Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation
Abstract
:1. Introduction
2. Results
2.1. Preparation and Optimization of Phl-TFs
2.1.1. Single-Factor Experiments
Ratio of SPC to CHOL
Phloretin Concentration
Hydration Time
2.1.2. Phl-TFs Optimized by BBD-RSM
2.1.3. Factorial Design to Optimize the Phl-TF Preparation
2.2. Preparation of PTG and PSG
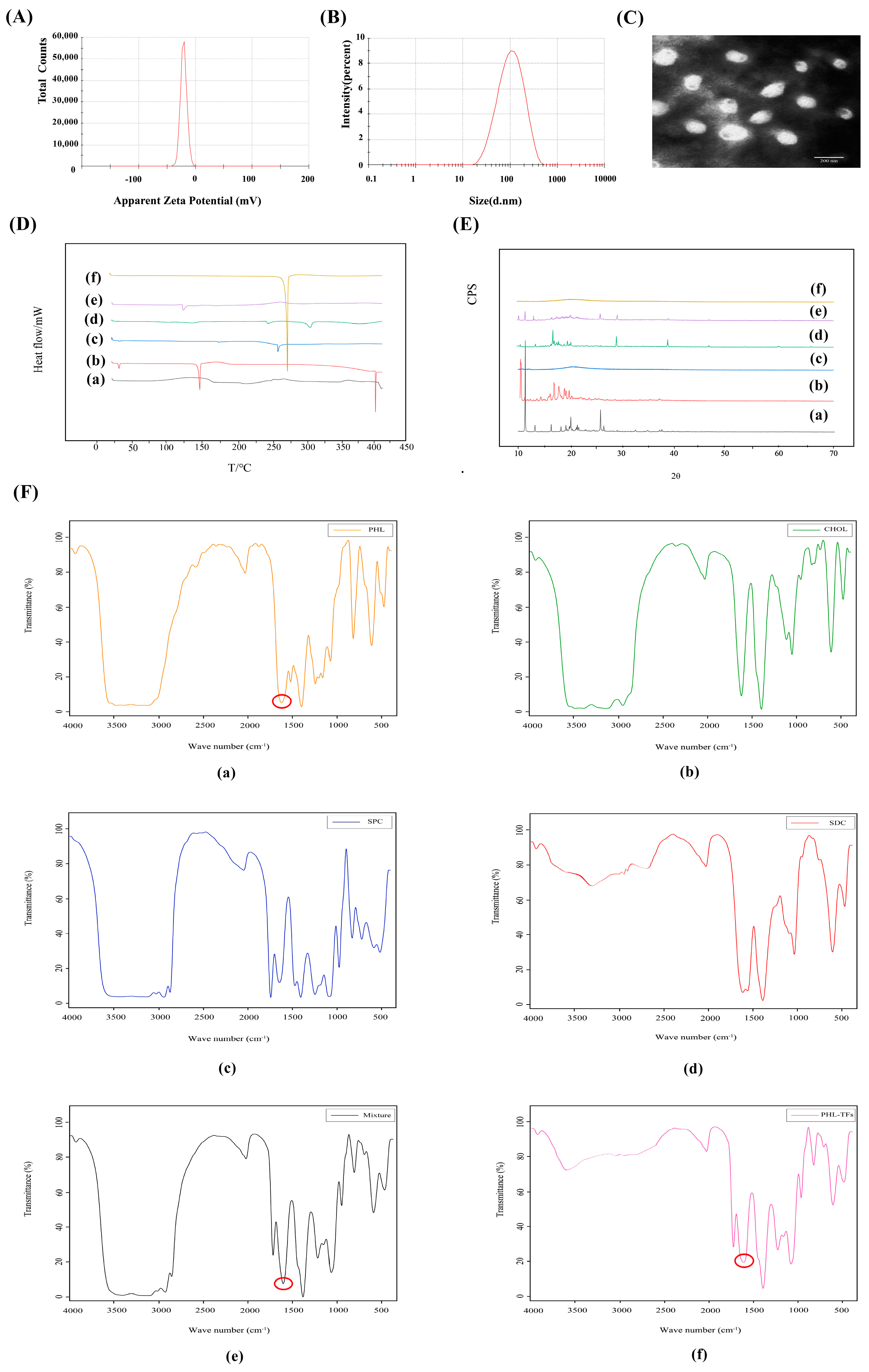
2.3. Characterization of Phl-TFs
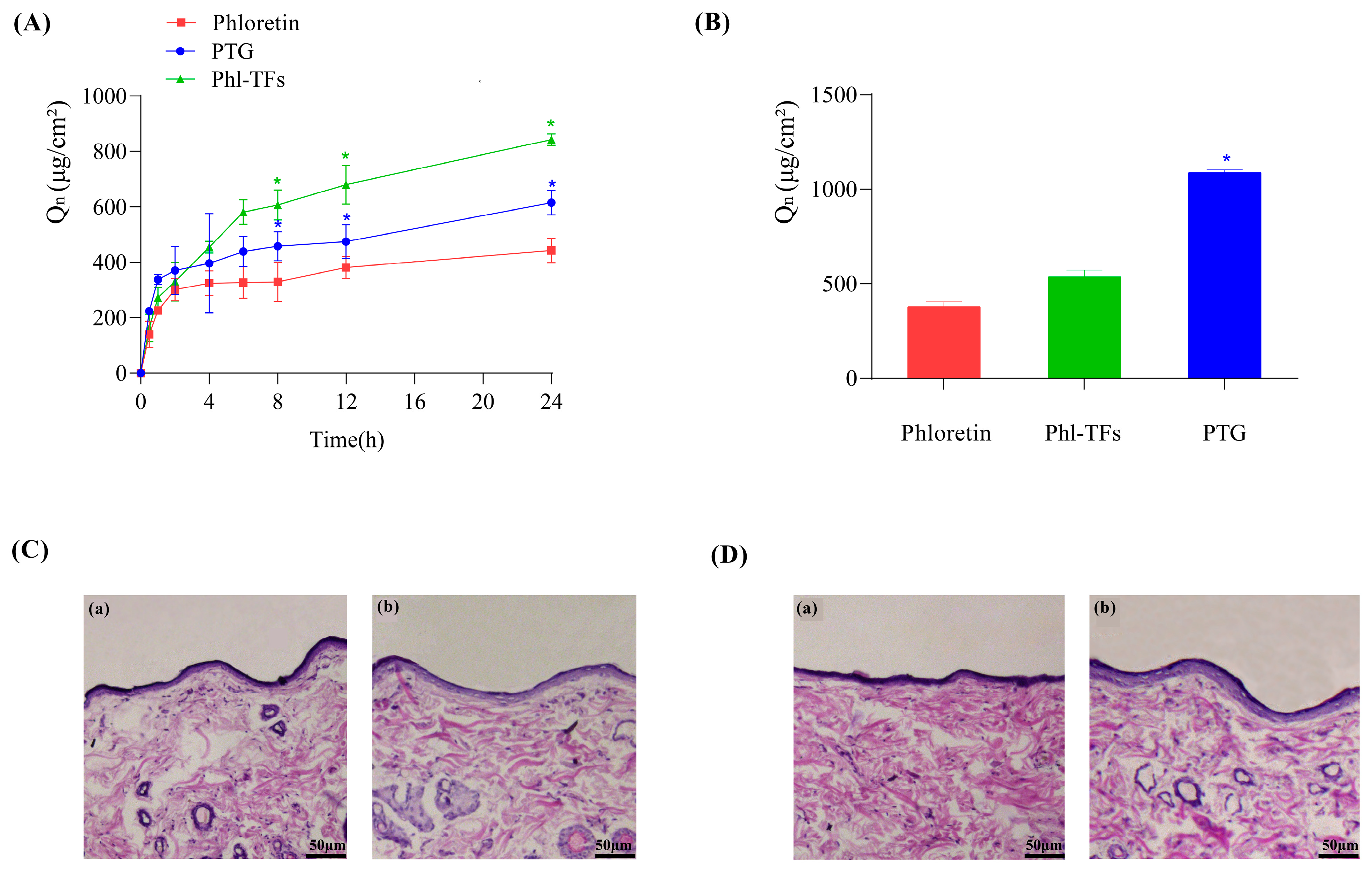
2.4. In Vitro Skin Permeation Studies
2.4.1. Transcutaneous Infiltration
2.4.2. Skin Retention
2.5. Skin Irritation Experiment
2.6. In Vivo Pharmacokinetics
2.7. Antioxidant Activity of PTG in a Rat Model of Aging Induced by D-gal
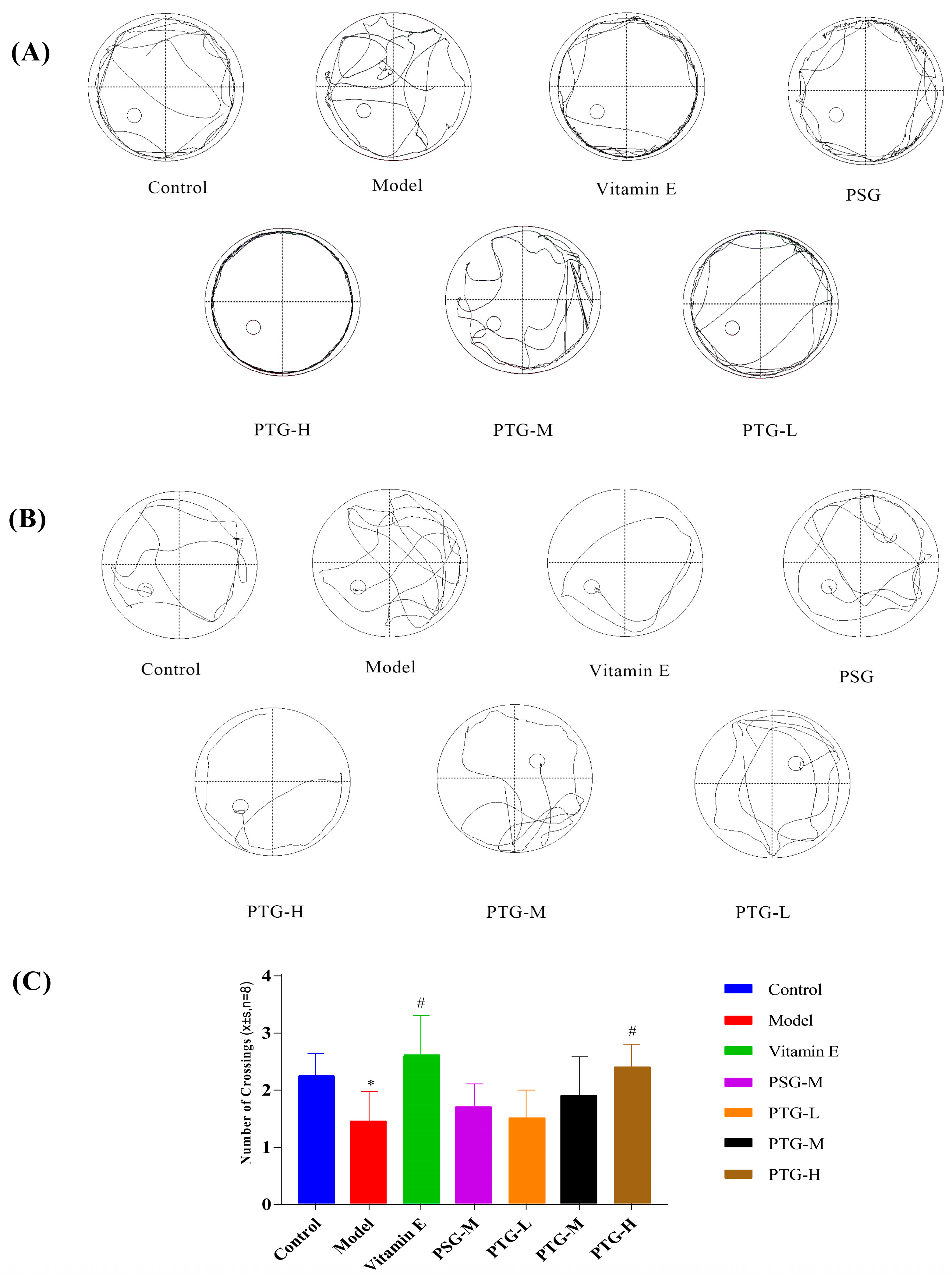
2.7.1. Morris Water Maze (MWM)
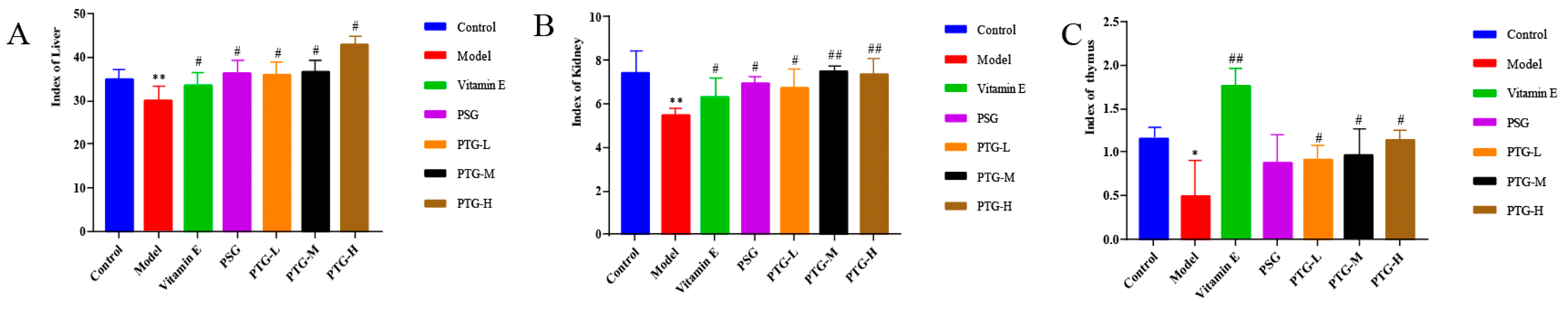
2.7.2. Organ Indices
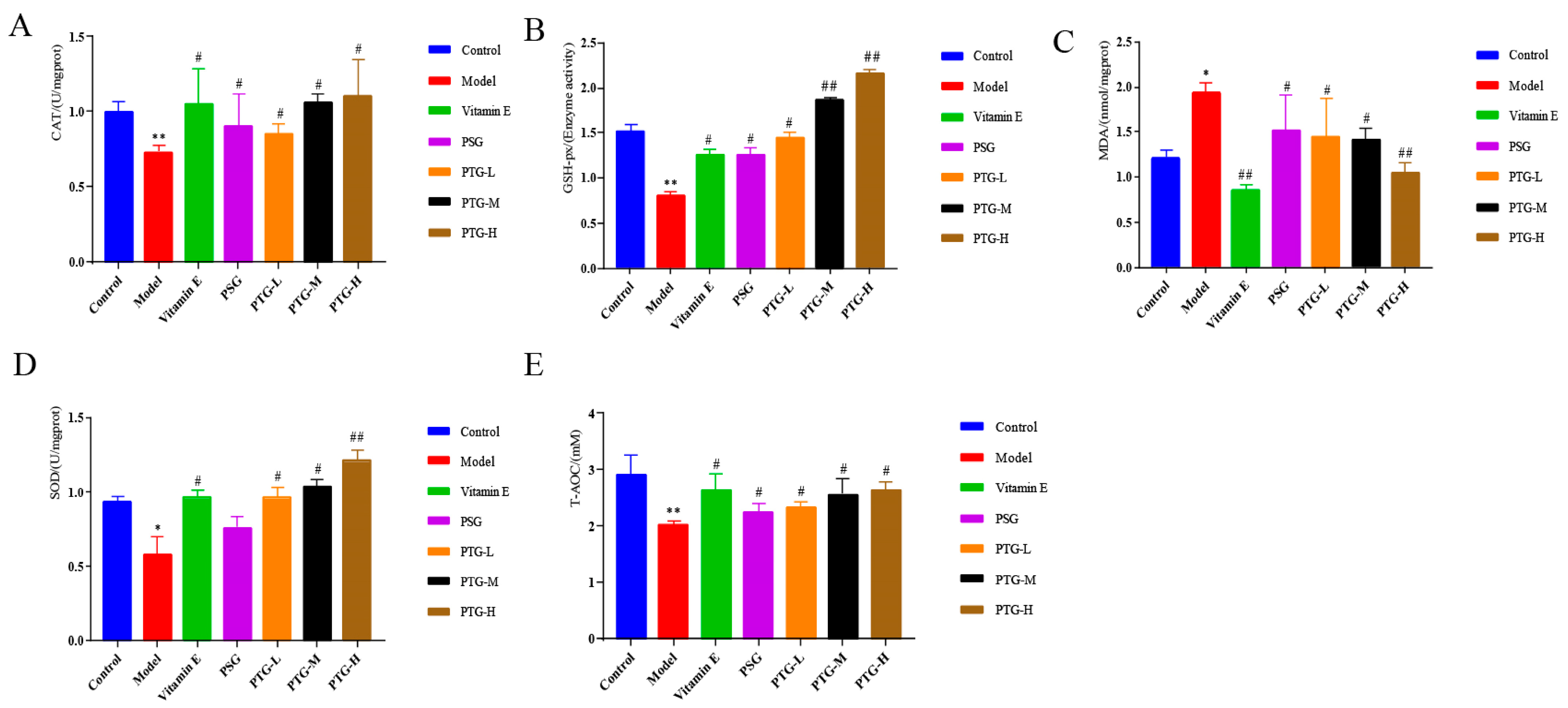
2.7.3. CAT, GSH-Px, MDA, SOD, and Content of T-AOC in Aging Rats
Content in the Serum
Content in the Skin Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of the Phl-TFs and Optimization of the Formulation
4.3.1. HPLC Analysis
4.3.2. Preparation of the Phl-TFs
Single-Factor Experiments
Box–Behnken Response Surface Method to Optimize Prescription
Factorial Design for Prescription Optimization
4.4. Characterization of the Phl-TFs
4.4.1. Transmission Electron Microscopy (TEM)
4.4.2. Analysis of Particle Size and Zeta Potential
4.4.3. Encapsulation Efficiency (EE%) and Drug Loading (DL%)
4.4.4. Differential Scanning Calorimetry (DSC)
4.4.5. X-ray Diffraction (XRD)
4.4.6. Fourier Transform Infrared (FTIR) Spectroscopy
4.4.7. Determination of the Oil–Water Partition Coefficient
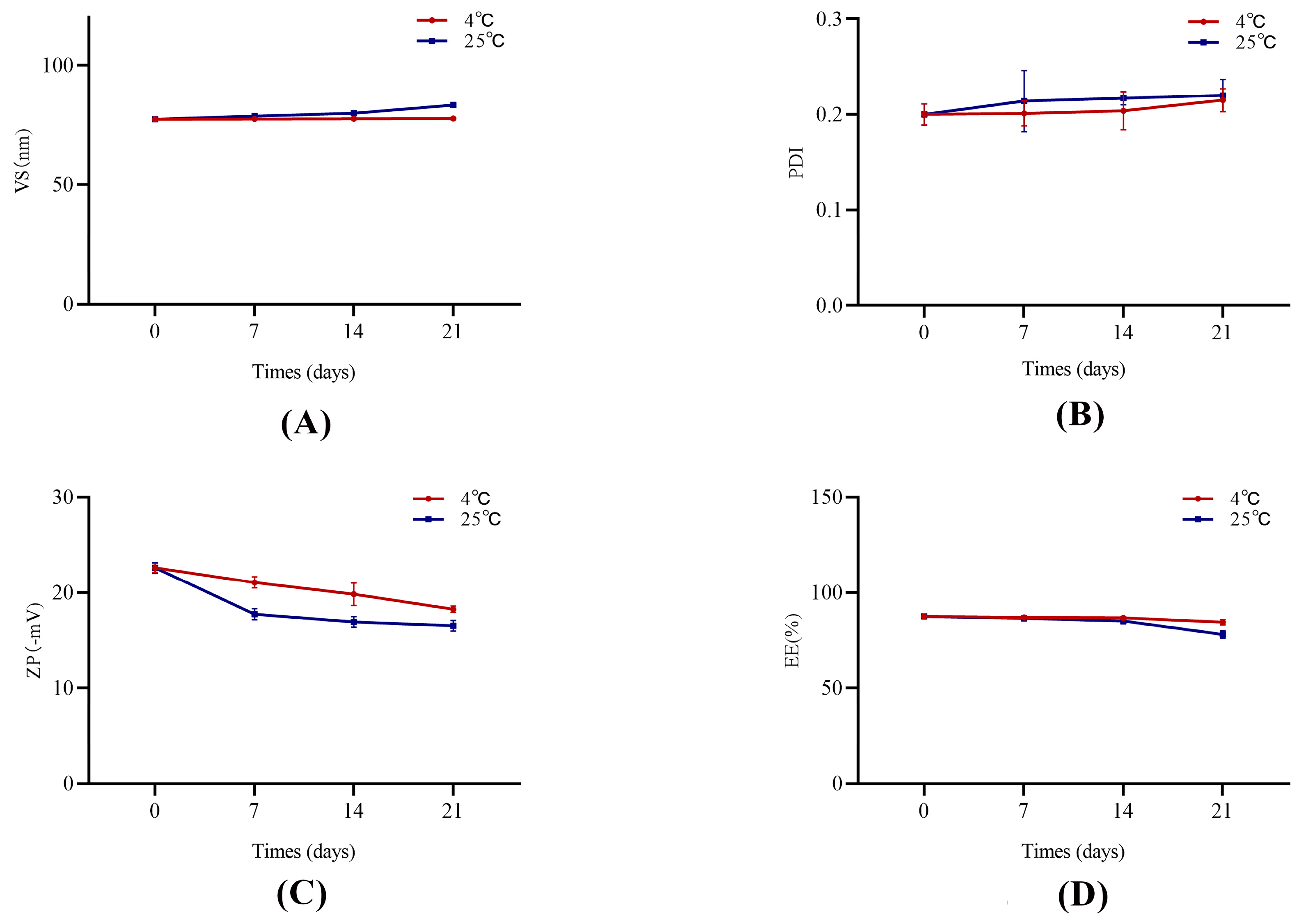
4.5. Stability Study
4.6. In Vitro Permeation of the Phl-TFs
4.6.1. Permeation through Rat Skin
4.6.2. Skin Retention Study
4.6.3. Skin Irritation
4.7. In Vivo Pharmacokinetic Studies
4.8. PTG Antioxidant Efficacy in D-gal-Induced Aging Rats
4.8.1. D-gal Model of Aging Rats
4.8.2. Morris Water Maze (MWM)
4.8.3. Organ Indices
4.8.4. Enzyme Immunoassay Assay of CAT, GSH-Px, MDA, SOD, and T-AOC
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boismal, F.; Serror, K.; Dobos, G.; Zuelgaray, E.; Bensussan, A.; Michel, L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. 2020, 36, 1163–1172. [Google Scholar]
- Rivero-Segura, N.A.; Bello-Chavolla, O.Y.; Barrera-Vázquez, O.S.; Gutierrez-Robledo, L.M.; Gomez-Verjan, J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020, 64, 101164. [Google Scholar] [CrossRef] [PubMed]
- Aging Atlas Consortium. Aging Atlas: A multi-omics database for aging biology. Nucleic Acids Res. 2021, 49, D825–D830. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxidative Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Anunciato Casarini, T.P.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Dermatological applications of the flavonoid phloretin. Eur. J. Pharmacol. 2020, 889, 173593. [Google Scholar] [CrossRef]
- Mao, W.; Fan, Y.; Wang, X.; Feng, G.; You, Y.; Li, H.; Chen, Y.; Yang, J.; Weng, H.; Shen, X. Phloretin ameliorates diabetes-induced endothelial injury through AMPK-dependent anti-EndMT pathway. Pharmacol. Res. 2022, 179, 106205. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Hu, X.; Xu, M.; Su, Y.; Zhang, C.; Yue, Y.; Zhang, X.; Wang, X.; Cui, W.; et al. Phloretin exhibits potential food-drug interactions by inhibiting human UDP-glucuronosyltransferases in vitro. Toxicol. Vitr. 2022, 84, 105447. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Lin, H.; Jiang, S.; Han, L.; Hou, S.; Lin, S.; Cheng, Z.; Bian, W.; Zhang, X.; et al. Enhanced oral bioavailability and bioefficacy of phloretin using mixed polymeric modified self-nanoemulsions. Food Sci. Nutr. 2020, 8, 3545–3558. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Alves, G.L.; Teixeira, F.V.; da Rocha, P.B.R.; Krawczyk-Santos, A.P.; Andrade, L.M.; Cunha-Filho, M.; Marreto, R.N.; Taveira, S.F. Preformulation and characterization of raloxifene-loaded lipid nanoparticles for transdermal administration. Drug Deliv. Transl. Res. 2022, 12, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, A. Transfersomes: The Ultra-Deformable Carrier System for Non-Invasive Delivery of Drug. Curr. Drug Deliv. 2021, 18, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.W.; Jamshaid, H.; Rehman, F.U.; Zaeem, M.; Khan, J.Z.; Zeb, A. Transfersomes: A Revolutionary Nanosystem for Efficient Transdermal Drug Delivery. AAPS PharmSciTech 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Miatmoko, A.; Marufah, N.A.; Nada, Q.; Rosita, N.; Erawati, T.; Susanto, J.; Purwantari, K.E.; Nurkanto, A.; Soeratri, W. The effect of surfactant type on characteristics, skin penetration and anti-aging effectiveness of transfersomes containing amniotic mesenchymal stem cells metabolite products in UV-aging induced mice. Drug Deliv. 2022, 29, 3443–3453. [Google Scholar] [PubMed]
- Yuan, M.; Niu, J.; Xiao, Q.; Ya, H.; Zhang, Y.; Fan, Y.; Li, L.; Li, X. Hyaluronan-modified transfersomes based hydrogel for enhanced transdermal delivery of indomethacin. Drug Deliv. 2022, 29, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Chatterjee, B. Identifying Underlying Issues Related to the Inactive Excipients of Transfersomes based Drug Delivery System. Curr. Pharm. Des. 2021, 27, 971–980. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, H.; Li, Z.W.; Tang, K.X.; Gao, H.Q.; Wang, W.L.; Cui, X.P.; Li, X.L. Low-dose phloretin alleviates diabetic atherosclerosis through endothelial KLF2 restoration. Biosci. Biotechnol. Biochem. 2020, 84, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.H. Phloretin Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species. Pathol. Oncol. Res. 2020, 26, 977–984. [Google Scholar] [CrossRef]
- Hytti, M.; Ruuth, J.; Kanerva, I.; Bhattarai, N.; Pedersen, M.L.; Nielsen, C.U.; Kauppinen, A. Phloretin inhibits glucose transport and reduces inflammation in human retinal pigment epithelial cells. Mol. Cell. Biochem. 2023, 478, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Caiyun, L.; Zhuoya, W.; Xinru, L.; Dairong, Z.; Gaowei, L.; Peicheng, W. Research on phyloretin antioxidants from microemulsion and microemulsion gelling. Flavour Fragr. Cosmet. 2017, 3, 49–53. [Google Scholar]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Li, Y.; Yang, Q.; Xie, L.; Zhang, Q.; Liu, Y.; Zhao, X.; Li, S. Phloretin loaded porous starch (Ph-PS): Preparation, characterization, in vitro release and protective effect against oxidative stress in vivo zebrafish model. Int. J. Biol. Macromol. 2021, 193 Pt B, 2047–2053. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Grivel, J.C.; Zhang, W.; Kaiser, A.; Pinelo, M. Alcohol dehydrogenase on inorganic powders: Zeta potential and particle agglomeration as main factors determining activity during immobilization. Colloids Surf. B Biointerfaces 2019, 175, 136–142. [Google Scholar] [CrossRef]
- Kitaoka, M.; Oka, A.; Goto, M. Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine. Pharmaceutics 2020, 12, 814. [Google Scholar] [CrossRef]
- Chen, B.H.; Inbaraj, B.S. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Schmieder, S.; Patel, P.; Krishnamurthy, K. Research Techniques Made Simple: Drug Delivery Techniques, Part 1: Concepts in Transepidermal Penetration and Absorption. J. Investig. Dermatol. 2015, 135, 1–5. [Google Scholar] [CrossRef]
- Hewlett, A.L.; Hohenberger, H.; Murphy, C.N.; Helget, L.; Hausmann, H.; Lyden, E.; Fey, P.D.; Hicks, R. Evaluation of the bacterial burden of gel nails, standard nail polish, and natural nails on the hands of health care workers. Am. J. Infect. Control. 2018, 46, 1356–1359. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.X.; Zou, J.B.; Zhang, X.F.; Shi, Y.J.; Guo, D.Y. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Sana, E.; Zeeshan, M.; Ain, Q.U.; Khan, A.U.; Hussain, I.; Khan, S.; Lepeltier, E.; Ali, H. Topical delivery of curcumin-loaded transfersomes gel ameliorated rheumatoid arthritis by inhibiting NF-κβ pathway. Nanomedicine 2021, 16, 819–837. [Google Scholar] [CrossRef]
- Qian, J.; Wang, X.; Cao, J.; Zhang, W.; Lu, C.; Chen, X. Dihydromyricetin attenuates D-galactose-induced brain aging of mice via inhibiting oxidative stress and neuroinflammation. Neurosci. Lett. 2021, 756, 135963. [Google Scholar] [CrossRef]
- Dinel, A.L.; Lucas, C.; Guillemet, D.; Layé, S.; Pallet, V.; Joffre, C. Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice. Nutrients 2020, 12, 1777. [Google Scholar] [CrossRef]
- Sun, K.; Yang, P.; Zhao, R.; Bai, Y.; Guo, Z. Matrine Attenuates D-Galactose-Induced Aging-Related Behavior in Mice via Inhibition of Cellular Senescence and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 7108604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Hong, W.; Yumin, W. Clinical observation of lung clearance, blood and phlegm removal in the treatment of acute exacerbation of chronic obstructive pulmonary disease with sputum stasis interconnection. Hebei J. Tradit. Chin. Med. 2019, 41, 1637–1642. [Google Scholar]
- Hongmiao, H.; Zhanjing, G.; Peizhuo, L.; Xiaoyan, L.; Lixia, Z. Protective effect of cinnamon water extract on whole brain ischemia reperfusion injury in rats. Chin. Tradit. Pat. Med. 2011, 33, 1788–1789. [Google Scholar]
- Daixiu, Y.; Zhizhong, Y.; Zhongzheng, L. Anti-aging effect of eucommia meal on D-galactose-induced aging mice. Lishizhen Med. Mater. Medica Res. 2012, 23, 2968–2970. [Google Scholar]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic. Biol. Med. 2003, 34, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Qiaoling, H. Research Progress of Flexible Liposome in Transdermal Drug Delivery System. Chin. J. Mod. Appl. Pharm. 2021, 38, 495–502. [Google Scholar]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates H(2)O(2)-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy-Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]


| Drug | Log P | RSD (%) |
|---|---|---|
| Phloretin | 1.89 | 4.92 |
| 1.95 | ||
| 2.08 | ||
| Phl-TFs | 2.26 | 4.86 |
| 2.37 | ||
| 2.15 |
| Parameter | Unit | PTG | PSG |
|---|---|---|---|
| AUC(0–t) | μg/mL·h | 7.07 ± 1.18 * | 3.63 ± 1.71 |
| AUC(0–∞) | μg/mL·h | 8.22 ± 2.15 * | 4.18 ± 2.35 |
| MRT(0–t) | h | 7.39 ± 0.76 | 6.31± 0.98 |
| MRT(0–∞) | h | 11.60 ± 2.53 | 9.99 ± 2.48 |
| VRT(0–t) | h2 | 42.12 ± 8.94 | 36.84 ± 6.22 |
| VRT(0–∞) | h2 | 162.79 ± 72.02 | 136.52 ± 48.23 |
| T1/2 | h | 8.06 ± 3.20 | 7.65 ± 1.20 |
| Tmax | h | 1.69 ± 0.59 | 2.13 ± 0.84 |
| Cmax | μg/mL | 0.85 ± 0.06 | 0.61 ± 0.26 |
| Incubation Period(s) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Control | 85.00 ± 0.01 | 87.14 ± 12.21 | 64.15 ± 20.20 | 45.90 ± 14.25 | 39.44 ± 13.21 |
| Model | 88.59 ± 31.42 | 88.09 ± 12.23 | 69.00 ± 14.58 | 62.20 ± 14.07 * | 54.93 ± 10.67 * |
| Vitamin E | 73.80 ± 18.13 # | 60.70 ± 13.61 # | 48.40 ± 16.50 ## | 37.10 ± 10.40 ## | 36.65 ± 11.54 # |
| PSG | 89.27 ± 23.35 | 83.37 ± 10.33 | 66.40 ± 16.57 | 65.00 ± 12.92 | 55.42 ± 18.03 |
| PTG-H | 80.88 ± 6.71 # | 80.60 ± 21.38 | 58.10 ± 10.17 # | 55.80 ± 12.48 # | 42.90 ± 10.30 # |
| PTG-M | 81.29 ± 27.5 | 84.35 ± 21.37 | 59.20 ± 19.10 # | 57.70 ± 21.92 # | 49.80 ± 5.68 # |
| PTG-L | 85.72 ± 9.18 | 84.94 ± 10.79 | 64.10 ± 25.45 | 57.94 ± 15.05 # | 49.75 ± 6.47 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, Y.; Zhai, B.; Cheng, J.; Sun, J.; Zhang, X.; Guo, D. Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation. Molecules 2023, 28, 6790. https://doi.org/10.3390/molecules28196790
Wang J, Zhao Y, Zhai B, Cheng J, Sun J, Zhang X, Guo D. Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation. Molecules. 2023; 28(19):6790. https://doi.org/10.3390/molecules28196790
Chicago/Turabian StyleWang, Jiawen, Yuanyuan Zhao, Bingtao Zhai, Jiangxue Cheng, Jing Sun, Xiaofei Zhang, and Dongyan Guo. 2023. "Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation" Molecules 28, no. 19: 6790. https://doi.org/10.3390/molecules28196790
APA StyleWang, J., Zhao, Y., Zhai, B., Cheng, J., Sun, J., Zhang, X., & Guo, D. (2023). Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation. Molecules, 28(19), 6790. https://doi.org/10.3390/molecules28196790





