Activation of SF5CF3 by the N-Heterocyclic Carbene SIMes
Abstract
1. Introduction
2. Results
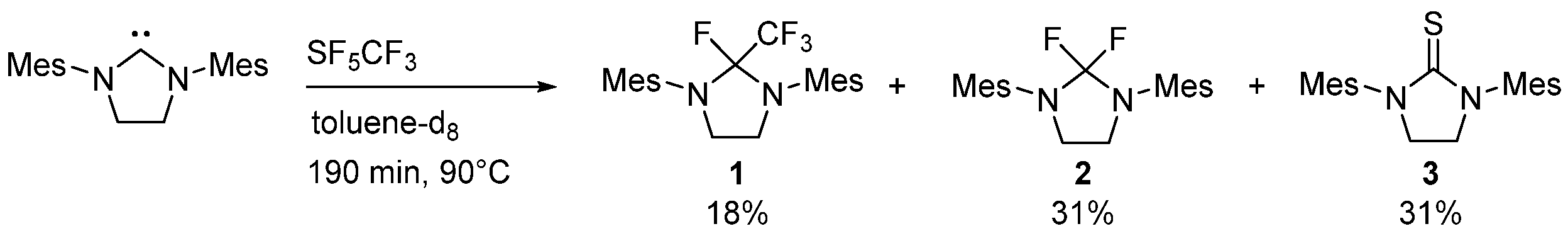
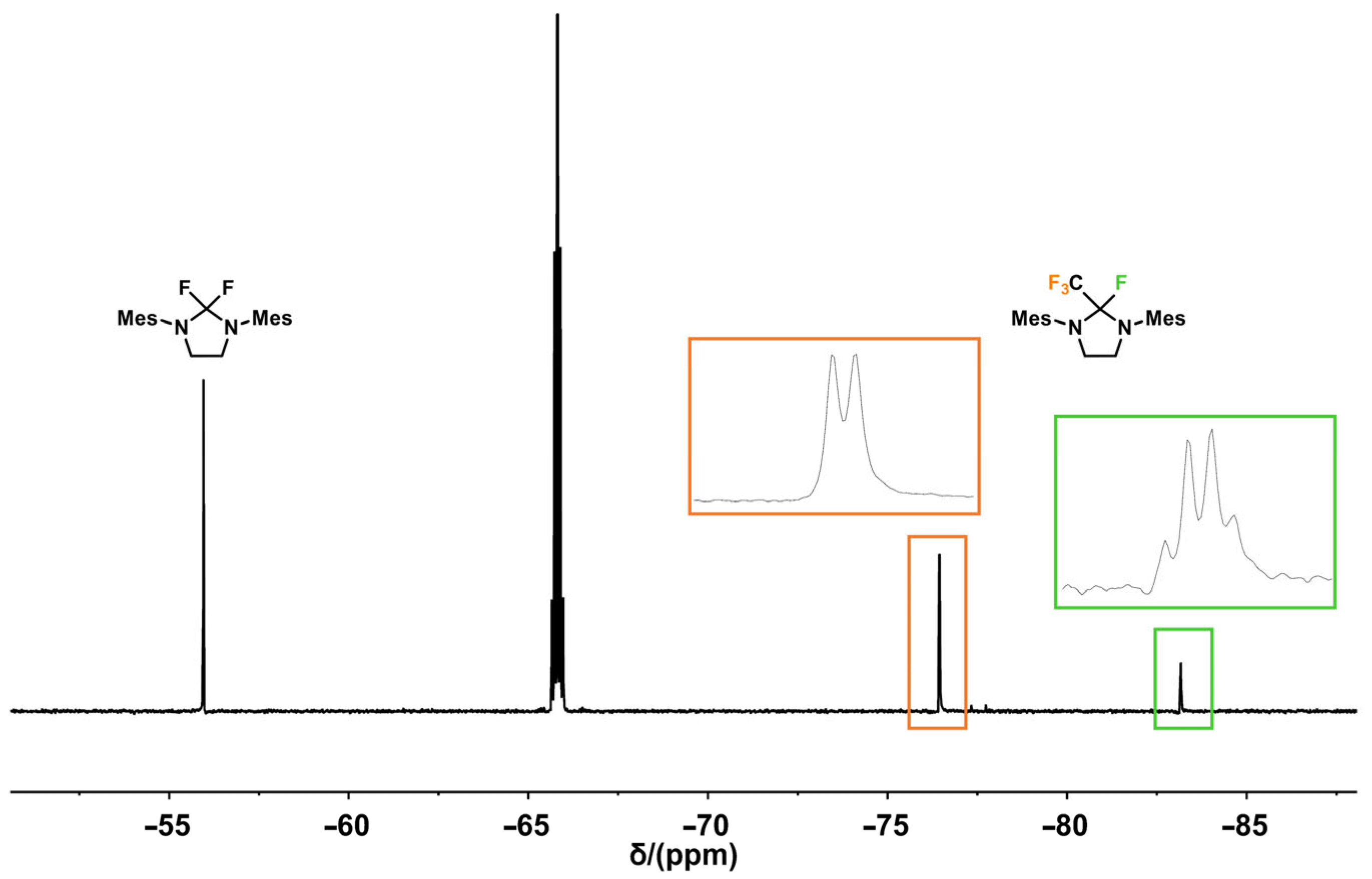
2.1. Thermal Activation of SF5CF3 with SIMes
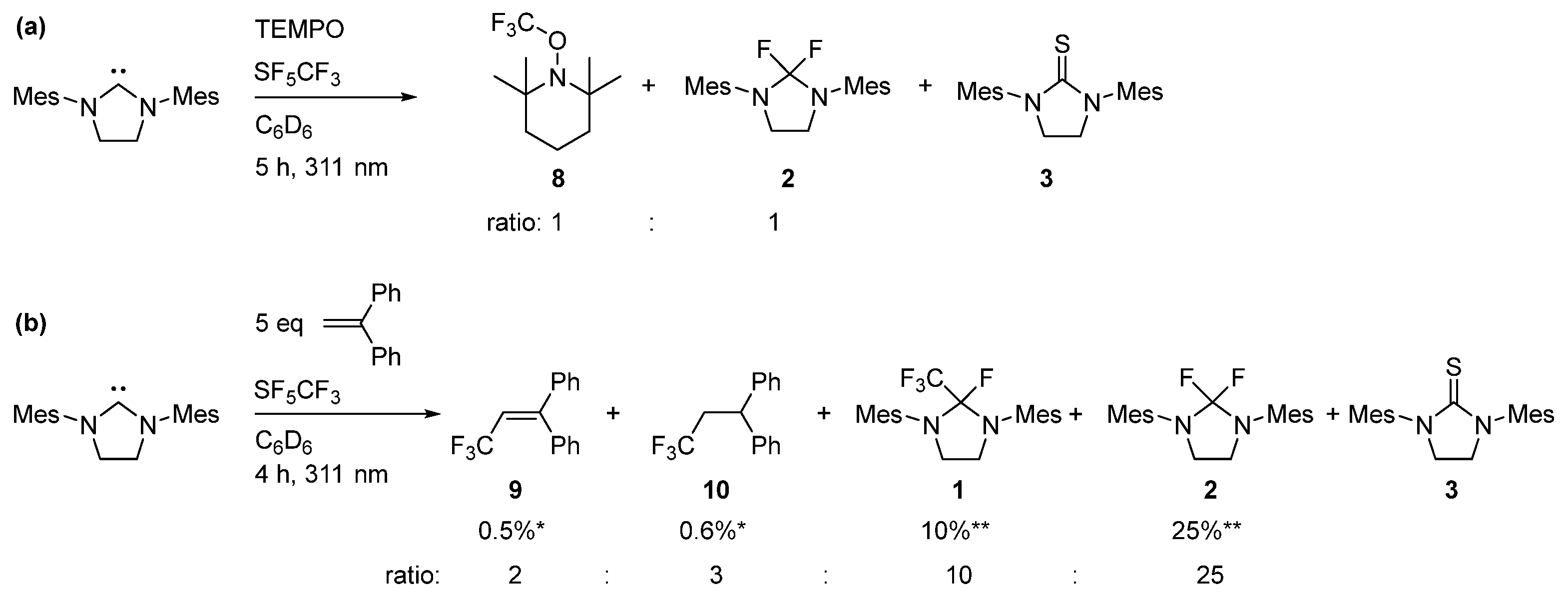
2.2. Photolytic Activation of SF5CF3 with SIMes
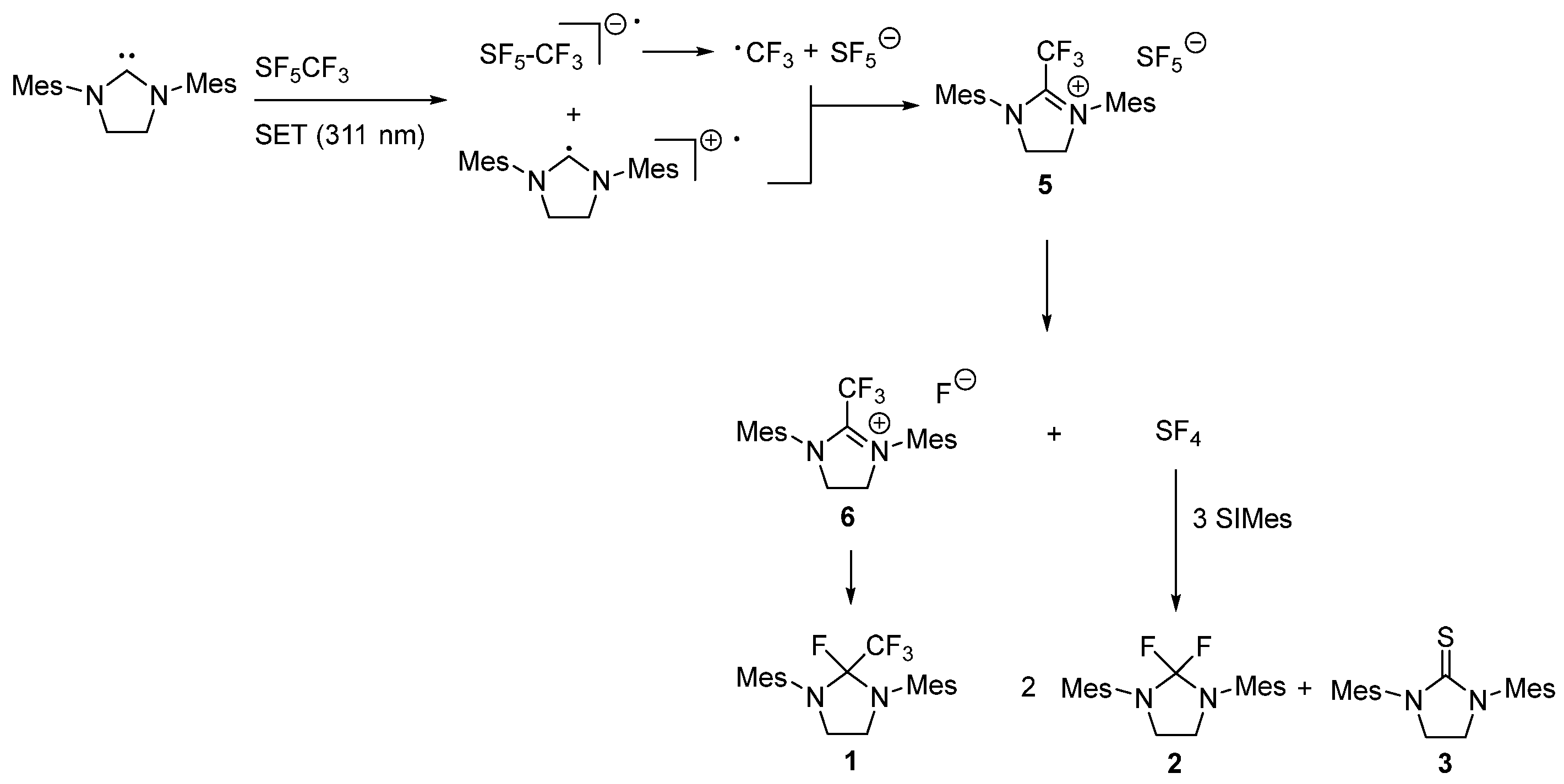
2.3. Mechanisms for the Activation of SF5CF3
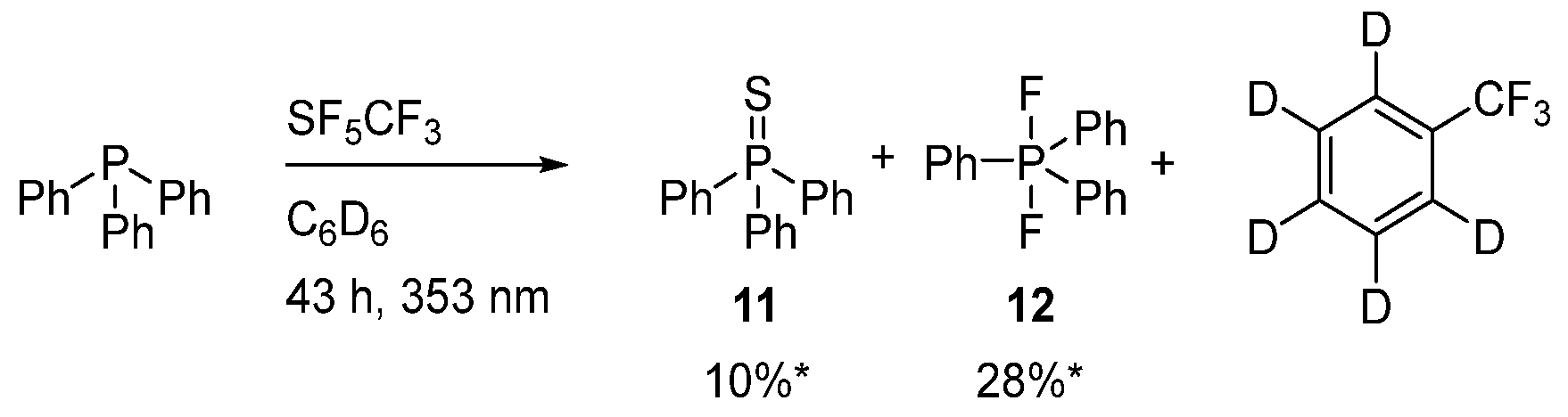
2.4. Activation of SF5CF3 with Triphenylphosphine
3. Materials and Methods
3.1. General Instruments, Methods, and Materials
3.2. Activation of SF5CF3 with SIMes by Heating
3.3. Photochemical Activation of SF5CF3 with SIMes
3.4. Experiments to Trap Radicals
3.4.1. Addition of TEMPO to Reaction Mixture
3.4.2. Addition of 1,1-Diphenylethylene
3.5. Activation of SF5CF3 with PPh3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sturges, W.; Wallington, T.J.; Hurley, M.D.; Shine, K.; Sihra, K.; Engel, A.; Oram, D.E.; Penkett, S.A.; Mulvaney, R.; Brenninkmeijer, C.A.M. A potent greenhouse gas identified in the atmosphere: SF5CF3. Science 2000, 289, 611–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savoie, P.R.; Welch, J.T. Preparation and utility of organic pentafluorosulfanyl-containing compounds. Chem. Rev. 2015, 115, 1130–1190. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Kumar, H.P.S.; Fye, J.L.; Sun Blanks, J.; Thrasher, J.S.; Willner, H.; Oberhammer, H. Synthesis of the Long Sought after Compound Pentafluoronitrosulfane, SF5NO2. Angew. Chem. Int. Ed. 2006, 118, 952–954. [Google Scholar] [CrossRef]
- Holze, P.; Horn, B.; Limberg, C.; Matlachowski, C.; Mebs, S. The Activation of Sulfur Hexafluoride at Highly Reduced Low-Coordinate Nickel Dinitrogen Complexes. Angew. Chem. Int. Ed. 2014, 126, 2788–2791. [Google Scholar] [CrossRef]
- Zámostná, L.; Braun, T. Catalytic Degradation of Sulfur Hexafluoride by Rhodium Complexes. Angew. Chem. Int. Ed. 2015, 127, 10798–10802. [Google Scholar] [CrossRef]
- Wozniak, M.; Braun, T.; Ahrens, M.; Braun-Cula, B.; Wittwer, P.; Herrmann, R.; Laubenstein, R. Activation of SF6 at a Xantphos-Type Rhodium Complex. Organometallics 2018, 37, 821–828. [Google Scholar] [CrossRef]
- Rueping, M.; Nikolaienko, P.; Lebedev, Y.; Adams, A. Metal-free reduction of the greenhouse gas sulfur hexafluoride, formation of SF5 containing ion pairs and the application in fluorinations. Green Chem. 2017, 19, 2571–2575. [Google Scholar] [CrossRef]
- Buß, F.; Mück-Lichtenfeld, C.; Mehlmann, P.; Dielmann, F. Nucleophilic Activation of Sulfur Hexafluoride: Metal-Free, Selective Degradation by Phosphines. Angew. Chem. Int. Ed. 2018, 130, 5045–5049. [Google Scholar] [CrossRef]
- Eder, T.; Buß, F.; Wilm, L.F.B.; Seidl, M.; Podewitz, M.; Dielmann, F. Oxidative Fluorination of Selenium and Tellurium Compounds using a Thermally Stable Phosphonium SF5− Salt Accessible from SF6. Angew. Chem. Int. Ed. 2022, 61, e202209067. [Google Scholar] [CrossRef]
- Huchenski, B.S.N.; Speed, A.W.H. Room-temperature reduction of sulfur hexafluoride with metal phosphides. Chem. Commun. 2021, 57, 7128–7131. [Google Scholar] [CrossRef]
- Weitkamp, R.F.; Neumann, B.; Stammler, H.-G.; Hoge, B. Non-Coordinated Phenolate Anions and Their Application in SF6 Activation. Chem. Eur. J. 2021, 27, 6460–6464. [Google Scholar] [CrossRef] [PubMed]
- Taponard, A.; Jarrosson, T.; Khrouz, L.; Médebielle, M.; Broggi, J.; Tlili, A. Metal-Free SF6 Activation: A New SF5 -Based Reagent Enables Deoxyfluorination and Pentafluorosulfanylation Reactions. Angew. Chem. Int. Ed. 2022, 61, e202204623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Khomutnyk, Y.; Bannykh, A.; Nagorny, P. Synthesis of Glycosyl Fluorides by Photochemical Fluorination with Sulfur(VI) Hexafluoride. Org. Lett. 2021, 23, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nagorny, P. Electrochemical Synthesis of Glycosyl Fluorides Using Sulfur(VI) Hexafluoride as the Fluorinating Agent. Org. Lett. 2022, 24, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Tomar, P.; Braun, T.; Kemnitz, E. Photochemical activation of SF6 by N-heterocyclic carbenes to provide a deoxyfluorinating reagent. Chem. Commun. 2018, 54, 9753–9756. [Google Scholar] [CrossRef] [PubMed]
- Zámostná, L.; Braun, T.; Braun, B. S-F and S-C activation of SF6 and SF5 derivatives at rhodium: Conversion of SF6 into H2S. Angew. Chem. Int. Ed. 2014, 53, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Rombach, D.; Wagenknecht, H.-A. Photoredoxkatalytische α-Alkoxypentafluorosulfanylierung von α-Methyl- und α-Phenylstyrol mithilfe von SF6. Angew. Chem. Int. Ed. 2020, 132, 306–310. [Google Scholar] [CrossRef]
- Dresdner, R. A New Synthesis of Simple Fluorocarbon Tertiary Amines. J. Am. Chem. Soc. 1956, 77, 69–70. [Google Scholar]
- Dresdner, R. The Pyrolysis of Trifluoromethyl Sulfur Pentafluoride and its Reaction with Perfluoropropylene. J. Am. Chem. Soc. 1955, 77, 6633–6634. [Google Scholar] [CrossRef]
- Dresdner, R.D.; Mao, T.J.; Young, J.A. Some Thermal Reactions of Perfluoroalkyl Derivatives of SF6 with Fluorocarbon Olefins. J. Am. Chem. Soc. 1957, 80, 3007–3009. [Google Scholar] [CrossRef]
- Huang, L.; Shen, Y.; Dong, W.; Zhang, R.; Zhang, J.; Hou, H. A novel method to decompose two potent greenhouse gases: Photoreduction of SF6 and SF5CF3 in the presence of propene. J. Hazard. Mater. 2008, 151, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Herbstritt, D.; Braun, T. Reduction of SF5CF3 via iridium catalysis: Radical trifluoromethylation of aromatics. Chem. Commun. 2023, 59, 3850–3853. [Google Scholar] [CrossRef] [PubMed]
- Rotering, P.; Mück-Lichtenfeld, C.; Dielmann, F. Solvent-free photochemical decomposition of sulfur hexafluoride by phosphines: Formation of difluorophosphoranes as versatile fluorination reagents. Green Chem. 2022, 24, 8054–8061. [Google Scholar] [CrossRef]
- Iakobson, G.; Pošta, M.; Beier, P. Reductive activation of sulfur hexafluoride with TEMPOLi: Addition of the pentafluorosulfanyl group and TEMPO to terminal alkenes. J. Fluor. Chem. 2018, 213, 51–55. [Google Scholar] [CrossRef]
- Akhgarnusch, A.; Höckendorf, R.F.; Beyer, M.K. Thermochemistry of the Reaction of SF6 with Gas-Phase Hydrated Electrons: A Benchmark for Nanocalorimetry. J. Phys. Chem. A 2015, 119, 9978–9985. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.A.; Mayhew, C. A study of low energy electron attachment to trifluoromethyl sulphur pentafluoride, SF5CF3: Atmospheric implications. Int. J. Mass Spectrom. 2001, 206, vii-x. [Google Scholar] [CrossRef]
- Sailer, W.; Drexel, H.; Pelc, A.; Grill, V.; Illenberger, N.; Skalny, J.D.; Mikoviny, T.; Scheier, P.; Märk, T.D. Low energy electron attachment to SF5CF3. Chem. Phys. Lett. 2002, 351, 71–78. [Google Scholar] [CrossRef]
- Tomar, P. N-Heterocyclic Carbene Derivatives for the Activation of Sulfur Fluorides. Ph.D. Thesis, Humboldt-Universität zu Berlin, Berlin, Germany, 2021. [Google Scholar]
- Herbstritt, D.; Tomar, P.; Müller, R.; Kaupp, M.; Braun, T. A 2,2-Difluoroimidazolidine Derivative for Deoxyfluorination Reactions: Mechanistic Insights by Experimental and Computational Studies. Chem. Eur. J. 2023, e202301556. [Google Scholar] [CrossRef]
- Nagib, D.A.; MacMillan, D.W.C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 2011, 480, 224–228. [Google Scholar] [CrossRef]
- Studer, A. A “Renaissance” in Radical Trifluoromethylation. Angew. Chem. Int. Ed. 2012, 124, 9082–9090. [Google Scholar] [CrossRef]
- Dong, Z.; Pezzato, C.; Sienkiewicz, A.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. SET processes in Lewis acid-base reactions: The tritylation of N-heterocyclic carbenes. Chem. Sci. 2020, 11, 7615–7618. [Google Scholar] [CrossRef] [PubMed]
- Ramnial, T.; McKenzie, I.; Gorodetsky, B.; Tsang, E.M.W.; Clyburne, J.A.C. Reactions of N-heterocyclic carbenes (NHCs) with one-electron oxidants: Possible formation of a carbene cation radical. Chem. Commun. 2004, 9, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Chim, R.; Kennedy, R.; Tuckett, R. The vacuum-UV absorption spectrum of SF5CF3; implications for its lifetime in the earth’s atmosphere. Chem. Phys. Lett. 2003, 367, 697–703. [Google Scholar] [CrossRef]
- Solovev, S.; Palmentieri, A.; Potekhina, N.D.; Madey, T.E. Mechanism for Electron-Induced SF5CF3 Formation in Condensed Molecular Films. J. Phys. Chem. C 2007, 111, 18271–18278. [Google Scholar] [CrossRef]
- Goettel, J.T.; Kostiuk, N.; Gerken, M. The Solid-State Structure of SF4: The Final Piece of the Puzzle. Angew. Chem. Int. Ed. 2013, 125, 8195–8198. [Google Scholar] [CrossRef]
- Kostiuk, N.; Goettel, J.T.; Gerken, M. Synthesis and Characterization of SF4 Adducts with Polycyclic Amines. Inorg. Chem. 2020, 59, 8620–8628. [Google Scholar] [CrossRef]
- Masiak, P.; Sobolewski, A.L. Theoretical study of the photophysics of SF5CF3. Chem. Phys. 2005, 313, 169–176. [Google Scholar] [CrossRef]
- Kivimäki, A.; Álvarez Ruiz, J.; Coreno, M.; Stankiewicz, M.; Fronzoni, G.; Decleva, P. Photoelectron spectroscopy of sulfur L levels in the SF5CF3 molecule. Chem. Phys. 2008, 353, 202–208. [Google Scholar] [CrossRef]
- Arnold, S.T.; Thomas, M.; Viggiano, A.A.; Mayhew, C.A. A temperature-dependent selected ion flow tube study of anions reacting with SF5CF3. Int. J. Mass Spectrom. IJMS 2003, 223–224, 403–409. [Google Scholar] [CrossRef]
- Kamigata, N.; Ohtsuka, T.; Fukushima, T.; Yoshida, M.; Shimizu, M. Direct perfluoroalkylation of aromatic and heteroaromatic compounds with perfluoroalkanesulfonyl chlorides catalysed by a ruthenium(II) phosphine complex. J. Chem. Soc. Perkin Trans. 1 1994, 10, 1339–1346. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Trifluoromethanesulfonic Anhydride as a Low-Cost and Versatile Trifluoromethylation Reagent. Angew. Chem. Int. Ed. 2018, 57, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.F.; Kuehner, C.S.; Bacsa, J.; Soper, J.D. Photoinduced Cobalt(III)-Trifluoromethyl Bond Activation Enables Arene C-H Trifluoromethylation. Angew. Chem. Int. Ed. 2018, 57, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Lonca, G.H.; Chiba, S. PhI(OAc)(2) -mediated radical trifluoromethylation of vinyl azides with Me3SiCF3. Angew. Chem. Int. Ed. 2014, 53, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, Y.; Su, Z.; Wu, C.; Chen, W.; Chen, Q.-Y. Deoxyfluorination of Carboxylic, Sulfonic, Phosphinic Acids and Phosphine Oxides by Perfluoroalkyl Ether Carboxylic Acids Featuring CF2O Units. Chin. J. Chem. 2021, 39, 1225–1232. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Feng, C.-C.; Chen, N.; Zhang, S.-L. Direct Arene Trifluoromethylation Enabled by a High-Valent CuIII -CF3 Compound. Angew. Chem. Int. Ed. 2022, 61, e202209029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-R.; Xiao, C.; Zhang, S.-L.; Zhang, X. Radical C−H Bond Trifluoromethylation of Alkenes by High-Valent Copper(III) Trifluoromethyl Compounds. Adv. Synth. Catal. 2019, 361, 5305–5310. [Google Scholar] [CrossRef]
- Wang, F.; Wang, D.; Mu, X.; Chen, P.; Liu, G. Copper-catalyzed intermolecular trifluoromethylarylation of alkenes: Mutual activation of arylboronic acid and CF3+ reagent. J. Am. Chem. Soc. 2014, 136, 10202–10205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, E. Activation of C-F bonds in fluoroarenes by N-heterocyclic carbenes as an effective route to synthesize abnormal NHC complexes. Chem. Commun. 2016, 52, 10922–10925. [Google Scholar] [CrossRef]
- Kolychev, E.L.; Bannenberg, T.; Freytag, M.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Reactivity of a frustrated lewis pair and small-molecule activation by an isolable Arduengo carbene-B{3,5-(CF3)2C6H3}3 complex. Chem. Eur. J. 2012, 18, 16938–16946. [Google Scholar] [CrossRef]
- Kronig, S.; Theuergarten, E.; Holschumacher, D.; Bannenberg, T.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Dihydrogen activation by frustrated carbene-borane Lewis pairs: An experimental and theoretical study of carbene variation. Inorg. Chem. 2011, 50, 7344–7359. [Google Scholar] [CrossRef]
- Kuhn, N.; Fahl, R.B.; Henkel, G. On the Reaction of 2,3-Dihydroimidazol-2-ylidenes with Pentafluoropyridine: Carbenes as Reactants in Nucleophilic Aromatic Substitution. Z. Naturforsch. 1998, 53, 881–886. [Google Scholar] [CrossRef]
- Leclerc, M.C.; Gorelsky, S.I.; Gabidullin, B.M.; Korobkov, I.; Baker, R.T. Selective Activation of Fluoroalkenes with N-Heterocyclic Carbenes: Synthesis of N-Heterocyclic Fluoroalkenes and Polyfluoroalkenyl Imidazolium Salts. Chem. Eur. J. 2016, 22, 8063–8067. [Google Scholar] [CrossRef]
- Paul, U.; Radius, U. Ligand versus Complex: C-F and C-H Bond Activation of Polyfluoroaromatics at a Cyclic (Alkyl)(Amino)Carbene. Chem. Eur. J. 2017, 23, 3993–4009. [Google Scholar] [CrossRef]
- Sen, S.; Roesky, H.W. Silicon-fluorine chemistry: From the preparation of SiF2 to C-F bond activation using silylenes and its heavier congeners. Chem. Commun. 2018, 54, 5046–5057. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): Stable carbenes on the rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef] [PubMed]

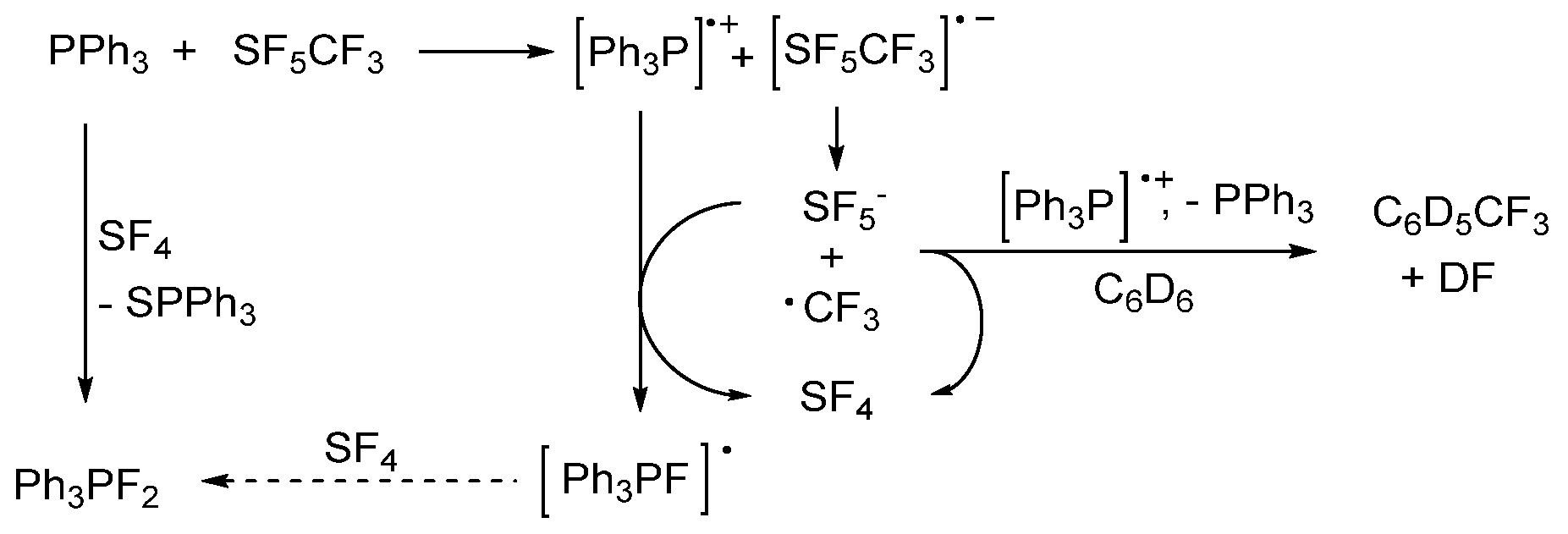
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbstritt, D.; Tomar, P.; Braun, T. Activation of SF5CF3 by the N-Heterocyclic Carbene SIMes. Molecules 2023, 28, 6693. https://doi.org/10.3390/molecules28186693
Herbstritt D, Tomar P, Braun T. Activation of SF5CF3 by the N-Heterocyclic Carbene SIMes. Molecules. 2023; 28(18):6693. https://doi.org/10.3390/molecules28186693
Chicago/Turabian StyleHerbstritt, Domenique, Pooja Tomar, and Thomas Braun. 2023. "Activation of SF5CF3 by the N-Heterocyclic Carbene SIMes" Molecules 28, no. 18: 6693. https://doi.org/10.3390/molecules28186693
APA StyleHerbstritt, D., Tomar, P., & Braun, T. (2023). Activation of SF5CF3 by the N-Heterocyclic Carbene SIMes. Molecules, 28(18), 6693. https://doi.org/10.3390/molecules28186693







