Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification and Isolation of Flavones
2.1.1. Identification of Flavones
2.1.2. Additional Constituents Present in Extracts of O. indicum
2.2. Quantitative Analysis of Flavone Contents
2.2.1. Method Validation
Linearity and Peak Purity
Precision
Accuracy
2.2.2. Quantitative Analysis of Flavone Contents
2.3. Quantitative Analysis of Multi-Components Using Single Marker (QAMS) Method for Simultaneous Determination of Flavonoids Contents
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Plant Extracts
3.3. Identification and Isolation of Flavones
3.3.1. Identification of Flavones via HPLC-MS
3.3.2. Isolation of Flavones in O. indicum Extract
3.3.2.1. Isolation and Identification of Compound OI1
3.3.2.2. Isolation and Identification of Compound OI2
3.4. Quantitative Analysis of Flavone Contents
3.5. Quantitative Analysis of Multi-Components Using Single Marker (QAMS) Method for Simultaneous Determination of Flavonoids Contents
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warrier, P.K.; Nambiar, V.P.K.; Ramankutty, C. Indian Medicinal Plant: A Compendium of 500 Species (4); Orient Longman: Hyderabad, India, 1993; pp. 186–190. [Google Scholar]
- Sharma, J.; Gairola, S.; Gaur, R.D.; Painuli, R.M.; Siddiqi, T.O. Ethnomedicinal plants used for treating epilepsy by indigenous communities of Sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2013, 150, 353–370. [Google Scholar] [CrossRef] [PubMed]
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of Peoples Republic of China; China Chemical Industry Press: Beijing, China, 2010.
- Rasadah, M.A. Oroxylum indicum (L.) Kurz. In Plant Resources of South-East Asia No.12(2), Medicinal and Poisonous Plants 2; van Valkenberg, J.L.C.H., Bunyapraphatsara, N., Eds.; Backhuys Publisher: Leiden, The Netherlands, 2001. [Google Scholar]
- Harminder, S.V.; Chaudhary, A.K. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian J. Pharm. Sc. 2011, 73, 483–490. [Google Scholar]
- Drury, H. Ayurvedic Useful Plants of India; Asiatic Publishing House: New Delhi, India, 2006. [Google Scholar]
- Bhushan, B.; Kumar, M. Ethnobotanically important medicinal plants of Tehsil Billawar, District Kathua, J&K, India. J. Pharmacogn. Phytochem. 2013, 2, 14–21. [Google Scholar]
- Limbu, D.K.; Rai, B.K. Ethno-medicinal practices among the Limbu community in Limbuwan, Eastern Nepal. Glob. J. Human Soc. Sci. Interdiscip. 2013, 13, 7–29. [Google Scholar]
- Jamir, N.S.; Limasemba, T. Traditional knowledge of Lotha-Naga tribes in Wokha district, Nagaland. Indian J. Tradit. Knowl. 2010, 9, 45–48. [Google Scholar]
- Libman, A.; Bouamanivong, S.; Southavong, B.; Sydara, K.; Soejarto, D.D. Medicinal plants: An important asset to healthcare in a region of central Laos. J. Ethnopharmacol. 2006, 106, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sithisarn, P.; Nantateerapong, P.; Rojsanga, P.; Sithisarn, P. Screening for antibacterial and antioxidant activities and phytochemical analysis of Oroxylum indicum fruit extracts. Molecules 2016, 21, 466. [Google Scholar] [CrossRef]
- Rojsanga, P.; Bunsupa, S.; Brantner, A.H.; Sithisarn, P. Comparative phytochemical profiling and in vitro antioxidant activity of extracts from raw materials, tissue-cultured plants, and callus of Oroxylum indicum (L.) Vent. Evid. Based Complement. Alternat. Med. 2017, 2017, 6853212. [Google Scholar] [CrossRef]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Flavone-rich fractions and extracts from Oroxylum indicum and their antibacterial activities against clinically isolated zoonotic bacteria and free radical scavenging effects. Molecules 2021, 26, 1773. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Mohanta, B.C.; Arima, S.; Sato, N.; Harigaya, Y. Flavonoids from the stem bark of Oroxylum indicum. Nat. Prod. Sci. 2007, 13, 190–194. [Google Scholar]
- Hari, B.T.; Manjulatha, K.; SureshKumar, G.; Hymavathi, A.; Tiwari, A.K.; Purohit, M.; MadhusudanaRao, J.; SureshBabu, K. Gastroprotective flavonoid constituents from Oroxylum indicum Vent. Bioorganic Med. Chem. Lett. 2010, 20, 117–120. [Google Scholar] [CrossRef]
- Ali, R.M.; Houghton, P.J.; Raman, A.; Hoult, J.R.S. Antimicrobial and anti-inflammatory activities of extracts and constituents of Oroxylum indicum (L.) Vent. Phytomedicine 1998, 5, 375–381. [Google Scholar]
- Khandhar, M.; Shah, M.; Santani, D.; Jain, S. Anti-ulcer activity of the rootbark of Oroxylum indicum against experimental gastriculcers. Pharm. Biol. 2006, 44, 363–370. [Google Scholar] [CrossRef][Green Version]
- Roy, M.K.; Nakahara, K.; Trakoontivakorn, G.; Takenaka, M.; Isobe, S.; Tsushida, T. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie 2007, 62, 149–153. [Google Scholar]
- Yan, R.Y.; Cao, Y.Y.; Chen, C.Y.; Dai, H.Q.; Yu, S.X.; Wei, J.L.; Li, H.; Yang, B. Antioxidant flavonoids from the seed of Oroxylum indicum. Fitoterapia 2011, 82, 841–848. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Nair, A.G.R. Flavonoids of the leaves of Oroxylum indicum and Pajanelia longifolia. Phytochemistry 1972, 11, 439–440. [Google Scholar] [CrossRef]
- Stavrianidi, A.; Stekolshchikova, E.; Porotova, A.; Rodin, I.; Shpigun, O. Combination of HPLC–MS and QAMS as a new analytical approach for determination of saponins in ginseng containing products. J. Pharm. Biomed. Anal. 2017, 132, 87–92. [Google Scholar] [CrossRef]
- Yadav, A.K.; Manika, N.; Bagchi, G.D.; Gupta, M.M. Simultaneous determination of flavonoids in Oroxylum indicum by RP-HPLC. Med. Chem. Res. 2013, 22, 2222–2227. [Google Scholar] [CrossRef]
- Arimboor, R.; Salini, K.; Arumughan, C. HPTLC estimation of oroxylin A in Oroxylum indicum herbal formulations. JPC 2013, 26, 274–278. [Google Scholar]
- Luitel, H.N.; Bhandari, M.R.; Kalani, S.K.; Awale, S.; Masuda, K.; Gewali, M.B. Chemical constituents from Oroxylum indicum (L) Kurz of Nepalese origin. Sci. World 2010, 8, 66–68. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Hung, T.; Atanasov, A.G.; Heiss, E.K.; Dirsch, V.M.; Stuppner, H. Screening of Vietnamese medicinal plants for NF-κB signaling inhibitors: Assessing the activity of flavonoids from the stem bark of Oroxylum indicum. J. Ethnopharmacol. 2015, 159, 36–42. [Google Scholar] [CrossRef]
- Majeed, M.; Pande, A.; Nagabhushanam, K.; Majeed, S.; Pande, A. Comparative chemical fingerprinting of Oroxylum indicum and Scutellaria baicalensis using liquid chromatography and mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 732–740. [Google Scholar] [CrossRef]
- Jagetia, G.C. A Review on the medicinal and pharmacological properties of traditional ethnomedicinal plant sonapatha, Oroxylum indicum. Sinusitis 2021, 5, 71–89. [Google Scholar] [CrossRef]
- Jiwajinda, S.; Santisopasri, V.; Murakami, A.; Kim, O.K.; Kim, H.W.; Ohigashi, H. Suppressive effects of edible Thai plants on superoxide and nitric oxide generation. Asian Pac. J. Cancer Prev. 2002, 3, 215–223. [Google Scholar] [PubMed]
- Peng, Q.; Shang, X.; Zhu, C.; Qin, S.; Zhou, Y.; Liao, Q.; Zhang, R.; Zhao, Z.; Zhang, L. Qualitative and quantitative evaluation of Oroxylum indicum (L.) Kurz by HPLC and LC-qTOF-MS/MS. Biomed. Chromatogr. 2019, 33, e4657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yang, L.L.; Lee, T.J.F. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor B-activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kakkar, P. Oroxylin A, a constituent of Oroxylum indicum inhibits adipogenesisand induces apoptosis in 3T3-L1 cells. Phytomedicine 2014, 21, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Kang, S.; Park, S.J.; Huang, J.; Im, D.S. Anti-allergic effect of oroxylin A from Oroxylum indicum using in vivo and in vitro experiments. Biomol. Ther. 2016, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, Y.; Wang, Y.; Liang, P.; Zhang, L.; Liu, R. Oroxylin A is a severe acute respiratory syndrome coronavirus 2-spiked pseudotyped virus blocker obtained from Radix scutellariae using angiotensin-converting enzyme II/cell membrane chromatography. Phytother. Res. 2021, 10, 3194–3204. [Google Scholar] [CrossRef]
- Gokhale, M.; Gautam, D.; Khanna, A. A Comparative GC-MS analysis of bioactive compounds in the different fractions of root extract of Oroxylum indicum (L.) Vent. Anal. Chem. Lett. 2017, 7, 410–420. [Google Scholar] [CrossRef]
- Zappalà, M.; Fallico, B.; Arena, A.; Verzera, A. Methods for the determination of HMF in honey: A comparison. Food Control 2005, 16, 273–277. [Google Scholar] [CrossRef]
- Boffo, E.F.; Tavares, L.A.; Tobias, A.C.T.; Ferreira, M.M.C.; Ferreira, A.G. Identification of components of Brazilian honey by 1H NMR and classification of its botanical origin by chemometric methods. Food Sci. Technol. 2012, 49, 55–63. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential antagonistic effects of acrylamide mitigation during coffee roasting on furfuryl alcohol, furan and 5-hydroxymethylfurfural. Toxics 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Rockville, ML, USA, 2022. [Google Scholar]
- Cao, Y.Y.; Yan, R.; Yang, L.; Guo, J.; Lui, H.; Zhang, J.; Yang, B.; Huang, L. Quality dvaluation of Semen Oroxyli based on the cetermination of multiple components with a single reference standard. J. Chromatogr. Sci. 2013, 51, 477–484. [Google Scholar] [CrossRef]
- Sinha, S.; Nailwal, T.K. Quality assessment and evaluation of Oroxylum indicum through HPLC fingerprint and QAMS for important flavonoid components. Eurasian Chem. Commun. 2021, 3, 45–55. [Google Scholar]
- Smitinand, T.; Larsen, K.; Nielsen, I. Flora of Thailand: Aristolochiaceae, Bignoniaceae, Droseraceae, Epacridaceae, Gentianaceae, Opiliaceae, Philydraceae, Proteaceae, Salicaceae, Thismiaceae, Valerianaceae, Xyridaceae; Flora of Thailand, 5, Part 1, Forest Herbarium; Royal Forest Department: Bangkok, Thailand, 1987; p. 138.
- Hemantha, H.P.; Ramanujam, R.; Majeed, M.; Nagabhushanam, K. An unambiguous and practical synthesis of oroxylin A: A commonly misidentified flavone. Nat. Prod. Res. 2021, 35, 1413–1420. [Google Scholar] [CrossRef]
- Galkin, K.I.; Krivodaeva, E.A.; Ananikov, V.P. Catalytic system for the selective conversion of cellulose to 5-hydroxymethylfurfural under mild conditions. Russ. Chem. Bull. 2015, 64, 2954–2957. [Google Scholar] [CrossRef]

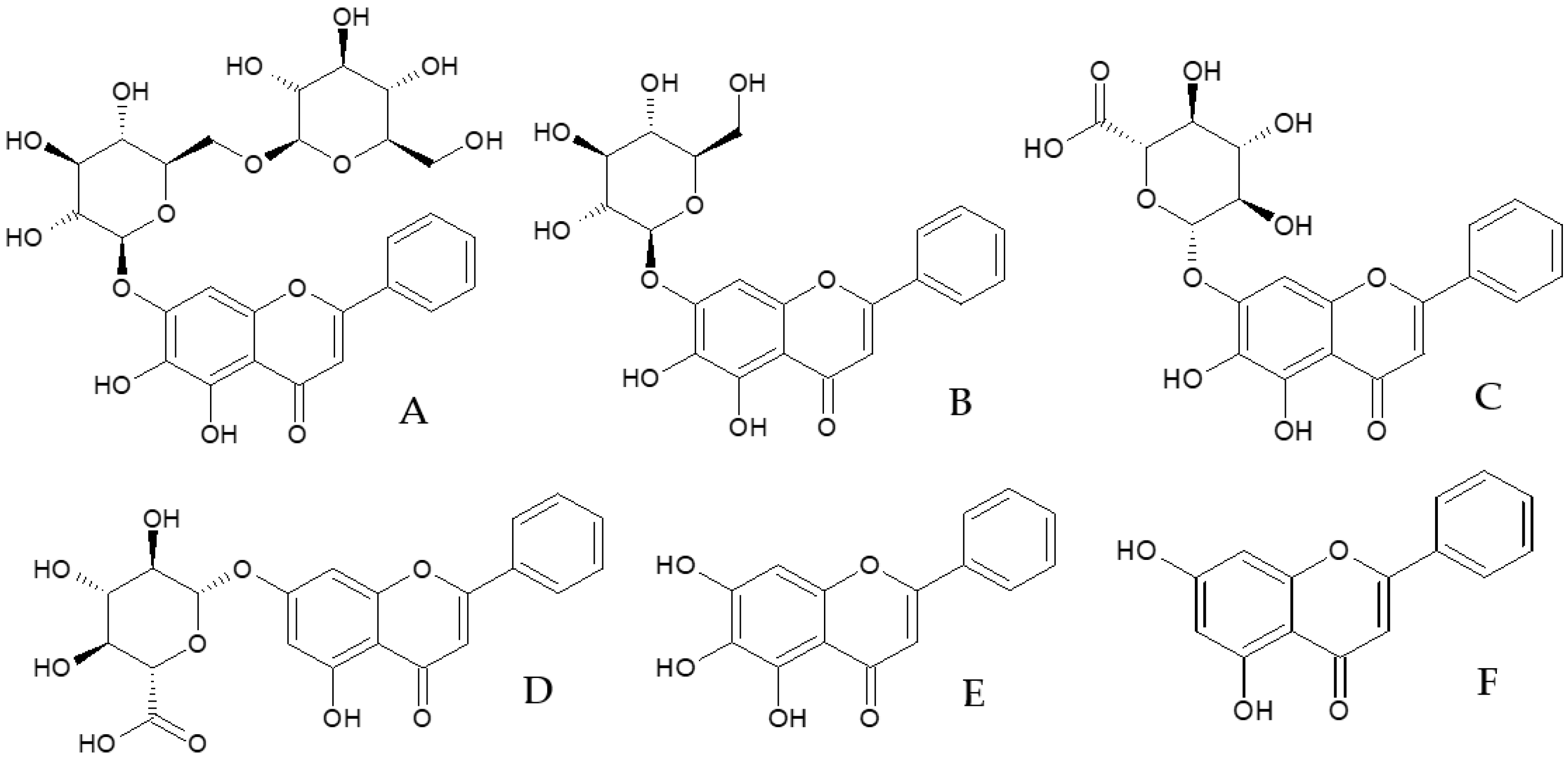
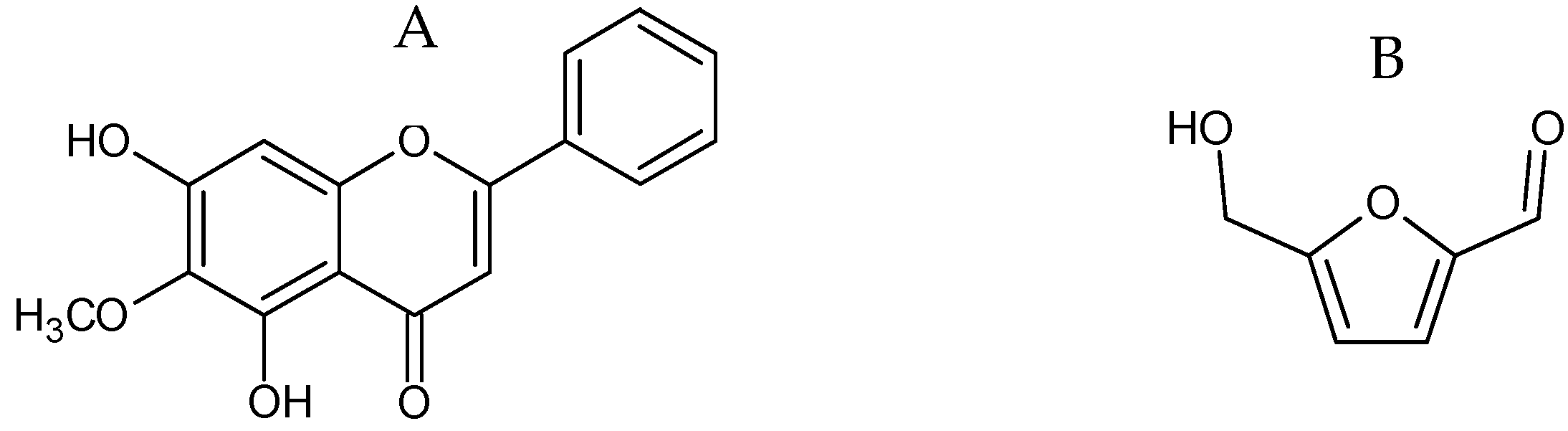
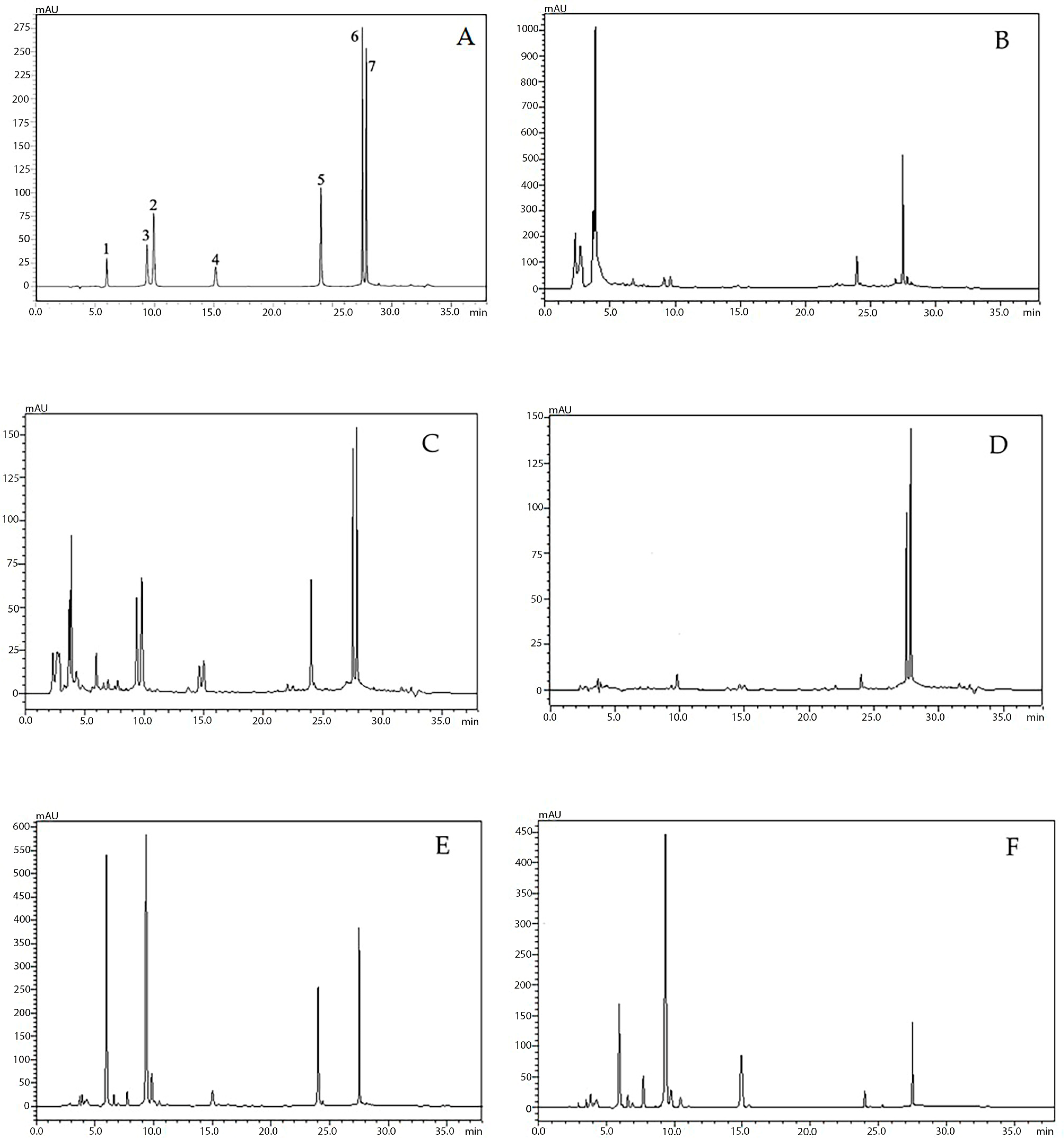
| Peak No. | Compound | RT (min.) | λmax (nm) | m/z [M+H]+/[M-H]− |
|---|---|---|---|---|
| 1 | Oroxin B (1) | 6.73 | 278, 316 | 595.2/593.0 |
| 2 | Oroxin A (3) | 8.81 | 278, 316 | 433.2/431.0 |
| 3 | Baicalin (2) | 10.91 | 276, 316 | 447.2/445.0 |
| 4 | Chrysin-7-O-glucuronide (4) | 14.67 | 268, 306 | 431.2/428.8 |
| 5 | Baicalein (5) | 17.78 | 272, 292sh, 318 | 271.2/268.8 |
| 6 | Chrysin (6) | 21.67 | 268, 314 | 255.0/252.8 |
| 7 | Oroxylin A (7) | 22.16 | 270, 316 | 285.2/283.0 |
| Compound | Range (µg/mL) | r | Added Concentration (µg/mL) | % Recovery | % RSD of Precision | |
|---|---|---|---|---|---|---|
| Repeatability | Intermediate Precision | |||||
| OB (1) | 0.65–500 | 0.9999 | 5.2–20 | 95–103 | 0.1–0.8 | 0.6 |
| BL (2) | 1–132 | 0.9978 | 13.2–52.8 | 96–101 | 0.1–0.2 | 0.3 |
| OA (3) | 0.7–500 | 0.9987 | 5.6–22.4 | 101–105 | 0.1–0.4 | 1.5 |
| C7 (4) | 0.7–56 | 0.9992 | 5.6–22.4 | 97–104 | 0.1–0.8 | 1.3 |
| BE (5) | 1–92 | 0.9994 | 9.2–36.8 | 97–101 | 0.2–0.9 | 0.8 |
| C (6) | 2–160 | 0.9979 | 16.0–24.0 | 101–104 | 0.2–0.3 | 0.7 |
| OX (7) | 2–160 | 0.9999 | 14.8–59.2 | 101–104 | 0.4–0.5 | 0.6 |
| Extract | Content (% w/w in the Extract) * | ||||||
|---|---|---|---|---|---|---|---|
| OB (1) | BL (2) | OA (3) | C7 (4) | BE (5) | C (6) | OXA (7) | |
| YFL | n.d. | 0.41 ± 0.00 a | 0.08 ± 0.00 b | n.d. | 0.53 ± 0.00 c | 0.96 ± 0.00 b | n.d. |
| GFC | 0.54 ± 0.00 a | 2.77 ± 0.00 c | 0.72 ± 0.00 e | 0.69 ± 0.00 b | 0.79 ± 0.00 d | 1.11 ± 0.00 d | 1.35 ± 0.00 b |
| DPC | n.d. | 1.44 ± 0.00 b | 0.06 ± 0.00 a | 0.04 ± 0.00 a | 0.09 ± 0.00 a | 0.66 ± 0.00 a | 1.20 ± 0.00 a |
| SC | 10.90 ± 0.07 c | 11.47 ± 0.03 e | 0.51 ± 0.01 d | 1.08 ± 0.01 c | 2.42 ± 0.02 e | 2.57 ± 0.02 e | n.d. |
| SL | 2.90 ± 0.01 b | 9.12 ± 0.01 d | 0.14 ± 0.00 c | 2.47 ± 0.00 d | 0.27 ± 0.00 b | 1.03 ± 0.00 c | n.d. |
| Other Compound | Reference Compound | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB (1) | BL (2) | OA (3) | C7 (4) | BE (5) | C (6) | OXA (7) | ||||||||
| RCF | SD | RCF | SD | RCF | SD | RCF | SD | RCF | SD | RCF | SD | RCF | SD | |
| OB (1) | - | 0.65 | 0.05 | 2.98 | 0.19 | 1.08 | 0.08 | 1.97 | 0.22 | 1.78 | 0.13 | 1.69 | 0.12 | |
| BL (2) | 1.54 | 0.11 | - | 4.56 | 0.31 | 1.65 | 0.13 | 3.01 | 0.35 | 2.73 | 0.21 | 2.6 | 0.2 | |
| OA (3) | 0.33 | 0.02 | 0.21 | 0.02 | - | 0.35 | 0.03 | 0.65 | 0.07 | 0.59 | 0.04 | 0.55 | 0.04 | |
| C7 (4) | 0.93 | 0.07 | 0.61 | 0.05 | 2.78 | 0.2 | - | 1.83 | 0.22 | 1.66 | 0.13 | 1.58 | 0.12 | |
| BE (5) | 0.52 | 0.06 | 0.34 | 0.04 | 1.53 | 0.18 | 0.55 | 0.07 | - | 0.92 | 0.11 | 0.87 | 0.11 | |
| C (6) | 0.56 | 0.04 | 0.37 | 0.03 | 1.67 | 0.11 | 0.61 | 0.05 | 1.1 | 0.13 | - | 0.96 | 0.07 | |
| OXA (7) | 0.59 | 0.04 | 0.39 | 0.03 | 1.77 | 0.11 | 0.64 | 0.05 | 1.16 | 0.13 | 1.06 | 0.08 | - | |
| Compound | Young Fruit | Green Fruit | Seed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Content, % | SMD, % | Content, % | SMD, % | Content, % | SMD, % | ||||
| EMS | QAMS | EMS | QAMS | EMS | QAMS | ||||
| Oroxin B (1) | n.d. | n.d. | - | 0.54 | 0.52 | 3.7 | 7.63 | 7.43 | 2.6 |
| Baicalin (2) | 0.41 | - a | - | 2.77 | - a | - | 9.01 | - a | - |
| Oroxin A (3) | 0.08 | 0.08 | 3.80 | 0.72 | 0.72 | 0.2 | 0.34 | 0.36 | 4.5 |
| Chrysin-7-O-glucuronide (4) | n.d. | n.d. | - | 0.68 | 0.67 | 1.7 | 0.74 | 0.73 | 2.5 |
| Baicalein (5) | 0.53 | 0.57 | 6.8 | 0.79 | 0.85 | 6.7 | 1.82 | 1.93 | 6.8 |
| Chrysin (6) | 0.92 | 0.96 | 5 | 1.11 | 1.18 | 6.1 | 1.91 | 1.90 | 0.4 |
| Oroxylin A (7) | n.d. | n.d. | - | 1.35 | 1.34 | 1.3 | n.d. | n.d. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojsanga, P.; Schwaiger, S.; Stuppner, H.; Sithisarn, P. Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods. Molecules 2023, 28, 6837. https://doi.org/10.3390/molecules28196837
Rojsanga P, Schwaiger S, Stuppner H, Sithisarn P. Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods. Molecules. 2023; 28(19):6837. https://doi.org/10.3390/molecules28196837
Chicago/Turabian StyleRojsanga, Piyanuch, Stefan Schwaiger, Hermann Stuppner, and Pongtip Sithisarn. 2023. "Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods" Molecules 28, no. 19: 6837. https://doi.org/10.3390/molecules28196837
APA StyleRojsanga, P., Schwaiger, S., Stuppner, H., & Sithisarn, P. (2023). Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods. Molecules, 28(19), 6837. https://doi.org/10.3390/molecules28196837







