Eb | 0.27 ± 0.02 Ab | 0.32 ± 0.01 Da | |
| 7.2 | 21.81 ± 0.20 E | 160 ± 1 Aa | 150± 3 Eb | 0.26 ± 0.03 Aa | 0.29 ± 0.02 Da | |
| MCP96 | 6.3 | 25.70 ± 0.05 D | 161 ± 1 Ab | 296 ± 5 Ca | 0.29 ± 0.00 Ab | 0.90 ± 0.07 Ba |
| 6.6 | 26.10 ± 0.00 CD | 161 ± 0 Aa | 156 ± 1 Ea | 0.32 ± 0.02 Aa | 0.35 ± 0.01 Db | |
| 6.9 | 25.90 ± 0.13 D | 161 ± 1 Aa | 154 ± 1 Eb | 0.31 ± 0.01 Aa | 0.32 ± 0.02 Db | |
| 7.2 | 24.53 ± 0.07 D | 162 ± 1 Aa | 149 ± 2 Eb | 0.26 ± 0.03 Aa | 0.33 ± 0.01 Db | |
| MCP100(Control) | 6.3 | 27.85 ± 0.08 C | 162 ± 0 Ab | 325 ± 6 Ba | 0.36 ± 0.03 Ab | 0.94 ± 0.02 Ba |
| 6.6 | 27.46 ± 0.05 C | 163 ± 1 Aa | 151 ± 1 Eb | 0.35 ± 0.04 Aa | 0.37 ± 0.02 Da | |
| 6.9 | 27.65 ± 0.05 C | 165 ± 1 Aa | 145 ± 1 Eb | 0.32 ± 0.02 Aa | 0.34 ± 0.01 Da | |
| 7.2 | 26.83 ± 0.55 C | 164± 1 Aa | 142 ± 2 Eb | 0.32 ± 0.02 Aa | 0.31 ± 0.00 Da | |
| MCP113 | 6.3 | 31.25 ± 0.18 A | 163 ± 1 Ab | 484 ± 3 Aa | 0.34 ± 0.02 Ab | 1.60 ± 0.04 Aa |
| 6.6 | 32.31 ± 0.12 A | 165 ± 1 Aa | 160 ± 1 Ea | 0.35 ± 0.00 Ab | 0.42 ± 0.01 Da | |
| 6.9 | 30.46 ± 0.02 B | 165 ± 1 Aa | 147 ± 1 Eb | 0.34 ± 0.01 Aa | 0.38 ± 0.03 Da | |
| 7.2 | 32.76 ± 0.12 A | 165 ± 1 Aa | 143 ± 1 Eb | 0.34 ± 0.02 Aa | 0.33 ± 0.03 Da |
| MCP-Adjusted Samples | pH | Milk Protein Concentration (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αs1-Casein | αs2-Casein | β-Casein | κ-Casein | α-Lactalbumin | β-Lactoglobulin | ||||||||
| Unheated | Heated | Unheated | Heated | Unheated | Heated | Unheated | Heated | Unheated | Heated | Unheated | Heated | ||
| MCP67 | 6.3 | 14.2 ± 0.1 Ba | 5.7 ± 0.2 Ab | 27.5 ± 0.3 Aa | 13.6 ± 0.5 Ab | 36.9 ± 0.1 Aa | 20.8 ± 0.2 Ab | 21.4 ± 0.1 Ab | 26.9 ± 0.1 Ga | 84.0 ± 0.3 Ba | 20.2 ± 0.02 Gb | 69.4 ± 0.4 Da | 44.6 ± 0.2 Bb |
| 6.6 | 14.0 ± 0.1 Bb | 5.8 ± 0.4 Ab | 20.3 ± 0.1 Ba | 11.1 ± 0.1 Bb | 36.3 ± 0.0 Aa | 18.6 ± 0.1 Bb | 15.1 ± 0.0 Cb | 24.9 ± 0.1 Ha | 79.1 ± 0.9 CDa | 24.2 ± 1.0 Fb | 70.0 ± 0.2 Da | 54.2 ± 0.3 Ab | |
| 6.9 | 14.5 ± 0.1 Ba | 5.8 ± 0.1 Ab | 16.1 ± 0.0 Ca | 1.3 ± 0.0 Db | 36.5 ± 0.1 Aa | 10.7 ± 0.1 Fb | 16.5 ± 0.1 Bb | 44.4 ± 0.1 Aa | 82.8 ± 0.2 Ba | 79.2 ± 0.4 Ab | 72.0 ± 0.2 Ca | 56.1 ± 0.8 Ab | |
| 7.2 | 16.1 ± 0.0 Aa | 5.8 ± 0.2 Ab | 13.1 ± 0.1 Da | 2.0 ± 0.1 Db | 36.3 ± 0.1 Aa | 11.6 ± 0.1 Fb | 15.8 ± 0.0 BCb | 43.7 ± 0.1 Ba | 80.4 ± 0.2 Ca | 60.4 ± 0.1 Eb | 70.8 ± 0.3 Da | 56.3 ± 0.1 Ab | |
| MCP96 | 6.3 | 5.2 ± 0.1 Ca | 3.8 ± 0.9 Bb | 16.1 ± 0.0 Ca | 8.6 ± 0.1 Cb | 17.4 ± 0.0 BCa | 16.8 ± 0.3 Cb | 7.6 ± 0.1 Db | 23.1 ± 0.1 Ja | 81.8 ± 0.1 Ca | 9.5 ± 0.1 Hb | 76.4 ± 0.1 BCa | 32.7 ± 0.3 Eb |
| 6.6 | 5.9 ± 0.1 Ca | 3.7 ± 0.3 Bb | 16.3 ± 0.1 Ca | 9.2 ± 0.1 Cb | 18.5 ± 0.1 Ba | 16.8 ± 0.1 Cb | 7.3 ± 0.1 Db | 23.2 ± 0.0 Ja | 81.7 ± 0.1 Ca | 24.2 ± 0.2 Fb | 77.8 ± 0.1 Ba | 32.4 ± 0.1 Eb | |
| 6.9 | 5.1 ± 0.1 Ca | 2.2 ± 0.1 Cb | 17.0 ± 0.2 Ca | 1.3 ± 0.0 Db | 19.0 ± 0.1 Ba | 7.3 ± 0.1 Hb | 5.9 ± 0.0 DEb | 39.6 ± 0.1 Da | 83.7 ± 1.6 Ba | 70.1 ± 0.3 Bb | 69.2 ± 0.2 Da | 44.8 ± 0.4 Bb | |
| 7.2 | 5.5 ± 0.4 Ca | 4.2 ± 0.1 Bb | 6.7 ± 0.1 Ea | 1.9 ± 0.1 Db | 17.6 ± 0.2 BCa | 9.4 ± 0.1 Gb | 5.6 ± 0.1 DEb | 37.1 ± 0.1 Ea | 81.5 ± 0.1 Ca | 63.5 ± 0.5 Db | 67.8 ± 0.1 Ja | 31.5 ± 0.2 Eb | |
| MCP100(Control) | 6.3 | 3.8 ± 0.1 Da | 3.7 ± 0.1 Ba | 11.1 ± 0.1 DEa | 8.3 ± 0.1 Cb | 18.0 ± 0.0 BCa | 12.8 ± 0.3 Eb | 4.2 ± 0.0 Eb | 19.7 ± 0.1 Ka | 83.0 ± 0.4 Ba | 10.5 ± 0.1 Hb | 76.3 ± 0.0 BCa | 39.8 ± 0.1 Cb |
| 6.6 | 3.6 ± 0.1 Db | 4.0 ± 0.5 Ba | 12.9 ± 0.0 Da | 8.9 ± 0.0 Cb | 17.9 ± 0.1 BCa | 13.2 ± 0.1 Eb | 6.7 ± 0.1 Db | 19.4 ± 0.1 Ka | 87.6 ± 0.2 Aa | 23.1 ± 0.1 Fb | 72.2 ± 0.0 Ca | 37.0 ± 0.2 Db | |
| 6.9 | 3.5 ± 0.0 Da | 2.4 ± 0.3 Cb | 15.6 ± 0.1 Ca | 1.2 ± 0.0 Db | 19.8 ± 0.0 Ba | 7.5 ± 0.0 Hb | 6.3 ± 0.1 Db | 35.8 ± 0.0 Fa | 86.5 ± 0.1 Aa | 67.0 ± 1.4 Cb | 73.8 ± 0.1 Ca | 43.9 ± 0.2 Bb | |
| 7.2 | 3.9 ± 0.1 Da | 3.7 ± 0.1 Bb | 8.3 ± 0.0 Ea | 1.5 ± 0.0 Db | 12.1 ± 0.0 CDa | 8.5 ± 0.1 GHb | 5.3 ± 0.0 DEb | 35.8 ± 0.1 Fa | 79.9 ± 1.3 Ca | 63.4 ± 0.1 Db | 72.5 ± 0.3 Ca | 32.7 ± 0.1 Eb | |
| MCP113 | 6.3 | 3.0 ± 0.1 Db | 4.1 ± 0.1 Ba | 5.5 ± 0.2 EFb | 7.5 ± 0.1 Ca | 13.3 ± 0.1 Cb | 15.1 ± 0.1 Da | 4.2 ± 0.1 Eb | 23.9 ± 0.1 Ia | 88.8 ± 0.2 Aa | 10.2 ± 0.0 Hb | 83.9 ± 0.2 Aa | 37.1 ± 0.1 Db |
| 6.6 | 3.4 ± 0.1 Db | 4.0 ± 0.1 Ba | 7.6 ± 0.1 Ea | 8.1 ± 0.1 Ca | 14.8 ± 0.1 Ca | 13.4 ± 0.1 Eb | 5.7 ± 0.1 DEb | 23.8 ± 0.1 Ia | 87.1 ± 0.9 Aa | 19.9 ± 0.2 Gb | 77.2 ± 0.2 BCa | 22.6 ± 0.1 Fb | |
| 6.9 | 3.6 ± 0.3 Da | 1.3 ± 0.1 Cb | 6.7 ± 0.1 Ea | 0.6 ± 0.0 DEb | 10.2 ± 0.1 Da | 6.3 ± 0.0 HIb | 3.5 ± 0.0 Eb | 40.8 ± 0.1 Ca | 88.7 ± 0.3 Aa | 63.6 ± 0.2 Db | 76.1 ± 0.1 BCa | 38.8 ± 0.0 Cb | |
| 7.2 | 3.0 ± 0.1 Da | 1.4 ± 0.1 Cb | 6.7 ± 0.0 Ea | 0.0 ± 0.0 Eb | 10.3 ± 0.1 Da | 7.3 ± 0.0 Hb | 3.8 ± 0.1 Eb | 37.0 ± 0.1 Ea | 86.1 ± 0.3 Aa | 60.3 ± 0.3 Eb | 78.8 ± 0.0 Ba | 24.6 ± 0.1 Fb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, E.; Vasiljevic, T.; Huppertz, T. Influence of pH on Heat-Induced Changes in Skim Milk Containing Various Levels of Micellar Calcium Phosphate. Molecules 2023, 28, 6847. https://doi.org/10.3390/molecules28196847
Ahmadi E, Vasiljevic T, Huppertz T. Influence of pH on Heat-Induced Changes in Skim Milk Containing Various Levels of Micellar Calcium Phosphate. Molecules. 2023; 28(19):6847. https://doi.org/10.3390/molecules28196847
Chicago/Turabian StyleAhmadi, Elaheh, Todor Vasiljevic, and Thom Huppertz. 2023. "Influence of pH on Heat-Induced Changes in Skim Milk Containing Various Levels of Micellar Calcium Phosphate" Molecules 28, no. 19: 6847. https://doi.org/10.3390/molecules28196847
APA StyleAhmadi, E., Vasiljevic, T., & Huppertz, T. (2023). Influence of pH on Heat-Induced Changes in Skim Milk Containing Various Levels of Micellar Calcium Phosphate. Molecules, 28(19), 6847. https://doi.org/10.3390/molecules28196847






