Natural Products as Mite Control Agents in Animals: A Review
Abstract
:1. Introduction
2. Acaricidal Activity of Plant Extracts
2.1. Plant Extracts against D. gallinae
2.2. Plant Extracts against P. cuniculi and S. scabiei
| Extracts | Main Components | Mite | Acaricidal Dose | Mechanism of Action | Reference |
|---|---|---|---|---|---|
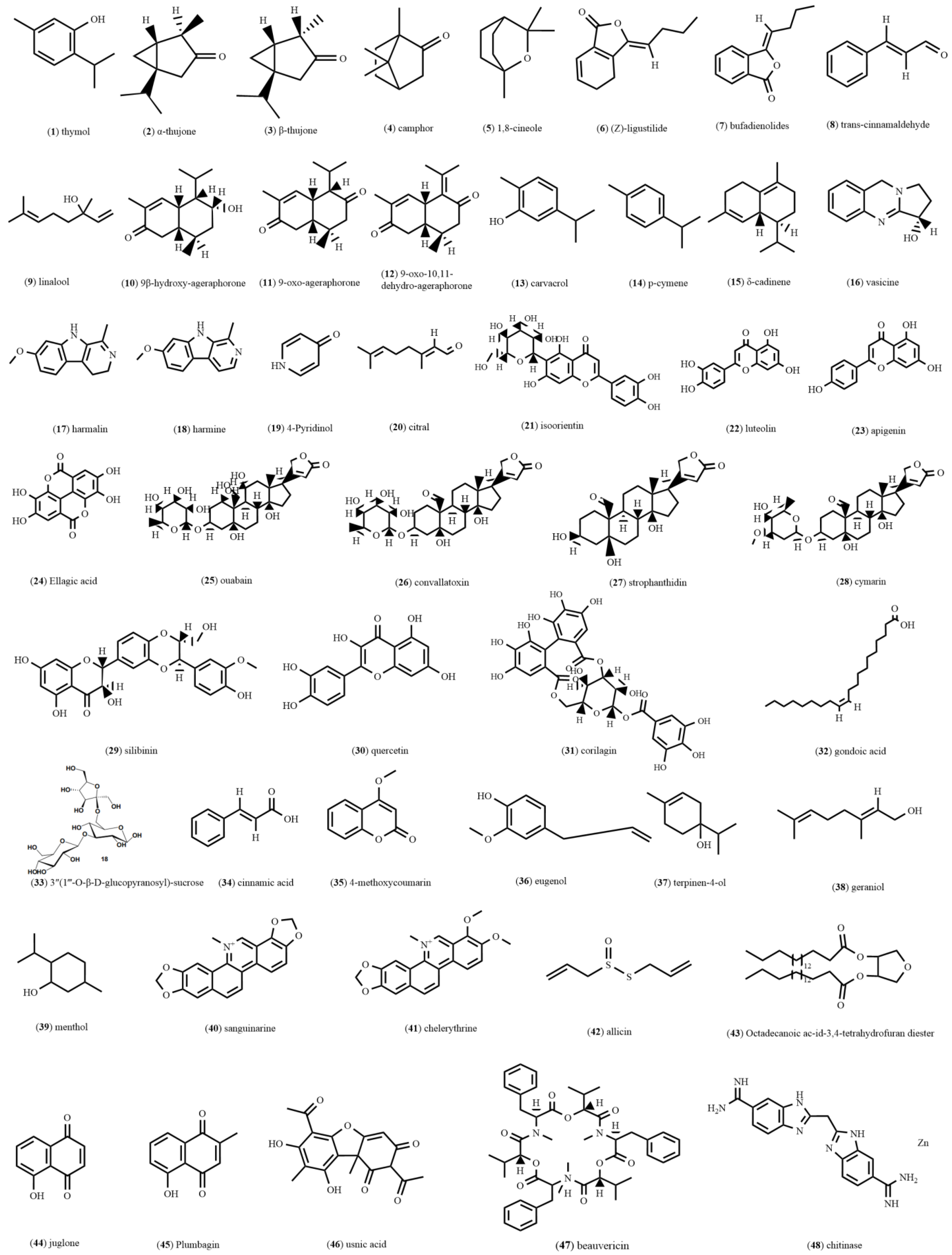
| EO and AE of Ajowan | Thymol | D. gallinae | At 24 h post-treatment, EO and AE both exceeded 90% mortality at 50 μg/cm2 and 150 μg/cm2, respectively | / | [13] |
| EO of A. sieberi | α-thujone (31.5%), β-thujone (11.92%), camphor (12.3%), 1,8-cineole (10.09%) | D. gallinae | LC50 15.85 μg/cm3 | / | [37] |
| ME of C. officinale | (Z)-ligustilide | D. gallinae | After 48 h of treatment, 100% mortality at 4000 ppm | / | [1] |
| ME of X. emarginata | Amides, alkaloids, phenolic, and terpenoids | D. gallinae | LC50 331.769 μg/cm2 | / | [38] |
| Acetonic extract of D. maritima bulbs | Bufadienolides | D. gallinae | At 100 mg/mL, the mortality was 100% after 24 h of exposure | / | [39] |
| EO of Syzygium aromaticum and Litchi chinensis | / | D. gallinae | LC50 8.9–24.7 μg/mL | / | [40] |
| Laurus nobilis essential oil | 1.8-cineole | D. gallinae | After 12 h of treatment, 100% mortality at 320 mg/mL | / | [41] |
| EO of C. cassia | Trans-cinnamaldehyde | D. gallinae | LC50 25.43 ± 1.0423 μg/cm3 | / | [42] |
| EO of C. camphora var. linalooliferum | Linalool | D. gallinae | LC50 39.84 ± 1.9635 μg/cm3 | / | [42] |
| E. adenophorum | 9-oxo-ageraphorone, 9-oxo-10,11-dehydro-ageraphorone, and 9β-hydroxy-ageraphorone | P. cuniculi and S. scabiei | 0.5% | / | [43] |
| Oregano oil | Carvacrol, thymol, and p-cymene | P. cuniculi | 0.05% and 0.02% (v/v) killed all mites within 1 and 6 h, respectively | / | [44] |
| EO of R. nivale | δ-cadinene | P. cuniculi | LT50 values of (33.33–4.17 mg/mL) of the EO ranged from 1.476 to 25.900 h | / | [45] |
| MAE extract of Peganum harmala L. | Vasicine, harmaline, and harmine | P. cuniculi | LT50 value of 100 mg/mL MAE extract against P. cuniculi was 17.322 h | / | [46] |
| AE of Ailanthus altissima bark | / | P. cuniculi | LT50 values at 1, 0.5, and 0.25 g/mL were 0.74, 1.29, and 3.33 h, respectively | / | [47] |
| AE of Ailanthus altissima bark | / | S. scabiei | LT50 values at 1, 0.5, and 0.25 g/mL were 0.60, 0.78, and 1.48 h, respectively | / | [47] |
| Cinnamomum zeylanicum and Ocimum sanctum EOs | / | S. scabiei | Most active at 10–0.1% | / | [48] |
| Clove oil and palmarosa oil | / | S. scabiei | 1% clove and palmarosa oil killed all mites within 20 and 50 min, respectively | / | [49] |
| AE of Ligularia virgaurea | / | S. scabiei | LC50 values were 1.388, 0.624, 0.310, and 0.213 g/mL at 1, 2, 4, and 6 h, respectively | / | [50] |
| EO of Elsholtzia densa (E. densa) Benth | 4-Pyridinol (28.16%) and thymol (26.58%) | S. scabiei | LC 50 values were 7.678–0.981 mg/mL at 1–24 h | / | [51] |
| Lemongrass oil | Citral | S. scabiei and S. scabiei eggs | S. scabiei: LC50 1.37%, 1.08%, 0.91%, 0.64%, and 0.48% at 1, 3, 6, 12, and 24 h, respectively; for eggs: 10%, 5%, 1%, 0.5%, and 0.1%, respectively | Decreases the hatching rate | [52] |
| Aqueous neem fruit extracts | / | S. scabiei | 25% | / | [53] |
| ME of Adonis coerulea Maxim | Isoorientin, luteolin, and apigenin | P. cuniculi | / | Inhibits AchE and Na+-K+-ATPase activities | [54] |
| ME of Adonis coerulea Maxim | Ellagic acid, ouabain, convallatoxin, strophanthidin, and cymarin | P. cuniculi | At 100 mg/mL, the mortality was 55.00% after 24 h | Inhibits Na+-K+-ATPase | [56] |
| ME of Adonis coerulea Maxim | Silibinin, quercetin, and corilagin | P. cuniculi | Inhibit AchE activity with IC50 values of 40.11, 46.15, and 50.98 μg/mL, respectively | Inhibits AchE | [57] |
| Coconut seed extract | Gondoic acid and 3″(1‴-O-β-d-glucopyranosyl)-sucrose | S. scabiei | / | Inhibits IL-1β, IL-6, IL-10, MMP-9, VEGF, and MCP-1; upregulates I-CAM-1, KGF, and TIMP-1 | [58] |
3. Acaricidal Activity of Natural Compounds
3.1. Phenylpropanoids
3.2. Terpenoids
3.3. Alkaloid Compounds
3.4. Other Active Substances
| Compound Name | Classification | Mite | Acaricidal Dose | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Trans-cinnamaldehyde | Phenylpropanoids | P. cuniculi | Up to 8 μg/mL | / | [60] |
| 4-methoxycoumarin | Phenylpropanoids | P. cuniculi | LC50 34.00 μg/mL | / | [63] |
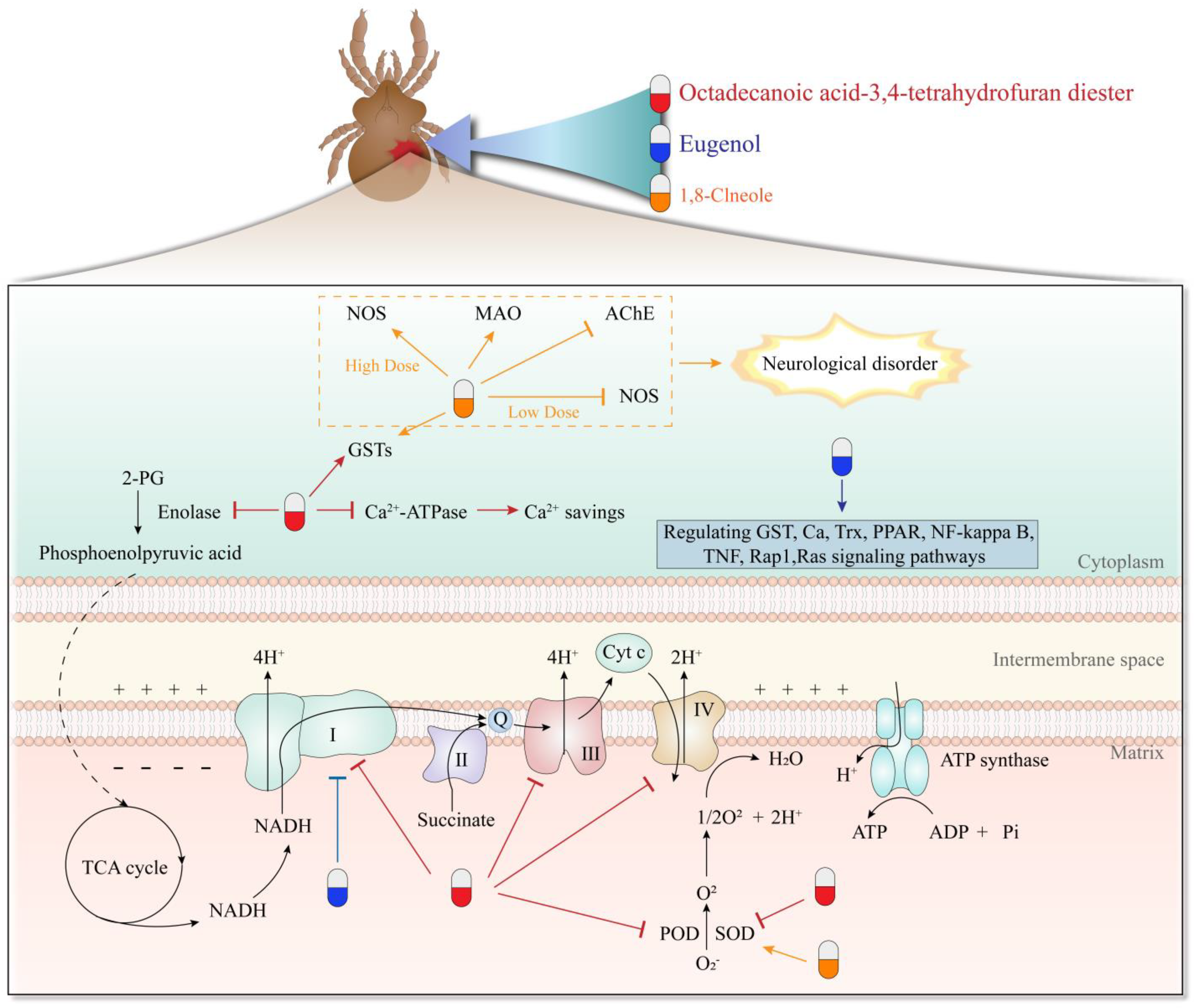
| Eugenol | Phenylpropanoids | P. cuniculi | LD50 values at 1–24 h after treatment were 1.564 ± 0.023 to 1.039 ± 0.009 mgmL−1 | Through PPAR, NF-kappa B, TNF, Rap1, and Ras signaling pathways | [5] |
| Eugenol | Phenylpropanoids | P. cuniculi | The inhibition rates were 37.89% for 50 μg/mL and 60.26% for 100 μg/mL, respectively | Inhibits complex I activity of the mitochondrial respiratory chain in the oxidative phosphorylation pathway | [55] |
| Eugenol, geraniol, citral, terpinen-4-ol, and linalool | Phenylpropanoids | P. cuniculi eggs | EC50 of egg hatching was 0.65–2.87% | / | [65] |
| Thymol | Monoterpene | S. scabiei | LC50 values were 3.829 mg/mL for S. scabiei in 4 h | Interference with the energy metabolism and nerve conduction of the mites | [66] |
| 1,8-Cineole | Monoterpene | S. scabiei | LC50 and LT50 values were 2.77 mg/mL and 3.606 h, respectively | Changes activity of SOD, NOS, and GSTs activity in the nervous system | [67] |
| Euptox A | Sesquiterpene | P. cuniculi and S. scabiei | LC50 values were 1.068 mg/mL for S. scabiei and 0.902 mg/mL for P. cuniculi in 2 h | / | [68,69] |
| Combinations of carvacml-thymol-menthol | Terpenes | D. gallinae | 100% killing at 0.5 μg/mL | / | [70] |
| Carvacrol, eugenol, geraniol | Terpenes | S. scabiei eggs | EC50 values were 0.5, 0.9, and 2.0% for carvacrol, eugenol, and geraniol, respectively | Penetrates through aeropyles on the egg surface | [71] |
| Carvacrol, eugenol, geraniol | Terpenes | S. scabiei | LC50 values at 30 min were 0.24, 0.79, and 0.91%, respectively | / | [9] |
| Vasicine, harmaline, harmine | Alkaloid | P. cuniculi | LT50 values at 2.5 mg/mL against P. cuniculi were 9.791, 10.095, and 9.273 h, respectively | / | [46] |
| Combinations of ivermectin-allicin | Organosulfur compound | D. gallinae | 0.25 mg/mL ivermectin + 1.00 mg/mL allicin | / | [74] |
| Octadecanoic acid-3,4-tetrahydrofuran diester | Esters | S. scabiei | LC50 0.082 mg/mL at 24 h | Suppresses SOD, POD, and Ca(2+)-ATPase and activates GSTs | [75,76] |
| Octadecanoic acid-3,4-tetrahydrofuran diester | Esters | S. scabiei | / | Interferes with energy metabolism, especially oxidative phosphorylation pathway | [77] |
| Juglone | Naphthoquinones | P. cuniculi | LC50 20.53 ppm at 24 h | Inhibits AchE and GST activity | [79] |
| Plumbagin | Naphthoquinones | P. cuniculi | LC50 17.96 ppm at 24 h | Inhibits AchE and GST activity | [79] |
4. Acaricidal Activity of Lichens and Algae
5. Acaricidal Activity of Microbial Metabolites
6. Conclusions and Future Research Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, H.K.; Lee, S.J.; Hwang, B.Y.; Yoon, J.U.; Kim, G.H. Acaricidal and repellent effects of Cnidium officinale-derived material against Dermanyssus gallinae (Acari: Dermanyssidae). Exp. Appl. Acarol. 2018, 74, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; Van Gorp, S.; Koopman, R.; Kempen, I.; Van Hoye, K.; De Baere, K.; Zoons, J.; De Herdt, P. Production losses in laying hens during infestation with the poultry red mite Dermanyssus gallinae. Avian Pathol. 2019, 48 (Suppl. S1), S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Valiente Moro, C.; De Luna, C.J.; Tod, A.; Guy, J.H.; Sparagano, O.A.; Zenner, L. The poultry red mite (Dermanyssus gallinae): A potential vector of pathogenic agents. Exp. Appl. Acarol. 2009, 48, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O. A nonexhaustive overview on potential impacts of the poultry red mite (Dermanyssus gallinae) on poultry production systems. J. Anim. Sci. 2020, 98 (Suppl. S1), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Fan, Y.; Liu, Z.; Hao, Y.; Mou, Y.; Liu, Y.; Zhang, W.; Song, X. The acaricidal activity and mechanism of eugenol on Psoroptes cuniculi. Vet. Parasitol. 2019, 266, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bernigaud, C.; Fischer, K.; Chosidow, O. The Management of Scabies in the 21st Century: Past, Advances and Potentials. Acta Derm. Venereol. 2020, 100, 225–234. [Google Scholar] [CrossRef]
- Richards, R.N. Scabies: Diagnostic and Therapeutic Update. J. Cutan. Med. Surg. 2021, 25, 95–101. [Google Scholar] [CrossRef]
- Karimkhani, C.; Colombara, D.V.; Drucker, A.M.; Norton, S.A.; Hay, R.; Engelman, D.; Steer, A.; Whitfeld, M.; Naghavi, M.; Dellavalle, R.P. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1247–1254. [Google Scholar] [CrossRef]
- Li, M.; Feng, S.; Huang, S.; Guillot, J.; Fang, F. In Vitro Efficacy of Terpenes from Essential Oils against Sarcoptes scabiei. Molecules 2023, 28, 3361. [Google Scholar] [CrossRef]
- Palopoli, M.F.; Fergus, D.J.; Minot, S.; Pei, D.T.; Simison, W.B.; Fernandez-Silva, I.; Thoemmes, M.S.; Dunn, R.R.; Trautwein, M. Global divergence of the human follicle mite Demodex folliculorum: Persistent associations between host ancestry and mite lineages. Proc. Natl. Acad. Sci. USA 2015, 112, 15958–15963. [Google Scholar] [CrossRef]
- Lee, Y.I.; Seo, M.; Cho, K.J. Demodex Blepharitis: An Analysis of Nine Patients. Korean J. Parasitol. 2022, 60, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Fromstein, S.R.; Harthan, J.S.; Patel, J.; Opitz, D.L. Demodex blepharitis: Clinical perspectives. Clin. Optom. 2018, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Imani Baran, A.; Jahanghiri, F.; Hajipour, N.; Sparagano, O.A.E.; Norouzi, R.; Moharramnejad, S. In vitro acaricidal activity of essential oil and alcoholic extract of Trachyspermum ammi against Dermanyssus gallinae. Vet. Parasitol. 2020, 278, 109030. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, J.U.; Park, G.H.; Kim, H.K.; Kim, G.H. Evaluation of susceptibility of red poultry mite, Dermanyssus gallinae (Acari: Dermanyssidae) in Five regions to 11 acaricides. Korean Soc. Appl. Entomol. 2017, 56, 427–434. [Google Scholar]
- Koç, N.; İnak, E.; Nalbantoğlu, S.; Alpkent, Y.N.; Dermauw, W.; Van Leeuwen, T. Biochemical and molecular mechanisms of acaricide resistance in Dermanyssus gallinae populations from Turkey. Pestic. Biochem. Physiol. 2022, 180, 104985. [Google Scholar] [CrossRef]
- Schiavone, A.; Price, D.R.; Pugliese, N.; Burgess, S.T.; Siddique, I.; Circella, E.; Nisbet, A.J.; Camarda, A. Profiling of Dermanyssus gallinae genes involved in acaricide resistance. Vet. Parasitol. 2023, 319, 109957. [Google Scholar] [CrossRef]
- Mounsey, K.E.; Holt, D.C.; McCarthy, J.; Currie, B.J.; Walton, S.F. Scabies: Molecular perspectives and therapeutic implications in the face of emerging drug resistance. Future Microbiol. 2008, 3, 57–66. [Google Scholar] [CrossRef]
- Bernigaud, C.; Fernando, D.D.; Lu, H.; Taylor, S.; Hartel, G.; Guillot, J.; Chosidow, O.; Fischer, K. In vitro ovicidal activity of current and under-development scabicides—Which treatments kill scabies eggs? Br. J. Dermatol. 2020, 182, 511–513. [Google Scholar] [CrossRef]
- Feng, S.; Shi, M.; Yin, Z.; Di, W.; Guillot, J.; Fang, F. Can Ivermectin kill Sarcoptes scabiei during the molting process? PLoS Negl. Trop. Dis. 2023, 17, e0011337. [Google Scholar] [CrossRef]
- Soler, P.; Germano, M.; Larroza, M. First report of in vitro resistance of Psoroptes ovis to ivermectin in Argentina. Exp. Parasitol. 2022, 235, 11. [Google Scholar] [CrossRef]
- Romero, T.; Javier Moya, V.; Fernández, N.; Althaus, R.; Reybroeck, W.; Molina, M.P. Interferences on microbial inhibitor tests related to ivermectin treatment in lactating dairy goats. J. Dairy Res. 2016, 83, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Al Khoury, C.; Nemer, N.; Nemer, G.; Kurban, M.; Bernigaud, C.; Fischer, K.; Guillot, J. In Vitro Activity of Beauvericin against All Developmental Stages of Sarcoptes scabiei. Antimicrob. Agents Chemother. 2020, 64, e02118–e02119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K.; Gao, J.M.; Yang, C.J.; Shang, X.F.; Zhao, Z.M.; Lawoe, R.K.; Zhou, R.; Sun, Y.; Yin, X.D.; Liu, Y.Q. Design, Synthesis, and Antifungal Evaluation of Neocryptolepine Derivatives against Phytopathogenic Fungi. J. Agric. Food Chem. 2020, 68, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Ibrahim, B.; Ahmad Bawadikji, A.; Lim, J.W.; Tong, W.Y.; Leong, C.R.; Khaw, K.Y.; Tan, W.N. Recent Developments in Metabolomics Studies of Endophytic Fungi. J. Fungi 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Sands, C.J.; Gómez-Romero, M.; Correia, G.; Chekmeneva, E.; Camuzeaux, S.; Izzi-Engbeaya, C.; Dhillo, W.S.; Takats, Z.; Lewis, M.R. Representing the Metabolome with High Fidelity: Range and Response as Quality Control Factors in LC-MS-Based Global Profiling. Anal. Chem. 2021, 93, 1924–1933. [Google Scholar] [CrossRef]
- Tampieri, A.; Szabó, M.; Medina, F.; Gulyás, H. A brief introduction to the basics of NMR spectroscopy and selected examples of its applications to materials characterization. Phys. Sci. Rev. 2021, 6, 20190086. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Nikolova, K.; Buhalova, D.; Andonova, V.; Gugleva, V.; Petkova, N.; Yotkovska, I.; Ivanova, N. Antioxidant Activity and Chemical Characteristics of Sambucus nigra L. Blossom from Different Regions in Bulgaria. Horticulturae 2022, 8, 309. [Google Scholar] [CrossRef]
- Plabon, M.E.A.; Mondal, S.C.; Or Rashid, M.M.; Chowdhury, M.K.; Saeid, A.; Althobaiti, F.; Dessok, E.S.; Rehmani, M.I.A.; Mustafa, S.K.; Islam, M.S. Chemical Composition and Anti-Microbial Activity of Hog Plum (Spondias mombin L.) Peel Oil Extracted from Different Regions of Tropical Climates. Horticulturae 2021, 7, 428. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Andryukov, B.G.; Kryzhanovsky, S.P.; Ermakova, S.P.; Kuznetsova, T.A.; Voronova, A.N.; Shchelkanov, M.Y. Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts. Mar. Drugs 2021, 19, 637. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Ait Akka, A.; Choubbane, A.; Akhazzane, M.; Aboul-Soud, M.A.; et al. Essential Oils from Leaves of Juniperus thurifera L.; Exhibiting Antioxidant, Antifungal and Antibacterial Activities against Antibiotic-Resistant Microbes. Horticulturae 2022, 8, 321. [Google Scholar] [CrossRef]
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Nagulapalli Venkata, K.C.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.; Pittalà, V.; et al. The analgesic potential of glycosides derived from medicinal plants. Daru 2020, 28, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Penson, P.E.; Banach, M. Natural compounds as anti-atherogenic agents: Clinical evidence for improved cardiovascular outcomes. Atherosclerosis 2021, 316, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- González Gutiérrez, F.H.; Rascón Valenzuela, L.A.; Meneses Sagrero, S.E.; Dias-Silva, M.J.; Antelo, O.V.; Velazquez, C.; Vilegas, W.; Zepeda, R.E.R. Antiproliferative activity of standardized herbal phytopreparation from Asclepias subulata. F1000Research 2022, 11, 527. [Google Scholar] [CrossRef]
- Kim, J.R.; Perumalsamy, H.; Lee, J.H.; Ahn, Y.J.; Lee, Y.S.; Lee, S.G. Acaricidal activity of Asarum heterotropoides root-derived compounds and hydrodistillate constitutes toward Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Exp. Appl. Acarol. 2016, 68, 485–495. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Benelli, G. Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): Toxic and repellent potential. Parasitol. Res. 2017, 116, 1545–1551. [Google Scholar] [CrossRef]
- Pares, R.B.; Alves, D.S.; Alves, L.F.A.; Godinho, C.C.; Gobbo Neto, L.; Ferreira, T.T.; Nascimento, M.M.; Ascari, J.; Oliveira, D.F. Acaricidal Activity of Annonaceae Plants for Dermanyssus gallinae (Acari: Dermanyssidae) and Metabolomic Profile by HPLC-MS/MS. Neotrop. Entomol. 2021, 50, 662–672. [Google Scholar] [CrossRef]
- Rhimi, W.; Ben salem, I.; Camarda, A.; Saidi, M.; Boulila, A.; Otranto, D.; Cafarchia, C. Chemical characterization and acaricidal activity of Drimia maritima (L) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019, 268, 61–66. [Google Scholar] [CrossRef]
- Tabari, M.A.; Rostami, A.; Khodashenas, A.; Maggi, F.; Petrelli, R.; Giordani, C.; Tapondjou, L.A.; Papa, F.; Zuo, Y.; Cianfaglione, K.; et al. Acaricidal activity, mode of action, and persistent efficacy of selected essential oils on the poultry red mite (Dermanyssus gallinae). Food Chem. Toxicol. 2020, 138, 111207. [Google Scholar] [CrossRef]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. In vitro acaricidal activity of essential oil and crude extracts of Laurus nobilis, (Lauraceae) grown in Tunisia, against arthropod ectoparasites of livestock and poultry: Hyalomma scupense and Dermanyssus gallinae. Vet. Parasitol. 2021, 298, 24. [Google Scholar] [CrossRef] [PubMed]
- Bordin, C.; Alves, D.S.; Alves, L.F.A.; de Oliveira, M.S.; Ascari, J.; Scharf, D.R. Fumigant activity of essential oils from Cinnamomum and Citrus spp. and pure compounds against Dermanyssus gallinae (De Geer) (Acari: Dermanyssidae) and toxicity toward the nontarget organism Beauveria bassiana (Vuill.). Vet. Parasitol. 2021, 290, 5. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.; Li, S.H.; Chen, F.Z.; Wang, J.H.; Xie, Y.; Fang, C.L.; Liu, T.F.; He, R.; Gu, X.B.; Peng, X.R.; et al. Isolation and identification of acaricidal compounds in Eupatorium adenophorum petroleum ether extract and determination of their acaricidal activity against Psoroptes cuniculi. Vet. Parasitol. 2014, 203, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, Y.; Zhou, X.; Guo, X.; Dong, S.; Wang, D.; Zhang, J.; Pan, H.; Zhang, Y.; Miao, X. Acaricidal activity of oregano oil and its major component, carvacrol, thymol and p-cymene against Psoroptes cuniculi in vitro and in vivo. Vet. Parasitol. 2016, 226, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shang, X.; Li, B.; Zhou, X.Z.; Wen, H.; Zhang, J. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, δ-cadinene against Psoroptes cuniculi. Vet. Parasitol. 2017, 236, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Guo, X.; Li, B.; Pan, H.; Zhang, J.; Zhang, Y.; Miao, X. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. J. Ethnopharmacol. 2016, 192, 350–361. [Google Scholar] [CrossRef]
- Gu, X.; Fang, C.; Yang, G.; Xie, Y.; Nong, X.; Zhu, J.; Wang, S.; Peng, X.; Yan, Q. Acaricidal properties of an Ailanthus altissima bark extract against Psoroptes cuniculi and Sarcoptes scabiei var. cuniculi in vitro. Exp. Appl. Acarol. 2014, 62, 225–232. [Google Scholar] [CrossRef]
- Andriantsoanirina, V.; Guillot, J.; Ratsimbason, M.; Mekhloufi, G.; Randriamialinoro, F.; Ranarivelo, L.; Ariey, F.; Durand, R. In vitro efficacy of essential oils against Sarcoptes scabiei. Sci. Rep. 2022, 12, 7176. [Google Scholar] [CrossRef]
- Fang, F.; Candy, K.; Melloul, E.; Bernigaud, C.; Chai, L.; Darmon, C.; Durand, R.; Botterel, F.; Chosidow, O.; Izri, A.; et al. In vitro activity of ten essential oils against Sarcoptes scabiei. Parasites Vectors 2016, 9, 594. [Google Scholar] [CrossRef]
- Luo, B.; Liao, F.; Hu, Y.C.; Liu, X.I.; He, Y.; Wu, L.; Tan, H.; Luo, L.; Zhou, Y.; Mo, Q.; et al. Acaricidal activity of extracts from Ligularia virgaurea against the Sarcoptes scabiei mite in vitro. Exp. Ther. Med. 2015, 10, 247–250. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, F.; Weng, J.; Mo, Q.; Xu, R.; Zhang, Y.; Ren, Z.; Zhong, Z.; Zuo, Z.; Peng, G.; et al. Composition and acaricidal activity of essential oil from Elsholtzia densa Benth against Sarcoptes scabiei mites in vitro. Vet. Med. 2019, 64, 178–183. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Bernigaud, C.; Fischer, K.; Guillot, J.; Fang, F. Lemongrass (Cymbopogon citratus) oil: A promising miticidal and ovicidal agent against Sarcoptes scabiei. PLoS Negl. Trop. Dis. 2020, 14, e0008225. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, C.N.; Tekedza, T.T.; Chatiza, F.P.; Gororo, E. Efficacy of neem (Azadirachta indica) aqueous fruit extracts against Sarcoptes scabiei var. suis in grower pigs. Trop. Anim. Health Prod. 2021, 53, 020–02545. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Guo, X.; Yang, F.; Li, B.; Pan, H.; Miao, X.; Zhang, J. The toxicity and the acaricidal mechanism against Psoroptes cuniculi of the methanol extract of Adonis coerulea Maxim. Vet. Parasitol. 2017, 240, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Dai, L.X.; Yang, C.J.; Guo, X.; Liu, Y.Q.; Miao, X.L.; Zhang, J.Y. A value-added application of eugenol as acaricidal agent: The mechanism of action and the safety evaluation. J. Adv. Res. 2020, 34, 149–158. [Google Scholar] [CrossRef]
- Shang, X.F.; Miao, X.L.; Dai, L.X.; Guo, X.; Li, B.; Pan, H.; Zhang, J.Y. The acaricidal mechanism and active compounds against Psoroptes cuniculi of the methanol extract of Adonis coerulea Maxim II: Integrated proteomics and SPR analysis. Vet. Parasitol. 2020, 287, 109267. [Google Scholar] [CrossRef]
- Dai, L.; Miao, X.; Li, B.; Zhang, J.; Pan, H.; Shang, X. The active compounds and AChE inhibitor of the methanol extract of Adonis coerulea maxim against Psoroptes cuniculi. Vet. Parasitol. 2020, 286, 109247. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdel-Maqsoud, N.M.R.; Tammam, O.Y.; Abdel-Rahman, I.M.; Elrehany, M.A.; Bakhsh, H.T.; Altemani, F.H.; Algehainy, N.A.; Alzubaidi, M.A.; Abdelmohsen, U.R.; et al. Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics 2022, 12, 43. [Google Scholar] [CrossRef]
- Usai, F.; Di Sotto, A. trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies. Antibiotics 2023, 12, 254. [Google Scholar] [CrossRef]
- Shen, F.; Xing, M.; Liu, L.; Tang, X.; Wang, W.; Wang, X.; Wu, X.; Wang, X.; Wang, X.; Wang, G.; et al. Efficacy of trans-cinnamaldehyde against Psoroptes cuniculi in vitro. Parasitol. Res. 2012, 110, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, C.; Li, W.; Cui, Z.; Chen, D.; Cao, F.; Miao, F.; Zhou, L. Ethyl cinnamate derivatives as promising high-efficient acaricides against Psoroptes cuniculi: Synthesis, bioactivity and structure-activity relationship. Chem. Pharm. Bull. 2015, 63, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Zhang, B.Y.; Liu, X.X.; Li, X.Q.; Yang, X.J.; Zhou, L. Bioactivity and structure-activity relationship of cinnamic acid derivatives and its heteroaromatic ring analogues as potential high-efficient acaricides against Psoroptes cuniculi. Bioorg. Med. Chem. Lett. 2018, 28, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F. Bioactivities and Modes of Action of Four Kinds of Natural Products; Lanzhou University: Lanzhou, China, 2019. [Google Scholar]
- Sharma, A.; Bhardwaj, G.; Sohal, H.S.; Gohain, A. Chapter 9—Eugenol. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 177–198. [Google Scholar]
- Fang, F.; Li, M.; Jiang, Z.; Lu, X.; Guillot, J.; Si, H. Comparing acaricidal and ovicidal activity of five terpenes from essential oils against Psoroptes cuniculi. Parasitol. Res. 2020, 119, 4219–4223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, F.; Fu, J.; Weng, J.; Mo, Q.; Xu, R.; Zhang, Y.; Sun, W.; Yue, D.; Ren, Z.; et al. In Vitro Acaricidal Activity of the Thymol against Sarcoptes scabiei and Regulating Effects on Enzyme Activity. J. Vet. Sci. Technol. 2018, 09, 554. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Z.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Zou, Y.; Liang, X.; Li, L.; He, C.; et al. In vitro acaricidal activity of 1,8-cineole against Sarcoptes scabiei var. cuniculi and regulating effects on enzyme activity. Parasitol. Res. 2015, 114, 2959–2967. [Google Scholar] [CrossRef]
- Liao, F.; Hu, Y.; Tan, H.; Wu, L.; Wang, Y.; Huang, Y.; Mo, Q.; Wei, Y. Acaricidal activity of 9-oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum in vitro. Exp. Parasitol. 2014, 140, 8–11. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, F.; Hu, Y.C.; Luo, B.; He, Y.; Mo, Q.; Zuo, Z.; Ren, Z.; Deng, J.; Wei, Y. Clinical efficacy of 9-oxo-10, 11-dehydroageraphorone extracted from Eupatorium adenophorum against Psoroptes cuniculi in rabbits. BMC Vet. Res. 2014, 10, 970. [Google Scholar] [CrossRef]
- Tabari, M.A.; Jafari, A.; Jafari, M.; Youssefi, M.R. Laboratory and field efficacy of terpene combinations (carvacrol, thymol and menthol) against the poultry red mite (Dermanyssus gallinae). Vet. Parasitol. 2023, 313, 109842. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Yin, Z.; Bernigaud, C.; Guillot, J.; Fang, F. Activity of terpenes derived from essential oils against Sarcoptes scabiei eggs. Parasites Vectors 2021, 14, 600. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Ma, Y.-N.; Zheng, F.; Song, X.P.; Zhou, L. Structural Modification of Sanguinarine and Chelerythrine and Their in Vitro Acaricidal Activity against Psoroptes cuniculi. Chem. Pharm. Bull. 2012, 60, 1508–1513. [Google Scholar] [CrossRef]
- Kang, J.; Chae, H.; Hossain, M.A. Poultry red mite eradication potential of ivermectin and allicin combination treatment. Vet. Med. Sci. 2023, 9, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chae, M.; Chae, H.; Kwon, Y.; Lee, J.; Hossain, M.A. In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite. Antibiotics 2023, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-H.; Li, J.-L.; Jia, R.-Y.; Yin, Z.-Q.; Li, X.-T.; Lv, C.; Ye, G.; Zhang, L.; Zhang, Y.-Q. Acaricidal activity of four fractions and octadecanoic acid-tetrahydrofuran-3,4-diyl ester isolated from chloroform extracts of neem (Azadirachta indica) oil against Sarcoptes scabiei var. cuniculi larvae in vitro. Vet. Parasitol. 2009, 163, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Z.; Deng, Y.-X.; Yin, Z.-Q.; Wei, Q.; Li, M.; Jia, R.-Y.; Xu, J.; Li, L.; Song, X.; Liang, X.-X.; et al. Studies on the acaricidal mechanism of the active components from neem (Azadirachta indica) oil against Sarcoptes scabiei var. cuniculi. Vet. Parasitol. 2014, 204, 323–329. [Google Scholar] [CrossRef]
- Song, X.; Chen, Z.; Jia, R.; Cao, M.; Zou, Y.; Li, L.; Liang, X.; Yin, L.; He, C.; Yue, G.; et al. Transcriptomics and proteomic studies reveal acaricidal mechanism of octadecanoic acid-3,4-tetrahydrofuran diester against Sarcoptes scabiei var. cuniculi. Sci. Rep. 2017, 7, srep45479. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Liu, T.; Xing, R.; Peng, S.; Song, X.; Zou, Y.; Zhao, X.; Jia, R.; Wan, H.; et al. Structural modification of octadecanoic acid-3,4-tetrahydrofuran diester and the acaricidal activity and mechanism of its derivatives against Sarcoptes scabiei var. Cuniculi. Front. Pharmacol. 2022, 13, 953284. [Google Scholar] [CrossRef]
- Shang, X.F.; Liu, Y.Q.; Guo, X.; Miao, X.L.; Chen, C.; Zhang, J.X.; Xu, X.S.; Yang, G.Z.; Yang, C.J.; Li, J.C.; et al. Application of Sustainable Natural Resources in Agriculture: Acaricidal and Enzyme Inhibitory Activities of Naphthoquinones and Their Analogs against Psoroptes cuniculi. Sci. Rep. 2018, 8, 1609. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Bansal, P. Algal lectins as promising biomolecules for biomedical research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef]
- Leite, Y.F.; Silva, L.M.; Amorim, R.C.; Freire, E.A.; de Melo Jorge, D.M.; Grangeiro, T.B.; Benevides, N.M.B. Purification of a lectin from the marine red alga Gracilaria ornata and its effect on the development of the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). Biochim. Biophys. Acta 2005, 20, 137–145. [Google Scholar] [CrossRef]
- De Medeiros, M.L.S.; de Moura, M.C.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Bezerra, A.C.D.S.; da Silva, M.D.C. Nematicidal activity of a water soluble lectin from seeds of Moringa oleifera. Int. J. Biol. Macromol. 2018, 108, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.L.; Alves, R.R.; Oliveira, B.F.; Napoleão, T.H.; Paiva, P.M.; Coelho, L.C.; Bezerra, A.C.; Silva, M.D. In vitro effects of Moringa oleifera seed lectins on Haemonchus contortus in larval and adult stages. Exp. Parasitol. 2020, 218, 19. [Google Scholar] [CrossRef] [PubMed]
- Basiouni, S.; Fayed, M.A.; Tarabees, R.; El-Sayed, M.; Elkhatam, A.; Töllner, K.R.; Hessel, M.; Geisberger, T.; Huber, C.; Eisenreich, W.; et al. Characterization of Sunflower Oil Extracts from the Lichen Usnea barbata. Metabolites 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, H.; Silva Júnior, J.G.D.; Albuquerque, M.C.P.D.A.; Coelho, L.C.B.B.; Aires, A.D.L. The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons. Molecules 2021, 26, 5995. [Google Scholar] [CrossRef]
- Shang, X.; Miao, X.; Lv, H.; Wang, D.; Zhang, J.; He, H.; Yang, Z.; Pan, H. Acaricidal activity of usnic acid and sodium usnic acid against Psoroptes cuniculi in vitro. Parasitol. Res. 2014, 113, 2387–2390. [Google Scholar] [CrossRef] [PubMed]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.; Nascimento, W.M.; Martins, M.C.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic acid potassium salt from Cladonia substellata (Lichen): Synthesis, cytotoxicity and in vitro anthelmintic activity and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Santos, V.H.B.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; Albuquerque, M.C.; Aires, A.L.; Lima, V.L. In vitro activity of usnic acid potassium salt against different developmental stages of Schistosoma mansoni: An ultrastructural study. Acta Trop. 2020, 201, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The Toxins of Beauveria bassiana and the Strategies to Improve Their Virulence to Insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Al Khoury, C.; Nemer, N.; Bernigaud, C.; Fischer, K.; Guillot, J. First evidence of the activity of an entomopathogenic fungus against the eggs of Sarcoptes scabiei. Vet. Parasitol. 2021, 298, 109553. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Krishnamoorthy, M.; Karuppiah, K.; Ethiraj, K.; Sekar, S. Chitinase from Streptomyces mutabilis as an Effective Eco-friendly Biocontrol Agent. Appl. Biochem. Biotechnol. 2023, 25, 023–04489. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, N.; Xie, Y.; Zheng, Y.; Chen, Y.; Zhou, X.; Li, X.; Zhong, Z.; He, R.; Yang, G. Metarhizium anisopliae CQMa128 regulates antioxidant/detoxification enzymes and exerts acaricidal activity against Psoroptes ovis var. cuniculi in rabbits: A preliminary study. Vet. Parasitol. 2020, 279, 21. [Google Scholar] [CrossRef] [PubMed]
- Dunstand-Guzmán, E.; Peña-Chora, G.; Hallal-Calleros, C.; Pérez-Martínez, M.; Hernández-Velazquez, V.M.; Morales-Montor, J.; Flores-Pérez, F.I. Acaricidal effect and histological damage induced by Bacillus thuringiensis protein extracts on the mite Psoroptes cuniculi. Parasites Vectors 2015, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Aguilar, J.A.; Arjona-Cambranes, K.; Torres-Acosta, J.F.J.; Rodríguez-Vivas, R.I.; Bolio-González, M.E.; Ortega-Pacheco, A.; Alzina-López, A.; Gutiérrez-Ruiz, E.J.; Gutiérrez-Blanco, E.; Aguilar-Caballero, A.J. Plant products and secondary metabolites with acaricide activity against ticks. Vet. Parasitol. 2017, 238, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Quadros, D.G.; Johnson, T.L.; Whitney, T.R.; Oliver, J.D.; Oliva Chávez, A.S. Plant-Derived Natural Compounds for Tick Pest Control in Livestock and Wildlife: Pragmatism or Utopia? Insects 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Candy, K.; Akhoundi, M.; Andriantsoanirina, V.; Durand, R.; Bruel, C.; Izri, A. Essential Oils as a Potential Treatment Option for Pediculosis. Planta Med. 2020, 86, 619–630. [Google Scholar] [CrossRef]
- Pan, H.; Deng, M.; Zhang, B.; Fang, T.; Liu, Y. Transcriptome analysis of Tetrahymena thermophila response to exposure with dihydroartemisinin. Heliyon 2023, 9, e14069. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Pan, Y.; Zheng, X.; Liang, X.; Sheng, L.; Zhang, D.; Sun, Q.; Wang, Q. Research advances on endophytic fungi and their bioactive metabolites. Bioprocess Biosyst. Eng. 2023, 46, 165–170. [Google Scholar] [CrossRef]
- Qin, M.; Li, Y.; Cai, L.; Yin, X.; He, Z.; Kang, J. Overexpression of the global regulator FnVeA upregulates antitumor substances in endophytic Fusarium nematophilum. Can. J. Microbiol. 2022, 68, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.R.; Santos, G.S.D.; Armstrong, L.; Colepicolo, P.; Debonsi, H.M. Antitumor Potential of Seaweed Derived-Endophytic Fungi. Antibiotics 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, F.; Liu, Y.; Abudoukerimu, A.; Zheng, Q.; Zhang, X.; Sun, Y.; Yimiti, D. Comparative Metabolomics Revealed the Potential Antitumor Characteristics of Four Endophytic Fungi of Brassica rapa L. ACS Omega 2020, 5, 5939–5950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Yao, S.C.; Wang, J.; Xie, X.Y.; Tan, X.M.; Huang, R.S.; Yang, X.F.; Tan, Y.; Yu, L.Y.; Fu, P. Cultivable endophytic fungal community associated with the karst endemic plant Nervilia fordii and their antimicrobial activity. Front. Microbiol. 2022, 13, 1063897. [Google Scholar] [CrossRef]
- Berestetskiy, A.; Hu, Q. The Chemical Ecology Approach to Reveal Fungal Metabolites for Arthropod Pest Management. Microorganisms 2021, 9, 1379. [Google Scholar] [CrossRef]
- Ormskirk, M.M.; Narciso, J.; Hampton, J.G.; Glare, T.R. Endophytic ability of the insecticidal bacterium Brevibacillus laterosporus in Brassica. PLoS ONE 2019, 14, e0216341. [Google Scholar] [CrossRef]
- Takao, K.; Toda, K.; Saito, T.; Sugita, Y. Synthesis of Amide and Ester Derivatives of Cinnamic Acid and Its Analogs: Evaluation of Their Free Radical Scavenging and Monoamine Oxidase and Cholinesterase Inhibitory Activities. Chem. Pharm. Bull. 2017, 65, 1020–1027. [Google Scholar] [CrossRef]
- Cao, F.-J.; Yang, R.; Lv, C.; Ma, Q.; Lei, M.; Geng, H.-L.; Zhou, L. Pseudocyanides of sanguinarine and chelerythrine and their series of structurally simple analogues as new anticancer lead compounds: Cytotoxic activity, structure-activity relationship and apoptosis induction. Eur. J. Pharm. Sci. 2015, 67, 45–54. [Google Scholar] [CrossRef]
- Qi, X.; Li, H.; Wang, B.; Meng, J.; Wang, X.; Sun, W.; Pan, B. Identification of guanine and hematin as arrestment pheromones of poultry red mites, Dermanyssus gallinae (Acari: Dermanyssidae) and their application in mite control. Vet. Parasitol. 2023, 313, 109843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.; Han, C.; Deng, Q.; Zhou, Z.; Bao, T.; Zhong, M.; Tao, G.; Li, R.; Han, B.; Qiao, Y.; et al. Natural Products as Mite Control Agents in Animals: A Review. Molecules 2023, 28, 6818. https://doi.org/10.3390/molecules28196818
Liao F, Han C, Deng Q, Zhou Z, Bao T, Zhong M, Tao G, Li R, Han B, Qiao Y, et al. Natural Products as Mite Control Agents in Animals: A Review. Molecules. 2023; 28(19):6818. https://doi.org/10.3390/molecules28196818
Chicago/Turabian StyleLiao, Fei, Changquan Han, Qingsheng Deng, Ziyao Zhou, Taotao Bao, Menghuai Zhong, Guangyao Tao, Renjun Li, Bo Han, Yanlong Qiao, and et al. 2023. "Natural Products as Mite Control Agents in Animals: A Review" Molecules 28, no. 19: 6818. https://doi.org/10.3390/molecules28196818
APA StyleLiao, F., Han, C., Deng, Q., Zhou, Z., Bao, T., Zhong, M., Tao, G., Li, R., Han, B., Qiao, Y., & Hu, Y. (2023). Natural Products as Mite Control Agents in Animals: A Review. Molecules, 28(19), 6818. https://doi.org/10.3390/molecules28196818







