Abstract
The bioconversion of agri-food waste into high-value products is gaining growing interest worldwide. Orange peel waste (OPW) is the main by-product of orange juice production and contains high levels of moisture and carbohydrates. In this study, the orange waste extract (OWE) obtained through acid hydrolysis of OPW was used as a substrate in the cultivation of the marine microalgae Nannochloropsis oculata. Photoheterotrophic (PH) and Photoautotrophic (PA) cultivations were performed in OWE medium and f/2 medium (obtained by supplementing OWE with macro- and micronutrients of f/2 medium), respectively, for 14 days. The biomass yields in PA and PH cultures were 390 mg L−1 and 450 mg L−1, while oil yields were 15% and 28%, respectively. The fatty acid (FA) profiles of PA cultures were mostly represented by saturated (43%) and monounsaturated (46%) FAs, whereas polyunsaturated FAs accounted for about 10% of the FAs. In PH cultures, FA profiles changed remarkably, with a strong increase in monounsaturated FAs (77.49%) and reduced levels of saturated (19.79%) and polyunsaturated (2.72%) FAs. Lipids obtained from PH cultures were simultaneously extracted and converted into glycerol-free biodiesel using an innovative microwave-assisted one-pot tandem protocol. FA methyl esters were then analyzed, and the absence of glycerol was confirmed. The FA profile was highly suitable for biodiesel production and the microwave-assisted one-pot tandem protocol was more effective than traditional extraction techniques. In conclusion, N. oculata used OWE photoheterotrophically, resulting in increased biomass and oil yield. Additionally, a more efficient procedure for simultaneous oil extraction and conversion into glycerol-free biodiesel is proposed.
1. Introduction
The bioconversion of agri-food waste into high-value products is gaining interest [] as such wastes have increased as the global population and economy have grown [,]. The incorrect disposal of food waste has several negative impacts on the environment, including bad smells, gas emissions, and groundwater contamination []. Due to this, efforts have been made in recent years to explore the conversion of food waste into value-added products and to develop downstream processes of converting feedstocks into end-products [,].
Among the various agri-food products, oranges represent one of the top five fruit commodities that dominate the worldwide market []. In addition, orange juice is one of the most consumed beverages, and large amounts of this fruit are used in the manufacture of juice and marmalade []. Wastes produced during orange juice production include seeds, peels, and membrane residues [], with a global estimated amount in the range of 15–25 million tons per year []. Among these wastes, orange peel waste (OPW) is the major constituent, accounting for approximately 44% of the fruit mass []. The exact chemical composition of oranges is affected by various factors []; however, the OPW usually contains 42.5% pectin, 16.9% soluble sugars, 10.5% hemicellulose, and 9.21% cellulose as the most relevant components []. The OPW is divided into organic and conventional. The first one is intended for producing feeds for organic livestock, while conventional pulp is exploited for biomethane production. Italian citrus production is estimated to be approximately 2.9 million tons per year, of which 12.6% is produced in Sicily, of which 55% is the corresponding overage waste (FaoStat, 2016). Herein, we have evaluated an alternative exploitation of conventional waste for biodiesel production, which could be considered complementary to anaerobic digestion in biomethane production. Traditional OPW recycling procedures such as landfilling, composting, pectin extraction, and animal feeding have many drawbacks and relatively high costs []. The disposal of OPW by bioconversion may be difficult because of the presence of several antimicrobial compounds (e.g., essential oils) and the high chemical complexity of the macromolecular content []. Thus, alternative biotechnological solutions are required for OPW recycling [].
Several species of microalgae have been exploited as innovative sources in a variety of biotechnological applications to produce food, additive feed ingredients, cosmetics, biopharmaceuticals, nutraceuticals, pigments, and biofuels []. From a long-term sustainable energy perspective, microalgae are considered the most suitable raw material for third-generation biofuel production [] since their lipid fraction can be extracted and converted into biodiesel []. In comparison to oleaginous plants, which are used as a traditional source of oil for biodiesel production, microalgae have several advantages such as high growth rate, high lipid content, ability to survive in adverse conditions, no competition for exploitation of agricultural land and food production, and the ability to sequester CO2 with higher efficiency [].
Microalgae belonging to the genus Nannochloropsis are considered promising candidates for biofuel production, as they can potentially accumulate lipids up to 28.7% of their dry weight, or 65–70% of their dry weight under nutrient deprivation, resulting in lipid productivity ranging from 25.8 to 60.9 mg L−1 d−1 []. Such productivity can be compared with other important known commercial microalgal species [] showing different growth rates and lipid content [], for example, Spirulina maxima (8.6 mg lipid L−1 d−1), Chlorella vulgaris (9.2 mg lipid L−1 d−1), and Dunaliella tertiolecta (20.0 mg lipid L−1 d−1) []. The unusually flexible photosynthetic apparatus and the genome sequence of Nannochloropsis species make them suitable for various purposes []. Interestingly, a genetic transformation method utilizing homologous recombination in Nannochloropsis has been performed, suggesting potential perspectives in biofuels research []. Nannochloropsis oculata is characterized by high productivity and high fatty acid and triglyceride content, representing an interesting bio-source for nutraceutical and biodiesel feedstock [,]. The ability of this marine microalgae to grow in ponds of saline, brackish, and hypersaline water suggests the possibility of conducting studies to explore further potential applications of this species []. Besides photosynthetic mechanisms, several microalgae can use organic carbon sources such as sugars, organic acids, alcohols, and phenolics for growth []. For these reasons, microalgae have been investigated for their ability to dispose of and recycle various carbon-rich agro-industrial wastes.
Current biodiesel production from microalgae is not economically viable due to the costs of biomass production, lipid extraction, and transesterification into fatty acid methyl esters (FAMEs) and purification of FAMEs from crude glycerol, which is a by-product of the transesterification process []. Regarding biodiesel production, most of the current industrial transesterification processes use alkaline chemical catalysts, which are inexpensive and result in high levels of conversion in short reaction times, even though they also produce glycerol as a by-product (about 10% of the produced biodiesel) that must be removed, with a consequential economic impact on the entire process []. Although it has been reported that crude glycerol can be a suitable substrate for microbial production of green chemicals [] such as biosurfactants [] and bioplastics [,], its bioconversion still has a considerable cost in terms of the energy required. In addition, even though glycerol is considered a key molecule in the preparation of many valuable organic compounds and several applications of glycerol and its derivatives are already well-known in the pharmaceutical, cosmetic, food, and beverage industries [], the potential growth in biodiesel demand can threaten the downgrading of glycerol to industrial waste. The reintegration of glycerol into the biodiesel production chain as a fuel additive is a desirable solution that could make the whole process much more competitive and sustainable []. Among the various derivatives, glycerol ethers have the optimal chemical–physical properties for this purpose and are already effectively used as fuel additives []. Among all lipid extraction techniques, microwave-assisted extraction (MAE) holds promise for its efficiency, high oil yield with superior quality, and reduced extraction times. Microwave heating uses a non-contact heat source that can penetrate biomass, interact with polar molecules such as water, and evenly heat the entire sample []. While non-polar organic solvents such as hexane are transparent to microwave radiation, they are suitable for selectively extracting the lipidic fraction. This solvent transparency improves the efficiency of microwave interaction within cells and the destruction of the membrane and consequently improves lipid extraction. In our previous study [], we reported a microwave-assisted one-pot transformation of vegetable oils into a mixture of FAMEs (biodiesel) and glycerol ether derivatives (biodiesel additives). The reaction requires the commercially available methyl tert-butyl ether (MTBE), which is catalyzed by a heterogeneous acid catalyst. In this process, MTBE is a single reagent suitable for both transesterification and transetherification (Figure 1).
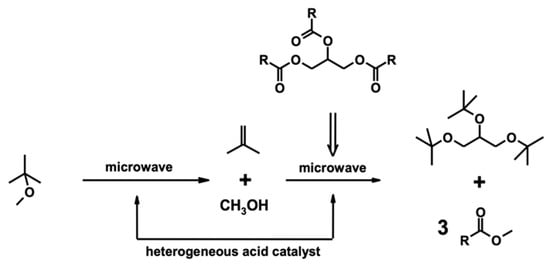
Figure 1.
Decomposition of methyl-tert-butyl ether, transesterification of triglycerides, and glycerol etherification.
The present study evaluated the potential recycling of the acid hydrolysis extract of OPW, called Orange Waste Extract (OWE), as a low-cost carbon source for growth and oil production in the marine unicellular microalgae Nannochloropsis oculata. OWEs were prepared by adding different aliquots of OPW (20, 30, 50, and 100 g) to 100 mL of 3% H2SO4 in natural seawater (OWE 20, 30, 50, and 100, respectively) and then supplementing with standard macro- and micronutrients (OWE 20, 30, 50, and 100 media). The microalgae were able to use OWE media photoheterotrophically, in particular OWE50 medium, with increased biomass and oil yields. Moreover, lipids were extracted and converted into biodiesel using an innovative microwave-assisted one-pot tandem protocol that combines lipid extraction from lyophilized cells and subsequent transformation into glycerol-free biodiesel. The residue is still rich in biomolecules that can be exploited in other processes. Furthermore, the final exhausted waste could be used for energy production (such as pyrolysis). To the best of our knowledge, this is the first report in which (i) the use of OPW as an organic carbon substrate for growth and oil production by microalgae and (ii) a unique procedure for microalgal oil extraction and simultaneous conversion to glycerol-free biodiesel are described.
2. Results
2.1. OWE50 Yield after OPW Treatment and Quantitative Determination of Carbohydrates Using HPAE-PAD
After the acidic treatment of fresh OPW, the OWE50 yield was about 50% (w/w) with respect to the acidic extraction mixture (i.e., 1 kg of fresh OPW mixed with 2 L of 3% H2SO4 in natural seawater). To obtain 1 L of OWE50, about 0.625 kg of OPW was subjected to acid hydrolysis. The main carbohydrates detected in the OWE50 extract are listed in Table 1. The results show that the total sugar content was 21.35 g L−1, of which 68.3% was glucose, the most commonly used organic carbon source in microalgae cultivation. Fructose was present at 18.5%, followed by arabinose (10.4%), and rhamnose (2.8%).

Table 1.
Carbohydrate content (g L−1) of OWE50.
2.2. Quantitative Determination of Ions in OWE50 Using Ion Chromatography
The main ions detected in OWE50 are listed in Table 2. Chloride (11,286.90 ± 0.73 mg L−1) and sodium (11,088.60 ± 2.34 mg L−1) were the most represented chemical species. The order of cations concentration was Na+ > Ca2+ > K+ > Mg2+, while that for anions was Cl− > SO42− > PO43− > NO3− > Br−. Comparing these values with the ionic content of the natural seawater used in OWE50, we observed an increase in potassium, calcium, and sulfate and the addition of nitrate and phosphate (useful for algae growth) attributable to the use of fresh OPW.

Table 2.
Ion content (mg L−1) of OWE50 and the natural seawater used in its preparation.
2.3. Growth Kinetics
The results of the growth of N. oculata in OWE20, 30, 50, and 100 culture media, obtained after acidic hydrolysis of different amounts of OPW (20, 30, 50, and 100 g; see Section 4 for details) are presented in Figure 2. Growth curves observed during photoautotrophic (PA) and photoheterotrophic (PH) batch processes are presented in Figure 3, and the values of growth kinetics variables, that is, specific growth rates (μ), biomass productivity (P), and biomass final yields, expressed as dry cell weight (DCW), are summarized in Table 3.
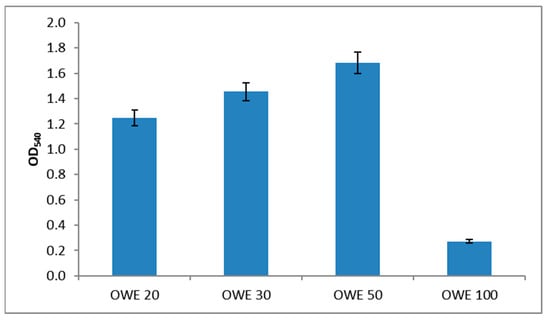
Figure 2.
Growth of N. oculata in different OWE media after 14 days of incubation. The graph shows data from triplicate experiments (mean ± SD).
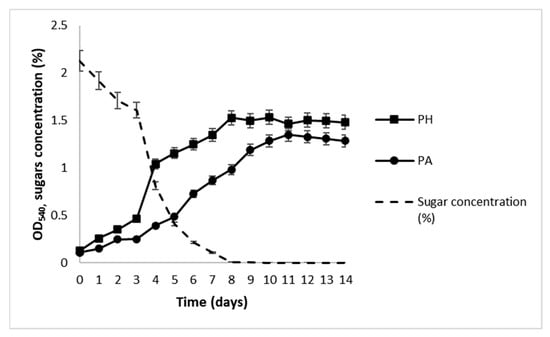
Figure 3.
Growth of N. oculata under PA in f/2 medium or PH in OWE50 medium and total sugar concentration (dotted line). The graph shows data from triplicate experiments (mean ± SD).

Table 3.
Values of specific growth rate (μ), biomass productivity (P), and biomass yield (expressed as DCW) in N. oculata cultivated under PA and PH batch processes.
A comparison of the culture media containing different amounts of OWE showed that the OWE50 medium was the most suitable for PH cultivation; thus, this medium was used for all subsequent experiments.
During PA batch cultivation, cultures showed a lag phase in the first two days. Growth started and continued progressively until the 14th day, and then stopped (Figure 3). The specific growth rate (μ) was 0.210 d−1, while biomass productivity and yield were 27.85 mg L−1 day−1 and 390 mg L−1, respectively (Table 3). During PH batch cultivation, growth started after three days and reached the highest value after 8 days, then entered the stationary phase. In parallel, a drastic decrease in the concentration of free sugars was observed between the 3rd and 5th days and reached 0 after 8 days (Figure 3). For PH batch cultivation, the specific growth rate (μ) was 0.308 d−1, while biomass productivity and yield were 32.14 mg L−1 day−1 and 450 mg L−1, respectively (Table 3).
2.4. Lipid Yield and Fatty Acid Composition
Lipids from all cultures were extracted from 1 g of each dried sample, and the total lipid yield was expressed as a percentage of DCW. Fatty Acids (FAs) characterized using gas chromatography (GC) are reported in Table 4.

Table 4.
Fatty acid composition of the microalgal lipids from PA and PH batch processes.
The lipid yield was 15% of DCW in PA cultures. Saturated FAs (SFAs, 42.90%) were mainly composed of palmitic acid (36.60%). Monounsaturated FAs (MUFAs) were present at a slightly higher percentage than SFAs (46.79%) and were mainly composed of palmitoleic (33.70%) and oleic (13.10%) acids. Polyunsaturated FAs (PUFAs) accounted for 10.31% of the FAs and were mainly represented by eicosapentaenoic acid. The lipid yield was 28% of DCW in PH cultures. SFAs represented 19.79% of the FAs, with palmitic acid (13.20%) being the most abundant. MUFAs were the predominant FA fraction (77.49%) and were principally composed of oleic (61.40%) and 10-octadecenoic (14.15%) acids. PUFAs accounted for 2.72% of the FAs and were exclusively represented by 9,11-octadecadienoic acid.
2.5. Conversion of Oil to Biodiesel Using Microwave-Assisted One-Pot Tandem Protocol and Analysis
For lipid extraction from N. oculata, different techniques for microalgal oil extraction were compared [,] using different solvents, reaction times, and temperatures of exposure (Table 5). Standard Soxhlet extraction gave the highest oil yield, followed by sonication-assisted extraction (SAE), with hexane as a solvent. SAE using MTBE/MeOH gave the worst performance. Interestingly, MAE efficiency was not influenced by the solvent used. We observed that oil yield using MTBE/MeOH (ratio of 9:1 v/v) was comparable to that obtained using hexane (Table 5). This result was particularly important as it allowed us to evaluate the possibility of directly converting microalgal oil into biodiesel using the microwave-assisted one-pot tandem protocol without any intermediate solvent exchange.

Table 5.
Oil yields obtained from N. oculata using different lipid extraction techniques.
The microalgal oil obtained using MAE with MTBE/MeOH was poured into a microwave vessel, Amberlyst®-15 (Rohm and Haas Company, PA, USA) dry form was added as a catalyst, and the mixture was then irradiated (pre-set at 20 W, maximum temperature limit was set at 130 °C). After approximately three hours, we observed a complete transformation of triglycerides to the corresponding FAMEs and a mixture of tert-butyl glycerol derivatives; as expected, no free glycerol was detected (Figure 4).
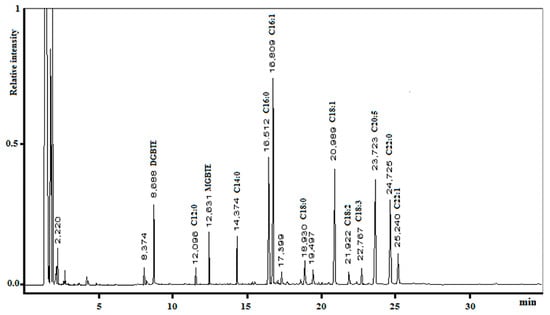
Figure 4.
Gas chromatography profiles of FAMEs and glycerol ethers produced using MAE with MTBE/MeOH.
3. Discussion
Microalgae products are used in the food and pharmaceutical industries as important sources of biomolecules such as PUFAs, proteins, vitamins, pigments, and so forth []. Microalgae also have applications in biofuel production as they are considered an alternative feedstock for third-generation biofuels [,]. The achievement of higher biomass and oil yields and growth using organic carbon sources has been described in several studies. Simple organic molecules such as acetate [], glycerol, glucose [], and ethanol [], as well as more complex carbon sources such as aqueous extracts of sugarcane bagasse, or vegetable ground biomass such as corn stover, sugarcane bagasse, or switchgrass [], are all suitable substrates for growing several Nannochloropsis species.
Agroindustry wastes are currently being explored as potential organic substrates for the cultivation of various species of microalgae. The recycling of these by-products represents an important strategy for reducing environmental pollution and obtaining valuable products, according to the unwritten economical “value from waste” rule [], improving the economic viability of microalgae-based industrial processes. This vision is the basis of the circular economy, defined as “a model of production and consumption, which involves sharing, leasing, reusing, repairing, refurbishing and recycling existing materials and products as long as possible” []. As a consequence, the biorefinery concept becomes a central model for driving the industry towards an eco-sustainable economy. A biorefinery is defined as “a facility (or network of facilities) that integrates biomass conversion processes and equipment to produce transportation biofuels, power, and chemicals from biomass” []. Biorefineries can use different kinds of feedstocks, including oil- and sugar-bearing crops (first-generation feedstocks), straw, bakery waste, and rotten fruits (second-generation feedstocks), or aquatic biomass such as microalgae (third-generation feedstocks).
In this study, we aimed to verify whether OPW could be used as a source of simple sugars for microalgal growth and oil production. The results demonstrate that OWE50 represents a low-cost carbon source for PH cultivation of N. oculata and can be bio-converted to microalgal biomass and lipids. It is possible to set up an eco-friendly, integrated process for OPW exploitation as a low-cost carbon source or microalgae cultivation and oil production. Data indicate that the OWE50 medium contains a remarkable concentration of free sugars, mainly glucose, and ions, mainly sodium and chloride, with OPW contributing nitrate and phosphate necessary for microalgal growth. The OWE50 medium facilitated N. oculata growth and oil production, with yields and growth parameters, i.e., specific growth rate, biomass yield, and productivity, higher than those obtained during the PA batch process. The FAs profiles of lipids produced by the same microalgal species under different culture conditions may vary significantly []. Similar results were obtained for N. oculata using the adopted nutritional regimens. The amounts of SFAs and MUFAs were almost similar (42.90% and 46.79%, respectively) in PA cultures, while eicosapentaenoic acid (EPA) was the most represented PUFA, accounting for 7.02% of the FAs. On the contrary, in PH cultures, MUFAs increased up to 77.49%, with a strong reduction in SFAs (19.79%), while EPA was not detected. Moreover, although the increase in biomass and lipid yields from PH cultures are mild, it is worth noting that the relative percentages of MUFAs, SFAs, and PUFAs produced by N. oculata in OWE50 medium are excellent for biodiesel production, as they fully satisfy UNE-EN 14214 [,]. The proportions of SFAs, MUFAs, and PUFAs influence several critical parameters of biodiesel, including cetane number, iodine values, and cold flow properties. In particular, the high content of MUFAs, the low content of SFAs, and, overall PUFAs have been reported as a very good compromise for good quality biodiesel []. Furthermore, the MUFAs produced by PH cultures of N. oculata are represented by oleic and 10-octadecenoic acids, both containing 18 carbon atoms, which positively influence viscosity, cetane number, and heat of combustion [].
Among the several extraction techniques evaluated (Table 5), MAE using a blend of MTBE/MeOH (ratio 9:1 v/v) was found to be a suitable combination for obtaining triglycerides from N. oculata biomass in terms of quality, time, and yield (almost 28%). The main advantage of this extraction technique includes reprocessing the mixture obtained directly in the microwave reactor for the transformation of triglycerides without any intermediate solvent exchange. Therefore, a complete transformation of the triglycerides to the corresponding FAMEs and a mixture of tert-butyl glycerol derivatives without any residual free glycerol is achieved. However, further pilot studies and variable optimization are necessary to evaluate the economic feasibility of the whole process. A similar research study was conducted by Park et al. [] using orange peel extracts as an inorganic and organic nutrient source for the mixotrophic cultivation of the microalgae Chlorella vulgaris. Park et al. [] reported increased production of biomass and FAMEs, suggesting that orange peel extract has the potential for use in the mixotrophic cultivation of microalgae for biodiesel production. Furthermore, several studies on species belonging to the genus Nannochloropsis have been conducted in recent years using different technological approaches [,,,,,,].
4. Materials and Methods
4.1. Seawater Collection Site and Orange Peel Waste Treatment
The natural seawater used for microalgae cultivation was sampled from the east coast of Sicily (37°34′30″ N 15°10′29″ E); its ionic composition is reported in Table 2. Fresh OPW was obtained using Oranfresh® S.r.l. (Catania, Italy). Before further processing, the OPW was ground in a food processor and transformed into small particles (<2 mm), and then subjected to heat-assisted acid hydrolysis. Briefly, different aliquots of OPW (20, 30, 50, and 100 g) were mixed with 100 mL of 3% H2SO4 in natural seawater and thermally treated in an autoclave at 121 °C for 30 min to hydrolyze polysaccharides and pectins. The solid fractions were separated via centrifugation at 5000× g for 20 min at 4 °C. The pH was then neutralized with 1 M sodium hydroxide, and the supernatants—orange waste extract (OWE) 20, OWE30, OWE50, and OWE100—were filtered at 0.22 µm to obtain a sterile and clear liquid. Finally, adequate aliquots of nutrient stock solutions used in the f/2 medium were added to all the OWEs to achieve the final millimolar concentrations present in the standard f/2 medium (nutrient-repleted OWEs) used to verify microalgae growth. The nutrient-repleted OWE50 (called OWE medium) showed the best result in terms of biomass yield (Figure 2) and was used in all the batch cultivations.
4.2. Quantitative Determination of Carbohydrates and Ions in OPW Hydrolysate
Quantification of the main carbohydrates in OWE50 was performed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD, Thermo Scientific Dionex ICS3000, Sunnyvale, CA, USA). The sample, diluted to 1:2000 with deionized water and then filtered using a 0.20 µm nylon filter, was analyzed using a chromatography system equipped with a quaternary gradient inert pump, a pulsed amperometric detector, and an AS40 automated sampler. The separation was carried out using a Dionex CarboPac PA10 analytical column (250 × 4 mm i.d.) and a CarboPac PA10 guard column (50 × 4 mm i.d.). The acquisition of chromatograms was performed using the Chromeleon chromatography management system. All experiments were carried out at 30 °C under isocratic elution using 100 mM NaOH, at a flow rate of 0.8 mL min−1. Analyses were performed in triplicate; analyte quantifications were conducted using external standards (calibration curve range 0.5–10.0 mg L−1 for glucose and fructose, 0.2–2.0 mg L−1 for arabinose and rhamnose; R2 ≥ 0.9978) and results are reported in g L−1. The percentage of relative standard deviations of peak retention times was <0.8%.
Quantification of the main ions present in OWE50 was determined using ion chromatography (Thermo Scientific, Dionex ICS3000, Sunnyvale, CA, USA) with suppressed conductivity detection. Anions were separated using anion exchange column (Dionex IonPac AS22 (250 × 4 mm i.d.) with IonPac AG22 guard column (50 × 4 mm i.d.)) and cations using cation exchange column (Dionex IonPac CS12A (250 × 4 mm i.d.) with IonPac CG12A guard column (50 × 4 mm i.d.)). An aqueous solution containing 20 mM methanesulfonic acid was used to elute cations. The mobile phase containing 4.5 mM sodium carbonate and 1.4 mM sodium bicarbonate was used to elute anions. Flow rates of 1.0 mL min−1 and 1.2 mL min−1 were used to separate cations and anions, respectively. The column temperature was maintained at 30 °C during analysis. The sample was appropriately diluted (1:1000 and 1:500) with deionized water and filtered with a nylon syringe filter (0.45 µm). Analyses were performed in triplicate; analyte quantifications were conducted using external standards (range 0.5–100 mg L−1; R2 > 0.999) and results are reported in mg L−1 (Table 1). The percentage of relative standard deviations of peak retention times ranged from 0.7% to 2.1%.
4.3. Microalgae Stock Cultures, Batch Cultivations, and Photobioreactor Management
Nannochloropsis oculata (K-1281) was obtained from the Scandinavian Culture Collection of Algae and Protozoa. The strain was grown in f/2-Si medium [,]. Stock cultures were maintained at room temperature (20 °C ± 2) under continuous shaking and illuminated by white LED light at 40 µmol photons m−2 s−1 under a 12 h:12 h light:dark cycle. Batch cultivations were performed in an ePBR cylindrical bench photobioreactor (Phenometrics, Lansing, MI, USA) with a 450 mL working volume. The photobioreactor was operated according to the manufacturer’s recommendations. PA or PH batch cultivations were conducted in f/2 or OWE media, respectively, for 14 days. For each process, an inoculum of 10% v/v of stock cultures was used. Cultures were irradiated using white LED light (100 µmol photons m−2 s−1) under a 16 h:8 h light:dark cycle, aerated with filtered air (0.22 μm filter) bubbled into the cultures and continuously stirred (130 rpm) under a temperature of 20 °C (± 2). The daily growth of all the cultures was measured spectrophotometrically as optical density at 540 nm (OD540). Moreover, in PH batch cultivations, the concentrations of free sugars were evaluated daily using the methods reported by Dubois et al. []. The results are expressed as the means ± standard deviations (SDs) of the values obtained from three replicates and graphically plotted as growth curves in a Microsoft Excel sheet. The specific growth rate (μ) was calculated as follows:
where N1 and N2 are biomass (OD540) at time 1 (t1) and time 2 (t2), respectively, and t1 and t2 are the days on which growth started and stopped, respectively []. Biomass productivity (P), expressed in mg L−1 d−1, was calculated according to the following equation:
where Ni and N0 are the biomass values (DCW, mg L−1) at time ti and t0, respectively [].
4.4. Lipid Extraction and Yield
At the end of each cultivation process, the biomass was harvested via centrifugation at 6000× g for 10 min at 4 °C, resuspended in a minimal volume of sterile MilliQ water, kept at −80 °C for 15 h, and then lyophilized using an Alpha1-2LD Plus freeze drier (Martin Christ GmBH, Osterode, Germany). Lipids were extracted from the lyophilized biomass (1.0 g) with the techniques thereinafter described to compare their efficiencies.
4.5. Soxhlet, Sonication-Assisted, and Microwave-Assisted Extractions
For the Soxhlet extraction, 1.0 g of lyophilized cells contained in a cellulose thimble was put inside the Soxhlet chamber. Then, 300 mL of pure n-hexane was used to extract the lipids for 6 h at a rate of 10 refluxes h−1 and a temperature of 90 °C. N-hexane was then eliminated by rotavapor and the total lipid extract was weighted.
Sonication-assisted extraction (SAE) of microalgal lipids was performed on lyophilized biomass (1.0 g) using hexane (20 mL) or methyl tert-butyl ether (MTBE) (20 mL) under cold sonication (35 Hz; 20 °C) condition through two sequential steps of 20 min each. The residual solid material from the first extraction step was removed by centrifugation and then re-extracted. The resulting organic solutions were combined and the solvent removed under reduced pressure.
For the microwave-assisted extraction (MAE), an aliquot (500 mg) of lyophilized biomass was dissolved in a mixture of MTBE (4.5 mL) and methanol (MeOH, 0.5 mL) and poured into a microwave tube. The resulting mixture was irradiated in a microwave reactor, pre-set at 20 watts (maximum temperature limit was set at 90 °C) for 20 min. After completion, the residual solid material was removed by centrifugation and the solvent was then evaporated under reduced pressure to obtain the crude oil. Lipid yields obtained using the above-mentioned extraction techniques were calculated as the ratio between the amounts of extracted lipid and the biomass yield, measured as DCW, and expressed as percentages.
4.6. Lipid Transesterification Methods and Procedures
For lipid transesterification, microalgal lipid extracts underwent two different transesterification protocols. The first (standard procedure) resulted in glycerol as a by-product, while the second (microwave-assisted procedure) did not. For the standard lipid transesterification procedure, quantitative lipid transesterification was carried out according to the following standard procedure: a 0.5 N methanolic sodium methoxide solution (8 mL) was slowly added to a stirred solution of oil (0.2 g) in toluene (4 mL). The resulting mixture was heated at 50 °C and left under stirring for almost 10 min before glacial acetic acid (0.4 mL) and water (20 mL) were added. The resulting mixture was poured into a separatory funnel, diluted with hexane (10 mL), and then extracted; the organic layer was collected while the aqueous phase was back-extracted with hexane (3 × 10 mL). The collected organic phase was dried on anhydrous sodium sulfate, filtered, and the solvent removed under reduced pressure. The final crude product, which is a mixture of FAMEs, was taken up using a known volume of hexane, filtered through a 0.45 μm PTFE membrane filter, and then subjected to GC/MS-FID analysis. For the microwave-assisted lipid transesterification procedure, a sample of microalgal lipid extract (250 mg) was dissolved in MTBE (1.6 mL, d 0.744 mg mL−1; 24.5 mmol) in a microwave tube and Amberlyst®-15 dry form catalyst (30 mg) added to the resulting solution. The reaction mixture was irradiated in a microwave reactor pre-set at 20 watts (the maximum temperature limit was set at 130 °C) until complete conversion of the substrate (3 h). The transformation of triglycerides into corresponding FAMEs (reaction progress) was monitored via HPLC analysis; once completed, the GC analysis of the final mixture showed the FAME composition and the presence of both di- and mono-tert-butyl glycerol ethers.
4.7. HPLC Analysis and FA Profiles Determined Using GC Analysis
HPLC analysis was conducted using a Varian 9010 instrument (Varian Inc., Palo Alto, CA, USA) equipped with an Alltech 3300 evaporative light scattering detector (ELSD) (BÜCHI Labortechnik, AG, Flawil, CH). A Luna C18 column (250 × 4.6 mm, 5 µm particle size) from Phenomenex (Torrance, CA, USA) was used for separating triglycerides and FAMEs. HPLC conditions were as follows: eluent A, MeOH; eluent B, CH2Cl2; gradient: 0–3 min (A-B/80:20), 3–18 min (A-B/30:70), 18–23 min (A-B/30:70); flow rate: 1 mL min−1. ELSD was set to a probe temperature of 40 °C and a gain of 16, and the nebulizer nitrogen gas was adjusted to 1.5 L min−1.
GC analyses were conducted using Shimadzu GC-17A (Shimadzu Corporation, Kyoto, Japan) equipped with a fused silica capillary column from J&W Scientific (INNOWAX, 30 m, 0.25 mm, 0.25 µm) (Agilent Technologies, Santa Clara, CA, USA); nitrogen was used as the carrier gas (flow rate of 1 mL min−1). GC conditions were as follows: the injector and detector temperatures were set at 250 and 280 °C, respectively; the aliquot of the reaction mixture was injected and analyzed using the following temp. prog.: 60 °C for 2 min, 60–200 °C at 10 °C min−1, 200–250 °C at 5 °C min−1, 250 °C for 5 min. Identification of different FAMEs and, eventually, the presence of tert-butyl glycerol ethers, was achieved by referring to the chromatograms of standard compounds.
4.8. Microwave-Assisted One-Pot Tandem Protocol for Lipid Extraction–Transesterification Process
A sample of lyophilized microalgal biomass (1 g) was dissolved in a mixture of MTBE (4.5 mL) and MeOH (0.5 mL) and poured into a microwave tube. The resulting mixture was irradiated in a microwave reactor pre-set at 20 watts (the maximum temperature limit was set at 90 °C) for 20 min. After completion, the residual solid material was removed via centrifugation, the resulting solution was poured into a microwave tube, and Amberlyst®-15 dry form catalyst (30 mg) added. The reaction mixture was irradiated in a microwave reactor pre-set at 20 watts (the maximum temperature limit was set at 130 °C) until complete conversion of the substrate as monitored using HPLC analysis (3 h). GC analysis of the final mixture showed the FAME composition and the presence of both di- and mono-tert-butyl glycerol ethers.
4.9. Statistical Analysis
Data from the different experimental groups were compared using one-way analysis of variance and the Tukey-b test for post hoc analysis. All statistical values were considered significant at a p-level of 0.05. Statistical analyses were performed using Instat version 2.10 for Microsoft Windows (GraphPad Software Inc., San Diego, CA, USA).
5. Conclusions
OPW is the most abundant waste generated during the production of orange juice. After heat-assisted acid hydrolysis, the extract obtained had a good content of free sugars (21.35 g L−1) and ions (15,694 mg L−1 of cations and 17,795 mg L−1 of anions), indicating that it is a suitable organic carbon source. In PH cultures, OWE50 medium supported microalgae growth and oil production. The experimental approach adopted in this study suggests that the use of OPW as a low-cost carbon source combined with the production of high-value-added compounds such as microalgal oils can be performed as a single integrated process. Interestingly, PH cultivation affected the fatty acid composition, acting on the global unsaturation degree of the mixture of the fatty acids. The oil obtained from photoautotrophic metabolism showed interesting levels of PUFAs, whereas that obtained from photoheterotrophic metabolism could be used for biodiesel production. Furthermore, we reported a versatile microwave-assisted one-pot tandem protocol for lipid extraction and transformation into glycerol-free biodiesel, using the same solvent media in both steps (28% yield). This result, while making the whole process very simple and practical to obtain biodiesel from microalgae, also offers the advantage of avoiding both biodiesel purification from crude glycerol and intermediate solvent exchange, which requires a distillation step, giving it a high added value in terms of cost, operation time, and sustainability. The absence of free glycerol in the final mixture makes the process advantageous as it allows the production of FAMEs without requiring steps to remove it, easy recovery and reuse of both the heterogeneous catalyst—through a simple filtration—and excess solvent—by simple distillation. Finally, the mixture obtained may be suitable for use directly in the energy chain as a biofuel. Further studies should be conducted to increase the yield of sugars extracted using standard and green technologies for fresh OPW treatment to set up economically viable, eco-friendly processes at a pre-industrial scale.
Author Contributions
G.T., M.S.N., C.D., C.G. (Claudia Genovese) and N.D. conceived and designed the experiments; G.T., M.S.N. and G.F. cultivated the microalgae; G.T., M.S.N., C.D. and C.G. (Claudia Genovese) managed the software; G.T., M.S.N., C.D. and C.G. (Claudia Genovese) performed the analysis; G.T., M.S.N., C.D., C.G. (Claudia Genovese), C.G. (Concetta Gugliandolo) and N.D. performed the data curation; G.T., M.S.N., C.D. and C.G. (Claudia Genovese) prepared and edited the paper; M.S.N. and N.D. co-supervised the project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was part of project BIO4BIO (Biomolecular and Energy valorization of residual biomass from Agroindustry and Fishing Industry), led by the Cluster Sicily Agrobio and Fishing Industry and funded by the Italian Research Fund (PON R&C 2007–2013, DD 713/Ric.—PON02 00451 3362376).
Institutional Review Board Statement
The authors declare that the strain Nannochloropsis oculata (K-1281) used in the present study was obtained from the Scandinavian Culture Collection of Algae and Protozoa. Neither wild species nor species at risk of extinction were used in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data for this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors gratefully acknowledge Tonia Strano and Antonio Greco for their assistance as expert technicians of the CNR–ICB, Luciano Falqui as scientific supervisor of the BIO4BIO project, and Nicola Brianti for providing the LED equipment used for microalgae stock culture maintenance. The authors dedicate this manuscript to the memory of Giovanni Nicolosi for his professional and human commitment.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds obtained in this study are available from the corresponding authors upon reasonable request.
References
- Kannah, R.Y.; Velu, C.; Rajesh Banu, J.; Heimann, K.; Karthikeyan, O.P. Food waste valorization by microalgae. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 319–342. [Google Scholar]
- Li, O.; Liang, J.; Chen, Y.; Tang, S.; Li, Z. Exploration of Converting Food Waste into Value-Added Products via Insect Pretreatment-Assisted Hydrothermal Catalysis. ACS Omega 2023, 8, 18760–18772. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef] [PubMed]
- Yang See, J.; Song, S.; Xiao, Y.; Trang Pham, T.; Zhao, Y.; Lapkin, A.; Yan, N. Transformation of Corn Lignin into Sun Cream Ingredients. Chem. Sus. Chem. 2021, 14, 1586–1594. [Google Scholar] [CrossRef]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of Biogas Production from Orange Peel Waste by Leaching of Limonene. BioMed Res. Int. 2015, 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martin, A. Biomethanization of orange peel waste. Biores. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Biores. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef]
- Dan, S.; Yang, S.X.; Tian, F.; Den, L. Classification of Orange Growing Locations Based on the Near-infrared Spectroscopy Using Data Mining. Intell. Autom. Soft Comput. 2015, 22, 229–236. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Negro, V.; Ruggeri, B.; Fino, D.; Tonini, D. Life cycle assessment of orange peel waste management. Resour. Conserv. Recy. 2017, 127, 148–158. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; Gonçalves de Oliveira, R.J.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Gugliandolo, C.; Rizzo, M.G.; Zammuto, V.; Cicero, N.; Dugo, G.; Guglielmino, S.P.P. Nutritional conditions of the novel freshwater Coccomyxa AP01 for versatile fatty acids composition. J. Appl. Microbiol. 2022, 132, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.C.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Biores. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Tamburic, B.; Guruprasad, S.; Radford, D.T.; Szabó, M.; Lilley, R.M.; Larkum, A.W.; Franklin, J.B.; Kramer, D.M.; Blackburn, S.I.; Raven, J.A.; et al. The effect of Diel temperature and light cycles on the growth of Nannochloropsis oculata in a photobioreactor matrix. PLoS ONE 2014, 9, e86047. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biot. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Kratky, L.; Sulc, R.; Jirout, T.; Aguedo, M.; Richel, A.; Goffin, D. Effect of Organic Solvents on Microalgae Growth, Metabolism and Industrial Bioproduct Extraction: A review. Int. J. Mol. Sci. 2017, 18, 1429. [Google Scholar] [CrossRef]
- Rahmat, A.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energ. Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Drago, C.; Liotta, L.F.; La Parola, V.; Testa, M.; Nicolosi, G. One-pot microwave assisted catalytic transformation of vegetable oil into glycerol-free biodiesel. Fuel 2013, 113, 707–711. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Franco, D.; Camarda, V.; Gullace, R.; Rizzo, M.G.; Fragalà, M.; Licciardello, G.; Catara, A.F.; Guglielmino, S.P.P. Integrated microbial process for bioconversion of crude glycerol from biodiesel into biosurfactants and PHAs. Chem. Eng. Trans. 2014, 38, 187–192. [Google Scholar]
- Nicolò, M.S.; Maria, G.; Cambria, M.G.; Impallomeni, G.; Rizzo, M.G.; Pellicorio, C.; Ballistreri, A.; Guglielmino, S.P.P. Carbon source effects on the mono/dirhamnolipid ratio produced by Pseudomonas aeruginosa L05, a new human respiratory isolate. New Biotechnol. 2017, 39, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Chines, V.; Franco, D.; Nicolò, M.S.; Guglielmino, S.P.P. The role of glutamine in Pseudomonas mediterranea in biotechnological processes. New Biotechnol. 2017, 37, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Nicolò, M.S.; Franco, D.; De Plano, L.M.; Chines, V.; Moscato, F.; Crea, G.; Gugliandolo, C.; Guglielmino, S.P.P. Glutamine-induced filamentous cells of Pseudomonas mediterranea CFBP-5447T as producers of PHAs. Appl. Microbiol. Biotechnol. 2019, 103, 9057–9066. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Sedghi, R.; Shahbeik, H.; Rastegari, H.; Rafiee, S.; Peng, W.; Nizami, A.S.; Gupta, V.K.; Chen, W.H.; Lam, S.S.; Pan, J.; et al. Turning biodiesel glycerol into oxygenated fuel additives and their effects on the behavior of internal combustion engines: A comprehensive systematic review. Renew. Sustain. Energy Rev. 2022, 167, 112805. [Google Scholar] [CrossRef]
- Pinto, B.P.; de Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of glycerol and ethanol as bioadditives for biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Kundu, A.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Synthesis of palm oil empty fruit bunch magnetic pyrolytic char impregnating with FeCl3 by microwave heating technique. Biomass. Bioenerg. 2014, 61, 265–275. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. A review on the extraction of lipid from microalgae for biodiesel production. Algal Res. 2015, 7, 117–123. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Matsakas, L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules 2018, 23, 1562. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Guglielmino, S.P.P.; Solinas, V.; Salis, A. Biodiesel from microalgae. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Lee, S.Y., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–20. [Google Scholar]
- Lin, W.; Li, P.; Liao, Z.; Luo, J. Detoxification of ammonium to Nannochloropsis oculata and enhancement of lipid production by mixotrophic growth with acetate. Biores. Technol. 2017, 227, 404–407. [Google Scholar] [CrossRef]
- Villanova, V.; Galasso, C.; Vitale, G.A.; Della Sala, G.; Engelbrektsson, J.; Strömberg, N.; Shaikh, K.M.; Andersson, M.X.; Palma Esposito, F.; Ekendahl, S.; et al. Mixotrophy in a local strain of Nannochloropsis granulata for renewable high-value biomass production on the West coast of Sweden. Mar. Drugs 2022, 20, 424. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wei, C.; Zhao-Ling, C.; Fan, O. Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Microchloropsis sp. J. Appl. Phycol. 2004, 16, 499–503. [Google Scholar] [CrossRef]
- Schambach, J.Y.; Finck, A.M.; Kitin, P.; Hunt, C.G.; Hanschen, E.R.; Vogler, B.; Starkenburg, S.R.; Barry, A.N. Growth, total lipid, and omega-3 fatty acid production by Nannochloropsis spp. cultivated with raw plant substrate. Algal Res. 2020, 51, 102041. [Google Scholar] [CrossRef]
- European Parliament News 2015. Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 17 June 2023).
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Abraham, C.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Biores. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Puhan, S.; Saravanan, N.; Nagarajan, G.; Vedaraman, N. Effect of biodiesel unsaturated fatty acid on combustion characteristics of a DI compression ignition engine. Biomass Bioenergy 2010, 34, 1079–1088. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Park, W.K.; Moon, M.; Kwak, M.S.; Jeon, S.; Choi, G.G.; Yang, J.W.; Lee, B. Use of orange peel extract for mixotrophic cultivation of Chlorella vulgaris: Increased production of biomass and FAMEs. Bioresour. Technol. 2014, 171, 343–349. [Google Scholar] [CrossRef]
- Castejón, N.; Marko, D. Fatty Acid Composition and Cytotoxic Activity of Lipid Extracts from Nannochloropsis gaditana Produced by Green Technologies. Molecules 2022, 27, 3710. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Muñoz, S.; Díaz, N.F.; Medina, A.; Bazaes, J.; Riquelme, C. Nutritional Enhancement of Farmed Salmon Meat via Non-GMO Nannochloropsis gaditana: Eicosapentaenoic Acid (EPA, 20:5 n-3), Docosapentaenoic Acid (DPA, 22:5 n-3) and Vitamin D3 for Human Health. Molecules 2020, 25, 4615. [Google Scholar] [CrossRef] [PubMed]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef]
- Zghaibi, N.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R.; Harun, R. Kinetics Study of Microwave-Assisted Brine Extraction of Lipid from the Microalgae Nannochloropsis sp. Molecules 2020, 25, 784. [Google Scholar] [CrossRef] [PubMed]
- Zghaibi, N.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R.; Harun, R. Microwave-Assisted Brine Extraction for Enhancement of the Quantity and Quality of Lipid Production from Microalgae Nannochloropsis sp. Molecules 2019, 24, 3581. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef]
- Elst, K.; Maesen, M.; Jacobs, G.; Bastiaens, L.; Voorspoels, S.; Servaes, K. Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition. Molecules 2018, 23, 1854. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve). Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- McKay, A.L.; Peters, A.C.; Wimpenny, J.W.T. Determining specific growth rates in different regions of Salmonella typhimurium colonies. Lett. Appl. Microbiol. 1997, 24, 74–76. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).