Investigation of the Mechanism of Action of Periploca forrestii Schltr. Extract on Adjuvant Collagen Rats Based on UPLC-Q-Orbitrap-HRMS Non-Targeted Lipidomics
Abstract
:1. Introduction
2. Results
2.1. Results of Pharmacodynamic Studies
2.1.1. Effect of P. forrestii in Adjuvant-Induced Arthritis (AA) Rats
Effect of P. forrestii on Body Weight, Joint Swelling in AA Rats
Effect of P. forrestii on Histopathological Examination of Joints in AA Rats
2.2. Non-Targeted Lipidomics Data Analysis
2.2.1. Multivariate Analysis of the Serum and Urine Profiles for Model Establishment
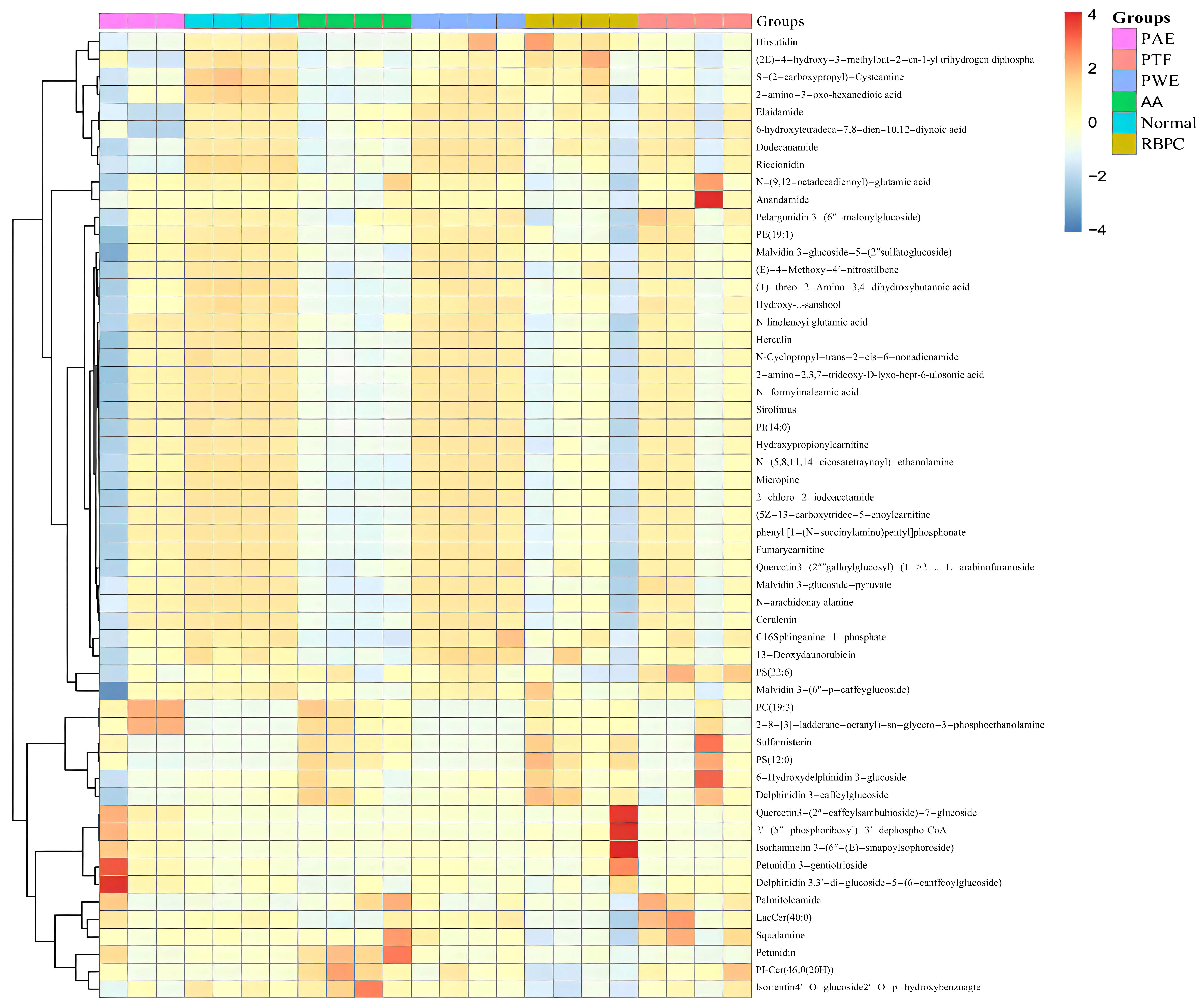
2.2.2. Identification of Potential Biomarkers
2.2.3. Evaluation of the Treatment Effect of P. forrestii on AA Rat
2.2.4. Lipid Metabolic Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Sample Preparation
4.2.1. P. forrestii Sample Preparation
4.2.2. Positive Drug Preparation
4.3. Establishment of Adjuvant-Induced Arthritis (AA) Rat and Treatment Regimen
4.3.1. Animals
4.3.2. Model Building of Adjuvant-Induced Arthritis (AA) Rat and Experimental Grouping
4.4. Pharmacodynamic Study
4.4.1. Evaluation of Arthritis
4.4.2. Collection of Serum, Urine
4.4.3. Preparation of Rat Serum Samples, Determination of Serum Cytokine Levels and PEG2, a Joint Inflammatory Substance
4.4.4. Histopathological Assessment of Joints
4.5. Non-Targeted Lipidomics Analysis
4.5.1. Preparation and Extraction of Lipid Samples
4.5.2. Chromatography and Mass Spectrometry
4.5.3. Multivariate Data Analysis and Biomarkers Identification
4.5.4. Pathway Analysis of Lipid Differentials
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- James, R.; O’Dell, M.D. Therapeutic Strategies for Rheumatoid Arthritis. N. Engl. J. Med. 2004, 2, 218–228. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, T.; Yin, C.; Zhou, J.; Huang, R.; Gao, S.; Zheng, L.; Wang, X.; Manyande, A.; Tian, X.; et al. Effects of the stem extracts of Schisandra glaucescens Diels on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol. 2016, 194, 1078–1086. [Google Scholar] [CrossRef]
- Xu, W.; Huang, M.; Zhang, Y.; Li, H.; Zheng, H.; Yu, L.; Chu, K. Extracts of Bauhinia championii (Benth.) Benth. inhibit NF-<kappa>B-signaling in a rat model of collagen-induced arthritis and primary synovial cells. J. Ethnopharmacol. 2016, 185, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.J.; Zeng, R.; Ren, X.D.; Dou, Q.L.; Yang, Q.B.; Dong, Y.; Qu, Y. Anti-rheumatoid arthritis effects in adjuvant-induced arthritis in rats and molecular docking studies of Polygonum orientale L. extracts. Immunol. Lett. 2018, 201, 59–69. [Google Scholar] [CrossRef]
- Cheng, W.J.; Yang, H.T.; Chiang, C.C.; Lai, K.H.; Chen, Y.L.; Shih, H.L.; Kuo, J.J.; Hwang, T.L.; Lin, C.C. Deer Velvet Antler Extracts Exert Anti-Inflammatory and Anti-Arthritic Effects on Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and Distinct Mouse Arthritis. Am. J. Chin. Med. 2022, 50, 1617–1643. [Google Scholar] [CrossRef]
- Chen, L.; Tang, S.; Li, X.; Kuang, Y.; Huang, H.; Fan, P.; Feng, F.; Liu, W. A review on traditional usages, chemical constituents and pharmacological activities of periploca forrestii schltr. J. Ethnopharmacol. 2021, 271, 113892. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Owusu Boadi, E.; Li, J.; Pang, X.; He, J.; Chang, Y.X. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Q.; Zhou, X.; Wang, X. Preparative separation of the flavonoid fractions from Periploca forrestii Schltr. ethanol extracts using macroporous resin combined with HPLC analysis and evaluation of their biological activities. J. Sep. Sci. 2019, 42, 650–661. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Wang, X.; Dong, Y.X.; Zheng, L.; Li, Y.J.; Ni, J.M. In vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii Schltr. Chin. J. Integr. Med. 2017, 23, 528–534. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; He, Q.; Yang, X.; Ruan, F.; Sun, G. Periploca forrestii Saponin Ameliorates Murine CFA-Induced Arthritis by Suppressing Cytokine Production. Mediat. Inflamm. 2016, 2016, 7941684. [Google Scholar] [CrossRef]
- Di Gioia, M.; Zanoni, I. Dooming Phagocyte Responses: Inflammatory Effects of Endogenous Oxidized Phospholipids. Front. Endocrinol. 2021, 12, 626842. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Chen, H.; Zhou, X. Untargeted lipidomics analysis of Mori Fructus polysaccharide on acute alcoholic liver injury in mice using ultra performance liquid chromatography-quadrupole-orbitrap-high resolution mass spectrometry. Int. Immunopharmacol. 2021, 97, 107521. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.B.; Nossent, J.C.; Preen, D.B.; Keen, H.I.; Inderjeeth, C.A. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-based Studies. J. Rheumatol. 2021, 48, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: A systematic review. Semin. Arthritis Rheum. 2006, 36, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Ke, X.; Sun, C.; Huang, X.; Jiang, P.; Feng, F.; Liu, W.; Zhang, J. Chemical profiling and the potential active constituents responsible for wound healing in Periploca forrestii Schltr. J. Ethnopharmacol. 2018, 224, 230–241. [Google Scholar] [CrossRef]
- Cui, P.; Qu, F.; Sreeharsha, N.; Sharma, S.; Mishra, A.; Gubbiyappa, S.K. Antiarthritic effect of chitosan nanoparticle loaded with embelin against adjuvant-induced arthritis in Wistar rats. IUBMB Life 2020, 72, 1054–1064. [Google Scholar] [CrossRef]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef]
- Gordon, D.A. Rheumatoid arthritis—New treatments, and a new look at medical ethics. J. Rheumatol. 1998, 25, 2049. [Google Scholar]
- Lee, H.C.; Yokomizo, T. Applications of mass spectrometry-based targeted and non-targeted lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 576–581. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, Y.Q. Metabolic effects of mulberry branch bark powder on diabetic mice based on GC-MS metabolomics approach. Nutr. Metab. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Katz, P. Causes and consequences of fatigue in rheumatoid arthritis. Curr. Opin. Rheumatol. 2017, 29, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A.; He, X.; Huang, J.; Fletcher, E.A.; Zaccardelli, A.; Friedlander, H.M.; Gill, R.R.; Hatabu, H.; Nishino, M.; Murphy, D.J.; et al. Rheumatoid Arthritis Disease Activity Predicting Incident Clinically Apparent Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Prospective Cohort Study. Arthritis Rheumatol. 2019, 71, 1472–1482. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.S.; Chen, X.Y.; Yang, X.; Yan, F.L.; Cao, F.; Yang, C.F.; Liang, J. Effect of Hei Gu Teng Zhui Feng Huo Luo Capsule on PI3K /AKT /HIF-1α protein signaling pathway in rheumatoid arthritis rats. Chin. J. Immunol. 2019, 35, 2206–2216. [Google Scholar]
- Li, S.; Zhou, X.; Chen, R.; Zhang, Q.; Sun, Y.; Chen, H. Effect of natural polysaccharides on alcoholic liver disease: A review. Int. J. Biol. Macromol. 2023, 251, 126317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, X.; Zhao, Y.; Xiao, J.; Lu, Y.; Shi, Q.; Wang, Y.; Wang, H.; Liang, Q. Polyphyllin I Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response in Macrophages Through the NF-κB Pathway. Front. Immunol. 2018, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive Compounds and Potential for the Treatment of Inflammatory and Metabolic Diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bao, Y.; Wang, S.; Li, T.; Chang, X.; Yang, G.; Meng, X. Anti-Inflammation Effects and Potential Mechanism of Saikosaponins by Regulating Nicotinate and Nicotinamide Metabolism and Arachidonic Acid Metabolism. Inflammation 2016, 39, 1453–1461. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.d.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Scher, J.U.; Abramson, S.B. The microbiome and rheumatoid arthritis. Nat. Rev. Rheumatol. 2011, 7, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Li, T.; Sun, W.; Tang, Z.; Wang, Y.; Zhou, K.; Li, J.; Ding, Q.; Liang, K.; et al. NAD+ and its possible role in gut microbiota: Insights on the mechanisms by which gut microbes influence host metabolism. Anim. Nutr. 2022, 10, 360–371. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holčapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for good practice in MS-based lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.C.; Zhou, X.; Gao, S. Purification of Periploca forrestii total flavonoids and assay for the in vitro inhibitory activity on xanthine oxidase. J. Int. Pharm. Res. 2019, 7, 527–531. [Google Scholar] [CrossRef]
- Lin, B.; Zhao, Y.; Han, P.; Yue, W.; Ma, X.Q.; Rahman, K.; Zheng, C.J.; Qin, L.P.; Han, T. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund’s adjuvant induced arthritis in rats. J. Ethnopharmacol. 2014, 155, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.W.; Kim, D.S.; Lee, J.Y.; Lee, S.; Oh, H.M.; Ha, Y.S.; Yoo, J.; Park, P.H.; Shin, T.Y.; et al. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol. Appl. Pharmacol. 2016, 290, 1–9. [Google Scholar] [CrossRef]
- Sultana, F.; Neog, M.K.; Rasool, M. Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf. B Biointerfaces 2017, 155, 349–365. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Li, H.; Yang, X.; Liu, H.; Chen, J. In vivo anti-inflammatory activities of the essential oil from Radix Angelicae dahuricae. J. Nat. Med. 2016, 70, 563–570. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Feng, J.; Zhou, S.; Li, C.; Zhao, S.; Shen, Y.; Hong, L.; Xuan, Q.; Liu, X.; et al. Untargeted Lipidomics Reveals Specific Lipid Abnormalities in Nonfunctioning Human Pituitary Adenomas. J. Proteome Res. 2020, 19, 455–463. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kawakami, M.; Robert, M.; Soga, T.; Tomita, M. Bioinformatics Tools for Mass Spectroscopy-Based Metabolomic Data Processing and Analysis. Curr. Bioinform 2012, 7, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
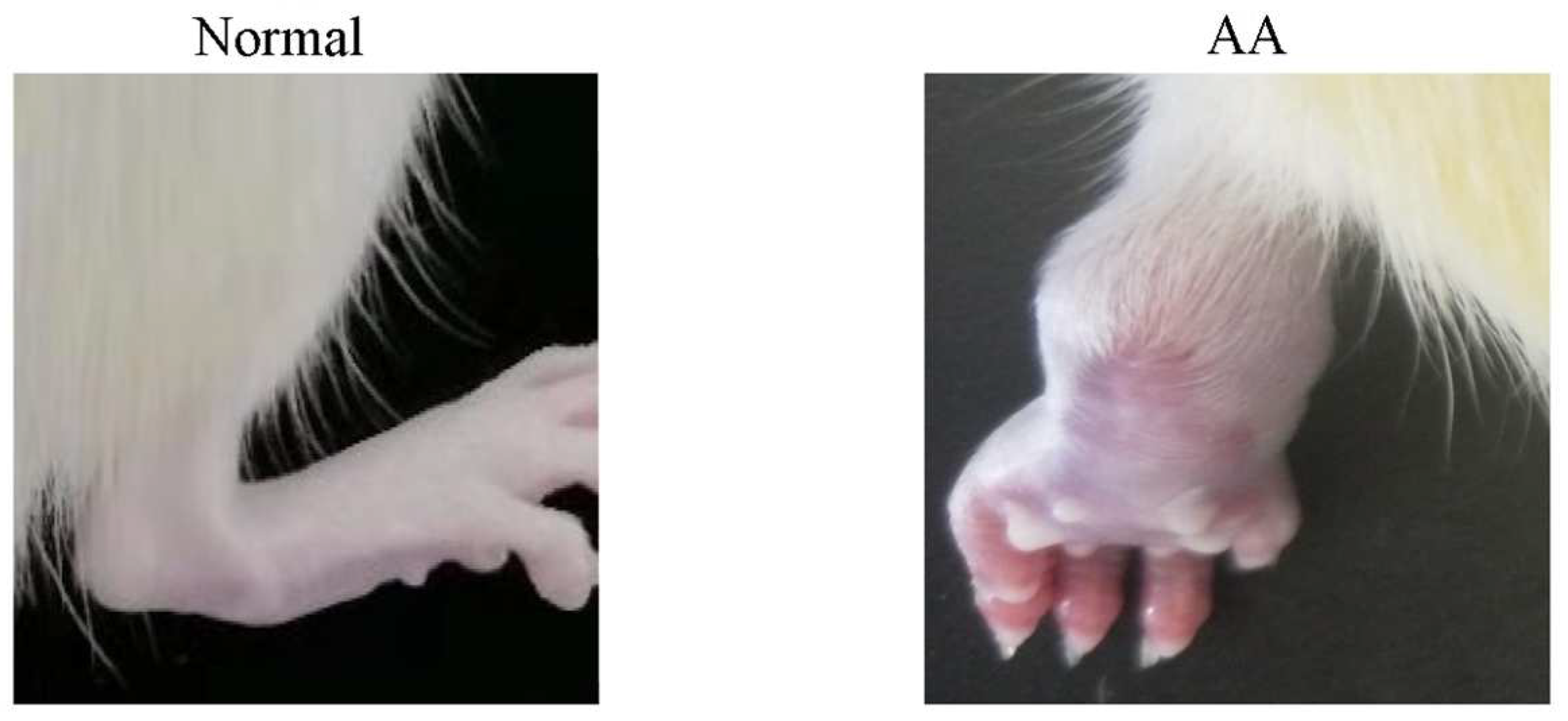

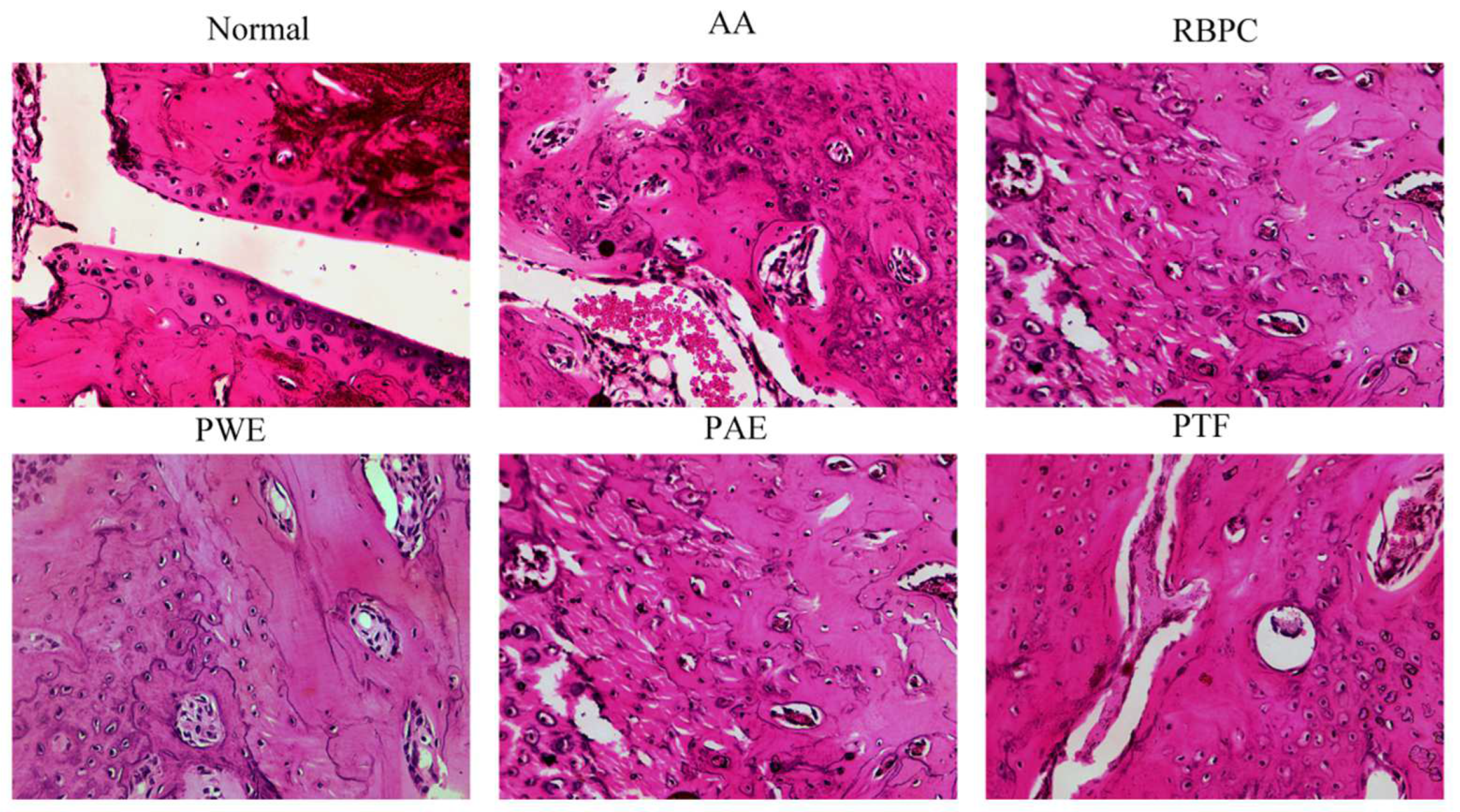
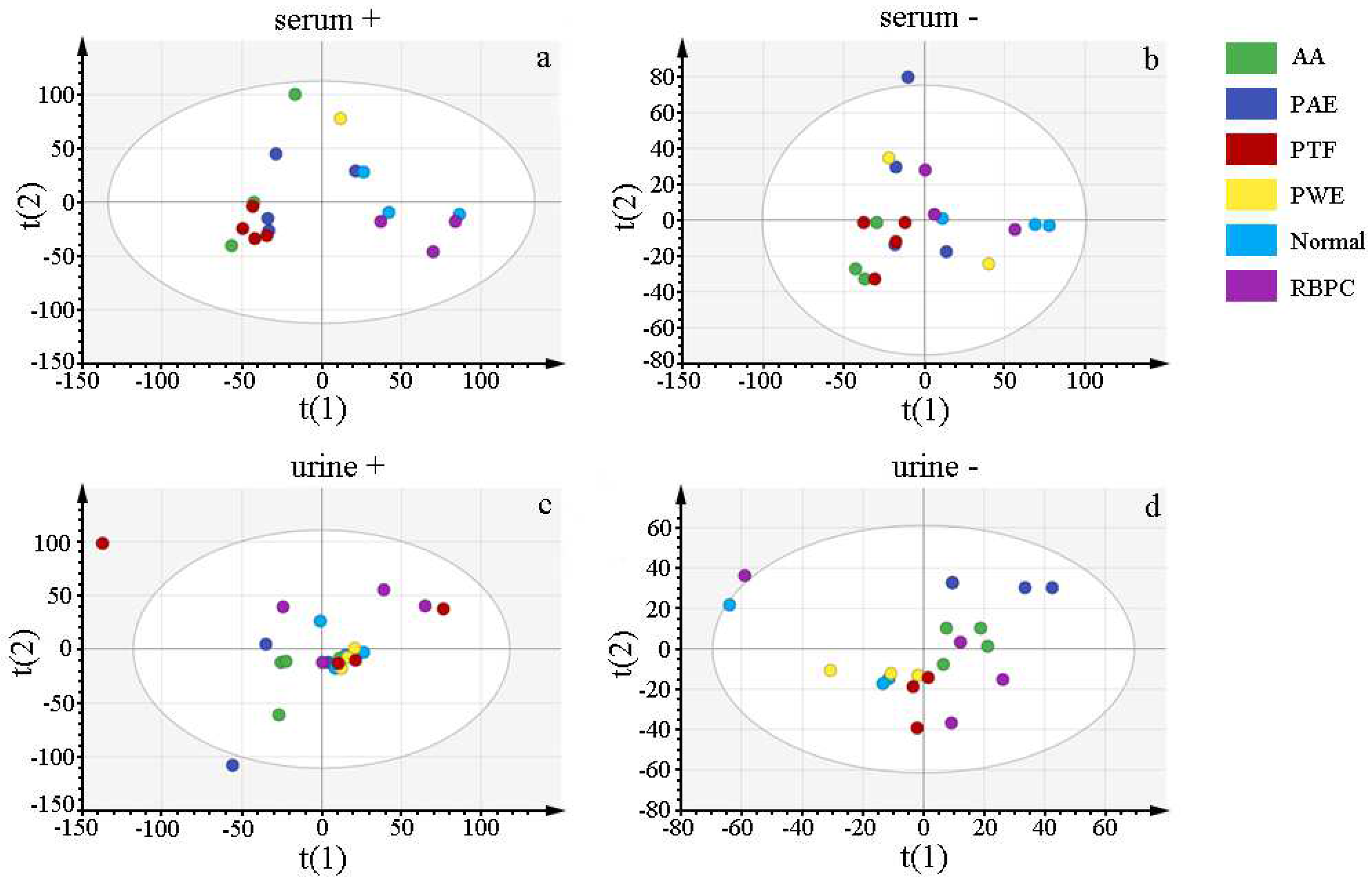



| Group | 0 h | 6 h | 24 h | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d |
|---|---|---|---|---|---|---|---|---|---|
| Normal | 6.19 ± 0.26 | 6.29 ± 0.2 * | 6.24 ± 0.11 * | 6.22 ± 0.17 * | 5.93 ± 0.16 * | 6.26 ± 0.31 * | 6.17 ± 0.12 * | 6.20 ± 0.11 * | 6.09 ± 0.11 * |
| AA | 6.20 ± 0.14 | 8.44 ± 0.47 | 8.69 ± 0.53 | 8.61 ± 0.64 | 8.37 ± 0.59 | 8.33 ± 0.43 | 8.39 ± 0.46 | 8.42 ± 0.48 | 8.33 ± 0.56 |
| RBPC | 6.24 ± 0.16 | 8.24 ± 0.72 | 8.67 ± 0.52 | 8.61 ± 0.51 | 8.59 ± 0.52 | 8.58 ± 0.43 | 8.50 ± 0.45 | 8.29 ± 0.36 | 8.10 ± 0.45 |
| PWE | 6.27 ± 0.09 | 8.44 ± 0.45 | 8.50 ± 0.53 | 8.12 ± 0.39 | 6.61 ± 0.32 # | 8.47 ± 0.38 | 8.47 ± 0.42 | 8.30 ± 0.48 | 8.20 ± 0.47 |
| PAE | 6.20 ± 0.26 | 8.46 ± 0.47 | 8.19 ± 0.46 | 8.13 ± 0.38 | 8.33 ± 0.39 | 8.24 ± 0.59 | 8.26 ± 0.51 | 8.18 ± 0.61 | 8.07 ± 0.61 |
| PTF | 6.22 ± 0.13 | 8.25 ± 0.54 | 8.28 ± 0.57 | 8.24 ± 0.51 | 8.33 ± 0.29 | 8.30 ± 0.31 | 8.12 ± 0.37 | 7.09 ± 0.47 # | 6.59 ± 0.45 # |
| Group | d 9 | d 11 | d 13 | d 15 | d 17 | d 19 | d 21 | d 23 |
|---|---|---|---|---|---|---|---|---|
| Normal | 6.19 ± 0.11 * | 5.90 ± 0.12 * | 6.16 ± 0.11 * | 6.18 ± 0.14 * | 6.07 ± 0.09 * | 6.13 ± 0.05 * | 6.10 ± 0.06 * | 6.05 ± 0.08 * |
| AA | 6.70 ± 0.16 | 6.69 ± 0.21 | 6.73 ± 0.20 | 6.73 ± 0.14 | 6.75 ± 0.10 | 6.69 ± 0.06 | 6.72 ± 0.08 | 6.71 ± 0.05 |
| RBPC | 6.50 ± 0.19 | 6.51 ± 0.26 | 6.63 ± 0.15 | 6.63 ± 0.10 | 6.48 ± 0.12 | 6.48 ± 0.08 | 6.45 ± 0.08 | 6.43 ± 0.12 |
| PWE | 6.48 ± 0.20 | 6.44 ± 0.22 | 6.52 ± 0.13 | 6.57 ± 0.11 | 6.39 ± 0.14 # | 6.45 ± 0.06 | 6.48 ± 0.04 | 6.46 ± 0.08 |
| PAE | 6.50 ± 0.14 | 6.21 ± 0.15 # | 6.65 ± 0.09 | 6.63 ± 0.06 | 6.58 ± 0.08 | 6.56 ± 0.07 | 6.26 ± 0.04 # | 6.47 ± 0.06 |
| PTF | 6.29 ± 0.18 # | 6.26 ± 0.17 # | 6.38 ± 0.08 # | 6.40 ± 0.11 # | 6.30 ± 0.05 # | 6.46 ± 0.07 | 6.24 ± 0.06 # | 6.24 ± 0.08 # |
| Group | Modeling | d 2 | d 5 | d 9 | d 13 | d 16 | d 20 | d 24 |
|---|---|---|---|---|---|---|---|---|
| Normal | 215.55 ± 0.87 | 222.47 ± 0.65 | 228.78 ± 0.32 | 232.11 ± 1.06 | 235.19 ± 0.96 | 241.11 ± 0.53 * | 247.68 ± 0.86 ** | 251.90 ± 0.72 ** |
| AA | 216.68 ± 0.82 | 219.76 ± 0.96 | 220.21 ± 1.02 | 223.71 ± 0.89 | 226.46 ± 1.32 | 221.46 ± 0.96 | 213.84 ± 0.56 | 201.02 ± 0.31 |
| RBPC | 217.68 ± 0.85 | 217.09 ± 0.98 | 220.79 ± 0.54 | 228.40 ± 0.68 | 231.19 ± 1.21 | 238.18 ± 0.36 | 241.55 ± 0.78 # | 243.20 ± 0.69 # |
| PWE | 214.36 ± 0.63 | 216.67 ± 0.85 | 220.19 ± 0.64 | 228.62 ± 0.89 | 230.59 ± 1.24 | 234.87 ± 1.33 | 236.79 ± 1.45 # | 240.90 ± 0.65 # |
| PAE | 215.90 ± 1.32 | 219.88 ± 0.98 | 222.39 ± 0.89 | 225.99 ± 1.01 | 230.41 ± 1.32 | 232.81 ± 0.78 | 236.17 ± 0.65 # | 239.80 ± 0.58 # |
| PTF | 213.97 ± 1.01 | 219.24 ± 0.65 | 220.54 ± 0.87 | 222.40 ± 0.85 | 230.93 ± 0.87 | 235.37 ± 0.89 | 237.58 ± 2.01 # | 242.50 ± 0.78 ## |
| Class | Lipid | MW | m/z | RT (Time) | FC | VIP | p-Value |
|---|---|---|---|---|---|---|---|
| Fatty acyls (FA) | N-Cyclopropyl-trans-2-cis-6-nonadienamide | 193.147 | 192.938 | 1.05 | 3.361 | 1.493 | ** |
| Herculin | 251.225 | 250.903 | 1.05 | 5.044 | 1.445 | ** | |
| Anandamide | 323.282 | 323.222 | 10.19 | 5.510 | 1.417 | ** | |
| Cerulenin | 223.121 | 223.028 | 16.34 | 3.193 | 1.474 | ** | |
| 2-chloro-2-iodoacetamide | 218.891 | 218.931 | 1.03 | 5.025 | 1.548 | ** | |
| N-arachidonoyl alanine | 375.277 | 375.193 | 1.93 | 5.859 | 1.428 | ** | |
| N-(9,12-octadecadienoyl)-glutamic acid | 409.283 | 409.210 | 13.09 | 7.823 | 1.353 | ** | |
| N-linolenoyl glutamic acid | 407.267 | 407.238 | 14.58 | 4.937 | 1.361 | ** | |
| Dodecanamide | 199.194 | 199.024 | 26.28 | 10.825 | 1.611 | ** | |
| 6-hydroxytetradeca-7,8-dien-10,12-diynoic acid | 232.110 | 232.022 | 29.61 | 0.412 | 1.652 | ** | |
| N-(5,8,11,14-eicosatetraynoyl)-ethanolamine | 339.219 | 339.120 | 13.92 | 0.243 | 1.612 | ** | |
| (5Z)-13-carboxytridec-5-enoylcarnitine | 399.262 | 399.196 | 14.31 | 0.253 | 1.614 | ** | |
| 2′-(5″-phosphoribosyl)-3′-dephospho-CoA | 899.157 | 899.210 | 18.11 | 8.556 | 1.657 | ** | |
| Hydroxy-α-sanshool | 263.188 | 263.188 | 2.00 | 0.035 | 1.632 | ** | |
| Fumarycarnitine | 259.106 | 259.049 | 3.33 | 1.668 | 3.613 | ** | |
| N-formylmaleamic acid | 143.002 | 142.995 | 14.30 | 5.843 | 1.683 | ** | |
| Palmitoleamide | 253.241 | 253.217 | 15.14 | 2.789 | 1.487 | ** | |
| Hydroxypropionylcarnitine | 233.126 | 233.036 | 1.62 | 3.20 | 1.631 | ** | |
| 2-amino-2,3,7-trideoxy-D-lyxo-hept-6-ulosonic acid | 191.079 | 191.106 | 4.90 | 4.284 | 1.641 | ** | |
| S-(2-carboxypropyl)-Cysteamine | 163.066 | 163.039 | 27.01 | 0.261 | 1.722 | ** | |
| (+)-threo-2-Amino-3,4-dihydroxybutanoic acid | 135.053 | 135.044 | 27.80 | 0.316 | 1.741 | ** | |
| phenyl [1-(N-succinylamino) pentyl]phosphonate | 343.118 | 343.167 | 11.6 | 1.391 | 9.689 | ** | |
| Elaidamide | 281.272 | 280.9828 | 21.77 | 0.444 | 0.647 | ** | |
| 2-amino-3-oxo-hexanedioic acid | 175.048 | 175.039 | 28.47 | 0.399 | 1.758 | ** | |
| C16Sphinganine-1-phosphate | 353.233 | 353.156 | 9.62 | 0.217 | 1.628 | ** | |
| Sphingolipids (SP) | Micropine | 265.204 | 265.252 | 13.79 | 0.244 | 1.631 | ** |
| Sulfamisterin | 461.206 | 461.224 | 13.48 | 3.346 | 1.347 | ** | |
| PI-Cer(46:0(2OH)) | 981.724 | 981.649 | 20.93 | 14.138 | 1.671 | ** | |
| LacCer(40:0) | 947.727 | 947.637 | 15.51 | 7.456 | 1.411 | ** | |
| Glycerophospholipids (GP) | PC(19:3) | 531.332 | 531.364 | 16.74 | 19.931 | 1.356 | ** |
| 2-(8-[3]-ladderane-octanyl)-sn-glycero-3-phosphoethanolamine | 487.306 | 487.339 | 16.60 | 10.786 | 1.399 | ** | |
| PS(12:0) | 455.192 | 455.124 | 13.50 | 2.808 | 1.299 | ** | |
| PS(22:6) | 879.505 | 879.409 | 19.39 | 23.384 | 1.527 | ** | |
| PE(19:1) | 477.322 | 477.391 | 22.11 | 8.005 | 1.659 | ** | |
| PI(14:0) | 544.265 | 544.272 | 15.44 | 4.174 | 1.680 | ** | |
| Polyketides (PK) | (E)-4-Methoxy-4′-nitrostilbene | 255.089 | 255.099 | 2.05 | 0.030 | 1.635 | ** |
| Malvidin 3-(6″-p-caffeyglucoside) | 655.166 | 655.069 | 13.47 | 4.752 | 1.444 | ** | |
| Petunidin 3-gentiotrioside | 803.225 | 803.196 | 15.13 | 10.825 | 1.610 | ** | |
| Delphinidin 3,3′-di-glucoside-5 -(6-caffeoylglucoside) | 951.241 | 951.234 | 15.91 | 24.67 | 1.236 | ** | |
| 6-Hydroxydelphinidin 3-glucoside | 481.098 | 481.133 | 13.48 | 4.068 | 1.291 | ** | |
| 13-Deoxydaunorubicin | 513.199 | 513.104 | 13.50 | 3.209 | 1.428 | ** | |
| Delphinidin 3-caffeylglucoside | 627.135 | 627.050 | 13.52 | 3.714 | 1.349 | ** | |
| Pelargonidin 3-(6″-malonylglucoside) | 519.114 | 519.149 | 15.14 | 20.063 | 1.537 | ** | |
| Quercetin3-(2‴-caffeylsambubioside)-7-glucoside | 920.222 | 920.250 | 17.09 | 14.725 | 1.551 | ** | |
| Petunidin | 317.066 | 317.065 | 26.24 | 3.515 | 1.655 | ** | |
| Malvidin 3-glucoside-pyruvate | 561.124 | 561.181 | 15.92 | 13.305 | 1.577 | ** | |
| Isorientin4’-O-glucoside2″-O-p-hydroxybenzoagte | 730.174 | 730.251 | 17.13 | 2.868 | 1.419 | ** | |
| Quercetin3-(2‴-galloylglucosyl)-(1->2)-α-L-arabinofuranoside | 748.149 | 748.246 | 14.36 | 2.676 | 1.491 | ** | |
| Hirsutidin | 345.097 | 345.097 | 15.26 | 0.260 | 1.671 | ** | |
| Malvidin3-glucoside-5-(2″-sulfatoglucoside) | 735.144 | 735.071 | 14.09 | 6.989 | 1.386 | ** | |
| Riccionidin A | 285.04 | 285.0493 | 27.25 | 0.319 | 1.645 | ** | |
| Isorhamnetin 3-(6″-(E)-sinapoylsophoroside) | 846.222 | 846.2310 | 16.53 | 11.717 | 1.659 | ** | |
| Sirolimus | 913.555 | 913.582 | 20.91 | 27.309 | 1.526 | ** | |
| Isoprenoids (PR) | (2E)-4-hydroxy-3-methylbut-2-en-1-yl trihydrogen diphosphate | 262.0007 | 261.9093 | 29.99 | 0.485 | 1.669 | ** |
| Sterol Lipids (ST) | Squalamine | 627.464 | 627.386 | 13.47 | 5.311 | 1.470 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Yan, X.; Chen, H.; Zhou, X. Investigation of the Mechanism of Action of Periploca forrestii Schltr. Extract on Adjuvant Collagen Rats Based on UPLC-Q-Orbitrap-HRMS Non-Targeted Lipidomics. Molecules 2023, 28, 6751. https://doi.org/10.3390/molecules28196751
An S, Yan X, Chen H, Zhou X. Investigation of the Mechanism of Action of Periploca forrestii Schltr. Extract on Adjuvant Collagen Rats Based on UPLC-Q-Orbitrap-HRMS Non-Targeted Lipidomics. Molecules. 2023; 28(19):6751. https://doi.org/10.3390/molecules28196751
Chicago/Turabian StyleAn, Silan, Xiaoting Yan, Huaguo Chen, and Xin Zhou. 2023. "Investigation of the Mechanism of Action of Periploca forrestii Schltr. Extract on Adjuvant Collagen Rats Based on UPLC-Q-Orbitrap-HRMS Non-Targeted Lipidomics" Molecules 28, no. 19: 6751. https://doi.org/10.3390/molecules28196751
APA StyleAn, S., Yan, X., Chen, H., & Zhou, X. (2023). Investigation of the Mechanism of Action of Periploca forrestii Schltr. Extract on Adjuvant Collagen Rats Based on UPLC-Q-Orbitrap-HRMS Non-Targeted Lipidomics. Molecules, 28(19), 6751. https://doi.org/10.3390/molecules28196751







