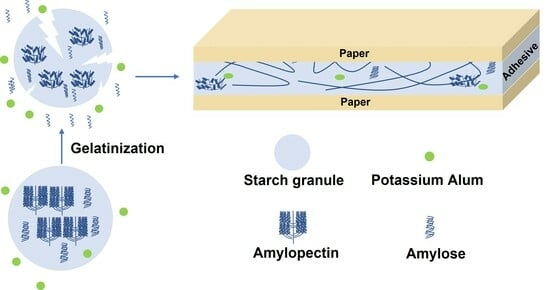
Thermal, Rheological, Structural and Adhesive Properties of Wheat Starch Gels with Different Potassium Alum Contents
Abstract
:1. Introduction
2. Results
2.1. Thermal Properties
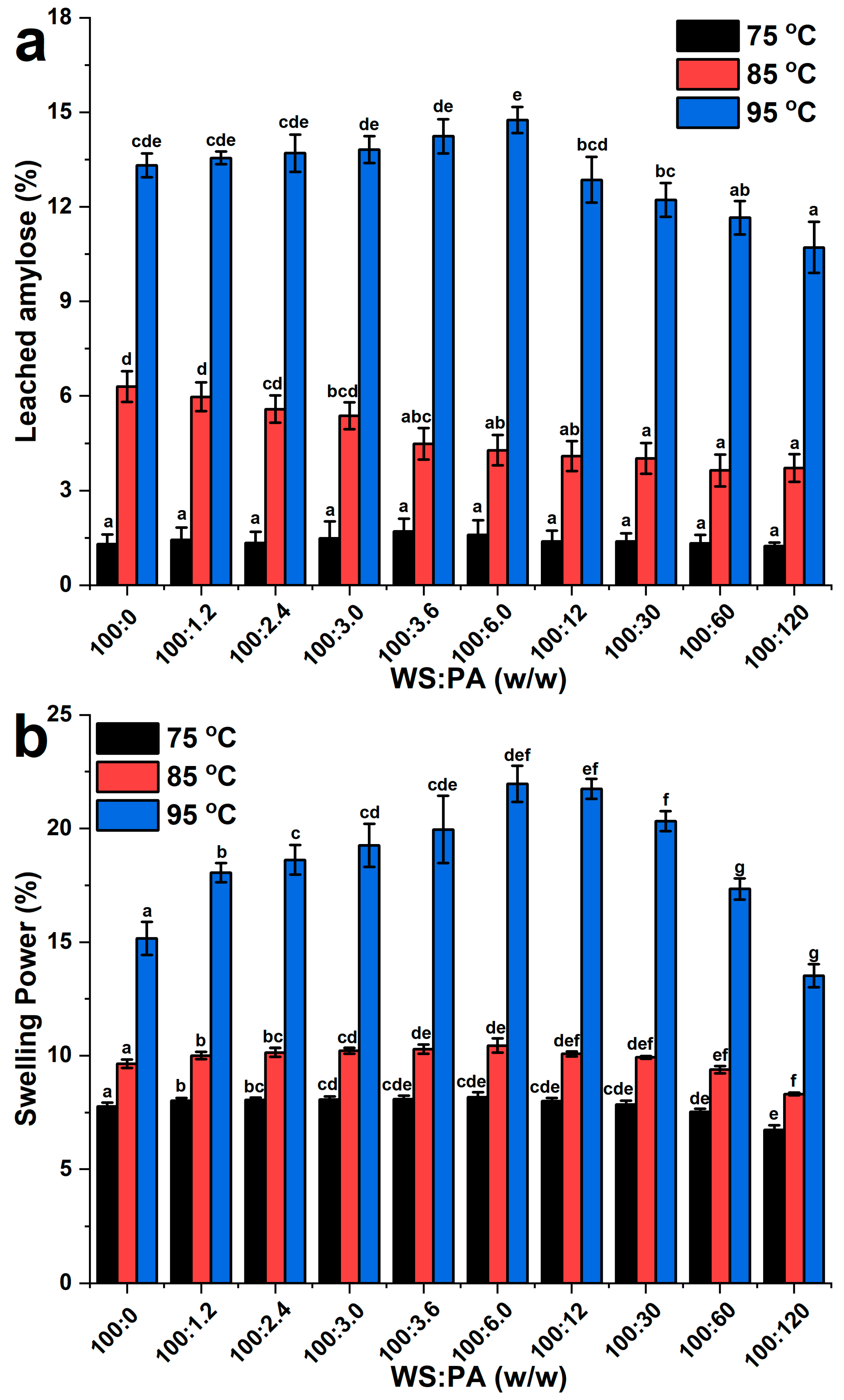
2.2. Leached Amylose and Swelling Power
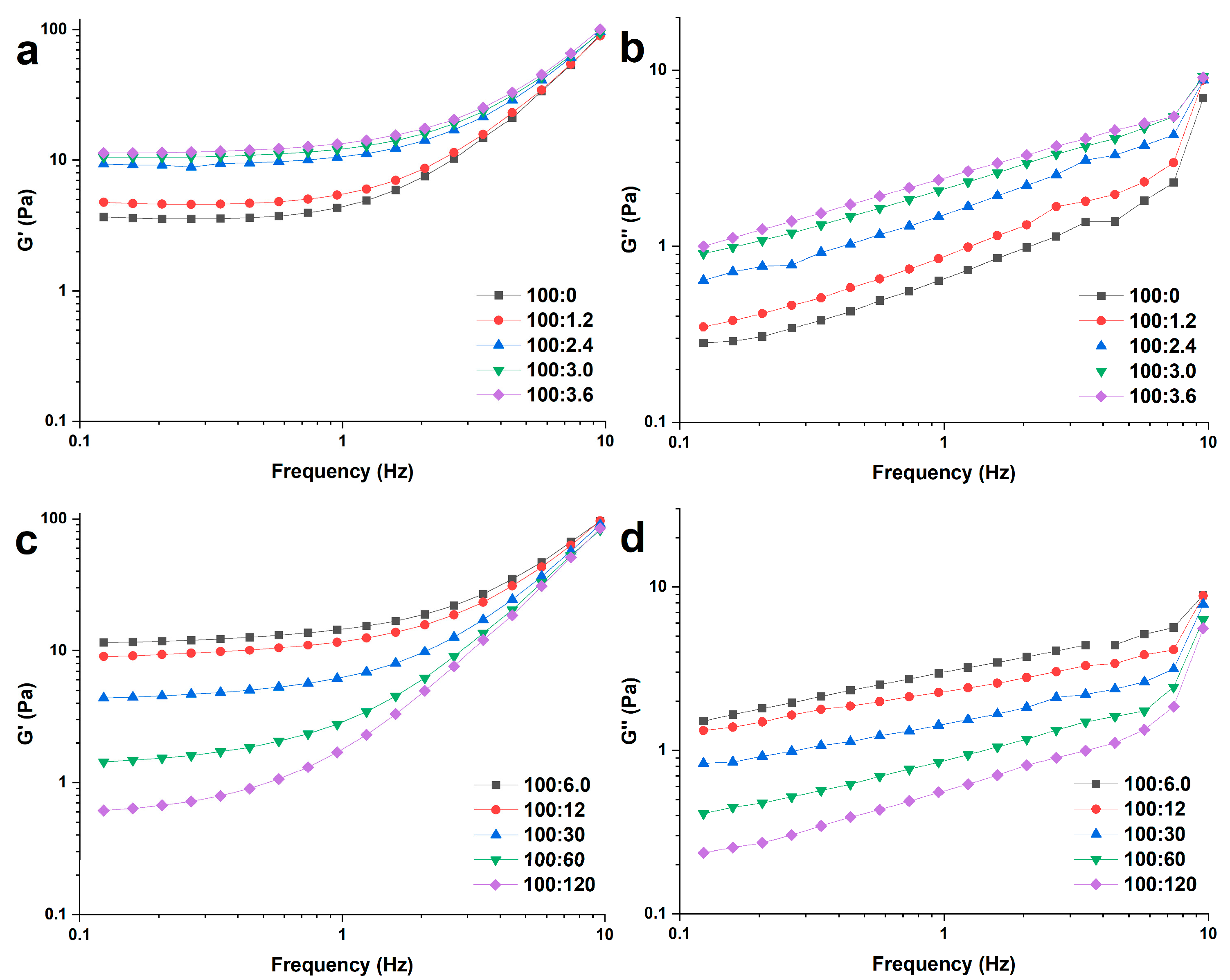
2.3. Rheological Measurements
2.3.1. Dynamic Rheological Measurements
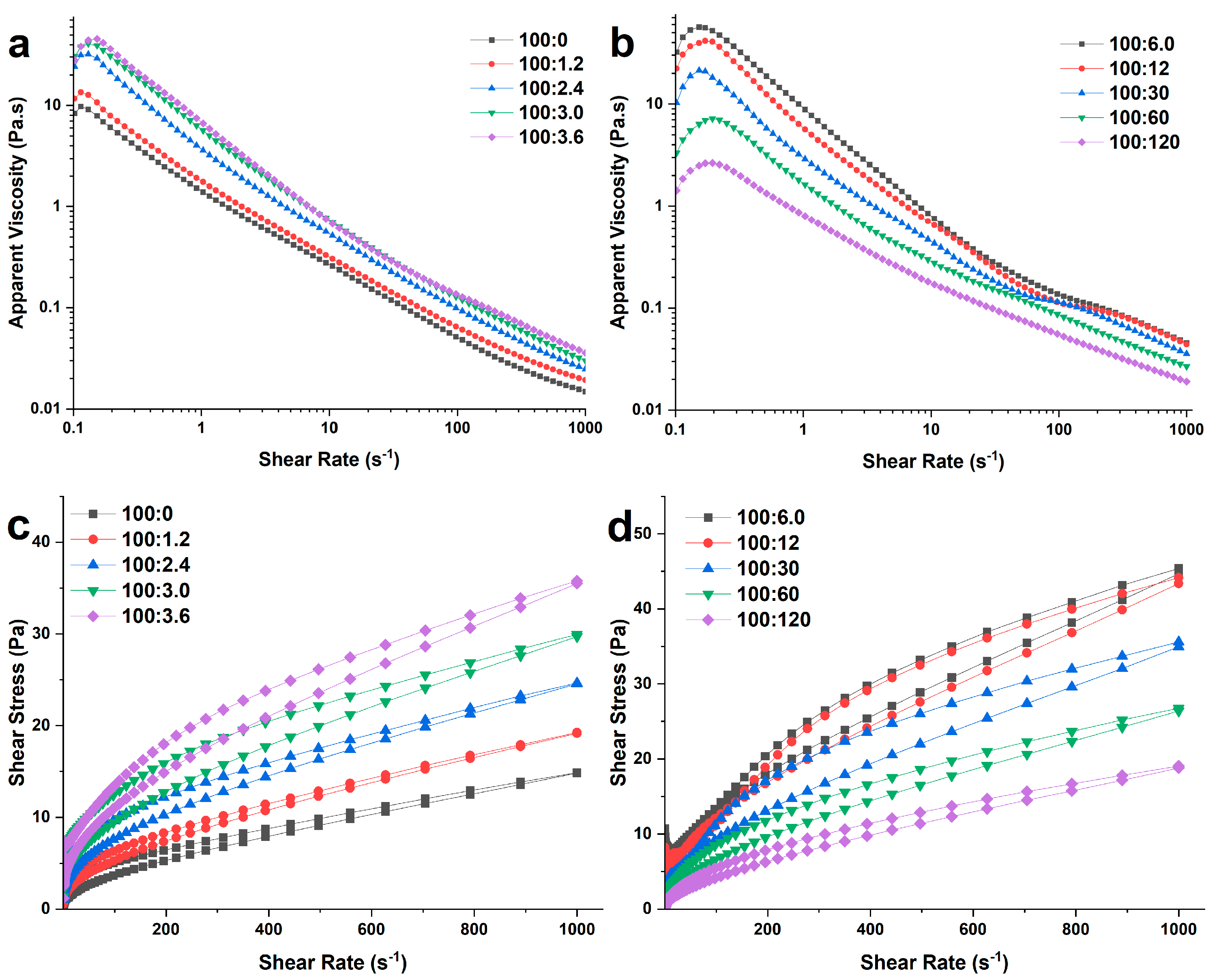
2.3.2. Steady Rheological Measurements
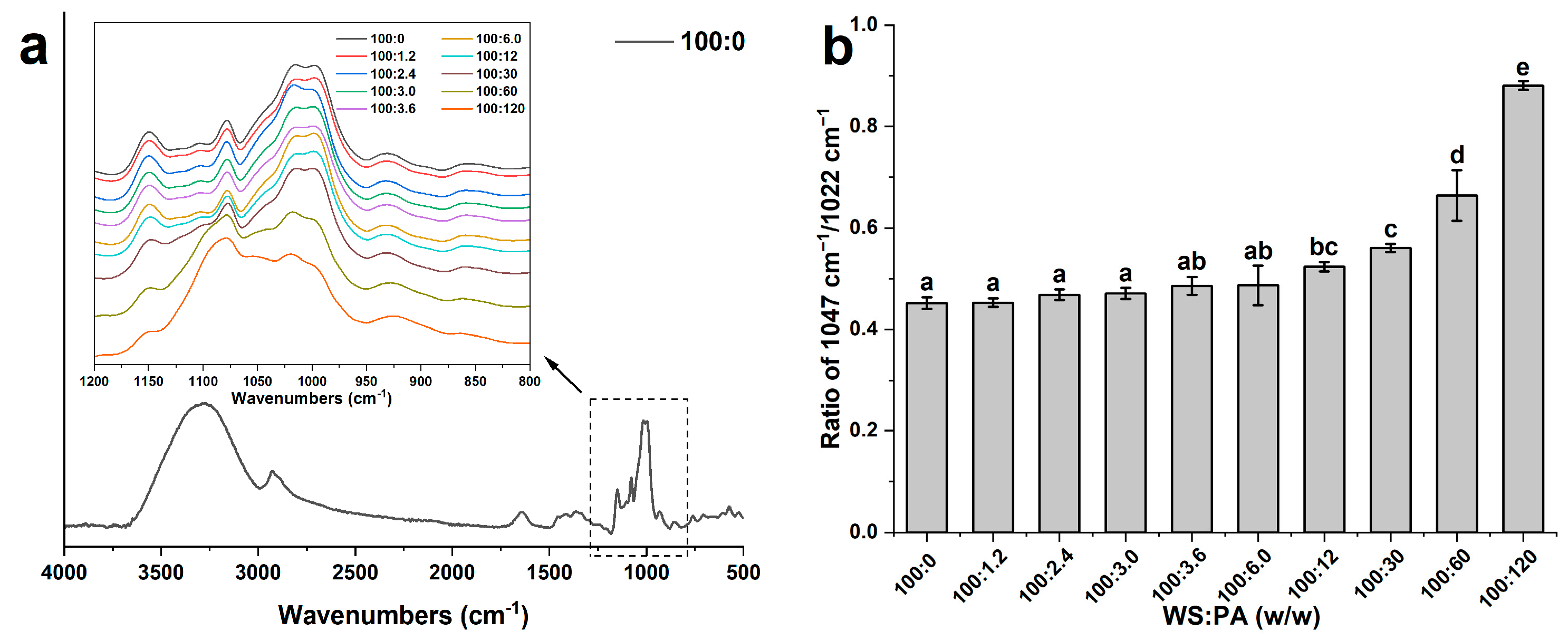
2.4. FT-IR
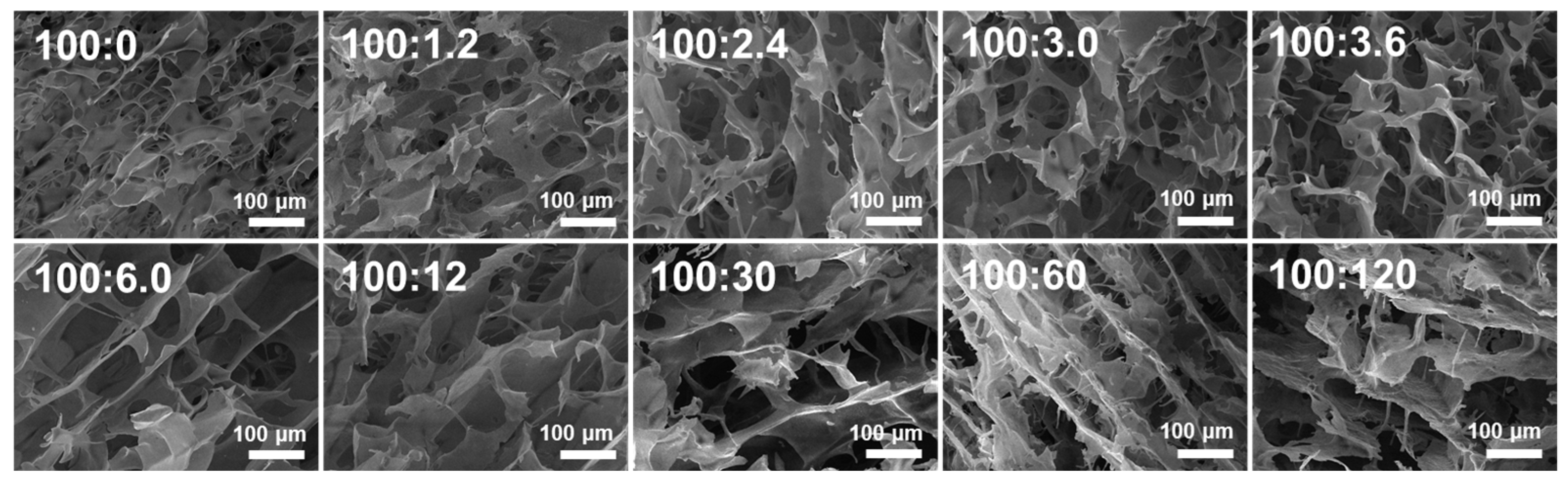
2.5. SEM
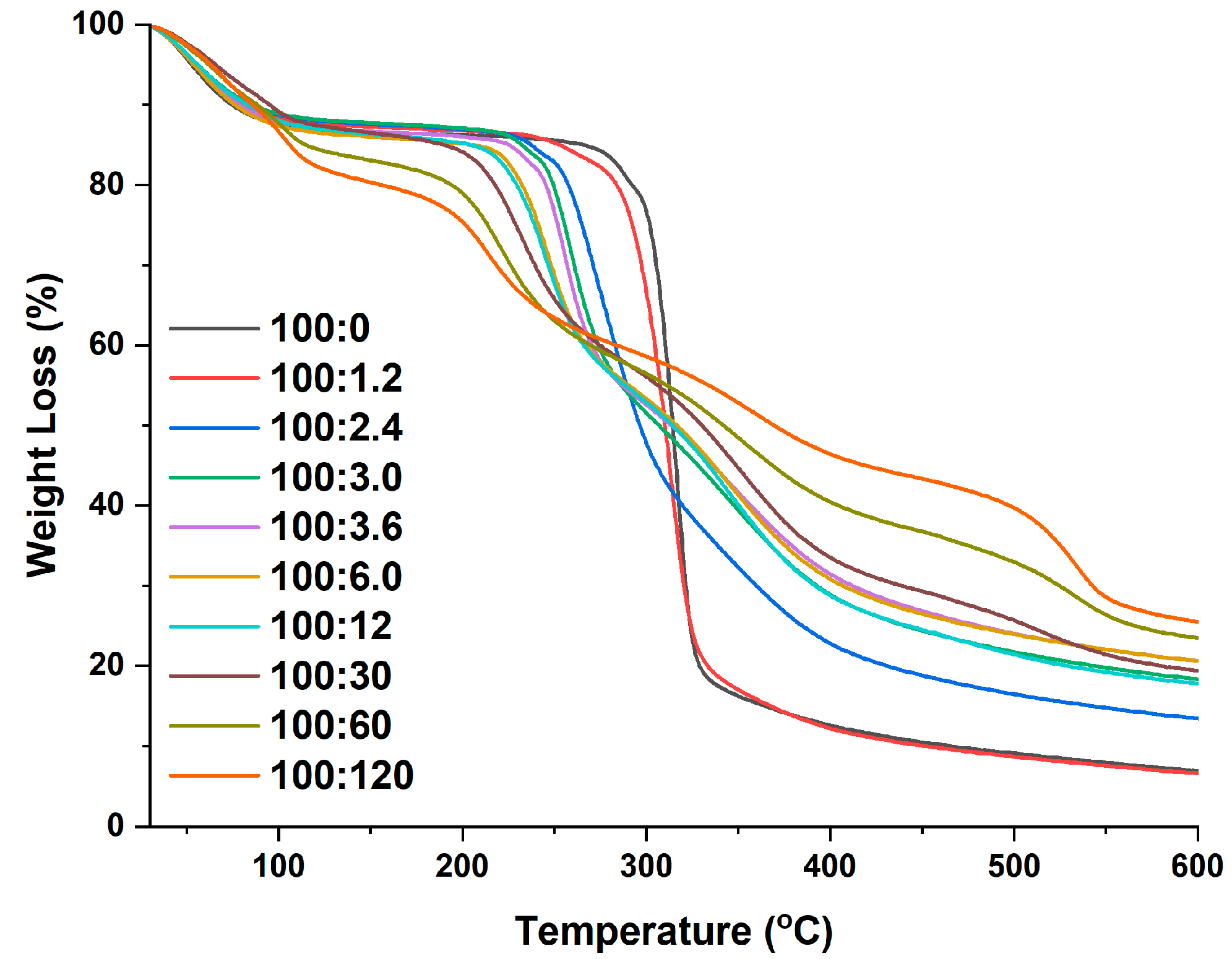
2.6. Thermogravimetry (TG)
2.7. Adhesive Properties
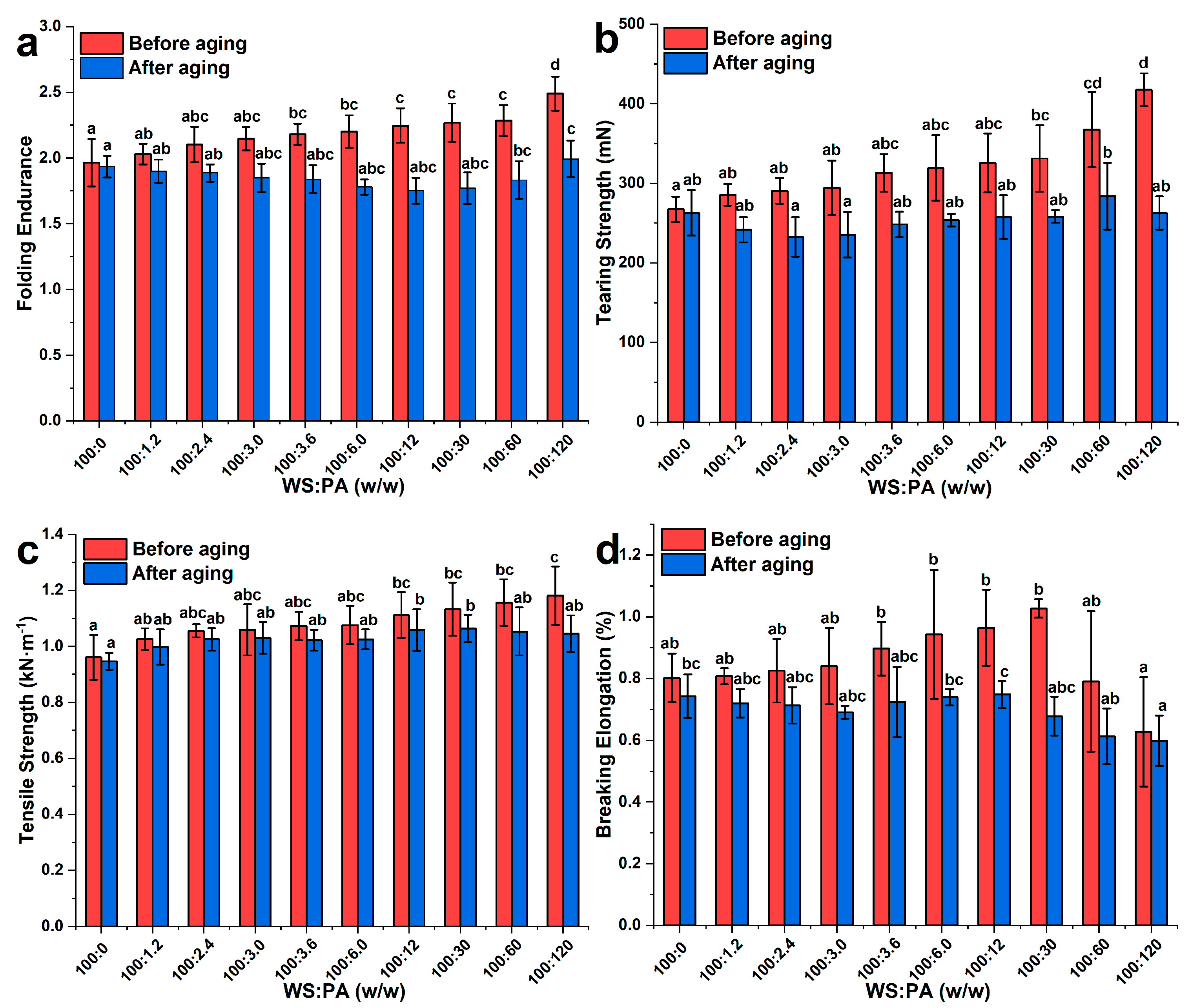
2.7.1. Mechanical Properties
2.7.2. Chromaticity Measurement
3. Materials and Methods
3.1. Materials
3.2. WS−PA Systems Preparation
3.3. Rheological Measurements
3.4. Leached Amylose and Swelling Power
3.5. DSC
3.6. TGA
3.7. ATR-FTIR
3.8. SEM
3.9. Paper Mechanical Properties Experiments
3.9.1. Tensile Strength Experiment
3.9.2. Folding Endurance Experiment
3.9.3. Tearing Strength Experiment
3.10. Color Evaluation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Biricik, Y.; Sonmez, S.; Ozden, O. Effects of surface sizing with starch on physical strength properties of paper. Asian J. Chem. 2011, 23, 3151–3154. [Google Scholar]
- Yu, J.; Wang, S.; Wang, J.; Li, C.; Xin, Q.; Huang, W.; Zhang, Y.; He, Z.; Wang, S. Effect of laboratory milling on properties of starches isolated from different flour millstreams of hard and soft wheat. Food Chem. 2015, 172, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Y.; Zhao, Y.; Chi, J.; Cheng, S. Starch and its derivatives for paper coatings: A review. Prog. Org. Coat. 2019, 135, 213–227. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Zhang, Z.; Tong, Q.; Rashed, M.M. Effect of pullulan on the short-term and long-term retrogradation of rice starch. Carbohydr. Polym. 2015, 115, 415–421. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, J.; Lu, H.; Chang, R.; Qiu, L.; Tian, Y. Amylopectin crystal seeds: Characterization and their effect on amylopectin retrogradation. Food Hydrocoll. 2021, 111, 106409. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Singh, A.; Geveke, D.J.; Yadav, M.P. Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT 2017, 80, 155–162. [Google Scholar] [CrossRef]
- Lian, C. Study on the Viscose for the Archives Decoration and Mount. J. Fujian Norm. Univ. 1999, 3, 63–67. [Google Scholar]
- Cao, F.; Liu, S.; Zheng, L.; Yu, J. Application of starch adhesive in mounting process of traditional Chinese painting and calligraphy. Adhesion 2008, 5, 47–50. [Google Scholar]
- Sasaki, A.; Kishigami, Y.; Fuchigami, M. Firming of cooked sweet potatoes as affected by alum treatment. J. Food Sci. 1999, 64, 111–115. [Google Scholar] [CrossRef]
- Baranova, L.I.; Syrkin, Y.K.; Kuchumova, L.M. Dielectric polarization of water in aluminum-potassium alums. J. Struct. Chem. 1966, 7, 467–469. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Chang, T.; Shi, L.; Yang, H.; Cui, M. Effect of salts on textural, color, and rheological properties of potato starch gels. Starch Stärke 2014, 66, 149–156. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Zhang, Q.; Hu, X.; Shen, Q. Effects of potassium alum addition on physicochemical, pasting, thermal and gel texture properties of potato starch. Int. J. Food Sci. Technol. 2011, 46, 1621–1627. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ai, Z.; Fan, H.; Wang, N.; Suo, B. Effect of potassium alum addition on the quality of potato starch noodles. J. Food Sci. Technol. 2019, 56, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Yang, H.; Zhao, S.; Liu, Y.; Liu, R. Effects of salts on the gelatinization and retrogradation properties of maize starch and waxy maize starch. Food Chem. 2017, 214, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef]
- Zhu, W.X.; Gayin, J.; Chatel, F.; Dewettinck, K.; Van der Meeren, P. Influence of electrolytes on the heat-induced swelling of aqueous dispersions of native wheat starch granules. Food Hydrocoll. 2009, 23, 2204–2211. [Google Scholar] [CrossRef]
- Jane, J. Mechanism of Starch Gelatinization in Neutral Salt Solutions. Starch Stärke 1993, 45, 161–166. [Google Scholar] [CrossRef]
- Beck, M.; Jekle, M.; Becker, T. Starch re-crystallization kinetics as a function of various cations. Starch Stärke 2011, 63, 792–800. [Google Scholar] [CrossRef]
- Zervos, S.; Alexopoulou, I. Paper conservation methods: A literature review. Cellulose 2015, 22, 2859–2897. [Google Scholar] [CrossRef]
- Liu, J.; Xing, H.; Zhou, Y.; Chao, X.; Li, Y.; Hu, D. An Essential Role of Polymeric Adhesives in the Reinforcement of Acidified Paper Relics. Polymers 2022, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C. Physicochemical, pasting and thermal properties of tuber starches as modified by guar gum and locust bean gum. Int. J. Food Sci. Technol. 2009, 44, 50–57. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Jensen, C.T.B.; Kristensen, P.G. Experimental and modelling studies of the flow properties of maize and waxy maize starch pastes. Chem. Eng. J. 1998, 70, 165–171. [Google Scholar] [CrossRef]
- Rosenholm, J.B. Critical evaluation of dipolar, acid-base and charge interactions II. Charge exchange within electrolytes and electron exchange with semiconductors. Adv. Colloid. Interface Sci. 2017, 247, 305–353. [Google Scholar] [CrossRef]
- Xie, J.; Ren, Y.; Xiao, Y.; Luo, Y.; Shen, M. Interactions between tapioca starch and Mesona chinensis polysaccharide: Effects of urea and NaCl. Food Hydrocoll. 2021, 111, 106268. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, M.; Han, X.; Wen, H.; Xie, J. Gelation characteristics of Mesona chinensis polysaccharide-maize starches gels: Influences of KCl and NaCl. J. Cereal Sci. 2020, 96, 103108. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, M.; Li, E.; Xiao, Y.; Wen, H.; Ren, Y.; Xie, J. Effect of Mesona chinensis polysaccharide on pasting, rheological and structural properties of corn starches varying in amylose contents. Carbohydr. Polym. 2020, 230, 115713. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, C.; Shi, L.; Chang, T.; Yang, H.; Cui, M. Effects of salts on physicochemical, microstructural and thermal properties of potato starch. Food Chem 2014, 156, 137–143. [Google Scholar] [CrossRef]
- Ren, Y.; Rong, L.; Shen, M.; Liu, W.; Xiao, W.; Luo, Y.; Xie, J. Interaction between rice starch and Mesona chinensis Benth polysaccharide gels: Pasting and gelling properties. Carbohydr. Polym. 2020, 240, 116316. [Google Scholar] [CrossRef]
- Kong, X.-R.; Zhu, Z.-Y.; Zhang, X.-J.; Zhu, Y.-M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Wagner, C.E.; Barbati, A.C.; Engmann, J.; Burbidge, A.S.; McKinley, G.H. Apparent shear thickening at low shear rates in polymer solutions can be an artifact of non-equilibration. Appl. Rheol. 2016, 26, 54091. [Google Scholar]
- Liu, S.; Lin, L.; Shen, M.; Wang, W.; Xiao, Y.; Xie, J. Effect of Mesona chinensis polysaccharide on the pasting, thermal and rheological properties of wheat starch. Int. J. Biol. Macromol. 2018, 118, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Deng, Y.; Han, D.; Tan, L.; Ding, Y.; Zhou, Z.; Xu, H.; Guo, Y. Exploring structural variations of hydrogen-bonding patterns in cellulose during mechanical pulp refining of tobacco stems. Carbohydr. Polym. 2019, 204, 247–254. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, Z.; Shen, M.; Rong, L.; Liu, W.; Xiao, W.; Xie, J. Improve properties of sweet potato starch film using dual effects: Combination Mesona chinensis Benth polysaccharide and sodium carbonate. LWT 2021, 140, 110679. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Xiao, W.; Li, J.; Xie, J. Effects of xanthan, guar and Mesona chinensis Benth gums on the pasting, rheological, texture properties and microstructure of pea starch gels. Food Hydrocoll. 2022, 125, 107391. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Atarés, L.; Vargas, M.; Chiralt, A. Physical and Antimicrobial Properties of Compression-Molded Cassava Starch-Chitosan Films for Meat Preservation. Food Bioprocess Technol. 2018, 11, 1339–1349. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Ye, Z.; Wang, S.; Tang, Y. Alkali-exchanged Y zeolites as superior deacidifying protective materials for paper relics: Effects of accessibility and strength of basic sites. Microporous Mesoporous Mater. 2020, 293, 109786. [Google Scholar] [CrossRef]
- He, Q.; Wang, L.; Zhang, Y. Study of Mechanism of Aluminum Sizing Precipitant on Xuan Paper Based on Spectral Analysis. Spectrosc. Spectr. Anal. 2018, 38, 418–423. [Google Scholar]
- Chen, G.; Zhu, Z.J.; Salminen, P.; Toivakka, M. Structure and Mechanical Properties of Starch/Styrene-Butadiene Latex Composites. Adv. Mater. Res. 2014, 936, 74–81. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Z.; Tanaka, H. Preparation and sizing mechanisms of neutral rosin size II: Functions of rosin derivatives on sizing efficiency. J. Wood Sci. 1999, 45, 475–480. [Google Scholar] [CrossRef]
- Gałkowska, D.; Pycia, K.; Juszczak, L.; Pająk, P. Influence of cassia gum on rheological and textural properties of native potato and corn starch. Starch Stärke 2014, 66, 1060–1070. [Google Scholar] [CrossRef]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, rheological, thermal and gel textural properties of pearl millet starch as modified by guar gum and its acid hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tong, Q.; Ren, F.; Zhu, G. Pasting and rheological properties of rice starch as affected by pullulan. Int. J. Biol. Macromol. 2014, 66, 325–331. [Google Scholar] [CrossRef]
- Chrastil, J. Improved colorimetric determination of amylose in starches or flours. Carbohydr. Res. 1987, 159, 154–158. [Google Scholar] [CrossRef]
- Ali, A.; Xie, F.; Yu, L.; Liu, H.; Meng, L.; Khalid, S.; Chen, L. Preparation and characterization of starch-based composite films reinfoced by polysaccharide-based crystals. Compos. Part B Eng. 2018, 133, 122–128. [Google Scholar] [CrossRef]
- Guo, P.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Mechanisms of starch gelatinization during heating of wheat flour and its effect on in vitro starch digestibility. Food Hydrocoll. 2018, 82, 370–378. [Google Scholar] [CrossRef]
- Krstonosic, V.; Dokic, L.; Nikolic, I.; Milanovic, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Z.; Zhang, H.; Tian, S.; Li, X.; Fan, H.; Fu, S. Superhydrophobic and deacidified cellulose/CaCO(3)-derived granular coating toward historic paper preservation. Int. J. Biol. Macromol. 2022, 207, 232–241. [Google Scholar] [CrossRef]
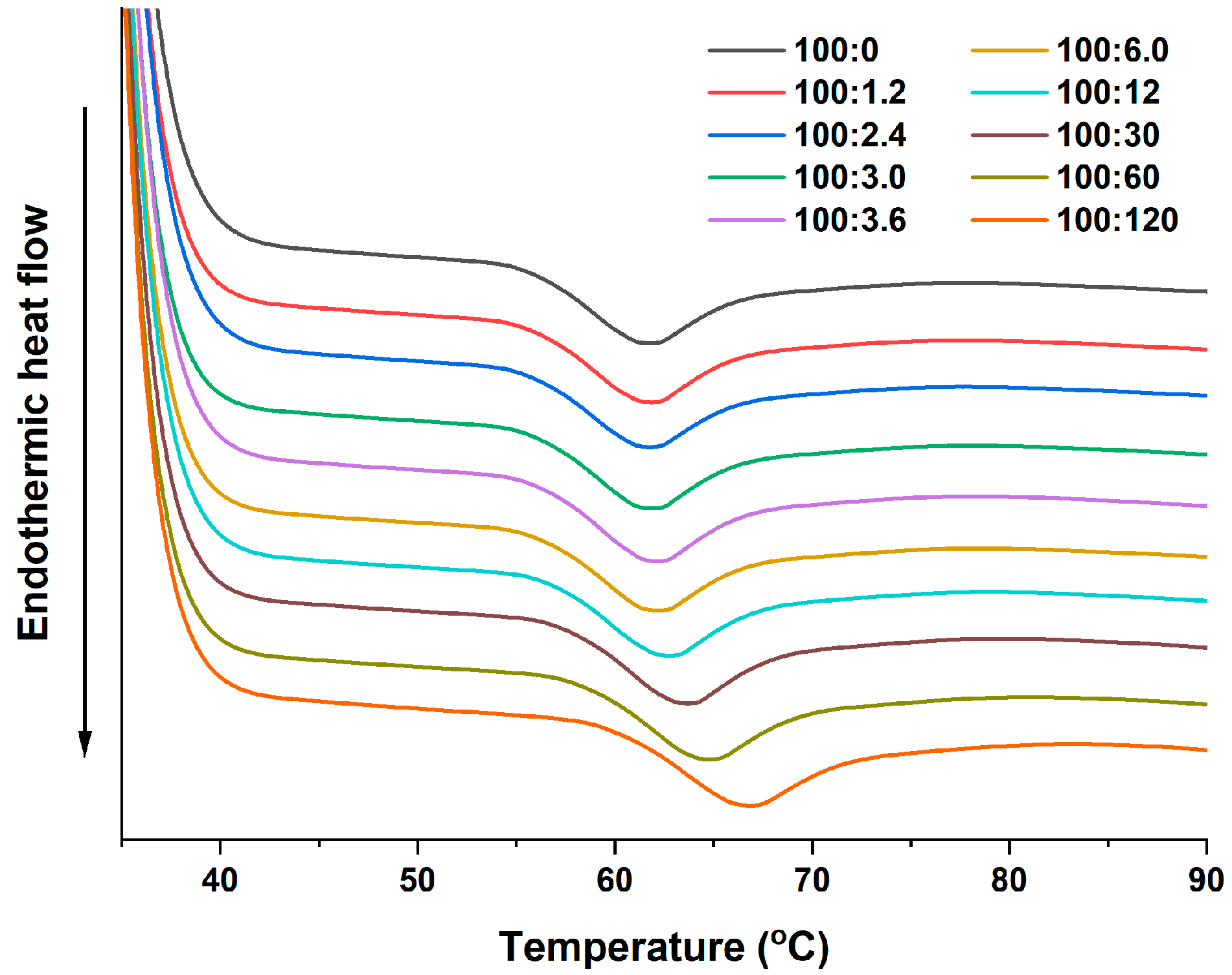
| WS:PA (w/w) | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| 100:0 | 55.98 ± 0.17 a | 61.30 ± 0.21 a | 65.91 ± 0.33 a | 1.525 ± 0.025 a |
| 100:1.2 | 56.08 ± 0.02 ab | 61.38 ± 0.05 ab | 66.00 ± 0.24 a | 1.542 ± 0.005 ab |
| 100:2.4 | 56.20 ± 0.05 bc | 61.53 ± 0.10 ab | 66.08 ± 0.19 ab | 1.548 ± 0.024 ab |
| 100:3.0 | 56.23 ± 0.03 bc | 61.59 ± 0.05 ab | 66.18 ± 0.20 ab | 1.558 ± 0.073 ab |
| 100:3.6 | 56.31 ± 0.10 cd | 61.65 ± 0.07 b | 66.27 ± 0.06 ab | 1.571 ± 0.003 ab |
| 100:6.0 | 56.45 ± 0.13 d | 61.66 ± 0.10 b | 66.42 ± 0.18 b | 1.578 ± 0.064 ab |
| 100:12 | 56.96 ± 0.07 e | 62.34 ± 0.12 c | 66.94 ± 0.12 c | 1.582 ± 0.015 ab |
| 100:30 | 57.75 ± 0.07 f | 63.21 ± 0.18 d | 67.87 ± 0.04 d | 1.602 ± 0.065 bc |
| 100:60 | 58.68 ± 0.12 g | 64.20 ± 0.27 e | 69.04 ± 0.26 e | 1.649 ± 0.082 c |
| 100:120 | 60.17 ± 0.19 h | 66.20 ± 0.37 f | 71.21 ± 0.36 f | 1.657 ± 0.046 c |
| WS:PA (w/w) | Up | Down | Hysteresis Loop | ||||||
|---|---|---|---|---|---|---|---|---|---|
| τ0 (Pa) | K (Pa•sn) | n (-) | R2 | τ0 (Pa) | K (Pa•sn) | n (-) | R2 | ||
| 100:0 | 1.14 ± 0.24 ab | 0.38 ± 0.12 bc | 0.51 ± 0.04 a | 0.992 | 0.55 ± 0.21 a | 0.16 ± 0.08 a | 0.65 ± 0.07 cd | 0.998 | 668 ± 82 ab |
| 100:1.2 | 1.48 ± 0.31 ab | 0.41 ± 0.11 bcd | 0.54 ± 0.03 ab | 0.995 | 0.66 ± 0.09 a | 0.31 ± 0.11 b | 0.58 ± 0.03 ab | 0.997 | 688 ± 97 a |
| 100:2.4 | 3.34 ± 0.76 c | 0.58 ± 0.04 def | 0.51 ± 0.01 a | 0.992 | 1.41 ± 0.05 b | 0.49 ± 0.11 c | 0.55 ± 0.02 a | 0.998 | 1213 ± 483 b |
| 100:3.0 | 4.97 ± 1.25 d | 0.73 ± 0.11 e | 0.51 ± 0.04 a | 0.989 | 2.23 ± 0.45 cd | 0.54 ± 0.11 c | 0.56 ± 0.01 a | 0.998 | 2069 ± 387 c |
| 100:3.6 | 5.76 ± 0.44 d | 0.48 ± 0.04 cde | 0.61 ± 0.01 b | 0.985 | 2.61 ± 0.22 de | 0.57 ± 0.08 c | 0.58 ± 0.01 ab | 0.999 | 2160 ± 120 c |
| 100:6.0 | 7.74 ± 1.34 e | 0.21 ± 0.06 a | 0.77 ± 0.05 c | 0.964 | 2.85 ± 0.23 e | 0.63 ± 0.05 c | 0.61 ± 0.01 ab | 0.998 | 2926 ± 292 d |
| 100:12 | 5.29 ± 1.96 d | 0.31 ± 0.16 ab | 0.73 ± 0.09 c | 0.974 | 2.07 ± 0.37 c | 0.61 ± 0.07 c | 0.61 ± 0.01 abc | 0.998 | 2856 ± 214 d |
| 100:30 | 2.19 ± 0.37 bc | 0.65 ± 0.11 ef | 0.58 ± 0.03 ab | 0.989 | 1.18 ± 0.14 b | 0.49 ± 0.01 c | 0.61 ± 0.01 abc | 0.998 | 2799 ± 230 d |
| 100:60 | 0.91 ± 0.32 ab | 0.62 ± 0.03 ef | 0.54 ± 0.02 ab | 0.998 | 0.58 ± 0.15 a | 0.35 ± 0.03 b | 0.62 ± 0.01 bc | 0.998 | 1719 ± 146 c |
| 100:120 | 0.37 ± 0.1 a | 0.39 ± 0.06 bc | 0.56 ± 0.02 ab | 0.999 | 0.27 ± 0.02 a | 0.18 ± 0.01 a | 0.66 ± 0.01 d | 0.999 | 1207 ± 176 b |
| WS:PA (w/w) | L* | a* | b* | ∆E |
|---|---|---|---|---|
| blank | 91.81 | −0.24 | 2.93 | |
| 100:0 | 90.67 ± 0.06 g | −0.2 ± 0.02 a | 3.18 ± 0.07 a | 1.17 ± 0.04 a |
| 100:1.2 | 90.53 ± 0.04 g | −0.17 ± 0.04 ab | 3.24 ± 0.09 a | 1.32 ± 0.02 b |
| 100:2.4 | 89.73 ± 0.11 f | −0.16 ± 0.07 ab | 3.3 ± 0.11 ab | 2.12 ± 0.09 c |
| 100:3.0 | 89.68 ± 0.09 ef | −0.07 ± 0.04 bc | 3.4 ± 0.12 b | 2.19 ± 0.06 c |
| 100:3.6 | 89.56 ± 0.08 de | −0.09 ± 0.05 c | 3.74 ± 0.04 c | 2.40 ± 0.06 d |
| 100:6.0 | 89.48 ± 0.04 d | 0.01 ± 0.03 d | 4.05 ± 0.06 d | 2.60 ± 0.01 e |
| 100:12 | 89.19 ± 0.12 c | 0.03 ± 0.03 de | 4.3 ± 0.1 e | 2.97 ± 0.06 f |
| 100:30 | 89.08 ± 0.05 c | 0.07 ± 0.02 def | 6.93 ± 0.12 f | 4.85 ± 0.07 g |
| 100:60 | 88.9 ± 0.1 b | 0.11 ± 0.07 ef | 7.07 ± 0.05 f | 5.07 ± 0.01 h |
| 100:120 | 88.6 ± 0.11 a | 0.13 ± 0.06 f | 7.43 ± 0.07 g | 5.54 ± 0.01 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhang, H.; Xu, Q.; Zhang, H.; Yang, Y. Thermal, Rheological, Structural and Adhesive Properties of Wheat Starch Gels with Different Potassium Alum Contents. Molecules 2023, 28, 6670. https://doi.org/10.3390/molecules28186670
Zhao H, Zhang H, Xu Q, Zhang H, Yang Y. Thermal, Rheological, Structural and Adhesive Properties of Wheat Starch Gels with Different Potassium Alum Contents. Molecules. 2023; 28(18):6670. https://doi.org/10.3390/molecules28186670
Chicago/Turabian StyleZhao, Haibo, Hongbin Zhang, Qiang Xu, Hongdong Zhang, and Yuliang Yang. 2023. "Thermal, Rheological, Structural and Adhesive Properties of Wheat Starch Gels with Different Potassium Alum Contents" Molecules 28, no. 18: 6670. https://doi.org/10.3390/molecules28186670
APA StyleZhao, H., Zhang, H., Xu, Q., Zhang, H., & Yang, Y. (2023). Thermal, Rheological, Structural and Adhesive Properties of Wheat Starch Gels with Different Potassium Alum Contents. Molecules, 28(18), 6670. https://doi.org/10.3390/molecules28186670