A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine
Abstract
:1. Introduction
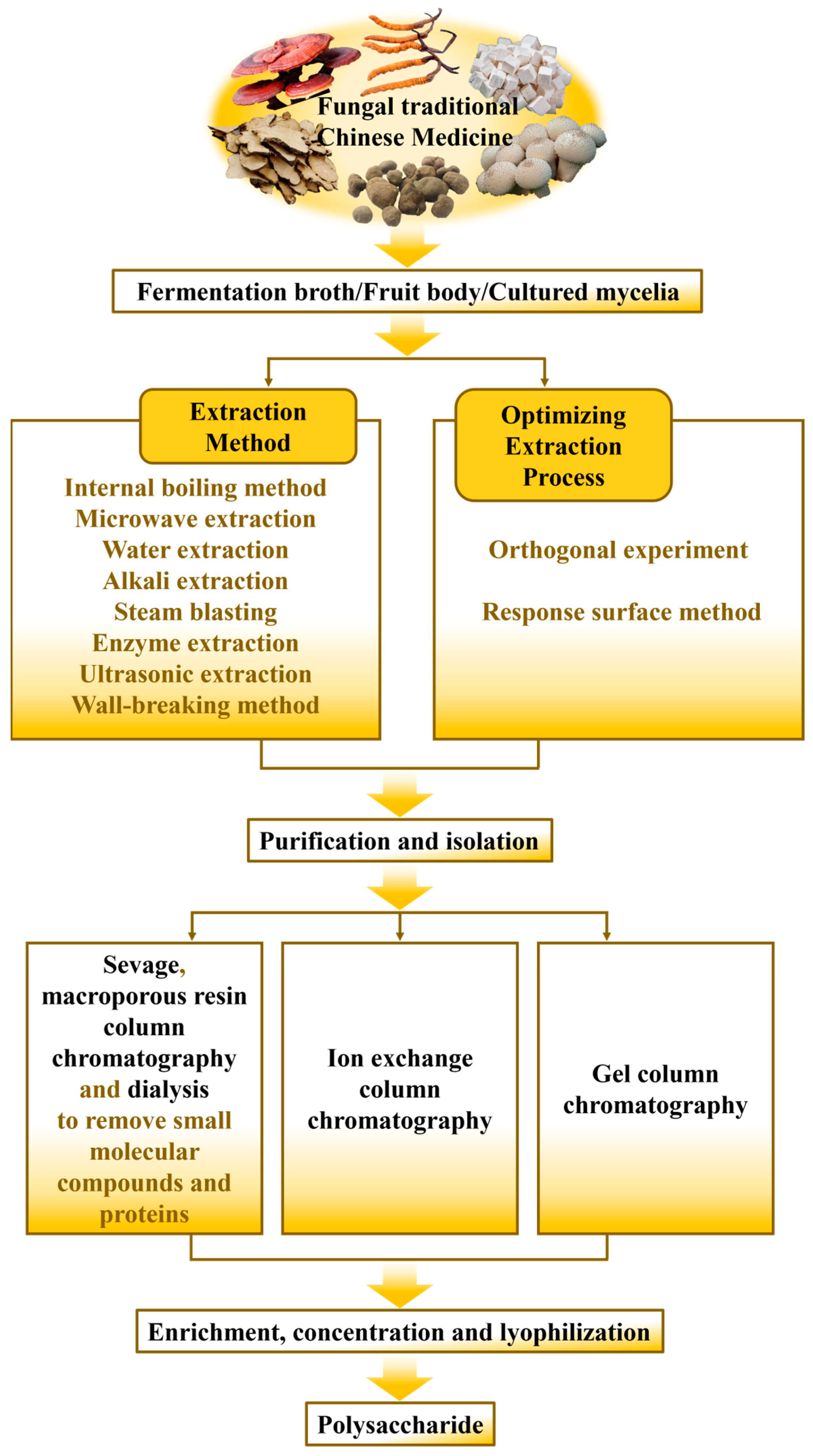
2. Preparation
2.1. Extraction of Polysaccharides from Fungal TCM
| Fungi Name | Polysaccharide Extraction Method | Parameters | Total Yield (%) | Reference |
|---|---|---|---|---|
| Ganoderma lucidum | Continuous phase transition extraction | The air-dried fruiting bodies of Ganoderma lucidum were extracted according to the following conditions: distilled water, 100 °C, 4 h, and flow rate of 28 L/h | 7.13% | [34] |
| Red Ganoderma lucidum | Ultrasound-assisted enzymatic extraction | Enzyme concentration, 3%; pH, 5.5; extraction temperature, 45 °C; extraction time, 30 min; ultrasonic power, 480 W | The highest content of polysaccharides was 32.08 mg/g | [35] |
| Cordeceps sinensis | Internal boiling method | Resolver concentration, 90%; parse time, 1 min; extractant volume, 60 mL; extract time, 4 min; 100 °C | 2.37% | [36] |
| Ophiocordyceps sinensis | Microwave extraction | Mesh number, 80; 1:26 (g/mL); 3 times; microwave power, 330 W; microwave time, 4 min | 9.06% | [37] |
| Cordyceps militaris | Water extraction | Shaking (150 rpm); 1: 10 (w/v); 24 h; twice | 4.95% | [38] |
| Cordyceps militaris | Alkali extraction | Extraction solvent, 0.5 M NaOH solution; 8 volumes; 95 °C; 3 h; twice | N/A | [39] |
| Poria | Steam blasting pretreatment, water extraction and alcohol precipitation | Steam blasting pressure, 2.0 MPa; dwell time, 60 s; 1:50 (g/mL); 60 °C; 120 min | 1.95% | [40] |
| Poria cocos (Schw.) Wolf | Dilute alkali leaching | 4 °C; 0.15 M NaOH solution | 72.656% (polysaccharide content) | [42] |
| Poria cocos | Combined enzyme extraction method | Cellulase, 2.5%; hemicellulose, 2.5%; beta-glucanase, 5%; 90 min; 50 °C; pH 5.0 | 6.13% | [43] |
| Poria cocos | Ultrasonic-assisted enzymatic extraction | Combined enzyme addition amount (cellulase:papain = 4:1), 7.60%; 1:44 (g/mL); pH 4.90 | 10.42% | [44] |
| Polyporus umbellatus | Ultrasonic-assisted extraction | Extraction temperature, 72 °C; extraction power, 300 W; extraction time, 65 min; liquid-to-solid ratio, 22 mL/g | 2.47% | [45] |
| Polyporus umbellatus | Microwave-assisted extraction | Extraction time, 2.5 min; microwave power, 614 W; pH 6.6; liquid-to-solid ratio, 30:1 | 6.75% | [46] |
| Polyporus umbellatus | Hot distilled water extraction | Pretreated with 80% ethanol for 24 h; hot distilled water, 90 °C; twice; 4 h | N/A | [47] |
| Polyporus umbellatus | Alkali solution extraction | 2% sodium hydroxide containing 2% of urea | N/A | [48] |
| Polyporus umbellatus | Enzymatic coupled with ultrasonic-assisted extraction | Cellulose:pectinase = 1:1; 50 °C; pH = 6.5, 60 min; additional 30 min of ultrasonic treatment | 7.48% | [49] |
| Omphalia | Water extraction | Extraction temperature, 100 °C; extraction time, 93 min; the water/solid ratio, 21:1; twice | 23.12% | [50] |
| Omphalia | Cellulose enzymolysis | Enzymolysis temperature of 40 °C; cellulose addition, 0.08%; enzymolysis time, 90 min | 3.57% | [51] |
| Lasiosphaera fenzlii | Ultrasound-assisted extraction | Liquid-to-solid ratio, 30:1; reflux time, 53.77 min; extraction temperature, 58.33 °C | 4.01% | [54] |
| Lasiosphaera puffball | Snailase-assisted extraction | Extraction temperature, 35 °C; extraction time, 120 min; snailase addition, 5.0%; pH 6.0 | 0.908% | [55] |
| Lasiosphaera puffball | High-temperature water extraction and alcohol precipitation as well as wall-breaking method | Breaking speed, 600 rpm; extraction time, 2.5 h; extraction temperature, 100 °C; liquid-to-solid ratio, 20:1 | 1.065% | [56] |
2.2. Purification and Isolation of Polysaccharides from Fungal TCM
| Fungi Name | Source | Polysaccharide Name | Extraction and Purification Method | Reference |
|---|---|---|---|---|
| Ganoderma lucidum | Fruiting body | GLPC2 | Water extraction and ethanol precipitation; fractioned using DEAE SepharoseTM FF column with different concentrations of NaCl solution; subsequently eluted using Sephacryl S-200 HR column with 0.5 M NaCl solution | [60] |
| Ganoderma lucidum | Fruiting body | FXM | The fruit body of Ganoderma lucidum was extracted with alkali solution after degreasing, and the alkaline extract was neutralized with acid and dialysis for 72 h. Fehling reagent was added to the solution, and the polysaccharide FXM was obtained after continuous washing with acid and alkali solution and dialysis. | [61] |
| Ganoderma lucidum | Fruiting body | RGLP-1 | The crude polysaccharides were injected into the cut-off ultrafiltration membrane to obtain different fractions (EGLP and RGLP) according to their molecular weight. RGLP was further purified through Sephacryl S-500 HR column and eluted with 0.2 mol/L NaCl resolution at the flow rate of 0.8 mL/min | [34] |
| Ganoderma lucidum | Spore | GLSP-I | Water extraction and ethanol precipitation; eluted by different concentrations of NaCl solution using middle-pressure liquid chromatography equipped with a DEAE Sepharose Fast Flow column; then purified on Sephadex G100 column | [62] |
| Ganoderma lucidum | Mycelia | SeMPN | Water extraction and ethanol precipitation; eluted with different concentrations of NaCl solution on a DEAE Sepharose Fast Flow column; then purified on Sephadex G100 column | [63] |
| Ganoderma lucidum | N/A | GLPs | Hot water extraction; graded by ultrafiltration membranes (100 kDa, 10 kDa and 1 kDa); transmembrane pressures of 0.6–1 MPa; flow speed of 700 r/min; deproteinized with Sevag method and precipitated with ethanol; eluted using DEAE Sepharose fast flow chromatography with different concentrations of NaCl solutions | [64] |
| Cordyceps militaris | Fruiting body | CM3-SII | Alkali extraction; water-soluble components were fractionated using Q-SepharoseTM Fast Flow column chromatography with NaCl; CM3-S was then purified on a Sephacryl S200HR column with 0.2 mol/L NH4HCO3 | [39] |
| Cordyceps cicadae | Fermentation medium | PACI-1 (an extracellular selenium-enriched polysaccharide) | PACI solution was loaded onto DEAE-52 column and eluted with pure water and a step gradient of 0.1 M to 0.3 M NaCl solution. Then, the main fraction was eluted with pure water on a Sephadex G-100 column and filtered through 8000 Da molecular mass membranes to desalt | [65] |
| Cordyceps cicadae | Fruiting body | JCH-a1 | Ultrasonically-assisted enzymatic extraction (cellulose:chitinase = 1:1); deproteinized with Sevage; the fractions were eluted with DEAE-32 column and Sephadex G-100 column, respectively | [66] |
| Cordyceps cicadae | Bacterium substance | BSP | Hot water bath extraction (78 °C); deproteinized with Sevage; eluted on DEAE-52 column chromatography with different concentrations of NaCl (0, 0.1, 0.2, 0.3, 0.4, 0.5 M NaCl) | [67] |
| Cordyceps cicadae | Spore powder | SPP | Hot water bath extraction (78 °C); deproteinized with Sevage; eluted on DEAE-52 column chromatography with different concentrations of NaCl (0, 0.1, 0.2, 0.3, 0.4, 0.5 M NaCl) | [67] |
| Cordyceps cicadae | Fruiting body | PPP | Hot water bath extraction (78 °C); deproteinized with Sevage; eluted on DEAE-52 column chromatography with different concentrations of NaCl (0, 0.1, 0.2, 0.3, 0.4, 0.5 M NaCl) | [67] |
| Cordyceps militaris | N/A | CMP | Hot water reflux extraction; eluted with 0, 0.1, 0.2, 0.3, 0.4 and 0.5 mol/L NaCl onto a DEAE-52 cellulose column | [68] |
| Cordyceps militaris | Culture broth | EPS-III (A homogenous exopolysaccharide) | The culture broth was centrifuged, collected and concentrated; then it was precipitated with ethanol absolute (1:4); Sevage method and macroporous absorption resin (AB-8) to remove protein and pigment; further purified using Sephadex G-200 with distilled water | [69] |
| Cordyceps militaris | Fermentation broth | AEPS-II (An acidic exopolysaccharide) | The fermentation broth was centrifuged, concentrated and mixed with anhydrous ethanol; NKA-9 macroporous adsorption resin was used to remove pigment and protein using Sevage method; the crude EPS was isolated and purified with DEAE-Sephacel and Sephadex G-200 column chromatography, respectively | [45] |
| Poria cocos | Powder | PCP-1 | The powder was extracted using deep eutectic solvent (ChoCl and oxalic acid in a molar ratio of 1:2); Sevage method was used for deproteinization; then the water solution was eluted on a Sephadex G-15 column | [71] |
| Poria cocos | Mycelial culture | FMGP | It was isolated from 49-day-old cultures of mycelia, after extraction with 0.1 M sodium acetate, centrifugation, precipitation and dialyzation, the supernatant was purified on a column of Fractogel BioSec | [72] |
| Wolfiporia cocos | Dried sclerotia | WIP (an acidic polysaccharide that is insoluble in water) | Dried sclerotia was extracted with NaOH solution (0.75 mol/L) and neutralized with HCl (1 mol/L); petroleum ether and hot water was applied to remove fat-soluble and water-soluble molecules; dialysis for removing inorganic salts | [73] |
| Poria cocos | Sclerotium | PCP-1C | The dried powder was extracted with ultrapure water and precipitated with ethanol; the Sevage method was used to remove protein; cellulose DEAE-52 column and Sephacryl S-500 column were applied to obtain PCP-1C | [74] |
| Poria cocos | Fermentation broth | EPS-0 M, EPS-0.1 M (exopolysaccharide) | Directly concentrated the supernatant of the fermentation broth; the water-soluble solution was dealt with using DEAE-52 cellulose anion exchange column and Sephadex G-100 gel column | [75] |
| Poria cocos | Lyophilized mycelium | IPS-0 M, IPS-0.1 M (intracellular polysaccharide) | Extract the lyophilized mycelium in hot water; the water-soluble solution was dealt with using DEAE-52 cellulose anion exchange column and Sephadex G-100 gel column | [75] |
| Polyporus umbellatus | Fruiting body | HPP | The Sevage method was used to remove protein; then the water solution was eluted using DEAE-52 cellulose column and Sephadex G-100 gel-filtration column, respectively | [76] |
| Polyporus umbellatus | Sclerotia | PUP-W-1 | Boiling water extraction; initial separation was completed using DEAE-Sepharose Fast-Flow column with water and different concentrations of NaCl; further separation was completed via SuperdexTM G-75 column | [77] |
| Polyporus grammocephalus | Fruit body | PGPS | The fruit bodies were boiled with 4% NaOH and precipitated with ethanol; the crude water soluble polysaccharide was fractionated with GPC on Sepharose-6B column | [78] |
| Omphalia lapidescens | Fruit body | OL-2 | The fruit body was extracted with hot water; the insoluble material was extracted with 0.1 M NaOH and 0.5 M NaOH, respectively; the 0.5 M NaOH soluble material was washed with water and 0.1 M NaOH, dissolved with 0.5 M NaOH and acidified with AcOH | [79] |
| Lasiosphaera fenzlii | Fruit body | TFP-1, TFP-2, TFP-3, TFP-4 | The fruit body was extracted with 0.2 M NaOH solution; trichloroacetic acid was used to remove free protein; DEAE cellulose column, SephacrylTM S-200 and SephacrylTM S-300 gel columns were used for further isolation and purification | [80] |
| Calvatia geigantea | N/A | CGP I-1 | Water extraction; DEAE-Sepharose fast flow ion-exchange column chromatography and Sephacryl S-300 gel filtration were used for isolation and purification | [81] |
3. Structural Identification of Polysaccharides from Fungal TCM
3.1. Ganoderma
3.2. Cordyceps
3.3. Poria Cocos
3.4. Polyporus
3.5. Omphalia lapidescens
3.6. Lasiosphaera fenzlii
4. Biological Functions
4.1. Anti-Tumor Activity
4.2. Anti-Oxidant Activity
4.3. Immunomodulatory Activity
4.4. Hypolipidemic Activity
4.5. Hypoglycemic Activity
4.6. Hepatoprotective Activity
4.7. Modulation on Gut Microbiota
4.8. Anti-Inflammatory Activity
4.9. Other Activities
| Bioactivity | Compound Name | Source | Subjects | Dose | Effects and Mechanism | Reference |
|---|---|---|---|---|---|---|
| Anti-tumor activity | Pachyman | Poria cocos | HepG2 human liver cancer cell and network pharmacology | 0, 25 and 50 μM | Pachyman exerted an anti-cancer activity by elevating the intracellular level of ALB protein and downregulating the cellular content of VEGFA protein | [85] |
| HPP | Polyporus | BBN-induced Fischer-334 rats and RAW 264.7, TPH-1 and T24 cells | 1, 10 and 100 μg/mL | HPP could inhibit bladder cancer in BBN-induced rats by ameliorating histological damages in bladder; improve the tumor inflammatory microenvironment by regulating TAM polarization and NF-κB/NLRP3 signaling pathway | [76] | |
| HPP | Polyporus | Phorbol myristate acetate-induced THP-1 human leukemic cell | 1, 10 and 100 μg/mL | HPP could confront bladder cancer through inhibiting the proliferation and progression of bladder cancer by the polarization of macrophages to M1 type, downregulating the JAK2/NF-κB signaling pathway | [86] | |
| CSP | Cordyceps sinensis | HCT116 cell line | 0–800 μg/mL | CSP could inhibit the proliferation of HCT116 cells by inducing apoptosis and autophagy flux blockage. It might be achieved by modulating PI3K-Akt-mTOR and AMPK-mTOR-ULK1 signaling pathways | [87] | |
| CCP | Cordyceps cicadae | Hela cells | 0, 25, 50, 100, 200, 400, 800 and 1600 μg/mL | CCP could inhibit the expression of Cyclin E, Cyclin A and CDK2, promote the expression of P53, activate Caspase cascade reaction, and up-regulate death receptor and the ratio of pro-apoptotic factor/anti-apoptotic factors to cause the cell cycle arrest and induce the apoptosis | [88] | |
| WCP | Wild Cordyceps | H22 tumor-bearing BALB/c mice | 100 and 300 mg/kg | The large molecular weight polysaccharide could inhibit the proliferation of H22 tumors by improving immune function and promoting the apoptosis of tumor cells, and mainly interfering with IL-10/STAT3/Bcl2 and Cytoc/Caspase8/3 signaling pathways | [89] | |
| WSG | Ganoderma lucidum | LLC1 cells induced lung cancer C57BL/6 mice | 75 mg/kg | WSG significantly prevented tumor growth and the formation of metastatic nodules in the lung tissue, and promoted the apoptotic responses mediated by cisplatin | [90] | |
| WSG | Ganoderma lucidum | Human tongue cancer SAS and HSC3 cells | 0–800 μg/mL | WSG increased subG1 and G2/M populations and elevated Bax/Bcl2 ratio to induce apoptosis; inhibited phosphorylation of EGFR and AKT | [91] | |
| GLPS | Ganoderma lucidum | Mouse RAW 264.7 macrophages and hepatocellular carcinoma cell line Hepa1–6 | 0–200 μg/mL | GLPS markedly prevented the growth of Hepa1–6 allograft; promoted the expression of M1 phenotype marker CD86, iNOS, and pro-inflammatory cytokines (IL-12a, IL-23a, IL-27 and TNF-α); blocked macrophage polarization towards the M2 phenotype; reduced the expression of CD206, Arg-1, IL-6 and IL-10; upregulated the phosphorylation of MEK and ERK, IκBα and P65 | [92] | |
| Anti-oxidant activity | PCPP | Poria cocos peels | In vitro | 1–5 mg/mL | PCPP has great anti-oxidant activity by scavenging DPPH radicals and reducing ABST radicals in a dose-dependent fashion | [93] |
| PPS | Polyporus umbellatus | In vitro | 1–8 mg/mL | PPS has the significant scavenging ability of DPPH free radicals and hydroxyl free radicals | [94] | |
| PPS | Polyporus umbellatus | In vitro | 0.5–8 mg/mL | PPS exhibits the significant scavenging ability on DPPH and other free radicals in a dose-dependent manner | [58] | |
| Immunomodulatory activity | CMP | Poria cocos | RAW 264.7 | 12.5, 25, 50, 100, 200 and 400 μg/mL | CMP plays a crucial role in immunoregulation by improving the secretions of iNOS, TNF-α and IL-6 through increasing the expression of iNOS, TNF-α and IL-6 mRNA | [95] |
| AESP-II | Cordyceps militaris | Cyclophosphamide-induced BALB/c mice | 25, 50 and 100 mg/kg | AESP-II could promote the proliferation of spleen T and B lymphocytes, increase the levels of cytokines and immunoglobulin secreted by T and B lymphocytes, and activate the MAPK signaling pathway to involve in the immunomodulatory function | [70] | |
| CSP | Cultured Cordyceps sinensis | Cyclophosphamide-induced female BALB/c mice | 25, 50 and 100 mg/kg | CSP inhibited immunosuppression in mice via stimulating cytokines secretion (IL-12, IFN-γ, IL-4, IL-13, IL-6, IL-10, IL-17, TGF-β3, TNF-α, IL-2, IL-21) and transcription factors production (T-bet, GATA-3, RORγt, Foxp3), upregulating TLRs and NF-κB pathway key proteins | [96] | |
| CCSP-2 | Cordyceps cicadae | Cyclophosphamide-induced immunosuppressive C57BL/6 mice | 50, 100 and 200 mg/kg | CCSP-2 significantly increased spleen and thymus indices, enhanced macrophage phagocytic activity, stimulated splenocyte proliferation, improved natural killer cytotoxicity and bone marrow suppression, regulated the secretion of cytokines and immunoglobulins and modulated antioxidant enzyme system | [97] | |
| Hypolipidemic activity | PCP | Poria cocos | High-fat diet-induced mice | 1.5 g/day | PCP significantly reduced serum and hepatic lipid levels, and altered metabolic pathways including fatty acid metabolism, bile acid metabolism and tricarboxylic acid cycle | [98] |
| CM3-SII | Cordyceps militaris | Heterozygous low-density lipoprotein receptor (LDLR)-deficient hamster | 25, 100 mg/kg | CM3-SII attenuated total plasma cholesterol, non-high-density lipoprotein cholesterol and triglyceride; enhanced the concentration of plasma apolipoprotein A1 and the expression of liver X receptor α/ATP-binding cassette transporter G8 mRNA pathway and suppressed the expression of Niemann-Pick C1-like 1; downregulated sterol regulatory element-binding protein 1c and upregulated peroxisome proliferator-activated receptor α; increased the abundance of Actinobacteria and Faecalibaculum and the ratio of Bacteroidetes/Firmicutes. | [99] | |
| CM1 | Cordyceps militaris | 3T3-L1 cell; LDLR(+/−) hamsters | 100 μg/mL; 100 mg/kg | CM1 alleviated hyperlipidemia by downregulating the plasma level of apolipoprotein B48, modulating the expression of key genes and proteins in liver, small intestine and epididymal fat, and inhibiting preadipocyte differentiation in 3T3-L1 cells by suppressing the key genes involved in lipid droplet formation | [100] | |
| SeCMP | Cordyceps militaris | High-fat diet-fed C57BL/6 mice | 50, 100 and 200 mg/kg | SeCMP-200 showed significantly hypolipidemic activity by decreasing serum triglyceride and low-density lipoprotein cholesterol, ameliorating obese-induced inflammation, decreasing the abundance of Dorea, Lactobacillus, Clostridium, Ruminococcus and increasing mucosal beneficial bacteria Akkermansia | [101] | |
| GLP | Ganoderma lucidum | High-fat diet-induced Kunming mice | 100, 200 and 400 mg/kg | GLP inhibited the body weight gain and excessive lipid levels, ameliorated tissue injury; activated Nrf2-Keap1 and suppressed NF-κB signaling pathway; facilitated cholesterol reverse transport by LXRα-ABCA1/ABCG1 pathway; promoted the expression of CYP7A1 and CYP27A1; inhibited intestinal FXR-FGF15 expressions | [102] | |
| Hypoglycemic activity | EPS-III | Cordyceps militaris | STZ-induced diabetic KM mice | 60, 120 and 225 mg/kg | EPS-III exerted significantly hypoglycemic effect through alleviating weight loss, reducing plasma glucose concentration, improving glucose tolerance, protecting immune organs and repairing dyslipidemia | [69] |
| AEPSa | Cordyceps militaris | High-fat diet and STZ-induced C57BL/6 mice | 400 mg/kg | AEPSa ameliorating diabetes through increasing Allobaculum, Alistipes, Lachnospiracae_NK4A136_group and norank_f_Muribaculaceae and decreasing Enterococcus and Ruminococcus_torques_group, inhibiting TLR4/NF-κB pathway | [103] | |
| SPP | Cordyceps cicadae | HepG2 cells and T2DM KM mice | 100, 200 and 400 mg/kg | SPP significantly increased glucose absorption and alleviated insulin resistance in HepG2 cells; SPP exerted hypoglycemic effect through activating PI3K/Akt signaling pathway to reduce hepatic insulin resistance | [67] | |
| CMP | Cordyceps militaris | High-fat/high-sucrose diet-induced C57BL/6 mice | N/A | CMP played a crucial role in the hypoglycemic effect by promoting the population of next generation probiotic Akkermansia muciniphila in the gut | [104] | |
| F31 | Ganoderma lucidum | High fat diet and STZ-induced type 2 diabetic Kunming mice | 60 and 180 mg/kg | F31 markedly decreased Firmicutes and enhanced the abundance of Bacteroidetes. Specifically, F31 may ameliorate glucose, insulin resistance and inflammation by inhibiting the release of endotoxins into the circulation from intestine, carbohydrate fermentation in gut and activation of intestine–brain axis | [105] | |
| F31 | Ganoderma lucidum | C57BL/c and db/db mice | N/A | F31 ameliorated hyperglycemia through different approaches: decreased adenosine, galactitol and glycerophosphocholine and increased arginine, proline, arachidonic acid, creatine, aspartic acid, leucine, phenylalanine and ornithine to protect kidney function; increased Caspase-3, Caspase-6 and Bax and inhibited Bcl-2 to promote apoptosis in epididymal fat; reduced mitochondrial membrane potential to induce adipocyte apoptosis | [106] | |
| Hepatoprotective activity | PCP-1C | Poria cocos | Alcohol-induced C57BL/6N mcie | 25, 50 and 100 mg/kg | PCP-1C exerted a hepatoprotective action by decreasing inflammatory factor release, inhibiting oxidative stress and apoptosis, and ameliorating intestinal barrier injury | [107] |
| GLP | Ganoderma lucidum | C57BL/6 mice and rat HSC-T6 hepatic stellate cell line | 150 and 300 mg/kg; 0, 1.25, 2.5, 5, 7.5 and 10 mg/mL | GLP dramatically ameliorated hepatic fibrogenesis and inflammation by TLR4/NF-κB/MyD88 signaling pathway; blocked HSCs activation by reducing collagen I and a-SMA expressions; suppressed cell cycle; induced S phase arrest; inhibited the ECM-receptor interaction-associated molecule expressions (ITGA6 and ITGA8); restrained TGF-β/Smad signaling pathway in mice; decreased TGF-β1, Smad2 and Smad3 phosphorylation and promoted Smad7 expression in HSC-T6 cells | [108] | |
| Modulation on gut microbiota | WIP | Wolfporia cocos | Alcohol-induced C57BL/6 mice | 1 g/kg | WIP significantly enhanced the ratio of Firmicutes to Proteobacteria, increased the abundance of Lachnospiraceae and inhibited the ethanol-induced fungal overgrowth. It activated the PPAR-γ signaling pathway and facilitated a hypoxic state that suppressed the overgrowth of fungi and Proteobacteria in the gut | [109] |
| PCP | Poria cocos | High-fat diet-induced nutritionally obese SD rats | 50, 100 and 200 mg/kg | PCP could regulate intestinal flora structure by increasing the relative abundance of Prevotella, Bacteroides and Sutteralla, and decreasing the ratio of Firmicutes/Bacteroidetes and the relative abundance of Morganella | [110] | |
| CSP | Cultured Cordyceps sinensis | Cyclophosphamide-induced female BALB/c mice | 25, 50 and 100 mg/kg | CSP regulated gut microbiota through recovering SCFAs levels, improving microbial community diversity, modulating the overall structure of gut microbiota, increasing the abundance of probiotics (Lactobacillus, Bifidobacterium and Bacteroides) and decreasing pathogenic bacteria (Clostridium and Flexispira) | [96] | |
| CMP | Cordyceps militaris | High-fat diet-induced C57BL/6 mice | 200 and 400 mg/kg | CMP significantly improved the high-fat diet-induced gut microbiota dysbiosis, increased the abundance of Alloprevotella, Parabacteroides, Butyricimonas and Alistipes, and decreased the abundance of Negativebacillus | [111] | |
| GLP | Ganoderma lucidum | C57BL/c mice | N/A | GLP elevated the abundances of probiotic bacteria including Lachnospiraceae NK4A136, Ruminococcaceae UGG-014, Lactobacillus and Parabacteroides. | [112] | |
| BSGLP | Sporoderm-broken spores of Ganoderma lucidum | C57BL/6J mice | 100 and 300 mg/kg | BSGLP improved gut microbiota dysbiosis; maintained intestinal barrier function; promoted short-chain fatty acid production and GPR43 expression; inhibited serum lipopolysaccharide level; augmented ileum expression of tight junction proteins and antimicrobial peptides; inhibited TLR4/MyD88/NF-κB signaling pathway in adipose tissue | [113] | |
| Anti-inflammatory activity | PCP | Poria cocos | Arteriosclerosis in ApoE−/− mice | 100, 200, 400 mg/kg | The serum inflammatory mediators and lipids were inhibited; the pathological changes of the aorta were improved and the activation of TLR4/NF-κB pathway of the aorta was inhibited | [115] |
| PPs | Poria cocos | Chronic nonbacterial prostatitis in SD rats | 100, 250, 500 mg/kg | PPs plays the role of anti-chronic nonbacterial prostatitis via alleviating inflammation and oxidative stress, regulating hormone production, modifying gut microbiota and remodeling the DNA methylome | [117] | |
| PPs | Poria cocos | Chronic nonbacterial prostatitis in SD rats | 250 mg/kg | PPs alleviates the chronic nonbacterial prostatitis by improving the histological damages in the inflamed prostate, inhibiting inflammation and regulating the gut microbiota by targeting Ruminococcaceae NK4A214 group | [118] | |
| PPs fermentation broth | Poria cocos | Chronic nonbacterial prostatitis in SD rats | 250 mg/kg | It is proved that the metabolites of PPs 7-ketodeoxycholic acid and haloperidol glucuronide may be the signal molecules of the “gut-prostate axis” | [119] | |
| CMP | Poria | Ulcerative colitis in ICR female mice | 300 mg/kg | CMP alleviated ulcerative colitis in mice through inhibiting colonic shortening and inflammation in colonic tissues, and regulating gut microbiota | [120] | |
| PCP | Poria cocos | Nonalcoholic steatohepatitis in C57BL/6 mice | 150 and 300 mg/kg | The mechanism of PCP in preventing the development of NASH may be associated with the modulation of intestinal microbiota and the downregulation of the NF-κB/CCL3/CCR1 axis | [121] | |
| PCP | Poria cocos | Nonalcoholic steatohepatitis in C57BL/6 J mice and zebrafish | 50, 100 and 200 mg/kg | PCP could slow down weight gain, hyperlipidemia and liver steatosis induced by high-fat diet; reduce the destruction of the gut-vascular barrier and the translocation of endotoxins; inhibit intestinal pyroptosis by regulating PARP-1 | [122] | |
| CM1 | Cordyceps militaris | Low-density lipoprotein receptor knockout (LDLR−/−) mice | 25, 50 and 100 mg/kg | CM1 could reduce plasma lipid level and formation of atherosclerotic plaques through multiple pathways, enhanced plasma level of apolipoprotein A-I, decreased the levels of triglyceride, apolipoprotein B and total cholesterol, inhibited sterol regulatory element binding protein 1c, increased the liver X receptor α/ATP-binding cassette G5 pathway, inhibited PPAR-γ and adipose triglyceride lipase in epididymal fat | [116] | |
| GLPs | Ganoderma lucidum | High-fat diet-induced Japanese big-ear white rabbits | 300 mg/kg | GLPs could prevent the progression of atherosclerosis through improving endothelial dysfunction and inflammatory polarization of macrophages, accelerating the apoptosis of foam cells | [123] | |
| GLP | Ganoderma lucidum | AOM/DSS-induced C57BL/6 mice | 200 and 300 mg/kg | GLP ameliorated microbiota dysbiosis; promoted short-chain fatty acid production; inhibited TLR4/MyD88/NF-κB signaling pathway; increased numbers of goblet cells, MUC2 secretion, tight junction protein expressions; inhibited macrophage infiltration and IL-1β, iNOS, COX-2 expressions; inhibited the activation of MAPK | [23] | |
| Other activities | PCPP | Poria cocos peels | AML-12 liver cell/60Co-γ induced KM mice | 50–400 μg/mL/5, 10 and 20 mg/kg | PCPP exerts a significantly radiation protection effect through ameliorating the damage of spleen and liver, improving the damage of hematopoietic system by regulating erythrocytes, platelets and hemoglobin and decreasing the degree of oxidative damage | [93] |
| PPS | Polyporus | Bleomycin-induced lung fibrosis C57BL/6 mice and human lung fibroblasts cell line | 100 mg/kg; 1 mg/mL | PPS significantly improved bleomycin-induced lung fibrosis in mice through ameliorating pathological damages of lung tissues; it exerted antifibrotic effects in vitro via inhibiting fibroblast-to-myofibroblast transition, suppressing ECM deposition, repressing lung fibroblast proliferation and migration, suppressing TGF-1β-induced Smad2/3 activating | [125] | |
| RLSP | Lasiophaere fenzlii | In vitro | 0.1, 0.01 and 0.001 mg/mL | RLSP possessed an inhibitory effect on both Staphylococcus aureus and Escherichia coil | [126] | |
| CPA-1 and CPB-2 | Cordyceps cicadae | High fructose/high fat diet induced obesity and metabolic disorders rats | 100 and 300 mg/kg | These two polysaccharides regulated metabolic disorders through inhibiting insulin and glucose tolerance, serum insulin and glucose levels, reducing serum and hepatic lipid profiles, liver function enzymes and pro-inflammatory cytokines, suppressing hepatic oxidative stress and hepatic lipid accumulation | [127] | |
| CMP | Cordyceps militaris | Ovalbumin-induced allergic asthma BALB/c mice | 50, 100 and 200 mg/kg | CMP showed significantly anti-allergic asthma effects through improving inflammatory cytokine levels, ameliorating the histopathological damages, regulating oxidative and inflammatory pathways, reversing gut dysbiosis and improving microbiota function | [128] | |
| CMP | Cordyceps militaris | High-fat diet-induced C57BL/6 mice | 400 mg/kg | CMP showed a promising ability to protect mice from obesity through ameliorating systematic inflammation, restoring the phylogenetic diversity of gut microbiota, increasing the relative abundance of short-chain fatty acid-producing bacteria, down-regulating the level of bacteria, which were positively related to the development of obesity | [129] | |
| CMPB | Cordyceps militaris | ICR mice | 400 and 800 mg/kg | CMPB significantly decreased fatigue metabolites and oxidative stress, increased the expression level of BDNF, PI3K, Nrf2 and HO-1 in the hippocampus | [130] | |
| GLP-1 | Ganoderma lucidum | Chronic cerebral hypoperfusion mice | N/A | GLP-1 improved cognitive impairment mice by elevating the levels of Foxp3+ Treg cell and inhibiting energy metabolism disorder | [26] | |
| GLP | Ganoderma lucidum | C57BL/6 mice and CD1 mice | 1, 5 and 12.5 mg/kg | GLP inhibited the expression of IL-1β and TNF-α; promoted the expression of IL-10 and BDNF; prevented the activation of microglia and proliferation of astrocytes in hippocampus; increased the expression of GluA1 S845 phosphorylation as well as GluA1 and GluA2 expression levels | [131] | |
| GLPs | Ganoderma lucidum | Ethanol-induced acute gastric injury SD rat | 100, 200 and 400 mg/kg | GLPs diminished the gastric injury in a dose-dependent manner through regulating anti-oxidation, inhibiting inflammation and decreasing the expression of histamine in serum | [132] | |
| GLP | Ganoderma lucidum | LPS-induced sepsis C57BL/6J mice | 25 mg/kg | GLP could elevate the expression of SIRT1, decreased inflammatory factors in serum and inflammatory cells in heart tissues, blocked apoptosis and facilitated proliferation of myocardial tissues | [133] | |
| Liz-H | Ganoderma lucidum | Cisplatin plus docetaxel induced cachexia C57BL/6J mice | 250 mg/mouse | Liz-H could block weight loss, muscle atrophy and neutropenia; downregulate muscle protein degradation-related genes (MuRF-1 and Atrogin-1); increase myogenic factors (MyoD and myogenin); restore the abundance of Ruminococcaceae and Bacteroides to normal levels | [134] | |
| GLP | Ganoderma lucidum | D-galactose-induced C57BL/6J mice | N/A | GLP increased the expression levels of AQP5, AQP4, AQP1; blocked the release of inflammatory factors; upregulated core clock genes and proteins; restored the co-localized expression of CLOCK and AQP5 | [135] | |
| GLP | Ganoderma lucidum | LPS-induced C57BL/6N mice | 25, 50 and 100 mg/kg | GLP inhibited inflammatory cell infiltration; reduced the expression levels of GM-CSF, IL-6, IL-1β, TNF-α and Saa3; blocked the activation of NRP1; promoted the expression of Bcl2/Bax and LC3; decreased the ratio C-Caspase 3/Caspase 3 and P62 expression | [124] | |
| GLP5 | Ganoderma lucidum | Human acute T cell leukemia cell line | 25 and 50 mg/L | GLP5 notably suppressed the proliferation of Jurkat cells; increased the expression levels of Caspase3; regulated the expression levels of Bax and Bcl-2 | [136] | |
| CSP | Cordyceps sinensis | Dextran sodium sulfate-induced C57BL/6J mice | N/A | CSP significantly increased the colon length; improved colon tissue damage; inhibited the activation of NF-κB pathway; decreased the expressions of inflammatory cytokines; augmented the number of goblet cells; regulated the expressions of intestinal tight junction proteins (Occludin and Claudin-1); promoted the formation of IgA-secretory cells and sIgA contents | [137] | |
| CMPS-80 | Cordyceps militaris | Apolipoprotein E-deficient mice | N/A | CMPS-80 dramatically blocked formation of atherosclerotic lesions and plasma lipid profiles; regulated multiple lncRNA-microRNA-mRNA axes | [138] |
5. Quality Control
6. Discussion and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current Advancements in Antitumor Properties and Mechanisms of Medicinal Components in Edible Mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Yu, M.A.; Pyun, Y.R.; Hwang, J.K.; Chu, D.C.; Juneja, L.R.; Mourão, P.A. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.S.; Krupodorova, T.A.; Barshteyn, V.Y.; Artamonova, A.B.; Shlyakhovenko, V.A. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66. [Google Scholar] [PubMed]
- Li, Z.Y.; Yao, X.P.; Liu, B.; Ha Nizaier, R.; Yang, G.; Zhan, S.; Qi, M.A. Auricularia auricular-judae polysaccharide attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and inflammation. Biomed. Rep. 2015, 3, 478–482. [Google Scholar]
- Bian, C.; Wang, Z.; Shi, J. Extraction Optimization, Structural Characterization, and Anticoagulant Activity of Acidic Polysaccharides from Auricularia auricula-judae. Molecules 2020, 25, 710. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Preparation, structure and activity of polysaccharide phosphate esters. Biomed. Pharmacother. 2021, 144, 112332. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Gong, H.; Li, W.; Sun, J.; Jia, L.; Guan, Q.; Guo, Y.; Wang, Y. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, H.; Liang, X.D.; Zhang, M.T.; Tang, Y.X.; Wang, J.H.; Mao, J.L. The isolation, structural features and biological activities of polysaccharide from Ligusticum chuanxiong: A review. Carbohydr. Polym. 2022, 285, 118971. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guo, Y.L.; Zhang, Y.; Li, Y.; Liang, J.; Kuang, H.X.; Xia, Y.G. Structure and immunological activity of an arabinan-rich acidic polysaccharide from Atractylodes lancea (Thunb.) DC. Int. J. Biol. Macromol. 2022, 199, 24–35. [Google Scholar] [CrossRef]
- Liu, X.Q.; Yan, X.H.; Liang, J.; Kuang, H.X.; Xia, Y.G. Microwave assisted free radical degradation of Schisandra polysaccharides: Optimization, identification and application. Int. J. Biol. Macromol. 2023, 237, 124107. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Liang, J.; Chai, J.H.; Kuang, H.X.; Xia, Y.G. Structure of a highly branched galacturonoglucan from fruits of Schisandra chinensis (Turcz.) Baill. Carbohydr. Polym. 2023, 313, 120844. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, S96–S115. [Google Scholar] [CrossRef]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, S.Y.; Yari, K.A.; Pourghassem, G.B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yu, L.S.; Liang, J.; Yang, B.Y.; Kuang, H.X. Chromatography and mass spectrometry-based approaches for perception of polysaccharides in wild and cultured fruit bodies of Auricularia auricular-judae. Int. J. Biol. Macromol. 2019, 137, 1232–1244. [Google Scholar] [CrossRef]
- Hu, Q.; Li, Y.; Liu, C.; Huang, L.P.; Zeng, L.; Wang, S.; Song, H.; Peng, H.; Huang, J.; Chen, C.; et al. Effects of polysaccharide from Portulaca oleracea L. on voltage-gated Na+ channel of INS-1 cells. Biomed. Pharmacother. 2018, 101, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, S.; Li, H.; Wang, X.; Song, L.; Xue, J. Polysaccharide from Ganoderma lucidum alleviates cognitive impairment in a mouse model of chronic cerebral hypoperfusion by regulating CD4+CD25+Foxp3+ regulatory T cells. Food Funct. 2022, 13, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, J.; Li, F.; Yu, H.; Lin, D.; Lin, S.; Li, M.; Zhou, H.; Yang, B. Ganoderma lucidum polysaccharide peptide alleviates hyperuricemia by regulating adenosine deaminase and urate transporters. Food Funct. 2022, 13, 12619–12631. [Google Scholar] [CrossRef]
- Liang, J.; Rao, Z.H.; Jiang, S.L.; Wang, S.; Kuang, H.X.; Xia, Y.G. Structure of an unprecedent glucuronoxylogalactoglucomannan from fruit bodies of Auricularia auricula-judae (black woody ear). Carbohydr. Polym. 2023, 315, 120968. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jiang, K. Antioxidant activity in vitro and in vivo of the polysaccharides from different varieties of Auricularia auricula. Food Funct. 2016, 7, 3868–3879. [Google Scholar] [CrossRef]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohydr. Polym. 2017, 172, 68–77. [Google Scholar] [CrossRef]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Zhang, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, G.; Xue, L.; Zhang, H.; Wang, J.; Xiang, H.; Li, J.; Zheng, K. Isolation, structural characterizations and bioactivities of exopolysaccharides produced by Bacillus licheniformis. Int. J. Biol. Macromol. 2019, 141, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mao, X.; Xu, B. Pulsed electric field extraction enhanced anti-coagulant effect of fungal polysaccharide from Jew’s ear (Auricularia auricula). Phytochem. Anal. 2013, 24, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, J.; Kan, Q.; Song, M.; Hou, T.; An, S.; Lin, H.; Chen, H.; Hu, L.; Xiao, J.; et al. Extraction, Structural Characterization, and Immunomodulatory Activity of a High Molecular Weight Polysaccharide from Ganoderma lucidum. Front Nutr. 2022, 9, 846080. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.; Lam, D.H.; Nguyen, T.; Phuong, M.T.; Phan, L.T.M.; Vuong, H.T.; Nguyen, D.V.; Linh, N.T.T.; Hoang, M.N.; Mai, T.P.; et al. Utilization of Response Surface Methodology in Optimization of Polysaccharides Extraction from Vietnamese Red Ganoderma lucidum by Ultrasound-Assisted Enzymatic Method and Examination of Bioactivities of the Extract. Sci. World J. 2021, 2021, 7594092. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhou, L.; Mei, Q.X. Research and optimization of internal boiling method for extracting polysaccharide from Ophiocordyceps sinensis. Shizhen Tradit. Chin. Med. 2018, 29, 2893–2895. [Google Scholar]
- Zha, Y.; Zhang, Z.H.; Li, X.Z.; Xu, H.; Liu, X.; Li, Y.L. Optimization of the extraction process of polysaccharide from Ophiocordyceps sinensis Mycelium by PB test combined with BBD response surface methodology. Chin. Edible Fungi 2020, 39, 25–31. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N. Cordyceps militaris polysaccharides: Preparation and topical product application. Fungal Biol. Biotechnol. 2023, 10, 3. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yang, X.; Lin, P.; Liu, N.; Li, X.; Zhang, B.; Guo, S. Purification, structural characterization, and PCSK9 secretion inhibitory effect of the novel alkali-extracted polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2021, 179, 407–417. [Google Scholar] [CrossRef]
- Liang, J.H.; Du, B. Optimization of Steam Explosion Extraction Process for Poria cocos Polysaccharides. Grain Oil 2021, 34, 73–77. [Google Scholar]
- Zhang, B.H.; Ma, C.; Zhang, M.; Yang, L.F.; Meng, X.F.; Wu, M.Y. Research on the Application Status of Steam Explosion Technology. Chin. Fruit Veg. J. 2020, 40, 31–34+38. [Google Scholar]
- Hu, C.T.; Xiao, Y.; Zhou, P.F.; Fu, M.; Hu, X. Extraction of Poria cocos polysaccharide and its bacteriostasis study. J. Huaihua Univ. 2012, 31, 15–18. [Google Scholar]
- Xiang, F.B.; Xiong, T.H.; Hu, S.W.; Chen, X.L.; Xiang, F. Extraction of Poria cocos polysaccharide from Jiuzihe River by complex enzyme method and its antioxidant activity. Food Stud. Dev. 2022, 43, 118–123. [Google Scholar]
- Huang, Q.; Wang, Y.; Wu, X.X.; Li, Y. Optimization of Ultrasound Assisted Enzymatic Extraction of Poria Cocos Polysaccharides Based on D-optimal Mixture Design and Response Surface Methodology. Chem. Res. Appl. 2022, 34, 2082–2087. [Google Scholar]
- Zheng, Y.Q.; Zhang, A.Q.; Zhang, X.J.; Mei, G.M.; He, P.F. Response Surface Optimization of Ultrasound Assisted Extraction Process and Antioxidant Activity Analysis of Polyporus umbellatus Sclerotinia Polysaccharides. Food Ind. Technol. 2023, 44, 255–263. [Google Scholar]
- Qiao, Y.M.; Chen, W.Q.; Deng, B.W.; Peng, H.; Xie, X.C.; Zhang, H.; Pan, J.W. Microwave-assisted extraction of polysaccharides from Polyporus umbellatus optimized by box-behnken design-response surface methodology. Food Sci. Biotechnol. 2015, 34, 986–994. [Google Scholar]
- Bi, Y.; Miao, Y.; Han, Y.; Xu, J.; Wang, Q. Biological and physicochemical properties of two polysaccharides from the mycelia of Grifola umbellate. Carbohydr. Polym. 2013, 95, 740–745. [Google Scholar] [CrossRef]
- Ueno, Y.; Okamoto, Y.; Yamauchi, R.; Kato, K. An antitumor activity of the alkali-soluble polysaccharide (and its derivatives) obtained from the sclerotia of Grifora umbellata (Fr.) Pilát. Carbohydr. Res. 1982, 101, 160–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Q.; Yang, X.F.; Gu, C.X.; Liu, H.M. Optimization of enzymatic coupled with ultrasonic-assisted extraction of polysaccharide from Polyporus umbellatus and its antioxidant activity. Nat. Prod. Res. Dev. 2015, 27, 1657–1662. [Google Scholar]
- Bi, C.H.; Shen, L.Q. Optimization of Extraction Process for Polysaccharides from Leiwan by Response Surface Analysis. Food Sci. Technol. 2010, 35, 217–221. [Google Scholar]
- Xu, M.F.; Shen, L.Q.; Wang, K.W.; Wang, X.Y. Extraction and separation of Leiwan polysaccharide and its antioxidant activity. J. China Foods Ltd. 2011, 11, 42–46. [Google Scholar]
- Li, Z.M.; Ke, C.L.; Wang, Y.B. The protective effect of crude polysaccharides from liquid fermented mycelium of Dahua Mabo on liver injury and in vitro antioxidant activity. J. Edible Fungi 2015, 22, 70–74. [Google Scholar]
- Zhu, Y.; Yuan, S.X.; Li, B. The scavenging effect of water-soluble polysaccharides from purple puffweed on oxygen free radicals. Anhui Agric. Sci. 2010, 38, 4053–4055. [Google Scholar]
- Shi, W.G.; Bai, G.D.; Zong, X.M.; Yuan, H.; Du, J.; Gao, Q.D. Optimization of Ultrasound Extraction Process for Crude Polysaccharides from Peeled Marble by Central Composite Design Response Surface Methodology. Wild Plant Resour. China 2018, 37, 4–7. [Google Scholar]
- Wang, H.; Wang, Y.; Zhan, C.J.; Wang, Y.; Xu, W. Study on the Process of Snail Enzyme Assisted Extraction of Marble Polysaccharides. China Feed 2017, 5, 24–26+30. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.C.; Zhao, W.; Ran, H.Q.; Han, Y. Study on Wall Breaking Method for Extracting Polysaccharides from Marble Tree. Chem. World 2021, 62, 741–745. [Google Scholar]
- Wei, T.; Dan, L.; Yin, J.Y.; Nie, S.P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar]
- Liu, G.K.; Yang, T.X.; Wang, J.R. Polysaccharides from Polyporus umbellatus: A review on their extraction, modification, structure, and bioactivities. Int. J. Biol. Macromol. 2021, 189, 124–134. [Google Scholar] [CrossRef]
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Chen, S.; Guan, X.; Yong, T.; Gao, X.; Xiao, C.; Xie, Y.; Chen, D.; Hu, H.; Wu, Q. Structural characterization and hepatoprotective activity of an acidic polysaccharide from Ganoderma lucidum. Food Chem. X 2022, 13, 100204. [Google Scholar] [CrossRef]
- da Silva, M.S.; de Lima, B.D.; Zavadinack, M.; Simas, F.F.; Smiderle, F.R.; Santana-Filho, A.P.; Sassaki, G.L.; Iacomini, M. Antimelanoma effect of a fucoxylomannan isolated from Ganoderma lucidum fruiting bodies. Carbohydr. Polym. 2022, 294, 119823. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Wen, L.; Yang, B. Structure identification of a polysaccharide in mushroom Lingzhi spore and its immunomodulatory activity. Carbohydr. Polym. 2022, 278, 118939. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Dong, G.; Lai, F.; Wu, H.; Zhan, Q. Purification and comparative study of bioactivities of a natural selenized polysaccharide from Ganoderma Lucidum mycelia. Int. J. Biol. Macromol. 2021, 190, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xing, H.; Tian, B.; Xu, J.; Li, Z.; Zhu, H.; Yang, K.; Sun, P. Characteristics and antifatigue activity of graded polysaccharides from Ganoderma lucidum separated by cascade membrane technology. Carbohydr. Polym. 2021, 269, 118329. [Google Scholar] [CrossRef]
- Zhuansun, W.; Xu, J.; Liu, H.; Zhao, Y.; Chen, L.; Shan, S.; Song, S.; Zhang, H.; Dong, T.; Zeng, H.; et al. Optimisation of the production of a selenium-enriched polysaccharide from Cordyceps cicadae S1 and its structure and antioxidant activity. Front. Nutr. 2022, 9, 1032289. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, W.; Chen, Y.; Feng, S.; Qin, Y.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Extraction, Purification, Physicochemical Properties, and Activity of a New Polysaccharide from Cordyceps cicadae. Front. Nutr. 2022, 9, 911310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, T.; Li, H.; Wang, Y.; Wang, J.; Yuan, H. Structural Characterization and Hypoglycemic Function of Polysaccharides from Cordyceps cicadae. Molecules 2023, 28, 526. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, J.; Jin, L.; Zong, T.; Duan, Y.; Sun, J.; Zhou, W.; Li, G. Preparation, Characterization and Anti-Complementary Activity of Three Novel Polysaccharides from Cordyceps militaris. Polymers 2022, 14, 4636. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, Q.; Song, A.; Liu, Y.; Wang, F.; Jiang, B. Isolation and immune activity of a new acidic Cordyceps militaris exopolysaccharide. Int. J. Biol. Macromol. 2022, 194, 706–714. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, W.; Pei, H.; Chen, G. Structure and physicochemical properties of polysaccharides from Poria cocos extracted by deep eutectic solvent. Glycoconj. J. 2022, 39, 475–486. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, M.K.; Chang, C.C. Structural identification of a fucose-containing 1,3-β-mannoglucan from Poria cocos and its anti-lung cancer CL1-5 cells migration via inhibition of TGFβR-mediated signaling. Int. J. Biol. Macromol. 2020, 157, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, S.; Feng, Y.; Cao, C.; Zhang, Y.; Cheng, S. Characteristics and properties of a polysaccharide isolated from Wolfiporia cocos as potential dietary supplement for IBS. Front Nutr. 2023, 10, 1119583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, Y.; Ge, J.C.; Wang, L.; Peng, D.Y.; Yu, N.J.; Zhang, Y.; Jiang, Y.H.; Luo, J.P.; Chen, W.D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Liu, S.T.; Gan, Q.; Zhang, J.; Chen, N.; Han, C.F.; Geng, W.J.; Wang, B.X.; Han, N.; Jia, S.R.; et al. Four polysaccharides isolated from Poria cocos mycelium and fermentation broth supernatant possess different activities on regulating immune response. Int. J. Biol. Macromol. 2023, 226, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Li, X.; Lai, G.N.; Li, J.H.; Jia, W.Y.; Cao, Y.Y.; Xu, W.X.; Tan, Q.L.; Zhou, C.Y.; Luo, M.; et al. Mechanisms of Macrophage Immunomodulatory Activity Induced by a New Polysaccharide Isolated from Polyporus umbellatus (Pers.) Fries. Front. Chem. 2020, 8, 581. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L.; Wu, J.; Xu, Y.; Qi, S.; Liu, W.; Liu, P.; Shi, S.; Wang, H.; Zhang, Q.; et al. Extraction, characterization, and anti-nonalcoholic steatohepatitis activity of a (1,3) (1,6)-β-D-glucan from the Polyporus umbellatus (Pers.) Fries. Int. J. Biol. Macromol. 2023, 230, 123252. [Google Scholar] [CrossRef]
- Patra, S.; Maity, P.; Chakraborty, I.; Sen, I.K.; Ghosh, D.; Rout, D.; Bhanja, S.K. Structural studies of immunomodulatory (1→3)-, (1→4)-α glucan from an edible mushroom Polyporus grammocephalus. Int. J. Biol. Macromol. 2021, 168, 649–655. [Google Scholar] [CrossRef]
- Tada, R.; Ohno, N.; Adachi, Y. Refinement and complete solution NMR analysis of the structure of a 6-branched 1,3-β-D-glucan (OL-2) isolate from Omphalialapidescens. Carbohydr. Res. 2023, 529, 108849. [Google Scholar] [CrossRef]
- Xia, L. Purification and Structural Study of Complement Inhibiting Active Marble Polysaccharides. Master’s Thesis, Suzhou University, Suzhou, China, 2016. [Google Scholar]
- Wu, C.L.; Deng, Y.K.; M, Y.F. Structural Study of Water Soluble Polysaccharides from Macrocephalum sibiricum. Nat. Prod. Res. Dev. 2008, 20, 1027–1030. [Google Scholar]
- Zhao, Y.; Li, Q.; Wang, M.; Wang, Y.; Piao, C.; Yu, H.; Liu, J.; Li, Z. Structural characterization of polysaccharides after fermentation from Ganoderma lucidum and its antioxidant activity in HepG2 cells induced by H2O2. Food Chem. X 2023, 18, 100682. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zhou, S.; Yan, M.; Tang, Q.; Zhang, J. Structure and chain conformation of bioactive β-D-glucan purified from water extracts of Ganoderma lucidum unbroken spores. Int. J. Biol. Macromol. 2021, 180, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Huang, D.; Huang, J.; Qin, F.; Huang, H. Integrated Analysis and Finding Reveal Anti-Liver Cancer Targets and Mechanisms of Pachyman (Poria cocos Polysaccharides). Front. Pharmacol. 2021, 12, 742349. [Google Scholar] [CrossRef]
- Jia, W.; Luo, S.; Lai, G.; Li, S.; Huo, S.; Li, M.; Zeng, X. Homogeneous polyporus polysaccharide inhibits bladder cancer by polarizing macrophages to M1 subtype in tumor microenvironment. BMC Complement. Med. Ther. 2021, 21, 150. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef]
- Tan, L.; Liu, S.; Li, X.; He, J.; He, L.; Li, Y.; Yang, C.; Li, Y.; Hua, Y.; Guo, J. The Large Molecular Weight Polysaccharide from Wild Cordyceps and Its Antitumor Activity on H22 Tumor-Bearing Mice. Molecules 2023, 28, 3351. [Google Scholar] [CrossRef]
- Qiu, W.L.; Hsu, W.H.; Tsao, S.M.; Tseng, A.J.; Lin, Z.H.; Hua, W.J.; Yeh, H.; Lin, T.E.; Chen, C.C.; Chen, L.S.; et al. WSG, a Glucose-Rich Polysaccharide from Ganoderma lucidum, Combined with Cisplatin Potentiates Inhibition of Lung Cancer In Vitro and In Vivo. Polymers 2021, 13, 4353. [Google Scholar] [CrossRef]
- Hsu, W.H.; Hua, W.J.; Qiu, W.L.; Tseng, A.J.; Cheng, H.C.; Lin, T.Y. WSG, a glucose-enriched polysaccharide from Ganoderma lucidum, suppresses tongue cancer cells via inhibition of EGFR-mediated signaling and potentiates cisplatin-induced apoptosis. Int. J. Biol. Macromol. 2021, 193, 1201–1208. [Google Scholar] [CrossRef]
- Li, G.L.; Tang, J.F.; Tan, W.L.; Zhang, T.; Zeng, D.; Zhao, S.; Ran, J.H.; Li, J.; Wang, Y.P.; Chen, D.L. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food. Funct. 2023, 14, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Q. Optimization of Extraction and Radiation Protection Effects of Poria Cocos Peel Polysaccharides. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2022. [Google Scholar]
- Yin, D.Y. Isolation, Purification, and Biological Activity of Polyporus umbellatus Polysaccharides. Master’s Thesis, Northwest A&F University, Xi’an, China, 2016. [Google Scholar]
- Liu, F.; Liu, Y.; Feng, X.; Ibrahim, S.A.; Huang, W. Structure characterization and in vitro immunomodulatory activities of carboxymethyl pachymaran. Int. J. Biol. Macromol. 2021, 178, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Li, C.; Shao, Y.; Chen, A. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Huang, F.; Zhao, A.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X.; et al. Theabrownin and Poria cocos Polysaccharide Improve Lipid Metabolism via Modulation of Bile Acid and Fatty Acid Metabolism. Front. Pharmacol. 2022, 13, 875549. [Google Scholar] [CrossRef]
- Yu, W.Q.; Wang, X.L.; Ji, H.H.; Miao, M.; Zhang, B.H.; Li, H.; Zhang, Z.Y.; Ji, C.F.; Guo, S.D. CM3-SII polysaccharide obtained from Cordyceps militaris ameliorates hyperlipidemia in heterozygous LDLR-deficient hamsters by modulating gut microbiota and NPC1L1 and PPARα levels. Int. J. Biol. Macromol. 2023, 239, 124293. [Google Scholar] [CrossRef]
- Yu, W.Q.; Yin, F.; Shen, N.; Lin, P.; Xia, B.; Li, Y.J.; Guo, S.D. Polysaccharide CM1 from Cordyceps militaris hinders adipocyte differentiation and alleviates hyperlipidemia in LDLR(+/−) hamsters. Lipids Health Dis. 2021, 20, 178. [Google Scholar] [CrossRef]
- Yu, M.; Yue, J.; Hui, N.; Zhi, Y.; Hayat, K.; Yang, X.; Zhang, D.; Chu, S.; Zhou, P. Anti-Hyperlipidemia and Gut Microbiota Community Regulation Effects of Selenium-Rich Cordyceps militaris Polysaccharides on the High-Fat Diet-Fed Mice Model. Foods 2021, 10, 2252. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Z.; Zhang, J.; Jia, L. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia. J. Ethnopharmacol. 2023, 309, 116321. [Google Scholar] [CrossRef]
- Zhao, H.; Li, M.; Liu, L.; Li, D.; Zhao, L.; Wu, Z.; Zhou, M.; Jia, L.; Yang, F. Cordyceps militaris polysaccharide alleviates diabetic symptoms by regulating gut microbiota against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 230, 123241. [Google Scholar] [CrossRef]
- Lee, B.H.; Chen, C.H.; Hsu, Y.Y.; Chuang, P.T.; Shih, M.K.; Hsu, W.H. Polysaccharides Obtained from Cordyceps militaris Alleviate Hyperglycemia by Regulating Gut Microbiota in Mice Fed a High-Fat/Sucrose Diet. Foods 2021, 10, 1870. [Google Scholar] [CrossRef]
- Shao, W.; Xiao, C.; Yong, T.; Zhang, Y.; Hu, H.; Xie, T.; Liu, R.; Huang, L.; Li, X.; Xie, Y.; et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef]
- Jiao, J.; Yong, T.; Huang, L.; Chen, S.; Xiao, C.; Wu, Q.; Hu, H.; Xie, Y.; Li, X.; Liu, Y.; et al. A Ganoderma lucidum polysaccharide F31 alleviates hyperglycemia through kidney protection and adipocyte apoptosis. Int. J. Biol. Macromol. 2023, 226, 1178–1191. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Wang, L.; Chen, W.D.; Duan, Y.T.; Sun, M.J.; Huang, J.J.; Peng, D.Y.; Yu, N.J.; Wang, Y.Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 9, 963598. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, J.; Wang, Y.; Fang, L.; Guo, C.; Sang, T.; Peng, H.; Zhao, Q.; Chen, S.; Lin, X.; et al. Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomedicine 2023, 110, 154626. [Google Scholar] [CrossRef]
- Sun, S.; Wang, K.; Sun, L.; Cheng, B.; Qiao, S.; Dai, H.; Shi, W.; Ma, J.; Liu, H. Therapeutic manipulation of gut microbiota by polysaccharides of Wolfiporia cocos reveals the contribution of the gut fungi-induced PGE2 to alcoholic hepatic steatosis. Gut Microbes 2020, 12, 1830693. [Google Scholar] [CrossRef]
- Wei, W.; Xuan, Y.; Huang, X. The lipid-lowering and gut microbiota regulating effects of Poria cocos polysaccharides on nutritional obese young rats. Mod. Food Technol. 2023. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Z.; Wu, S.; Wang, J.; Chen, M.; Liu, W.; Huang, A.; Zhang, J.; Wu, Q.; Ding, Y. Polysaccharides from Cordyceps militaris prevent obesity in association with modulating gut microbiota and metabolites in high-fat diet-fed mice. Food Res. Int. 2022, 157, 111197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, L.; Zhai, Q.; Chu, C.; Wang, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Combined Ganoderma lucidum polysaccharide and ciprofloxacin therapy alleviates Salmonella enterica infection, protects the intestinal barrier, and regulates gut microbiota. Food Funct. 2023, 14, 6896–6913. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Zhao, J.; Xiao, X.; Li, W.; Zang, L.; Yu, J.; Liu, H.; Niu, X. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE-/- mice by inhibiting inflammation. Phytother. Res. 2021, 35, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lin, P.; Yu, W.Q.; Shen, N.; Li, Y.; Guo, S.D. The Cordyceps militaris-Derived Polysaccharide CM1 Alleviates Atherosclerosis in LDLR(-/-) Mice by Improving Hyperlipidemia. Front. Mol. Biosci. 2021, 8, 783807. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, J.; Peng, X. Poria cocos Polysaccharides Alleviates Chronic Nonbacterial Prostatitis by Preventing Oxidative Stress, Regulating Hormone Production, Modifying Gut Microbiota, and Remodeling the DNA Methylome. J. Agric. Food Chem. 2020, 68, 12661–12670. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhang, G.; Peng, X. Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis. Int. J. Biol. Macromol. 2021, 189, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, Q.; Liu, J.; Luo, J.; Liu, L.; Peng, X. Metabolites of gut microbiota fermenting Poria cocos polysaccharide alleviates chronic nonbacterial prostatitis in rats. Int. J. Biol. Macromol. 2022, 209, 1593–1604. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, Q.; Zhao, R.; Huang, T.; Tian, Y.; Lin, Y. A Comparative Study on the Effects of Different Sources of Carboxymethyl Poria Polysaccharides on the Repair of DSS-Induced Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 9034. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Yue, S.R.; Lu, A.P.; Zhang, L.; Ji, G.; Liu, B.C.; Wang, R.R. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis. Phytomedicine 2022, 103, 154208. [Google Scholar] [CrossRef]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Gao, H.; Xu, Y.; Han, Y.; Shang, H.; Lu, Y.; Qin, C. Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1929–1938. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Tian, Y.; Chen, X.; Lan, J.; Wei, F.; Li, Y.; Luo, Y.; Sun, X. Ganoderma lucidum polysaccharides ameliorate lipopolysaccharide-induced acute pneumonia via inhibiting NRP1-mediated inflammation. Pharm. Biol. 2022, 60, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, F.; Luo, A.; Lin, S.; Feng, X.; Yan, W.; Shi, Y.; Zhang, Q.; Gu, X.; Cui, G.; et al. Polyporus Polysaccharide Ameliorates Bleomycin-Induced Pulmonary Fibrosis by Suppressing Myofibroblast Differentiation via TGF-β/Smad2/3 Pathway. Front. Pharmacol. 2020, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.D. Ultrasound Extraction and Liquid Fermentation Process Optimization and Antibacterial Activity Study of Polysaccharides from Mabo. Master’s Thesis, Jiamusi University, Jiamusi, China, 2020. [Google Scholar]
- Zhang, X.; Li, J.; Yang, B.; Leng, Q.; Li, J.; Wang, X.; Lu, J.; Olatunji, O.J.; Tang, J. Alleviation of Liver Dysfunction, Oxidative Stress, and Inflammation Underlines the Protective Effects of Polysaccharides from Cordyceps cicadae on High Sugar/High Fat Diet-Induced Metabolic Syndrome in Rats. Chem. Biodivers. 2021, 18, e2100065. [Google Scholar] [CrossRef]
- Song, L.; Yang, J.; Kong, W.; Liu, Y.; Liu, S.; Su, L. Cordyceps militaris polysaccharide alleviates ovalbumin-induced allergic asthma through the Nrf2/HO-1 and NF-κB signaling pathways and regulates the gut microbiota. Int. J. Biol. Macromol. 2023, 238, 124333. [Google Scholar] [CrossRef]
- Huang, S.; Zou, Y.; Tang, H.; Zhuang, J.; Ye, Z.; Wei, T.; Lin, J.; Zheng, Q. Cordyceps militaris polysaccharides modulate gut microbiota and improve metabolic disorders in mice with diet-induced obesity. J. Sci. Food Agric. 2023, 103, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Tan, C.; Ren, J.; Liu, J.; Zou, W.; Liu, G.; Sheng, Y. Cordyceps militaris acidic polysaccharides improve learning and memory impairment in mice with exercise fatigue through the PI3K/NRF2/HO-1 signalling pathway. Int. J. Biol. Macromol. 2023, 227, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Y.; Han, L.; Jia, Y.; Luo, S.; Zhang, D.; Zhang, L.; Wu, P.; Xiao, C.; Kan, W.; et al. Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system. Brain Res. Bull. 2021, 171, 16–24. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, Q.; Xing, H.; Xu, J.; Li, Z.; Zhu, H.; Yang, K.; Sun, P.; Cai, M. Gastroprotective Effects of Ganoderma lucidum Polysaccharides with Different Molecular Weights on Ethanol-Induced Acute Gastric Injury in Rats. Nutrients 2022, 14, 1476. [Google Scholar] [CrossRef]
- Xu, Z.H.; Su, X.; Yang, G.; Qin, T.; Liu, Y. Ganoderma lucidum polysaccharides protect against sepsis-induced cardiac dysfunction by activating SIRT1. J. Pharm. Pharmacol. 2022, 74, 124–130. [Google Scholar] [CrossRef]
- Wu, S.Y.; Ou, C.C.; Lee, M.L.; Hsin, I.L.; Kang, Y.T.; Jan, M.S.; Ko, J.L. Polysaccharide of Ganoderma lucidum Ameliorates Cachectic Myopathy Induced by the Combination Cisplatin plus Docetaxel in Mice. Microbiol. Spectr. 2023, 11, e0313022. [Google Scholar] [CrossRef]
- Wu, M.; Huang, B.; Hu, L.; Zhang, T.; Zhang, B.; Zhao, X.; Lu, R.; Xiong, W.; Zhang, S.; Li, J.; et al. Ganoderma lucidum polysaccharides ameliorates D-galactose-induced aging salivary secretion disorders by upregulating the rhythm and aquaporins. Exp. Gerontol. 2023, 175, 112147. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, J.; Mao, P.; He, C.; Yuan, D.; Chen, C.; Zhang, H.; Hu, J.; Zhang, J. Ganoderma lucidum polysaccharide inhibits the proliferation of leukemic cells through apoptosis. Acta Biochim. Pol. 2022, 69, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Dong, N.; Fang, Q.; Zhang, Y.; Chen, C.; Cui, S.W.; Nie, S. Polysaccharides from natural Cordyceps sinensis attenuated dextran sodium sulfate-induced colitis in C57BL/6J mice. Food Funct. 2023, 14, 720–733. [Google Scholar] [CrossRef]
- Li, Y.; Miao, M.; Yin, F.; Shen, N.; Yu, W.Q.; Guo, S.D. The polysaccharide-peptide complex from mushroom Cordyceps militaris ameliorates atherosclerosis by modulating the lncRNA-miRNA-mRNA axis. Food Funct. 2022, 13, 3185–3197. [Google Scholar] [CrossRef]
- Suárez, E.R.; Kralovec, J.A.; Grindley, T.B. Isolation of phosphorylated polysaccharides from algae: The immunostimulatory principle of Chlorella pyrenoidosa. Carbohydr. Res. 2010, 345, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, Y.; Wang, Y.; Liu, J. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Vet. Res. 2014, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.L.; Liang, J.; Zhou, F.Y.; Kuang, H.X.; Xia, Y.G. Discrimination and characterization of Panax polysaccharides by 2D COS-IR spectroscopy with chemometrics. Int. J. Biol. Macromol. 2021, 183, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, B.; Peng, X.; Li, S.; Zhao, J. Quality Evaluation of Ophiopogon japonicus from Two Authentic Geographical Origins in China Based on Physicochemical and Pharmacological Properties of Their Polysaccharides. Biomolecules 2022, 12, 1491. [Google Scholar] [CrossRef]
- Wei, W.; Li, Z.; Li, S.; Wu, S.; Zhang, D.; An, Y.; Li, Y.; Wu, M.; Zhang, J.; Yao, C.; et al. Fingerprint profiling and gut microbiota regulation of polysaccharides from Fritillaria species. Int. J. Biol. Macromol. 2023, 237, 123844. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.D.; Yang, F.F.; Chen, B.X.; Li, X.; Huang, Q.X.; Li, J.; Li, X.Y.; Li, Z.; Yu, H.S.; et al. Multi-level fingerprinting and cardiomyocyte protection evaluation for comparing polysaccharides from six Panax herbal medicines. Carbohydr. Polym. 2022, 277, 118867. [Google Scholar] [CrossRef]
- Sun, W.; Xu, J.D.; Zhang, W.; Guo, M.F.; Kong, M.; Zhu, H.; Zhou, S.S.; Wu, C.Y.; Li, S.L.; Mao, Q. Holistic quality evaluation of Callicarpae formosanae Folium by multi-chromatography-based qualitative and quantitative analysis of polysaccharides and small molecules. J. Pharm. Biomed. Anal. 2023, 227, 115282. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Ahmadigol, A.; Khubber, S.; Altintas, Z. Whey protein isolate/jujube polysaccharide-based edible nanocomposite films reinforced with starch nanocrystals for the shelf-life extension of banana: Optimization and characterization. Int. J. Biol. Macromol. 2022, 222, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.G.; Li, X.; Yu, L.S.; Liang, J.; Sun, H.M.; Kuang, H.X. Structural-fingerprinting of polysaccharides to discern Panax species by means of gas-liquid chromatography and mass spectrometry. Int. J. Biol. Macromol. 2020, 151, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 11th ed.; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhao, H.; Lai, C.J.; Yu, Y.; Wang, Y.N.; Zhao, Y.J.; Ma, F.; Hu, M.; Guo, J.; Wang, X.; Guo, L. Acidic hydrolysate fingerprints based on HILIC-ELSD/MS combined with multivariate analysis for investigating the quality of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2020, 163, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xu, W.; Jin, H.Y.; Jian, N.I.; Ma, H.C. Preliminary study on quality control methods for polysaccharides in Ganoderma lucidum extract. J. Pharm. Anal. 2018, 38, 1442–1447. [Google Scholar]
- Liu, J.; Zhang, J.; Feng, J.; Tang, C.; Yan, M.; Zhou, S.; Chen, W.; Wang, W.; Liu, Y. Multiple Fingerprint-Activity Relationship Assessment of Immunomodulatory Polysaccharides from Ganoderma lucidum Based on Chemometric Methods. Molecules 2023, 28, 2913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Q.; Li, W.Q.; Huang, Y.J.; Zhou, J.L.; He, Y.; Mei, Q.X.; Qian, Z.M. Comparative analysis of polysaccharide components of Ophiocordyceps sinensis of different specifications. Shizhen Tradit. Chin. Med. 2023. Available online: https://kns.cnki.net/kcms/detail/42.1436.R.20230703.1710.002.html (accessed on 19 September 2023).
- Zhang, Y.; Wu, M.; Xi, J.; Pan, C.; Xu, Z.; Xia, W.; Zhang, W. Multiple-fingerprint analysis of Poria cocos polysaccharide by HPLC combined with chemometrics methods. J. Pharm. Biomed. Anal. 2021, 198, 114012. [Google Scholar] [CrossRef]
- Yi, Y.; Hua, H.; Sun, X.; Guan, Y.; Chen, C. Rapid determination of polysaccharides and antioxidant activity of Poria cocos using near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118623. [Google Scholar] [CrossRef]
- Xie, J.; Huang, J.; Ren, G.; Jin, J.; Chen, L.; Zhong, C.; Cai, Y.; Liu, H.; Zhou, R.; Qin, Y.; et al. Determination of Cultivation Regions and Quality Parameters of Poria cocos by Near-Infrared Spectroscopy and Chemometrics. Foods 2022, 11, 892. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Li, S.; Qi, E.R.; Meng, J.; Ching Lam, K.Y.; Dong, X.; Xu, J.; Chen, H.; Zhao, Z. Qualitative and quantitative characterization of carbohydrate profiles in three different parts of Poria cocos. J. Pharm. Biomed. Anal. 2020, 179, 113009. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Nan, T.G.; Yuan, Y.Y.; Jin, Y.; Yang, Q.; Zhang, M.; Hu, K.Y. Study on the Content of Polyporus umbellatus Polysaccharides from Different Origins and Monosaccharide Composition in Polyporus umbellatus Polysaccharides. Chin. J. Tradit. Chin. Med. 2019, 44, 3608–3614. [Google Scholar]
- Guo, N.; Bai, Z.; Jia, W.; Sun, J.; Wang, W.; Chen, S.; Wang, H. Quantitative Analysis of Polysaccharide Composition in Polyporus umbellatus by HPLC-ESI-TOF-MS. Molecules 2019, 24, 2526. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | Source | Molecular Weight | Monosaccharide Composition | Proposed Structure | Reference |
|---|---|---|---|---|---|
| GLP | Ganoderma lucidum | 112 kDa | Xylose:mannose:galactose: glucose:glucuronic acid = 1.53:15.64:1.84:80.6:0.39 | β-1,6-Glcp and β-1,3-Glcp linkages were the main ones that existed; the apparent structure was porous and loose | [82] |
| GLPC2 | Ganoderma lucidum fruiting body | 20.6 kDa | Mannose:glucuronic acid:glucose:galactose:xylose:fucose = 5.9:9.0:80.4:1.8:1.8:0.9 | GLPC2 was mainly composed of D-Glcp-(1→, →3)-D-Glcp-(1→, →4)-D-Glcp-(1→, →6)-D-Glcp-(1→, →3,6)-D-Glcp-(1→, and→4)-D-GlcpA-(1→ | [60] |
| FGLP | Fermented Ganoderma lucidum | 88.9 kDa | Arabinose:xylose:mannose:galactose:glucose:glucuronic acid = 0.42:0.82:34.31:3.32:59.47:1.66 | β-1,6-Glcp and β-1,3-Glcp linkages were the main ones that existed; the glucose content was decreased, and the uronic acid content was increased; the apparent structure was smooth and hard | [82] |
| GLSP | Ganoderma lucidum | 4–5485 kDa | Arabinose:glucose:galactose = 1.23:90.82:7.95 | The types of glycosidic linkages that existed in GLSP were as follows: β-Glcp-(1→; →3)-β-D-Glcp-(1→; β-Glcp-(1→or→6)-β-D-Glcp-(1→; →5)-α-Araf-(1→; →6)-α-Galp-(1→; →4)-α-Galp-(1→; →6)-α-Galp-(1→4)-α-Galp-(1→ | [83] |
| FXM | Ganoderma lucidum | 35.9 kDa | Fucose:xylose:mannose:glucose = 24:25:48.3:2.7 | The main chain of FXM was α-D-Manp-(1→4)-linked units, and some of them were branched at O-6 position with α-L-Fucp-(1→2)-β-D-Xylp groups | [61] |
| RGLP-1 | Ganoderma lucidum | 3978 kDa | Fucose:mannose:glucose:galactose = 0.13:0.05:0.72:0.10 | RGLP-1 contained 12 linkage forms: (1→3)-linked glucose; (1→)-linked glucose; (1→3,6)-linked glucose; (1→4)-linked glucose; (1→6)-linked glucose; (1→3)-linked galactose; (1→2,3)-linked galactose; (1→)-linked galactose; (1→6)-linked galactose; (1→4,6)-linked galactose; (1→3)-linked mannose; and (1→2)-linked mannose | [34] |
| GLSP-I | Ganoderma lucidum spore | 128 kDa | Glucose | The backbone was (1→3)-β-D-glucan, and three side chains including Glc-(1→3)-Glc-(1→3)-Glc-(1→6)-Glc, Glc-(1→6)-Glc-(1→6)-Glc-(1→6)-Glc and Glc-(1→3)-Glc-(1→3)-Glc-(1→3)-Glc-(1→3)-Glc were linked at O-6 | [62] |
| SeMPN | Ganoderma lucidum mycelia | 9.7 kDa | Glucose | The backbone was 1,4-linked Glcp, and the other types of linkage contained T-linked Glcp and 1,4,6-linked Glcp | [63] |
| GLSB50A-III-1 | Ganoderma lucidum spore | 193 kDa | Glucose | The backbone of GLSB50A-III-1 was (1→3), (1→4), (1→6)-linked β-D-glucose, and the side chains consisted of β-(1→3) and β-(1→4)-linked residues, which were attached at O-6 | [84] |
| CM3-SII | Cordyceps militaris fruiting body | 25.2 kDa | Mannose:glucose:galactose = 10.6:1.0:3.7 | →4)-β-D-Manp(1→, →6)-β-D-Manp(1→, and→6)-α-D-Manp(1→glycosyls, and branching at the O-4 positions of →6)-β-D-Manp(1→ glycosyls with β-D-Galp, (1→2) linked-β-D-Galf, and →2,6)-α-D-Manp(1→ residues, O-6 and O-2 positions of the →2,6)-α-D-Manp(1→ residues were substituted with methyl and β-D-Galp | [39] |
| PACI-1 | Cordyceps cicadae fermentation medium | 10 kDa | A homopolysaccharide composed of fructose | NMR spectrum indicated that PACI-1 mainly contained β-configured pyranoside bonds | [65] |
| JCH-a1 | Cordyceps cicadae fruiting body | 60.7 kDa | Galactose:glucose:mannose = 0.89:1.0:0.39 | It has a triple helix with more α-glycosides and has strong thermal stability | [66] |
| BSP | Cordyceps cicadae bacterium substance | N/A | Arabinose:galactose:glucose:xylose = 7.60:1.80:76.10:14.50 | Araf-(1→, Xylp-(1→, →5)-Araf-(1→, Manp-(1→, Galp-(1→, →3, 5)-Araf-(1→, →2)-Manp-(1→, →6)-Manp-(1→, →4, 6)-Glcp-(1→, →4, 6)-Galp-(1→, →2, 6)-Manp-(1→ | [67] |
| SPP | Cordyceps cicadae spore powder | N/A | Arabinose:galactose:glucose:xylose:mannose = 9.10:15.40:41.20:17.50:16.80 | Araf-(1→, →5)-Araf-(1→, Glcp-(1→, →3, 5)-Araf-(1→, →3)-Glcp-(1→, →4)-Glcp-(1→, →3, 4)-Glcp-(1→, →4, 6)-Glcp-(1→ | [67] |
| PPP | Cordyceps cicadae fruiting body | N/A | Arabinose:galactose:glucose:xylose:mannose = 4.50:11.90:62.20:10.70:10.70 | Araf-(1→, Xylp-(1→, →5)-Araf-(1→, Manp-(1→, Galp-(1→, →3, 5)-Araf-(1→, →2)-Manp-(1→, →3)-Glcp-(1→, →4)-Galp-(1→, →4)-Glcp-(1→, →6)-Glcp-(1→, →6)-Manp-(1→, →3, 4)-Glcp-(1→, →4, 6)-Glcp-(1→, →2, 6)-Manp-(1→ | [67] |
| CMP-1 | Cordyceps militaris | 2.2 × 103 kDa | Mannose:Glucosamine:Ribose:Rhamose:Glucuronic acid:Galacturonic acid:Glucose:Galactose:Xylose:Arabinose:Fucose = 39.35:4.03:3.98:2.56:1.62:1.52:70.52:26.90:1.00:3.23:4.23 | It has three weak absorption peaks (1109 cm−1, 1125 cm−1 and 1151 cm−1) in the range of 1110~1160 cm−1, which may be contributed to side chains on α-carb\zon from the second derivative of infrared spectrum. And it has three broad absorption bands in the range of 990~860 cm−1 | [68] |
| CMP-2 | Cordyceps militaris | 2.8 × 103 kDa | Mannose:Glucosamine:Ribose:Rhamose:Glucuronic acid:Galacturonic acid:Glucose:Galactose:Xylose:Fucose = 13.62:86.70:7.80:6.22:1.47:2.99:17.80:9.26:1.00:3.02 | It has two weak absorption peaks (1109 cm−1 and 1151 cm−1) in the range of 1110~1160 cm−1 and four absorption peaks in the range of 990~860 cm−1 | [68] |
| CMP-3 | Cordyceps militaris | 1.7 × 103 kDa | Mannose:Glucosamine:Ribose:Rhamose:Glucuronic acid:Galacturonic acid:Glucose:Galactose:Xylose:Fucose = 33.61:44.67:27.34:31.84:7.32:8.39:102.23:38.27:1.00:5.79 | It has two weak absorption peaks (1109 cm−1 and 1125 cm−1) in the range of 1110~1160 cm−1 and six absorption peaks in the range of 990~860 cm−1 | [68] |
| EPS-III | Cordyceps militaris culture broth | 1.6 × 103 kDa | Mannose:Glucose:Galactose = 1.68:1:1.83 | The backbone was mainly consisted of →4)-α-D-Galp-(1→, while →3, 6)-α-D-Manp-(1→, →4)-α-D-Manp-(1→, →3)-β-D-Galp-(1→ and →3)-α-D-Glcp-(1→ | [69] |
| AEPS-II | Cordyceps militaris fermentation broth | 61.5 kDa | Mannose:Glucuronic acid:rhamnose:galactose acid:N-acetyl-galactosamine:Glucose:galactose:Arabinose = 1.07:5.38:1:3.14:2.23:15:6.09:4.04 | The backbone contained →4)-α-D-Glcp-(1→, 4, 6-α-D-Glcp-(1→, 2, 4)-β-D-Glcp-(1→, →3)-α-D-Glcp-(1→, →3)-β-D-Glcp-(1→, →4)-β-D-Galp-(1→, →4)-α-D-GalpA-(1→, →4)-β-D-Glcp-(1→, →6)-β-D-GalNAc-(1→; the linkages of branches were mainly composed of 4, 6-α-D-Glcp-(1→, →3)-α-D-Araf-(1→, →2, 4)-β-D-GlcpA-(1→ | [70] |
| PCP-1 | Poria cocos | 3.2 kDa | Nearly 100% glucose | The main linkages of backbone structure were 1, 3-linked glucose | [71] |
| FMGP | Poria cocos cultures mycelia | 31.7 kDa | Glucose:Galactose:Mannose:Fucose = 16:7:3:2 | The main skeleton was a 1,4-α-Man-interlaced-1,3-β-Glucan with interlaced 6-O-α-L-fucosyl 1,4-α-Glc and 1,4-α-Gal branches | [72] |
| WIP | Wolfiporia cocos dried sclerotia | 8.1 kDa | Glucose | It was a kind of pyranose form with β anomeric configuration; the main chain was 1,3-β-Glucan with amorphous structure | [73] |
| PCP-1C | Poria cocos sclerotium | 17 kDa | Galactose:Glucose:Mannose:Fucose = 43.5:24.4:17.4:14.6 | The backbone consisted of 1,6-α-D-Glcp, whose branches composed of 1,3-β-D-Glcp, 1,4-β-D-Glcp, 1,6-β-D-Glcp, T-β-D-Glcp, T-α-D-Manp, T-α-L-Fucp, 1,3-α-L-Fucp | [74] |
| EPS-0M | Poria cocos fermentation broth | 1.8 × 103 kDa | Glucose:Mannose:Galactose:Fucose:Rhamnose = 17.3:46.3:19.9:8.7:5.0 | It possessed β-type glycosidic bonds and exhibited loose fragmentary aggregation with an amorphous spherical structure | [75] |
| EPS-0.1M | Poria cocos fermentation broth | 2.0 × 103 kDa | Glucose:Mannose:Galactose:Fucose:Rhamnose = 11.5:46.5:21.9:10.7:5.6 | It possessed β-type glycosidic bonds and exhibited loose fragmentary aggregation with an amorphous spherical structure | [75] |
| IPS-0M | Poria cocos mycelium | 30 kDa | Glucose:Mannose:Galactose:Fucose:Rhamnose = 79.7:8.9:5.5:1.7:3.1 | It possessed β-type glycosidic bonds and it was mainly filamentous and rod-like, with a relatively regular structure | [75] |
| IPS-0.1M | Poria cocos mycelium | 5.0 × 103 kDa | Glucose:Mannose:Galactose:Fucose:Rhamnose = 50.3:20.9:16.1:6.0:4.0 | It possessed β-type glycosidic bonds and it was mainly filamentous and rod-like, with a relatively regular structure | [75] |
| HPP | Polyporus umbellatus | 6.9 kDa | Only glucose | It has a backbone of 1,4-linked α-D-glucan with a (1→6)-α-D-glucopyranosyl side-branching unit | [76] |
| PUP-W-1 | Polyporus umbellatus sclerotia | 41.1 kDa | Only glucose | The backbone consisted of a repeating chain of eight →3)-β-D-Glcp-(1→ units, with branched chains of four β-D-Glcp residues, joined by repeating 1,6-linkage units at the O-6 position of the backbone | [77] |
| PGPS | Polyporus grammocephalus | 140 kDa | Only glucose | The repeating unit of the glucan was →3)-α-D-Glcp(1→[4) -α-D-Glcp(1]2→ | [78] |
| OL-2 | Omphalia lapidescens | N/A | Only glucose | It consisted of a 1,3-β-Glucan backbone chain decorated with a single six-branched β-glucosyl side unit on every fourth residue | [79] |
| TFP-1 | Lasiosphaera fenzlii fruit body | 500 kDa | Glucose:Mannose:Galactose = 6.3:1.03:2.07 | Total sugar content of 96.94%; Terminal Glcp:1,3-linked Galp:1,2-linked Manp:1,3-linked Glcp:1,6-linked Glcp:1,2,3,6-linked Glcp = 28.48:10.31:26.69:25.86:8.21:8.25 | [80] |
| TFP-2 | Lasiosphaera fenzlii fruit body | 500 kDa | Glucose:Mannose:Galactose = 8.68:0.69:0.63 | Total sugar content of 97.24%; Terminal Glcp:1,3-linked Galp:1,2-linked Manp:1,3-linked Glcp:1,6-linked Glcp:1,2,3,6-linked Glcp = 14.42:6.27:9.71:30.85:34.03:8.75 | [80] |
| TFP-3 | Lasiosphaera fenzlii fruit body | 600 kDa | Glucose:Mannose:Galactose = 7.46:1.25:1.29 | Total sugar content of 95.24%; 1,3-linked Galp:1,2-linked Manp:Terminal Glcp:1,3,6-linked Glcp:1,2,3,6-linked Glcp = 0.132:0.12:0.402:0.261:0.075 | [80] |
| TFP-4 | Lasiosphaera fenzlii fruit body | 1000 kDa | Glucose:Mannose:Galactose = 5.89:1.22:2.89 | Total sugar content of 56.94%; uronic acid content of 47.95%; protein content of 34.89%; due to the high content of protein and uronic acid, methylation was not successful | [80] |
| CGP I-1 | Calvatia geigantea | N/A | Glucose:Mannose:Galactose = 11.28:1.22:3.92 | The CGP I-1 molecules was a linear molecules with branches; the main chain is composed of mannose and glucose, and there were 1→3, 1→2,3, 1→3,6 bond types that were not oxidized by periodate acid; the branch chain or end residues of the main chain were composed of β-Gal(1→4), β-Glc(1→6) and α-Glc(1→4) | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.; Su, F.; Zhang, W.; Kuang, H. A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine. Molecules 2023, 28, 6816. https://doi.org/10.3390/molecules28196816
Bai C, Su F, Zhang W, Kuang H. A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine. Molecules. 2023; 28(19):6816. https://doi.org/10.3390/molecules28196816
Chicago/Turabian StyleBai, Chenxi, Fazhi Su, Wensen Zhang, and Haixue Kuang. 2023. "A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine" Molecules 28, no. 19: 6816. https://doi.org/10.3390/molecules28196816
APA StyleBai, C., Su, F., Zhang, W., & Kuang, H. (2023). A Systematic Review on the Research Progress on Polysaccharides from Fungal Traditional Chinese Medicine. Molecules, 28(19), 6816. https://doi.org/10.3390/molecules28196816





