Merging of Azulene and Perylene Diimide for Optical pH Sensors
Abstract
1. Introduction
2. Results and Discussion
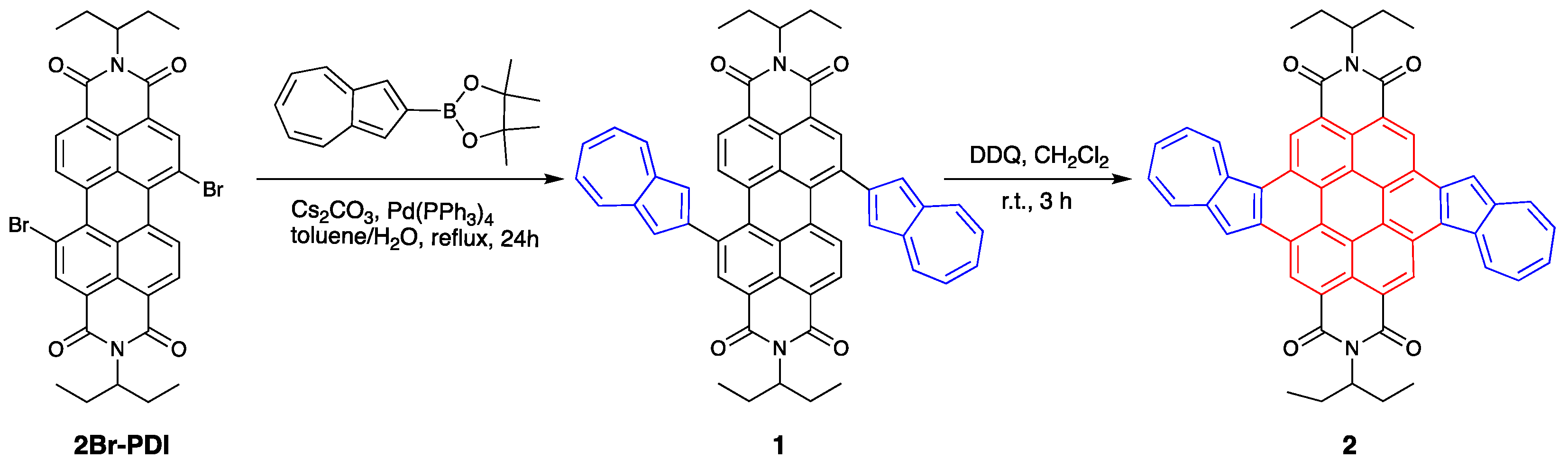
2.1. Synthesis
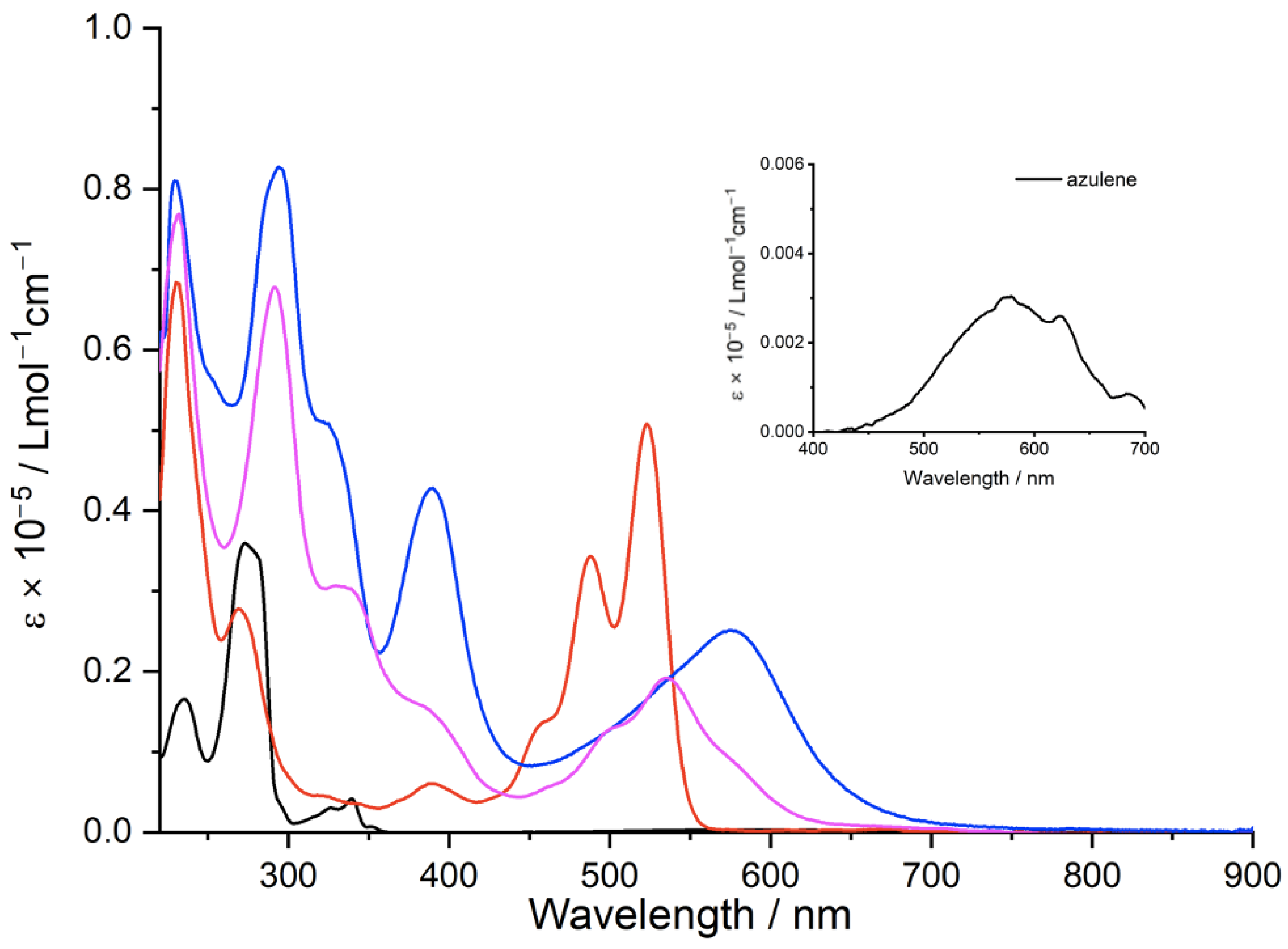
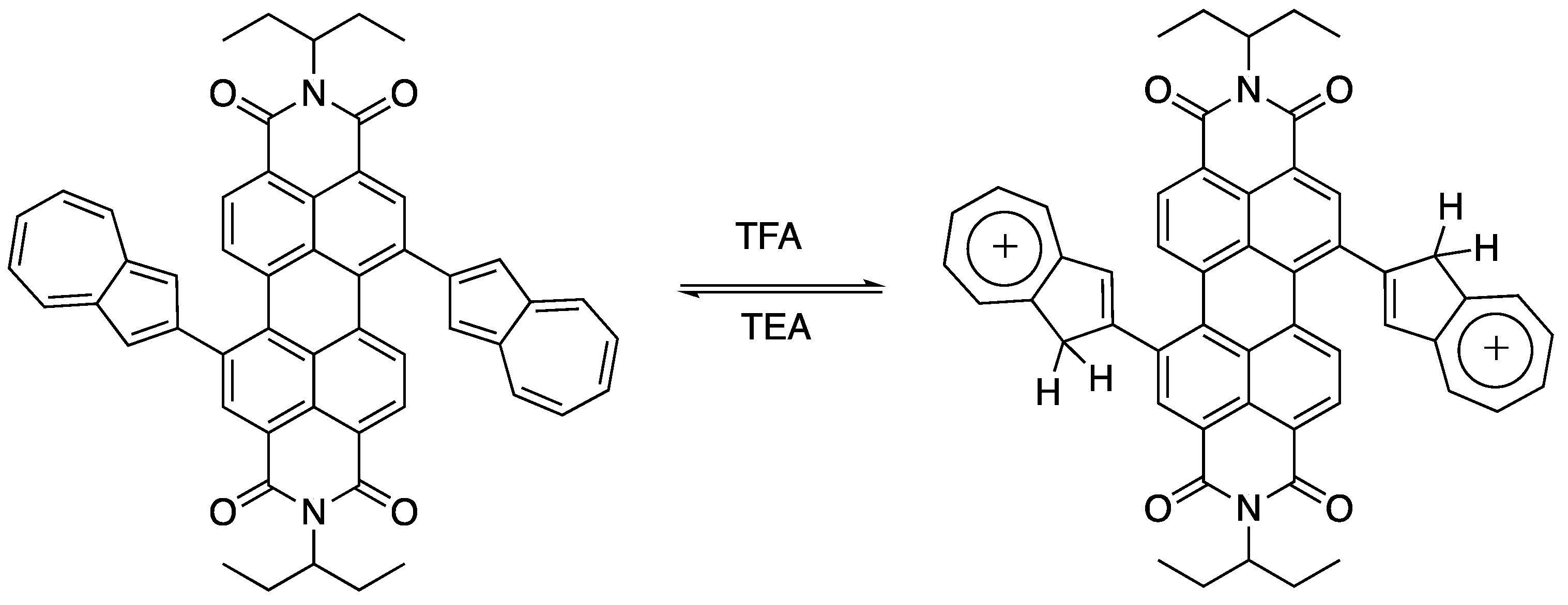
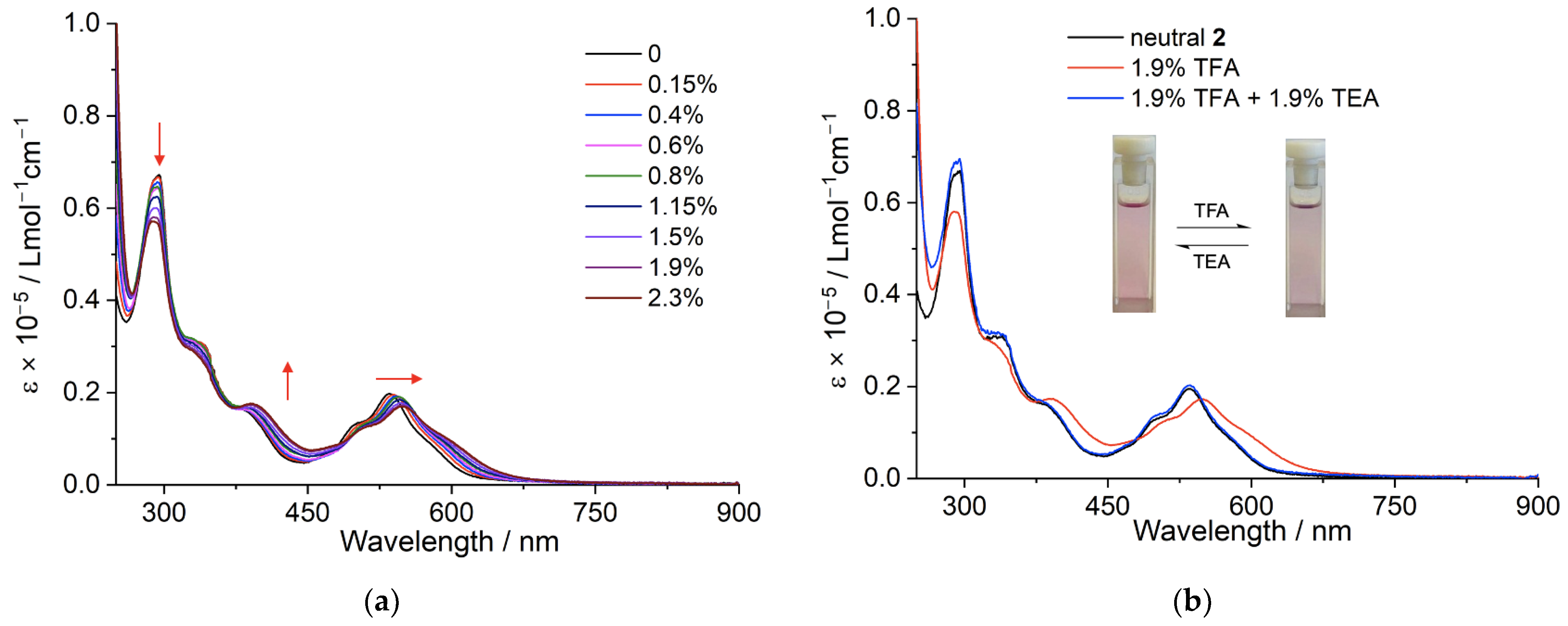
2.2. Optical Properties
2.3. Electrochemical Properties
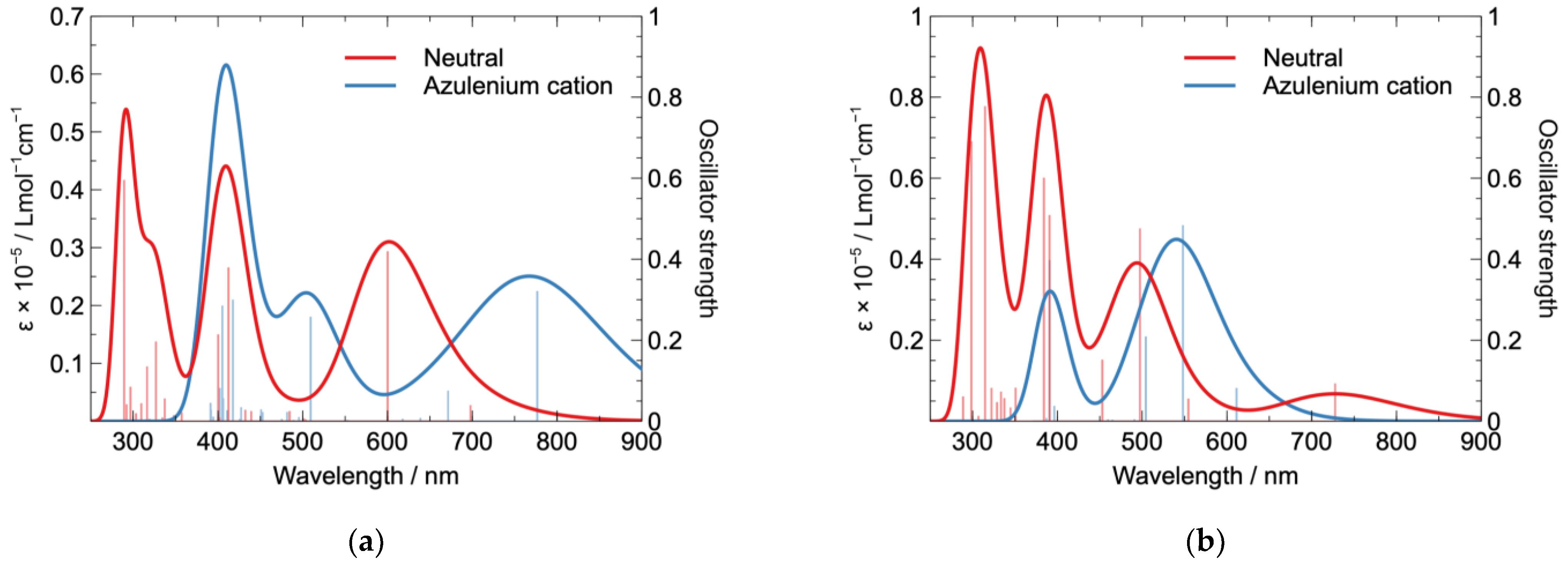
2.4. Computational Study
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hiroto, S. Heteroatoms in Bowl-shaped Polycyclic Aromatic Hydrocarbons: Synthesis and Structures. Chem. Lett. 2021, 50, 1146–1155. [Google Scholar] [CrossRef]
- Luo, J.; Xu, X.; Mao, R.; Miao, Q. Curved Polycyclic Aromatic Molecules That Are π-Isoelectronic to Hexabenzocoronene. J. Am. Chem. Soc. 2012, 134, 13796–13803. [Google Scholar] [CrossRef]
- Pascal, R.A. Twisted Acenes. Chem. Rev. 2006, 106, 4809–4819. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Huang, Y.-Y.; Zhang, J.; Meng, W.; Peng, Q.; Kong, R.; Xiao, Z.; Liu, J.; Huang, M.; Yi, Y.; et al. Dicyclohepta [ijkl,uvwx] rubicene with Two Pentagons and Two Heptagons as a Stable and Planar Non-benzenoid Nanographene. Angew. Chem. Int. Ed. 2020, 59, 3529–3533. [Google Scholar] [CrossRef]
- Ball, M.; Zhong, Y.; Wu, Y.; Schenck, C.; Ng, F.; Steigerwald, M.; Xiao, S.; Nuckolls, C. Contorted Polycyclic Aromatics. Acc. Chem. Res. 2015, 48, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Hou, B.; Gao, X. Azulene-Based π-Functional Materials: Design, Synthesis, and Applications. Acc. Chem. Res. 2021, 54, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.N.; Png, Z.M.; Xu, J. Azulene in Polymers and Their Properties. Chem. Asian J. 2020, 15, 1904–1915. [Google Scholar] [CrossRef]
- Huang, J.; Huang, S.; Zhao, Y.; Feng, B.; Jiang, K.; Sun, S.; Ke, C.; Kymakis, E.; Zhuang, X. Azulene-Based Molecules, Polymers, and Frameworks for Optoelectronic and Energy Applications. Small Methods 2020, 4, 2000628. [Google Scholar] [CrossRef]
- Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. Incorporation of 1,3-Free-2,6-Connected Azulene Units into the Backbone of Conjugated Polymers: Improving Proton Responsiveness and Electrical Conductivity. ACS Macro Lett. 2019, 8, 1360–1364. [Google Scholar] [CrossRef]
- Xin, H.; Gao, X. Application of Azulene in Constructing Organic Optoelectronic Materials: New Tricks for an Old Dog. ChemPlusChem 2017, 82, 945–956. [Google Scholar] [CrossRef]
- Koch, M.; Blacque, O.; Venkatesan, K. Impact of 2,6-connectivity in azulene: Optical properties and stimuli responsive behavior. J. Mater. Chem. C 2013, 1, 7400–7408. [Google Scholar] [CrossRef]
- Ran, H.; Li, F.; Zheng, R.; Zhang, H.; Xie, F.; Jin, P.; Lei, Z.; Wang, X.-T.; Hu, J.-Y. Polarity change of OFETs based on Dithienocoronene Diimide (DTCDI)-Derived isomeric triads end-capped with Azulene. Dyes Pigm. 2022, 203, 110311. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.-I.; Koganezawa, T.; Katagiri, H. Terazulene Isomers: Polarity Change of OFETs through Molecular Orbital Distribution Contrast. J. Am. Chem. Soc. 2016, 138, 11335–11343. [Google Scholar] [CrossRef]
- Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Tuning the Semiconducting Behaviors of New Alternating Dithienyldiketopyrrolopyrrole-Azulene Conjugated Polymers by Varying the Linking Positions of Azulene. Macromolecules 2015, 48, 2039–2047. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. Terazulene: A High-Performance n-Type Organic Field-Effect Transistor Based on Molecular Orbital Distribution Control. J. Am. Chem. Soc. 2013, 135, 19095–19098. [Google Scholar] [CrossRef]
- Wang, X.; Ng, J.K.-P.; Jia, P.; Lin, T.; Cho, C.M.; Xu, J.; Lu, X.; He, C. Synthesis, Electronic, and Emission Spectroscopy, and Electrochromic Characterization of Azulene-Fluorene Conjugated Oligomers and Polymers. Macromolecules 2009, 42, 5534–5544. [Google Scholar] [CrossRef]
- Chen, L.; Wu, B.; Qin, L.; Huang, Y.-Y.; Meng, W.; Kong, R.; Yu, X.; ChenChai, K.; Li, C.; Zhang, G.; et al. A perylene five-membered ring diimide for organic semiconductors and π-expanded conjugated molecules. Chem. Commun. 2022, 58, 5100–5103. [Google Scholar] [CrossRef]
- Xin, H.; Li, J.; Lu, R.-Q.; Gao, X.; Swager, T.M. Azulene-Pyridine-Fused Heteroaromatics. J. Am. Chem. Soc. 2020, 142, 13598–13605. [Google Scholar] [CrossRef]
- Sasaki, Y.; Takase, M.; Okujima, T.; Mori, S.; Uno, H. Synthesis and Redox Properties of Pyrrole- and Azulene-Fused Azacoronene. Org. Lett. 2019, 21, 1900–1903. [Google Scholar] [CrossRef]
- Jiang, Q.; Tao, T.; Phan, H.; Han, Y.; Gopalakrishna, T.Y.; Herng, T.S.; Li, G.; Yuan, L.; Ding, J.; Chi, C. Diazuleno-s-indacene Diradicaloids: Syntheses, Properties, and Local (anti)Aromaticity Shift from Neutral to Dicationic State. Angew. Chem. Int. Ed. 2018, 57, 16737–16741. [Google Scholar] [CrossRef] [PubMed]
- Hieulle, J.; Carbonell-Sanroma, E.; Vilas-Varela, M.; Garcia-Lekue, A.; Guitian, E.; Pena, D.; Pascual, J.I. On-Surface Route for Producing Planar Nanographenes with Azulene Moieties. Nano Lett. 2018, 18, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Takesue, M.; Murafuji, T.; Satomi, K.; Suzuki, Y.; Kawamata, J.; Terai, K.; Suzuki, M.; Yamada, H.; Shiota, Y.; et al. An Azulene-Fused Tetracene Diimide with a Small HOMO-LUMO Gap. ChemPlusChem 2017, 82, 1010–1014. [Google Scholar]
- Pigulski, B.; Shoyama, K.; Würthner, F. NIR-Absorbing π-Extended Azulene: Non-Alternant Isomer of Terrylene Bisimide. Angew. Chem. Int. Ed. 2020, 59, 15908–15912. [Google Scholar] [CrossRef]
- Murai, M.; Iba, S.; Ota, H.; Takai, K. Azulene-Fused Linear Polycyclic Aromatic Hydrocarbons with Small Bandgap, High Stability, and Reversible Stimuli Responsiveness. Org. Lett. 2017, 19, 5585–5588. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Tang, M.; Wu, L.; Bian, L.; Jiang, L.; Tang, Z.-B.; Liu, J.; Guan, A.; Liu, Z. Cascade Synthesis of Benzotriazulene with Three Embedded Azulene Units and Large Stokes Shifts. Angew. Chem. Int. Ed. 2023, 62, e202218839. [Google Scholar] [CrossRef]
- Xin, H.; Li, J.; Yang, X.; Gao, X. Azulene-Based BN-Heteroaromatics. J. Org. Chem. 2020, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kurotobi, K.; Kim, K.S.; Noh, S.B.; Kim, D.; Osuka, A. A quadruply azulene-fused porphyrin with intense near-IR absorption and a large two-photon absorption cross section. Angew. Chem. Int. Ed. 2006, 45, 3944–3947. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef]
- Barendt, T.A.; Myers, W.K.; Cornes, S.P.; Lebedeva, M.A.; Porfyrakis, K.; Marques, I.; Félix, V.; Beer, P.D. The Green Box: An Electronically Versatile Perylene Diimide Macrocyclic Host for Fullerenes. J. Am. Chem. Soc. 2020, 142, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Mallia, A.R.; Muraleedharan, K.; Hariharan, M. Enhanced intersystem crossing in core-twisted aromatics. Chem. Sci. 2017, 8, 1776–1782. [Google Scholar] [CrossRef]
- Lee, K.J.; Woo, J.H.; Kim, E.; Xiao, Y.; Su, X.; Mazur, L.M.; Attias, A.J.; Fages, F.; Cregut, O.; Barsella, A.; et al. Electronic energy and electron transfer processes in photoexcited donor–acceptor dyad and triad molecular systems based on triphenylene and perylene diimide units. Phys. Chem. Chem. Phys. 2016, 18, 7875–7887. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Xu, K.; Kucukoz, B.; Cui, X.; Zhao, J.; Wang, Z.; Karatay, A.; Yaglioglu, H.G.; Hayvali, M.; Elmali, A. DiiodoBodipy-Perylenebisimide Dyad/Triad: Preparation and Study of the Intramolecular and Intermolecular Electron/Energy Transfer. J. Org. Chem. 2015, 80, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.J.; Spaenig, F.; Rodriguez-Morgade, M.S.; Ohkubo, K.; Fukuzumi, S.; Guldi, D.M.; Torres, T. A Tightly Coupled Bis(zinc(II) phthalocyanine)-Perylenediimide Ensemble To Yield Long-Lived Radical Ion Pair States. Org. Lett. 2007, 9, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Wescott, L.D.; Mattern, D.L. Donor-σ-Acceptor Molecules Incorporating a Nonadecyl-Swallowtailed Perylenediimide Acceptor. J. Org. Chem. 2003, 68, 10058–10066. [Google Scholar] [CrossRef]
- Volland, M.; Zhou, P.; Wibmer, L.; Häner, R.; Decurtins, S.; Liu, S.X.; Guldi, D.M. Nanographene favors electronic interactions with an electron acceptor rather than an electron donor in a planar fused push-pull conjugate. Nanoscale 2019, 11, 1437–1441. [Google Scholar] [CrossRef]
- Pfattner, R.; Pavlica, E.; Jaggi, M.; Liu, S.-X.; Decurtins, S.; Bratina, G.; Veciana, J.; Mas-Torrent, M.; Rovira, C. Photo-induced intramolecular charge transfer in an ambipolar field-effect transistor based on a pi-conjugated donor-acceptor dyad. J. Mater. Chem. C 2013, 1, 3985–3988. [Google Scholar] [CrossRef][Green Version]
- El-Khouly, M.E.; Jaggi, M.; Schmid, B.; Blum, C.; Liu, S.-X.; Decurtins, S.; Ohkubo, K.; Fukuzumi, S. Annulation of Tetrathiafulvalene to the Bay Region of Perylenediimide: Fast Electron-Transfer Processes in Polar and Nonpolar Solvents. J. Phys. Chem. C 2011, 115, 8325–8334. [Google Scholar] [CrossRef]
- Jaggi, M.; Blum, C.; Marti, B.S.; Liu, S.-X.; Leutwyler, S.; Decurtins, S. Annulation of Tetrathiafulvalene to the Bay Region of Perylenediimide. Org. Lett. 2010, 12, 1344–1347. [Google Scholar] [CrossRef]
- Jaggi, M.; Blum, C.; Dupont, N.; Grilj, J.; Liu, S.-X.; Hauser, J.; Hauser, A.; Decurtins, S. A Compactly Fused pi-Conjugated Tetrathiafulvalene-Perylenediimide Donor-Acceptor Dyad. Org. Lett. 2009, 11, 3096–3099. [Google Scholar] [CrossRef][Green Version]
- Zhang, A.; Jiang, W.; Wang, Z. Fulvalene-Embedded Perylene Diimide and Its Stable Radical Anion. Angew. Chem. Int. Ed. 2020, 59, 752–757. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Y.; Yu, Q.; Liu, Y.; Jiang, W.; Wang, D.; Wang, Z. Fuller-Rylenes: Cross-Dimensional Molecular Carbons. CCS Chem. 2020, 2, 271–279. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; McAfee, S.M.; Sun, J.-P.; McCormick, T.M.; Hill, I.G.; Welch, G.C. Phthalimide-based π-conjugated small molecules with tailored electronic energy levels for use as acceptors in organic solar cells. J. Mater. Chem. C 2015, 3, 8904–8915. [Google Scholar] [CrossRef]
- Vollbrecht , J.; Bock, H.; Wiebeler, C.; Schumacher, S.; Kitzerow, H. Polycyclic Aromatic Hydrocarbons Obtained by Lateral Core Extension of Mesogenic Perylenes: Absorption and Optoelectronic Properties. Chem. Eur. J. 2014, 20, 12026–12031. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Amir, R.J.; Campos, L.M.; Hawker, C.J. Stimuli-Responsive Azulene-Based Conjugated Oligomers with Polyaniline-like Properties. J. Am. Chem. Soc. 2011, 133, 10046–10049. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Amir, E.; Amir, R.J.; Hawker, C.J. Azulene-based conjugated polymers: Unique seven-membered ring connectivity leading to stimuli-responsiveness. Chem. Sci. 2012, 3, 2721–2725. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Y.-H.; Han, M.-Y. Stimuli-Responsive Conjugated Copolymers Having Electro-Active Azulene and Bithiophene Units in the Polymer Skeleton: Effect of Protonation and p-Doping on Conducting Properties. Macromolecules 2004, 37, 3222–3230. [Google Scholar] [CrossRef]
- Murai, M.; Ku, S.-Y.; Treat, N.D.; Robb, M.J.; Chabinyc, M.L.; Hawker, C.J. Modulating structure and properties in organic chromophores: Influence of azulene as a building block. Chem. Sci. 2014, 5, 3753–3760. [Google Scholar] [CrossRef]
- Mahmood, Z.; Sukhanov, A.A.; Rehmat, N.; Hu, M.; Elmali, A.; Xiao, Y.; Zhao, J.; Karatay, A.; Dick, B.; Voronkova, V.K. Intersystem Crossing and Triplet-State Property of Anthryl- and Carbazole-[1,12]fused Perylenebisimide Derivatives with a Twisted π-Conjugation Framework. J. Phys. Chem. B 2021, 125, 9317–9332. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.; Liu, J.; Chen, Y.; Wu, J.; Pang, Z.; Lu, Z.; Zhao, S.; Huang, Y. Marked effects of azulenyl vs. naphthyl groups on donor-π-acceptor-π-donor small molecules for organic photovoltaic cells. Dyes Pigm. 2021, 187, 109079. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
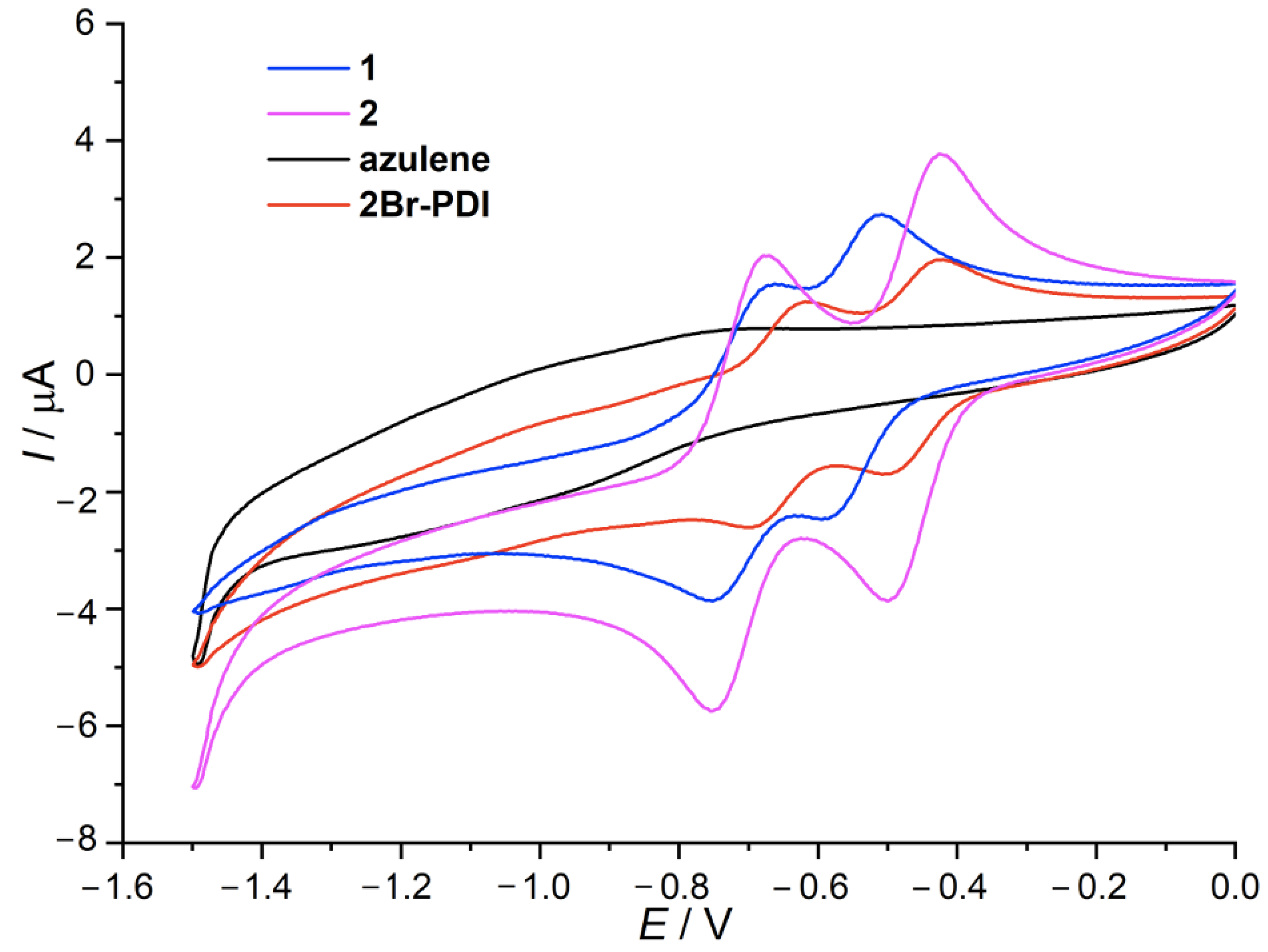


| Compounds | Eox (V) | E1/2red1 (V) | E1/2red2 (V) | HOMO a (eV) | LUMO a (eV) | EgCV (eV) | Egopt (eV) |
|---|---|---|---|---|---|---|---|
| azulene | 1.19 | −5.31 | 1.74 c | ||||
| 2Br-PDI | −0.47 | −0.67 | −3.90 | 2.27 c | |||
| 1 | 1.19 | −0.55 | −0.72 | −5.36 | −3.81 | 1.55 b | 1.90 c |
| 2 | 1.32 | −0.46 | −0.71 | −5.43 | −3.90 | 1.53 b | 2.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Aschauer, U.; Decurtins, S.; Feurer, T.; Häner, R.; Liu, S.-X. Merging of Azulene and Perylene Diimide for Optical pH Sensors. Molecules 2023, 28, 6694. https://doi.org/10.3390/molecules28186694
Zhou P, Aschauer U, Decurtins S, Feurer T, Häner R, Liu S-X. Merging of Azulene and Perylene Diimide for Optical pH Sensors. Molecules. 2023; 28(18):6694. https://doi.org/10.3390/molecules28186694
Chicago/Turabian StyleZhou, Ping, Ulrich Aschauer, Silvio Decurtins, Thomas Feurer, Robert Häner, and Shi-Xia Liu. 2023. "Merging of Azulene and Perylene Diimide for Optical pH Sensors" Molecules 28, no. 18: 6694. https://doi.org/10.3390/molecules28186694
APA StyleZhou, P., Aschauer, U., Decurtins, S., Feurer, T., Häner, R., & Liu, S.-X. (2023). Merging of Azulene and Perylene Diimide for Optical pH Sensors. Molecules, 28(18), 6694. https://doi.org/10.3390/molecules28186694







