Abstract
An efficient, straightforward, and metal-free methodology to rapidly access functionalised pyrazolo-[1,5-c]quinazolinones via a [3 + 2] dipolar cycloaddition and regioselective ring expansion process was developed. The synthesised compounds were characterised by methods such as NMR, HRMS, and HPLC. The in vitro antiproliferative activity against A549 cells (non-small cell lung cancer) was significant for compounds 4i, 4m, and 4n with IC50 values of 17.0, 14.2, and 18.1 μM, respectively. In particular, compounds 4t and 4n showed inhibitory activity against CDK9/2. Predicted biological target and molecular modelling studies suggest that the compound 4t may target CDKs for antitumour effects. The synthesised derivatives were considered to have moderate drug-likeness and sufficient safety in silico. In summary, a series of pyrazolo-[1,5-c]quinazolinone derivatives with antitumour activity is reported for the first time. We provide not only a simple and efficient synthetic method but also helpful lead compounds for the further development of novel cyclin-dependent kinase (CDK) inhibitors.
1. Introduction
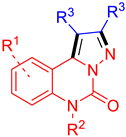
Quinazolinone [1,2] moieties are widely recognised as crucial structural components in the development of antineoplastic drugs. These compounds have been incorporated into various drug classes and have demonstrated efficacy through a diverse range of mechanisms. Research has shown that some compounds with a quinazoline core can inhibit cell proliferation, induce apoptosis, and impede angiogenesis, and they have the ability to modulate signal transduction pathways and target specific proteins [3,4,5,6,7,8,9]. As shown in Figure 1A, ispinesib [10,11,12] is a potent inhibitor of kinesin spindle protein (KSP), which plays a role as a key regulator of cell mitosis. By inhibiting KSP, ispinesib disrupts cell division and hinders the growth and spread of cancer cells. It is evaluated in phase II clinical trials for the treatment of various cancers. Raltitrexed [13,14] is a folate analogue thymidylate synthase inhibitor used in the treatment of advanced colorectal cancer. It inhibits thymidylate synthase (TS) leading to DNA fragmentation and cell death. Idelalisib [15,16] is a phosphoinositide 3-kinase (PI3K) inhibitor used to treat certain types of cancer such as chronic lymphocytic leukaemia (CLL), relapsed follicular B-cell non-Hodgkin lymphoma (FL), and relapsed small lymphocytic lymphoma (SLL). It works by slowing or stopping the growth of cancer cells. As such, these compounds containing quinazolinone moieties have played a significant role in the development of effective antineoplastic drugs.
Figure 1.
(A) Representative structures of quinazolinone derivatives as anticancer agents previously reported. (B) Some biologically active molecules containing a pyrazolo-[1,5-c]quinazolinone skeleton. (C) The design idea and synthesis strategy of the pyrazolo-[1,5-c]quinazolinone skeleton.
In light of the significant antitumour activities demonstrated by quinazolinone-based compounds, our research endeavours have been directed towards the development of a lead compound incorporating pyrazolo-[1,5-c]quinazolinones, formed through the N-fusion of quinazolinones with pyrazoles [17,18]. It is noteworthy that quinazolinone–pyrazole hybrids have exhibited a range of biological activities, which may extend to potential therapeutic applications in neurodegenerative disorders [19,20,21], bacterial infections [22,23], inflammatory diseases [21,24], and neoplasia [23], among others [25]. Molecular hybridisation has recently emerged as a potent approach for addressing multifaceted diseases such as cancer and neurodegenerative disorders [26,27,28,29]. This innovative drug discovery strategy involves the integration of two or more pharmacophores into a single hybrid molecule, with the intent of generating novel chemical entities that display superimposed pharmacological effects. Our hope is to capitalise on the putative synergistic effects and enhanced pharmacological properties arising from the combination of quinazolinones and pyrazoles, in the pursuit of innovative antineoplastic agents. It is crucial to recognise that, to the best of our knowledge, the pyrazolo-[1,5-c]quinazolinone scaffold has been infrequently reported to exhibit antitumour activity. Consequently, in an effort to establish a novel foundation for the development of prospective anticancer therapeutics, we have embarked upon a preliminary investigation of an array of pyrazolo-[1,5-c]quinazolinones.
Our primary aim is to develop an easy and efficacious synthetic strategy for the expeditious assembly of the pyrazolo-[1,5-c]quinazolinone framework. Simultaneously, we strive to identify pyrazolo-[1,5-c]quinazolinone derivatives that display potent antitumour activity and serve as valuable lead compounds targeting CDK9/2, thereby unveiling new opportunities for progress in the realm of anticancer drug discovery. Meanwhile, the further chemical modification and biological exploration of these compounds are underway in our laboratory.
2. Results and Discussion
2.1. Chemistry Efforts of the Synthesised Compounds
2.1.1. Design and Optimisation of Synthesis
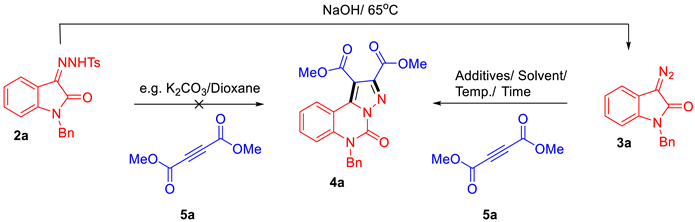
The development of efficient strategies for the synthesis of diverse and complex heterocyclic compounds has been a longstanding goal in synthetic organic chemistry due to their widespread applications across various scientific disciplines. While several approaches for the synthesis of the pyrazolo-[1,5-c]quinazolinone scaffold have been reported, many are limited by their reliance on metal catalysts, low atom economy, or cumbersome isolation procedures [30,31,32,33]. Inspired by the diazo compounds that have emerged as valuable synthetic platforms for the total synthesis of bioactive natural products [34,35], the N-Bn-protected isatin-derived tosylhydrazone was designed for the synthesis strategy of pyrazolo-[1,5-c]quinazolinones. The synthesis route in Scheme 1 shows that indole derivatives, which are inexpensive and readily available, were reacted with benzyl bromide and 4-methylbenzenesulfonhydrazide to yield the N-Bn-protected isatin-derived tosylhydrazone intermediates 2. Unfortunately, no desired products were obtained when intermediates 2 reacted with dimethyl but-2-ynedioate using different Lewis base catalysts via an anticipated one-pot method of tandem reaction (Table 1, entries 1–4), although the reactions were processed under an argon atmosphere and the dry solvent conditions.
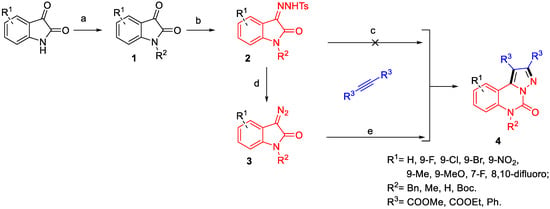
Scheme 1.
Design of synthesis route. (a). R2Br, K2CO3, DMF, 100 °C; (b). 4-methylbenzenesulfonhydrazide, 100 °C; (c) corresponding alkyne, K2CO3, 1,4-dioxane (for details, see Table 1, entries 1–4); (d) NaOH, H2O, 60 °C; (e) corresponding alkyne, toluene, 85 °C.

Table 1.
Optimisation of the conditions for the formation of 4a a.
Despite initial setbacks, further investigation was conducted to better understand this reaction. The purified diazo isatin 3a was isolated from the intermediate 2a under NaOH conditions. It was then reacted with dimethyl but-2-ynedioate under heated conditions to successfully produce the desired product 4a. To enhance the production of the target compound, we commenced our investigation with the model reaction of diazo isatin 3a with dimethyl but-2-ynedioate (5a). As illustrated in Table 1, the high reactivity of product 4a was observed after a series of chemistry attempts under different conditions. Interestingly, the reactions were tolerated under aqueous solvent, open-air, or acidic solvent conditions (entries 5–8). The influence of various solvents and temperature conditions were examined to further improve the yield of the reaction (entries 9–17). Among the solvents tested, most non-polar solvents were found to be suitable for the reaction particularly toluene and tetrahydrofuran. Based on these attempts and observations, optimal reaction conditions were established as being carried out in toluene at 85 °C without treatment with an anhydrous solvent or protection by inert gas. The structure of product 4a was established by spectroscopic analysis and mass spectrometry.
2.1.2. Scope of the Reaction
To explore the scope of the reaction and further obtain diverse pyrazolo-[1,5-c]quinazolinone derivatives for the study of pharmacological activity, several different kinds of the diazo isatins were prepared and applied to synthesise the corresponding derivatives of esterified pyrazolo-[1,5-c]quinazolinones with the optimised conditions (Figure 2). Gratifyingly, the electronic property of the substituents on the benzene rings did not have significant impact on the reaction yields. The cyclisation reaction worked efficiently to afford the products 4a–4g in excellent yields (over than 90% yields) whether the substituents were electron-withdrawing or electron-donating groups. Not surprisingly, the ethyl-esterified products 4h–4p were synthesised in good to excellent yields when the raw material was replaced with diethyl but-2-ynedioate, which suggested that the reactions tolerated different electronic properties and the position of their benzene rings.
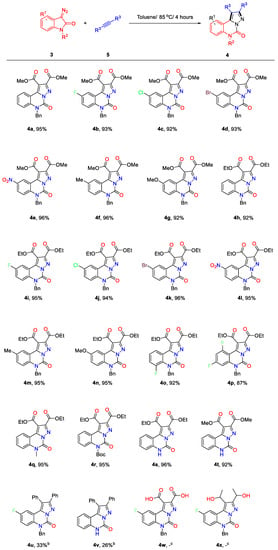
Figure 2.
Chemical structures and synthesis yields of pyrazolo-[1,5-c]quinazolinone derivatives. Reaction conditions: the reactions were performed in a round-bottomed flask on a 0.25 mmol scale of 3 and 0.3 mmol scale of 5 in toluene (3 mL) at 85 °C for 4–6 h. b Yield of 140 °C/72 h. c The desired product was not observed.
The presence of the highly reactive NH bond is responsible for the formation of the complex mixture, however, the NH position of the isatin substrates without any N-protecting groups did not inhibited the formation of the product 4s/4t with the optimised conditions. The reactions of isatins with N-electron-withdrawing group (4r) were inferior to those of isatins with N-electron-donating groups (4q/4h); it appears that the electronic nature of the N-protecting groups had a slight impact on the reaction yields.
Although the products 4u and 4v could be observed weakly by this synthesis method, the reaction inefficiently worked after increasing the reaction temperature to 140 °C and extending the reaction time to 48 h. In view of the medium drug-like properties predicted by the derivative 4v (see Table 5, violation of Lipinski rules), we have not prepared more of these derivatives. Regrettably, the expected products 4w or 4x were not observed while but-2-ynedioic acid or hex-3-yne-2,5-diol was used as the reactant instead of dimethyl but-2-ynedioate (5a). This novel method took advantage of high atom economy, metal-free process, and simple operation. Most of the products (4a–4t) were isolated by simple recrystallisation and filtration, which did not require any column chromatography technique for purification.
2.1.3. Scale-Up and Green Chemistry Applications
Impressively, the formation of the products 4s and 4t was not prevented by the presence of the highly reactive NH bond of the starting materials 3l. To demonstrate the reliability and practicality of the present synthetic methodology, a gram-scalable preparation was investigated using starting materials 3l (2.54 g, 16.0 mmol) and 5a (2.73 g, 19.2 mmol) to give the corresponding product 4t in 88% yield (4.22 g, Scheme 2). This high yield confirms the practicality of this approach for large-scale synthesis.
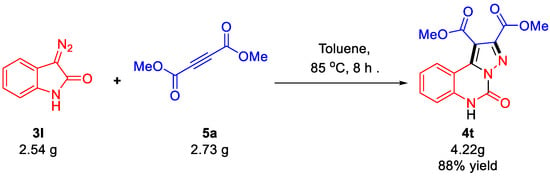
Scheme 2.
Gram-scale synthesis of 4t. Reaction conditions: the reactions were performed in a round-bottomed flask on a 16.0 mmol scale of 3l and 19.2 mmol scale of 5a in toluene (30 mL) at 85 °C for 8 h.
In view of the fact that the reactions were tolerated under the conditions of aqueous and open-air atmosphere (Table 1, entry 6), we attempted the reaction in H2O at 85 °C to make the reaction much greener and to avoid the toluene solvent. It is regrettable that the reaction was not efficient at yielding the product 4t within a short time, due to insolubility of the starting materials (Table 2, entry 1). Attempts were made to improve the solubility of the starting materials by performing the reaction in the various of mixed solvents. To our delight, the reaction proceeded, and an excellent improvement in the yield was observed in a H2O/THF (v/v = 2:1) mixture as solvent (Table 2, entry 2–4). A similar conclusion can be observed when the reactions of product 4k and 4o are used with a H2O/THF (v/v = 2:1) mixture as solvent (Table S1). This reaction provided the advantage of gram-scale synthesis and was environment friendly, indicating the broad application of the pharmaceutical industry.

Table 2.
Green synthesis of 4t a.
2.1.4. Plausible Mechanism of the Reaction
On the basis of the experimental results and previous reports on bis(diazo)indolin-2-one chemistry, we proposed a plausible mechanism to rationalise the reaction pathways for the formation of 4a (Scheme 3). A [3 + 2] dipolar cycloaddition of diethyl but-2-ynedioate and 3-diazoindolin-2-one proceeded to generate the intermediate spiro[indoline-3,3’-pyrazol]-2-one, which underwent acyl migration and rearrangement of ring expansion to form thermodynamically stable pyrazolo-[1,5-c]quinazolin-5(6H)-one under thermal conditions.
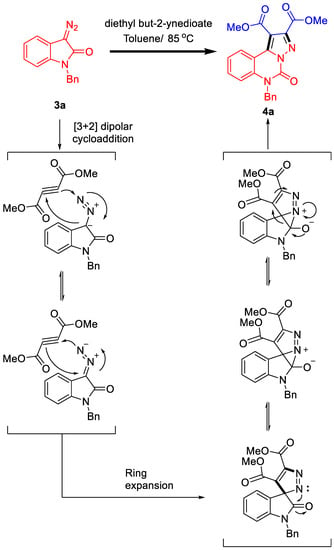
Scheme 3.
Plausible reaction mechanism.
2.2. Biological Studies
2.2.1. In Vitro Cytotoxicity Evaluation and Structure–Activity Relationship (SAR)
Previously, the widespread presence of quinazolinone moieties in antineoplastic drugs reflected their versatility and effectiveness in the treatment of cancer [3,4,5,7]. Therefore, the compounds of pyrazolo-[1,5-c]quinazolinones were selected and evaluated for in vitro antiproliferative activity against A549, MDA-MB-231, U-87, and HepG2 using the standard CCK8 assay, and 5-fluorouracil and abemaciclib (Abe) were used as positive controls.
As demonstrated in Figure 3 and Table 3 and Table S2, A549 cells exhibit a higher sensitivity to the majority of compounds at a concentration of 30 μM compared to MDA-MB-231 cells, U-87 cells, or HepG2 cells. Further evaluation of the half-maximal inhibitory concentration (IC50) of the compounds was conducted on A549 and MDA-MB-231 cells. For A549 cells, compounds 4a–4g (R3 = COOEt), which contain ethoxycarbonyl groups at the C1 and C2 positions of quinazolino, generally exhibit superior antiproliferative activity compared to compounds 4h–4s (R3 = COOMe). Further analysis reveals that when R1 position of quinazolino is 9-methyl (4m) or 9-methoxy (4n), their antiproliferative activity is superior to H or 9-F/ 9-Cl/ 9-Br (4h–4k). The compound 4l (R1 = 9-NO2) has little antitumour activity, indicating that the introduction of an electron-rich group on the benzene ring of quinazolino is significantly beneficial for improving cellular activity. When the R2 position of quinazolino is tert-butoxycarbonyl (4r), benzyl (4h), or methyl (4q), cellular activity is marginally superior to hydrogen (4s). However, when comparing benzyl substitution (4a) at R2 position with hydrogen substitution (4t), no significant improvement in cellular activity is observed. Additionally, it is believed that the tert-butoxycarbonyl group may have drawbacks such as a rapid metabolic rate during drug metabolism. Therefore, the R2 position may not be a suitable structural site for optimising activity. Importantly, compounds 4i, 4m, and 4n (IC50 = 17.0, 14.2, and 18.1 μM, respectively) exhibit relatively potent antitumour activities compared with 5-fluorouracil (IC50 = 21.2 μM) against A549 cells. The inhibitory activities of compounds 4n and 4v on MDA-MB-231 cells were found to be comparable to the positive control drug, 5-fluorouracil. For U87 and HepG2 cells, only compound 4j/4k (for U87 cells) and 4v (for HepG2 cells) exhibit an inhibition rate greater than 40%, while the rest of the compounds were found to be insensitive. In summary, the structure-activity relationships of the synthesized derivatives are depicted in Figure 4. These results suggest that the pyrazolo-[1,5-c]quinazolinone framework has the potential to serve as a new template for the design of novel anticancer molecules.
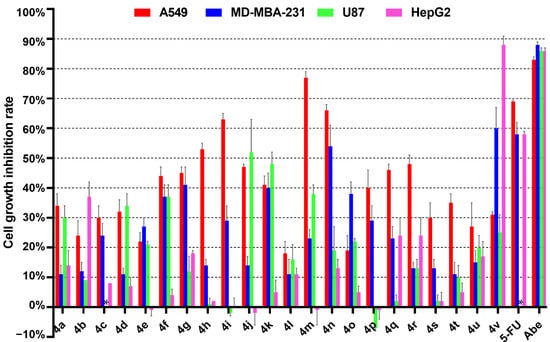
Figure 3.
Cell growth inhibition rate of the pyrazolo-[1,5-c]quinazolinone derivatives on A549 cells, MDA-MB-231 cells, U87 cells, and HepG2 cells (30 μM).Cells were treated with compounds at the concentration of 30 μM for 48 h, the data represent mean ± SD (n = 3). 5-Fluorouracil (5-Fu) and abemaciclib (Abe) were used as positive controls at a concentration of 30 μM. * Not determined.

Table 3.
Cytotoxic activities of compounds 4a–4v against cancerous cell lines a.
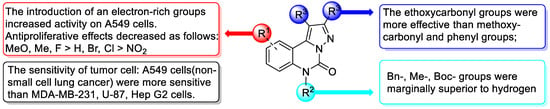
Figure 4.
Structure–activity relationship (SAR) of a series of pyrazolo-[1,5-c]quinazolinone derivatives.
2.2.2. In Silico Studies of Biological Target and Molecular Modelling
Recent advances in deep-learning algorithms have made the accessible profiling of small molecules potential drug targets through in silico modelling approaches possible. The platform of ProfKin based on a kinase–ligand-focused database (KinLigDB) has been exploited as a multifunctional website for structure-based kinase target profiling [37]. The website (http://www.lilab-ecust.cn/profkin, accessed on 22 December 2022.) can be used to predict the possible binding modes, which can be helpful for target prediction and mechanistic study. To better understand the potential drug targets for which the compound exhibits antitumour effects, the assay of ProfKin was used to estimate the most probable kinases targets and binding conformations of compound 4t. The results were displayed as the phylogenetic tree picture (Figure 5, the detailed data are provided in the Supplementary Materials). In short, compound 4t can be used to identify probable drug targets through the inspection of top-ranked kinases, including CDK2, CK2, TYK, IRAK, JAK, NTRK, GSK3, CHK, PIM, MAPK, Aurora, RAF, and DYRK, among others.
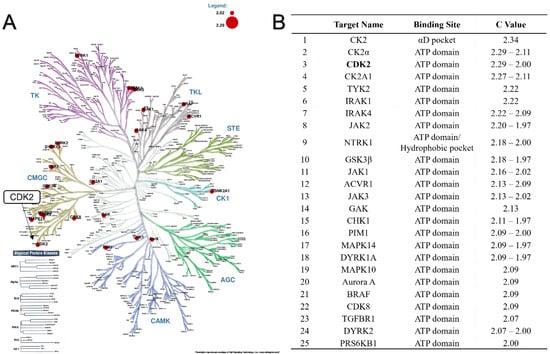
Figure 5.
(A) The results of kinases target profiling were performed as the picture of phylogenetic tree; the red circle code means the predicted kinase targets. (B) The detailed data were ranked by their C value, with a higher C value indicating greater reliability of the calculated results.
As one of cyclin-dependent kinase (CDK) family members, CDK2 is an important regulatory factor of various carcinogenic signalling pathways. The inhibition of CDK2 provides a potential therapeutic benefit against certain tumour cells [38,39]. We focus on analysing the predicted binding poses of compound 4t to CDK2 for developing the drug use of the pyrazolo-[1,5-c]quinazolinones backbone. As shown in Figure 6A, compound 4t is located in a narrow cleft of the ATP-binding site between the N-terminal and C-terminal lobes. The pyrazolo-[1,5-c]quinazolinone core forms two hydrogen bonds with the leucine at the eighty-third position (Leu83) of CDK2. The binding poses of the compound 4t in the ATP pocket are similar to the thiazolylpyrimidine ligand 19K by the visual inspection of binding modes (Figure 6B,C), which predicts the rationality and feasibility of the analysis of the binding mode. The C8 and C9 positions of quinazolino are located in the entranceway of the ATP-binding pocket and extend into the solvent-accessible area. Other research in the literature has reported that chemical structural modifications in solvent-accessible areas improve cellular activity [21,40], which is similar to our previous finding that the introduction of different groups on the benzene ring at this region leads to significant changes in antitumour cell activity. Thus, the pyrazolo-[1,5-c]quinazolinone compounds have the potential to exhibit antitumour effects by targeting CDK2, and could be used as a starting skeleton for the development of CDK inhibitors via a structure-based drug design, for which further research is already underway.
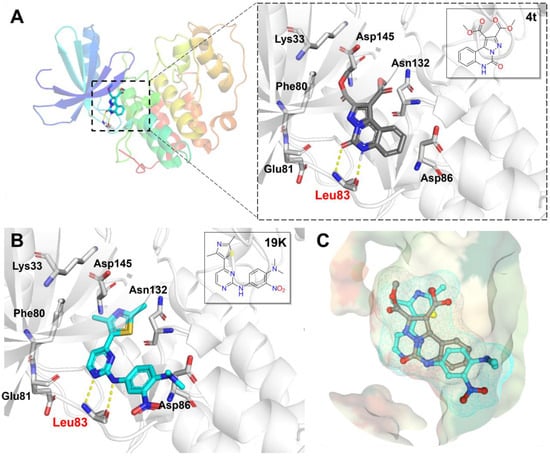
Figure 6.
Binding mode analysis of compound 4t within CDK2 kinase (ribbon representation, PDB code: 3SW7). The docking images were generated by the PyMOL molecular graphics system. The key amino acids of CDK2 are labelled, and hydrogen bonds in all panels are depicted by yellow dotted lines. (A) The binding pose of compound 4t (grey stick model) in the CDK2. (B) The binding pose of the ligand 19K (cyan stick model) in the CDK2. (C) Comparison of the binding poses of 4t and the ligand 19K within CDK2. The electron density of studied compounds is surfaced by the mesh.
2.2.3. CDK2/7/9 Enzyme Activity Assay
Considering the high degree of homology and conservation among the amino acid sequences of various CDK family kinase subtypes reported in the literature [38,39], we conducted a preliminary exploration of the CDK2/7/9 enzyme activity of selected compounds 4h, 4n, and 4t with different structural characteristics. The results (Table 4) demonstrate that the compounds do not exhibit strong inhibitory activity against CDK2 at a concentration of 1.0 μM, which may be due to their lack of efficacy at low concentrations. Surprisingly, the compounds exhibit superior inhibitory activity against CDK9 compared to CDK2 or CDK7. Half-maximal inhibitory concentration assays of CDK9 reveal that the compounds fall within the micromolar concentration range (4t: 4.7 μM; 4n: 9.8 μM). Notably, the previous literature has reported that the CDK9 inhibitor can induce apoptosis and death in A549 cells [41,42]. Therefore, the ability of pyrazolo-[1,5-c]quinazolinone derivatives to inhibit CDK9 kinase may contribute to the inhibition of A549 cell growth. This finding highlights the potential of targeting CDK9 as a therapeutic strategy for cancer treatment.

Table 4.
The CDK2/7/9 activity of representative compounds 4h, 4n, and 4t a.
2.3. Drug-Likeness Studies In Silico
2.3.1. Pharmacokinetic and Drug-Likeness Prediction
The development of new drugs requires not only good pharmacological activity, but also suitable physical and chemical properties and pharmacokinetic properties such as absorption, distribution, metabolism, and excretion (ADME), as well as acceptable toxicity effects. The calculation and prediction of the drug-like properties of these compounds in silico is valuable to improve the success of drug discovery.
In order to ensure that the compounds have good drug properties in the bioactivity study of this project, drug-likeness aspects prediction and pharmacokinetics of the studied pyrazolo-[1,5-c]quinazolinone derivatives were carried out in the available Swiss ADME [43]. The result (Figure 7) of BOILED-EGG chart indicates that most of the target compounds have a high probability of being passively absorbed by the gastrointestinal tract and do not permeate the blood–brain barrier (BBB). Additionally, these compounds may not be substrates for P-glycoprotein (P-gp) and, thus, eliminate the possibility of tumour cell line resistance through efflux mechanisms. However, compound 4v was predicted to have a high probability of permeating through the BBB to access the central nervous system (CNS), while also having the ability to be a substrate for P-glycoprotein. Moreover, most of the compounds do not violate Lipinski rules and do not belong to PAINS compounds. The detailed drug-likeness parameters of the studied compounds are listed in Table 5. Most compounds are estimated to have negligible activity on cytochrome P450 isomers (e.g., CYP3A2 and CYP2D6) and are, therefore, considered to have no drug–drug interactions upon administration. Additionally, moderate bioavailability (score = 0.55) was predicted for the target molecules based on their compliance with five rule-based drug filters (Lipinski, Ghose, Veber, Egan, and Muegge rules).
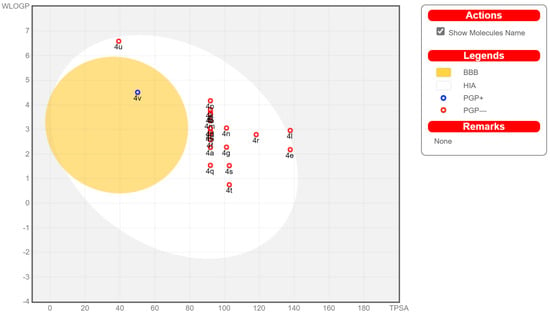
Figure 7.
BOILED-EGG chart of the target molecules 4a–4v. Results are presented as a 2D plot using the calculated TPSA and WLogP properties of the target molecules. The different points in the BOILED-EGG chart represent the target compounds 4a–4v. The points located in the white ellipse represent compounds with high probability to be passively absorbed by the gastrointestinal tract, and located in the yellow yolk ellipse are for compounds with high probability to permeate through the BBB to access the CNS. The red point predicts no effluxing via P-glycoprotein (PGP−). If a predicted substrate of the P-glycoprotein (PGP+), the point colour is in blue.

Table 5.
Pharmacokinetic aspects and drug-likeness parameters of compounds 4a–4v a.
2.3.2. Toxicity Risk Assessment
The toxicity of chemicals should be known to avoid unwanted events. To anticipate the potential toxicity of compounds, the chemical structures of molecules were evaluated using the available OSIRIS property explorer to determine probable toxicity risks such as mutagenicity, carcinogenicity, tumorigenicity, and teratogenicity [44]. The OSIRIS property explorer calculates these risks based on functional group similarities between the investigated compound and those in its database with known in vitro and in vivo studies. The results (Table 6) indicate that the majority of the studied compounds are deemed safe and exhibit no or low toxicity concerning mutagenicity, tumorigenicity, and irritant effects. However, compound 4r may pose a high risk of tumorigenicity and irritant effects, and compounds 4u and 4v may have a high risk to the reproductive system, while others exhibit moderate toxic effects on it.

Table 6.
Toxicity risks of compounds 4a–4v.
The acute toxicity prediction of pyrazolo-[1,5-c]quinazolinone derivatives in this study were performed using the ProTox-II webserver [45,46,47]. Most of the compounds have similar LD50 values in the range of 564–1140 mg/kg and are predicted to belong to a medium toxicity class (Table 6). This toxicity level was categorised as toxicity class number 4 from 6 classes and turned to moderate toxicity, especially for oral consumption. The detailed toxicity profiles calculated with the help of ProTox-II are shown in Supplementary Information (Figure S2). In summary, further research into the optimisation and pharmacological investigation of these compounds holds significant potential and is highly encouraged.
3. Conclusions
In conclusion, our study presents the first report on the utilisation of pyrazolo-[1,5-c]quinazolinone derivatives for antitumour pharmacological applications. We have developed an efficient and straightforward methodology to rapidly access functionalised pyrazolo-[1,5-c]quinazolinones via a [3 + 2] dipolar cycloaddition and regioselective ring expansion process. This reaction strategy offers several advantages including high yield, high atom economy, metal-free process, simple operation, gram-scale synthesis, and environmental friendliness. In a series of in vitro studies, we found that some of the compounds demonstrated antitumour activities and inhibitory activity against CDK9/2. In silico studies indicate that the majority of the synthesised derivatives exhibit good drug-like properties and safety profiles. Our study not only provides a convenient and efficient synthetic method but also provides helpful structure-based guided drug design for further development of novel cyclin-dependent kinase (CDK) inhibitors. Further research into the application of these types of compounds in medicine would be valuable and meaningful.
4. Experimental Section
4.1. General Methods and Materials
All reactions were monitored by analytical thin-layer chromatography (TLC), which was visualised by ultraviolet light (254 nm or 356 nM). All solvents were obtained from commercial sources and were purified according to standard procedures. Purification of the products was accomplished by recrystallisation or flash chromatography using silica gel (100–200 mesh). Melting points were determined by WRR-Y drug melting point apparatus (Shanghai Yidian Physical and Optical Instruments Co., Ltd., Shanghai, China.). All NMR spectra were recorded with JEOL JNM-ECZ400S/L1 spectrometer at 400 MHz in DMSO or CDCl3: chemical shifts (δ) are given in ppm, coupling constants (J) in Hz, and the following abbreviations are used to indicate the multiplicity in NMR spectra: s (singlet); d (doublet); t (triplet); q (quartet); m (multiplet). The solvent signals were used as references (residual DMSO in DMSO-d6: δH = 2.50 ppm, δc = 39.5 ppm; CHCl3 in CDCl3: δH = 7.26 ppm, δc = 77.0 ppm). Mass spectra were obtained on the Agilent 1100 LC/MSD mass spectrometer (Agilent, Santa Clara, CA, USA) and Q-tofmicro MS (micromass company). High resolution mass spectrometry (HRMS) was recorded on TOF primer for ESI+. The purity of biologically evaluated compounds was >95% as determined by HPLC analysis (Waters e2695, C18 column (50 × 2.00 mm), with CH3OH and H2O as the mobile phase, monitored by UV absorption at 231 nm).
4.1.1. General Procedure for the Synthesis of Isatin-Derived Tosylhydrazone 2 and the Diazo Isatin 3
The isatin-derived tosylhydrazone 2 and the diazo isatin 3 are prepared according to a known procedure starting from substituted isatins [48].
The diazo isatin 3 are synthesised by compounds 2 with a sodium hydroxide aqueous solution condition. The corresponding isatin-derived tosylhydrazone 2 (10 mmol) and NaOH (1.0 g, 25 mmol) were suspended in water (30 mL), and the suspension was stirred at 50 °C for 2 h until the completion of the reaction as monitored by TLC. The reaction mixture was cooled down, then the residue was collected by the filtration funnel and was washed with water (10 mL × 3). After air-drying, the diazo product 3 appears as a red or orange solid.
1-benzyl-3-diazoindolin-2-one (3a). Orange solid. 1H NMR (400 MHz, chloroform-d) δ = 7.48 (dt, J = 9.2, 4.7, 1H), 7.42–7.24 (m, 5H), 7.16–7.08 (m, 1H), 7.08–6.98 (m, 2H), 5.00 (s, 2H). 13C NMR (100 MHz, chloroform-d) δ 167.2, 136.3, 133.9, 129.1, 128.0, 127.6, 125.7, 122.5, 118.6, 117.1, 109.9, 44.6.
4.1.2. General Procedure for the Synthesis of 4
To a 25 mL round-bottomed flask was added the diazo isatin 3 (0.25 mmol), alkyne 5 (0.30 mmol), and the toluene (3 mL). The resulting mixture was heated at 85 °C in air for a period of time (usually 4–6 h). After completion of the reaction as monitored by TLC, the mixture was cooled to room temperature. The solvent was evaporated under reduced pressure and the residue was purified by recrystallisation (ethyl acetate/n-hexane) or chromatography (ethyl acetate/petroleum ether) on silica gel to afford products 4.
4.1.3. Characterisation of Products 4a–4v
Dimethyl 6-benzyl-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4a). Pale pink solid, 95% yield, mp: 224–225 °C. 1H NMR (400 MHz, chloroform-d) δ 8.7 (d, J = 8.0 Hz, 1H), 7.5 (t, J = 8.0 Hz, 1H), 7.3 (d, 1H), 7.3–7.3 (m, 3H), 7.3–7.2 (m, 3H), 5.6 (s, 2H), 4.0 (s, 6H). 13C NMR (100 MHz, chloroform-d) δ 163.5, 162.2, 148.1, 145.3, 140.0, 135.7, 134.8, 132.3, 129.2(2C), 128.0, 126.9, 126.7(2C), 124.3, 115.9, 112.6, 110.53, 53.0(2C), 48.2. HRMS (ESI) calcd. for C21H18N3O5 (M+H)+: 392.1241, found 392.1243. HPLC retention time 3.49 min, purity 95.7%.
Dimethyl 6-benzyl-9-fluoro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4b). Pale pink solid, 93% yield, mp: 186–187 °C. 1H NMR (400 MHz, chloroform-d) δ 8.6 (d, J = 9.2 Hz, 1H), 7.3 (t, 1H), 7.3 (d, J = 7.4 Hz, 2H), 7.3–7.2 (m, 3H), 7.2 (d, J = 8.3 Hz, 1H), 5.6 (s, 2H), 4.0 (s, 3H), 4.0 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 163.0, 162.2, 148.7, 144.9, 138.9, 135.1, 134.7, 134.4, 129.6, 129.3(2C), 128.2, 126.6(2C), 117.5, 117.4, 114.2, 110.81, 53.1(2C), 48.4. HRMS (ESI) calcd. for C21H17FN3O5 (M+H)+: 410.1147, found 410.1157. HPLC retention time 3.73 min, purity 99.5%.
6-benzyl-9-chloro-1,2-bis(methoxycarbonyl)-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazolin-3-ium (4c). Light yellow solid, 92% yield, mp: 161–162 °C. 1H NMR (400 MHz, chloroform-d) δ 8.8 (d, J = 2.4 Hz, 1H), 7.4 (dd, J = 9.0, 2.4 Hz, 1H), 7.3–7.3 (m, 2H), 7.3–7.2 (m, 4H), 5.6 (s, 2H), 4.0 (s, 3H), 4.0 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 163.0, 162.1, 148.6, 144.9, 139.0, 134.4, 134.3, 132.3, 129.9, 129.2(2C), 128.2, 126.6(2C), 126.5, 117.4, 113.7, 110.7, 53.1(2C), 48.4. HRMS (ESI) calcd. for C21H17ClN3O5 (M+H)+: 426.0851, found 426.0849. HPLC retention time 4.21 min, purity 99.6%.
Dimethyl 6-benzyl-9-bromo-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4d). Pale pink solid, 93% yield, mp: 196–198 °C. 1H NMR (400 MHz, chloroform-d) δ 8.9 (s, 1H), 7.6 (d, J = 9.1 Hz, 1H), 7.3 (d, J = 7.3 Hz, 2H), 7.3–7.2 (m, 3H), 7.2 (d, J = 8.8 Hz, 1H), 5.6 (s, 2H), 4.0 (s, 3H), 4.0 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 163.0, 162.2, 148.7, 144.9, 138.9, 135.1, 134.7, 134.4, 129.6, 129.3(2C), 128.2, 126.6(2C), 117.5, 117.4, 114.2, 110.81 53.1(2C), 48.4. HRMS (ESI) calcd. for C21H17BrN3O5 (M+H)+: 470.0346, found 470.0353. HPLC retention time 4.16 min, purity 98.4%.
Dimethyl 6-benzyl-9-nitro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4e). Light yellow solid, 96% yield, mp: 226–228 °C. 1H NMR (400 MHz, chloroform-d) δ 9.9 (d, J= 2.5 Hz, 1H), 8.3 (dd, J = 9.4 Hz, 2.5 Hz, 1H), 7.4 (d, J = 9.3 Hz, 1H), 7.4–7.3 (m, 3H), 7.3 (dd, J = 7.3 Hz, 1.3 Hz, 2H), 5.7 (s, 2H), 4.0 (s, 3H), 4.0 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 162.5, 161.9, 149.3, 144.7, 143.5, 139.9, 138.9, 133.8, 129.5(2C), 128.5, 126.8, 126.6(2C), 123.4, 116.8, 112.9, 111.41, 53.3, 53.3, 48.9. HRMS (ESI) calcd. for C21H17N4O7 (M+H)+: 437.1092, found 437.1093. HPLC retention time 3.72 min, purity 97.0%.
Dimethyl 6-benzyl-9-methyl-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4f). Pale pink solid, 96% yield, mp: 165–167 °C. 1H NMR (400 MHz, chloroform-d) δ 8.4 (s, 1H), 7.3 (d, J = 7.1 Hz, 1H), 7.3 (d, 2H), 7.2–7.2 (m, 3H), 7.1 (d, J = 9.8 Hz, 1H), 5.5 (s, 2H), 4.0 (s, 3H), 4.0 (s, 3H), 2.4 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 163.5, 162.3, 148.0, 145.2, 140.0, 134.9, 134.2, 133.6, 133.3, 129.1(2C), 128.0, 126.7(2C), 126.6, 115.7, 112.4, 110.29, 53.0(2C), 48.1, 21.0. HRMS (ESI) calcd. for C22H20N3O5 (M+H)+: 406.1397, found 406.1390. HPLC retention time 3.97 min, purity 98.6%.
Dimethyl 6-benzyl-9-methoxy-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4g). Grey–white solid, 92% yield, mp: 172–174 °C. 1H NMR (400 MHz, chloroform-d) δ 8.4 (s, 1H), 7.3 (d, J = 7.5 Hz, 2H), 7.2–7.2 (m, 3H), 7.2 (d, J = 8.3 Hz, 1H), 7.0 (d, J = 9.0 Hz, 1H), 5.5 (s, 2H), 4.0 (s, 3H), 3.9 (s, 3H), 3.8 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 163.3, 162.5, 155.9, 148.6, 145.1, 140.2, 135.0, 129.7, 129.1(2C), 128.0, 126.7(2C), 120.5, 117.1, 113.3, 110.0, 109.5, 55.8, 53.0, 52.9, 48.2. HRMS (ESI) calcd. for C22H20N3O6 (M+H)+: 422.1347, found 422.1352. HPLC retention time 3.80 min, purity 98.8%.
Diethyl 6-benzyl-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4h). Yellow solid, 92% yield, mp: 168–169 °C. 1H NMR (400 MHz, chloroform-d) δ 8.8 (d, J = 8.0 Hz, 1H), 7.5 (t, J = 7.2 Hz, 1H), 7.3 (d, J = 7.4 Hz, 1H), 7.3–7.3 (m, 3H), 7.3–7.3 (m, 3H), 5.6 (s, 2H), 4.5 (qd, J = 7.1, 3.9 Hz, 4H), 1.4 (dt, J = 11.3, 7.1 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 163.0, 162.1, 145.4, 140.0, 135.7, 134.9, 132.2, 129.2(2C), 128.0, 127.1, 126.7(2C), 124.3, 115.8, 112.8, 62.4, 62.1, 48.2, 14.3, 14.1. HRMS (ESI) calcd. for C23H22N3O5 (M+H)+: 420.1554, found 420.1557. HPLC retention time 3.05 min, purity 97.8%.
Diethyl 6-benzyl-9-fluoro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4i). White–pink solid, 95% yield, mp: 137–139 °C. 1H NMR (400 MHz, chloroform-d) δ 8.6 (dd, J = 9.5, 2.9 Hz, 1H), 7.2 (d, 1H), 7.2–7.2 (m, 1H), 7.2–7.1 (m, 3H), 7.1–7.1 (m, 2H), 5.5 (s, 2H), 4.4 (dq, J = 9.9, 7.1 Hz, 4H), 1.3 (dt, J = 9.9, 7.1 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.3, 162.0, 149.3, 144.9, 139.24, 134.6, 132.1, 129.1(2C), 128.0, 126.6(2C), 120.0, 119.7, 117.8, 117.7, 113.4, 110.5, 62.4, 62.2, 48.3, 14.2, 13.9. HRMS (ESI) calcd. for C23H21FN3O5 (M+H)+: 438.1460, found 438.1463. HPLC retention time 4.42. min, purity 98.4%.
Diethyl 6-benzyl-9-chloro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4j). White solid, 94% yield, mp: 165–166 °C. 1H NMR (400 MHz, chloroform-d) δ 8.8 (s, 1H), 7.3 (d, J = 9.2 Hz, 1H), 7.2 (d, J = 7.8 Hz, 1H), 7.2 (d, 2H), 7.2 (t, 3H), 5.5 (s, 2H), 4.4 (dq, J = 7.4, 3.7 Hz, 4H), 1.4 (q, J = 6.6, 6.1 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.3, 162.0, 149.3, 145.0, 138.9, 134.5, 134.2, 132.2, 129.8(2C), 129.2, 128.1, 126.6(3C), 117.3, 113.7, 110.7, 62.5, 62.3, 48.3, 14.2, 14.0. HRMS (ESI) calcd. for C23H21ClN3O5 (M+H)+: 454.1164, found 454.1176. HPLC retention time 4.59 min, purity 97.6%.
Diethyl 6-benzyl-9-bromo-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4k). White solid, 96% yield, mp: 167–168 °C. 1H NMR (400 MHz, chloroform-d) δ 8.9 (s, 1H), 7.5 (d, J = 9.0 Hz, 1H), 7.2 (d, J = 6.8 Hz, 2H), 7.2–7.1 (m, 3H), 7.1 (d, J = 9.1 Hz, 1H), 5.5 (s, 2H), 4.4 (qd, J = 7.2, 3.4 Hz, 4H), 1.4 (td, J = 7.0, 3.5 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.3, 161.9, 149.3, 144.9, 138.7, 135.0, 134.6, 134.5, 129.5, 129.2(2C), 128.1, 126.6(2C), 117.6, 117.2, 114.1, 110.7, 62.5, 62.3, 48.2, 14.2, 14.0. HRMS (ESI) calcd. for C23H21BrN3O5 (M+H)+: 498.0659, found 498.0657. HPLC retention time 4.76 min, purity 98.4%.
Diethyl 6-benzyl-9-nitro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4l). Light yellow solid, 95% yield, mp: 168–169 °C. 1H NMR (400 MHz, chloroform-d) δ 9.9 (s, 1H), 8.3 (d, J = 9.2 Hz, 1H), 7.4 (d, J = 9.5 Hz, 1H), 7.3–7.3 (m, 3H), 7.2 (d, J = 7.3 Hz, 2H), 5.6 (s, 2H), 4.5–4.4 (m, 4H), 1.4 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.0, 161.7, 149.9, 144.8, 143.5, 139.9, 138.7, 133.8, 129.4(2C), 128.5, 126.7, 126.6(2C), 123.5, 116.8, 112.9, 111.5, 62.7, 62.6, 48.9, 14.2, 14.0. HRMS (ESI) calcd. for C23H21N4O7 (M+H)+: 465.1405, found 465.1412. HPLC retention time 4.44 min, purity 98.1%.
Diethyl 6-benzyl-9-methyl-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4m). White solid, 95% yield, mp: 141–143 °C. 1H NMR (400 MHz, chloroform-d) δ 8.5 (s, 1H), 7.2 (d, J = 8.0 Hz, 1H), 7.2 (d, 2H), 7.2–7.1 (m, 3H), 7.1 (d, J = 8.8 Hz, 1H), 5.5 (s, 2H), 4.4 (q, J = 7.3 Hz, 4H), 2.3 (s, 3H), 1.4 (q, J = 6.4 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.9, 162.1, 148.7, 145.3, 139.8, 135.0, 134.1, 133.4, 133.2, 129.0(2C), 127.9, 126.7(3C), 115.7, 112.4, 110.3, 62.3, 62.0, 47.9, 20.9, 14.2, 14.1. HRMS (ESI) calcd. for C24H24N3O5 (M+H)+: 434.1710, found 434.1716. HPLC retention time 4.69 min, purity 98.8%.
Diethyl 6-benzyl-9-methoxy-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4n). White solid, 95% yield, mp: 116–117 °C. 1H NMR (400 MHz, chloroform-d) δ 8.4 (s, 1H), 7.2 (d, J = 7.6 Hz, 2H), 7.2–7.1 (m, 3H), 7.1 (d, J = 9.3 Hz, 1H), 6.9 (d, J = 9.3 Hz, 1H), 5.5 (s, 2H), 4.4 (dq, J = 19.4, 6.7 Hz, 4H), 3.8 (s, 3H), 1.4 (dt, J = 14.9, 7.0 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.7, 162.3, 155.8, 149.2, 145.1, 140.1, 135.0, 129.5, 129.0(2C), 127.9, 126.7(2C), 120.3, 117.0, 113.3, 109.9, 109.5, 62.3, 62.0, 55.7, 48.0, 14.2, 14.0. HRMS (ESI) calcd. for C24H24N3O6 (M+H)+: 450.1660, found 450.1660. HPLC retention time 4.39 min, purity 98.5%.
Diethyl 6-benzyl-7-fluoro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4o). White–pink solid, 92% yield, mp: 149–150 °C. 1H NMR (400 MHz, chloroform-d) δ 8.5 (d, J = 5.5 Hz, 1H), 7.2 (d, J = 2.2 Hz, 1H), 7.2–7.2 (m, 5H), 7.2 (d, J = 2.2 Hz, 1H), 5.7 (s, 2H), 4.5–4.3 (m, 4H), 1.4 (dt, J = 13.2, 7.4 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.9, 161.7, 148.8, 145.5, 136.2, 128.7(2C), 127.7, 126.6(2C), 125.3, 125.2, 122.8, 122.8, 120.3, 120.0, 115.2, 111.3, 62.4, 62.3, 51.0, 14.2, 14.0. HRMS (ESI) calcd. for C23H21FN3O5 (M+H)+: 438.1460, found 438.1456. HPLC retention time 4.32 min, purity 97.0%.
Diethyl 6-benzyl-8,10-difluoro-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4p). Light yellow solid, 87% yield, mp: 194–196 °C. 1H NMR (400 MHz, chloroform-d) δ 7.4 (d, J = 7.8 Hz, 1H), 7.3–7.3 (m, 2H), 7.3 (d, J = 3.7 Hz, 2H), 6.9 (d, J = 11.4 Hz, 1H), 6.8–6.8 (m, 1H), 5.5 (s, 2H), 4.5 (q, J = 7.3 Hz, 4H), 1.5–1.4 (m, 6H). 13C NMR (100 MHz, chloroform-d) δ 163.5, 160.6, 160.3, 157.79, 145.9, 145.0, 137.8, 134.0, 132.5, 129.3(2C), 128.4, 126.6(2C), 115.0, 100.5, 100.3, 99.9, 62.4, 62.3, 48.9, 14.2, 14.1. HRMS (ESI) calcd. for C23H20F2N3O5 (M+H)+: 456.1366, found 456.1363. HPLC retention time 4.09 min, purity 97.6%.
Diethyl 6-methyl-5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4q). Orange solid, 95% yield, mp: 117–119 °C. 1H NMR (400 MHz, chloroform-d) δ 8.8 (d, J = 8.2 Hz, 1H), 7.6 (t, J = 7.9 Hz, 1H), 7.4–7.3 (m, 2H), 4.5–4.4 (m, 4H), 3.8 (s, 3H), 1.4 (td, J = 8.2, 6.0 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.9, 162.1, 148.7, 144.8, 139.8, 136.4, 132.3, 127.0, 124.2, 114.8, 112.5, 110.3, 62.3, 62.0, 31.8, 14.2, 14.0. HRMS (ESI) calcd. for C17H18N3O5 (M+H)+: 344.1241, found 344.1244. HPLC retention time 3.22 min, purity 97.0%.
6-(tert-butyl) 1,2-diethyl 5-oxopyrazolo[1,5-c]quinazoline-1,2,6(5H)-tricarboxylate (4r). Light yellow solid, 95% yield, mp: 181–182 °C. 1H NMR (400 MHz, chloroform-d) δ 8.8 (d, J = 8.2 Hz, 1H), 7.6 (t, J = 7.9 Hz, 1H), 7.4 (t, J = 7.7 Hz, 1H), 7.2 (d, J = 8.4 Hz, 1H), 4.4 (q, J = 7.2 Hz, 4H), 1.7 (s, 9H), 1.4 (q, J = 7.2 Hz, 6H). 13C NMR (100 MHz, chloroform-d) δ 162.8, 161.8, 149.1, 148.3, 142.22, 140.1, 133.3, 132.3, 127.0, 125.0, 114.6, 111.9, 111.25, 88.6, 62.4, 62.2, 27.6(3C), 14.2, 14.0. HRMS (ESI) calcd. for C21H24N3O7 (M-100+H)+: 330.1084, found 330.1087. HPLC retention time 2.28 min, purity 98.9%.
Diethyl 5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4s). White solid, 96% yield, mp: 184–186 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.34 (s, 1H), 8.46 (dd, J = 8.1, 1.3 Hz, 1H), 7.61 (ddd, J = 8.4, 7.3, 1.4 Hz, 1H), 7.38 (d, J = 7.2 Hz, 1H), 7.32 (ddd, J = 8.3, 7.3, 1.2 Hz, 1H), 4.37 (qd, J = 7.1, 5.1 Hz, 4H), 1.32 (dt, J = 11.2, 7.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.1, 162.3, 147.2, 144.1, 141.1, 136.2, 132.5, 125.7, 123.9, 116.7, 111.7, 109.8, 62.4, 62.2, 14.5, 14.3. HRMS (ESI) calcd. for C16H16N3O5 (M+H)+: 330.1084, found 330.1089. HPLC retention time 4.23 min, purity 95.0%.
Dimethyl 5-oxo-5,6-dihydropyrazolo[1,5-c]quinazoline-1,2-dicarboxylate (4t). Yellow solid, 92% yield, mp: 248–250 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.3 (s, 1H), 8.4 (d, J = 8.1 Hz, 1H), 7.6 (td, J = 7.8, 7.2, 1.3 Hz, 1H), 7.4 (d, J = 8.3 Hz, 1H), 7.3 (t, J = 7.7 Hz, 1H), 3.9 (s, 3H), 3.9 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 163.6, 162.6, 146.7, 144.0, 141.1, 136.1, 132.5, 125.5, 123.9, 116.7, 111.6, 109.8, 53.5, 53.4. HRMS (ESI) calcd. for C14H12N3O5 (M+H)+: 302.0771, found 302.0774. HPLC retention time 3.26 min, purity 98.1%.
6-benzyl-9-fluoro-1,2-diphenylpyrazolo[1,5-c]quinazolin-5(6H)-one (4u). White–pink solid, 33% yield, mp: 232–233 °C. 1H NMR (400 MHz, chloroform-d) δ 7.6 (d, J = 7.1 Hz, 2H), 7.5–7.5 (m, 3H), 7.5–7.4 (m, 2H), 7.4–7.3 (m, 4H), 7.3–7.3 (m, 4H), 7.2 (dd, J = 8.9, 4.3 Hz, 1H), 7.0 (q, J = 9.0 Hz, 2H), 5.6 (s, 2H). 13C NMR (100 MHz, chloroform-d) δ 154.9, 146.1, 135.5, 132.2, 131.7, 131.6, 130.6(2C), 129.7(2C), 129.1(2C), 128.9, 128.8(2C), 128.3(2C), 127.9, 126.7(2C), 118.2, 117.5(2C), 117.4, 117.3, 110.3, 110.0, 48.1. HRMS (ESI) calcd. for C29H21FN3O (M+H)+: 446.1663, found 446.1660. HPLC retention time 5.07 min, purity 99.3%.
1,2-diphenylpyrazolo[1,5-c]quinazolin-5(6H)-one (4v). Yellow solid, 26% yield, mp: 189–190 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.0 (s, 1H), 7.6–7.5 (m, 3H), 7.5–7.4 (m, 5H), 7.3 (d, J = 8.3 Hz, 1H), 7.3 (d, J = 5.9 Hz, 3H), 7.1 (d, J = 8.1 Hz, 1H), 7.0 (t, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 153.2, 144.9, 137.7, 135.3, 133.0, 132.4, 131.1(2C), 130.6, 130.0(2C), 129.2, 129.1, 128.9(2C), 128.4(2C), 123.3, 122.9, 117.1, 116.5, 113.1. HRMS (ESI) calcd. for C22H16N3O (M+H)+: 338.1288, found 338.1286. HPLC retention time 4.52 min, purity 98.4%.
4.2. Biology and In Silico Study Methods
The experimental details are presented in Supplementary Materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28186606/s1. Table S1. Green synthesis of 4k/4o (page 2). Table S2. Cell growth inhibition of the pyrazolo-[1,5-c]quinazolinone derivatives on A549 cells, MDA-MB-231 cells, U87 cells, and HepG2 cells (30 μM) (page 3). Figure S1. Compound IC50 data for CDK9 inhibition (page 5). Figure S2. The ProTox-II toxicity profiles for compounds 4a–4v (page 6). Figure S3. Copies of NMR spectra and HRMS spectra for compounds 4a–4v (page 14). Figure S4. Copies of HPLC analysis for products 4a–4v (page 58). Biology methods (page 69). In silico study methods (page 70).
Author Contributions
Conceptualisation, Y.L. and J.X.; synthetic work and data curation, D.Z., C.Y. and Y.Z.; biological experiments and interpretation of results, X.L., D.L. and Y.W.; chemical characterisation, Y.T. and X.W.; project administration and supervision, X.Z.; validation, Y.W.; writing—original draft, D.Z. and C.Y.; writing—review and editing, Y.L. and J.X.; funding acquisition, C.Y. and J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the National Natural Science Foundation of China (no. 82003587), Natural Science Foundation of Hainan province (no. 820QN265), Research start-up funding project of Hainan Medical College and College Student Innovation and Entrepreneurship Training Program of Hainan province (S202111810010), and the Hainan Graduate of College Innovation and Entrepreneurship Training Program (no. Qhys2022-299 and Qhys2022-297).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Wuxi Biortus Biosciences Co. Ltd. and Li Zhang of Jinan University for their invaluable technical guidance and assistance in conducting biological testing.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compound are available from the authors.
References
- Haider, K.; Das, S.; Joseph, A.; Yar, M.S. An appraisal of anticancer activity with structure–activity relationship of quinazoline and quinazolinone analogues through EGFR and VEGFR inhibition: A review. Drug Dev. Res. 2022, 83, 859–890. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinasab, R.; Jafari, E.; Khodarahmi, G. Quinazolinone-based hybrids with diverse biological activities: A mini-review. J. Res. Med. Sci. 2022, 27, 68. [Google Scholar] [CrossRef]
- Nowar, R.M.; Osman, E.E.A.; Abou-Seri, S.M.; El Moghazy, S.M.; El Ella, D.A.A. Design, synthesis and biological evaluation of some novel quinazolinone derivatives as potent apoptotic inducers. Future Med. Chem. 2018, 10, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, C.; Tang, L.; Liu, Z. Discovery of novel quinazolinone derivatives as high potent and selective PI3Kδ and PI3Kδ/γ inhibitors. Eur. J. Med. Chem. 2018, 151, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Resende, D.I.S.P.; Kijjoa, A.; Silva, A.M.S.; Pina, A.; Fernández-Marcelo, T.; Vasconcelos, M.H.; Sousa, E.; Pinto, M.M.M. Antitumor Activity of Quinazolinone Alkaloids Inspired by Marine Natural Products. Mar. Drugs 2018, 16, 261. [Google Scholar] [CrossRef]
- Sun, J.; Fang, Z.-Y.; Tao, Y.-N.; Zhang, Y.-H.; Zhang, Y.; Sun, H.-Y.; Zhou, Y.; Wu, Y.-F. Design, Synthesis and Antitumor Activity of FAK/PLK1 Dual Inhibitors with Quinazolinone as the Skeleton. Chem. Biodivers. 2023, 20, e202300146. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Gan, Y.; Fu, Y.; Liu, J.; Li, W.; Zou, X.; Zhou, Z.; Wang, Z.; Ouyang, G.; Yan, L. Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J. Enzyme Inhib. Med. Chem. 2020, 35, 555–564. [Google Scholar] [CrossRef]
- Osman, E.O.; Emam, S.H.; Sonousi, A.; Kandil, M.M.; Abdou, A.M.; Hassan, R.A. Design, synthesis, anticancer, and antibacterial evaluation of some quinazolinone-based derivatives as DHFR inhibitors. Drug Dev. Res. 2023, 84, 888–906. [Google Scholar] [CrossRef]
- Wenthur, C.J.; Morrison, R.D.; Daniels, J.S.; Conn, P.J.; Lindsley, C.W. Synthesis and SAR of substituted pyrazolo[1,5-a]quinazolines as dual mGlu2/mGlu3 NAMs. Bioorg. Med. Chem. Lett. 2014, 24, 2693–2698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Purcell, J.W.; Davis, J.; Reddy, M.; Martin, S.; Samayoa, K.; Vo, H.; Thomsen, K.; Bean, P.; Kuo, W.L.; Ziyad, S.; et al. Activity of the Kinesin Spindle Protein Inhibitor Ispinesib (SB-715992) in Models of Breast Cancer. Clin. Cancer Res. 2010, 16, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Shahin, R.; Aljamal, S. Kinesin spindle protein inhibitors in cancer: From high throughput screening to novel therapeutic strategies. Future Sci. OA 2022, 8, FSO778. [Google Scholar] [CrossRef] [PubMed]
- Chamariya, R.; Suvarna, V. Role of KSP Inhibitors as Anti-Cancer Therapeutics: An Update. Anticancer. Agents Med. Chem. 2022, 22, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Rigo, R.; Hannouf, M.B.; Cheung, W.Y. Real-world Safety and Efficacy of Raltitrexed in Patients With Metastatic Colorectal Cancer. Clin. Color. Cancer 2021, 20, E75–E81. [Google Scholar] [CrossRef] [PubMed]
- Avallone, A.; Gennaro, E.D.; Silvestro, L.; Iaffaioli, V.R.; Budillon, A. Targeting thymidylate synthase in colorectal cancer: Critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin. Drug Saf. 2014, 13, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, B.J.; Meadows, S.A.; Herman, S.E.M.; Kashishian, A.; Steiner, B.; Johnson, A.J.; Byrd, J.C.; Tyner, J.W.; Loriaux, M.M.; Deininger, M.; et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011, 117, 591–594. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.H.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.M.; Hassan, A.M.A.; Elkaeed, E.B.; Alesawy, M.S.; Al-Karmalawy, A.A. Design, synthesis, and SAR studies of novel 4-methoxyphenyl pyrazole and pyrimidine derivatives as potential dual tyrosine kinase inhibitors targeting both EGFR and VEGFR-2. Bioorg. Chem. 2022, 123, 105770. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Colotta, V.; Calabri, F.R.; Lenzi, O.; Filacchioni, G.; Galli, A.; Costagli, C.; Deflorian, F.; Moro, S. 1-Substituted pyrazolo[1,5-c]quinazolines as novel Gly/NMDA receptor antagonists: Synthesis, biological evaluation, and molecular modeling study. Biorg. Med. Chem. 2005, 13, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Colotta, V.; Filacchioni, G.; Galli, A.; Costagli, C.; Carlà, V. Synthesis and Biological Evaluation of a New Set of Pyrazolo[1,5-c]quinazoline-2-carboxylates as Novel Excitatory Amino Acid Antagonists. J. Med. Chem. 2002, 45, 1035–1044. [Google Scholar] [CrossRef]
- Catarzi, D.; Colotta, V.; Varano, F.; Poli, D.; Squarcialupi, L.; Filacchioni, G.; Varani, K.; Vincenzi, F.; Borea, P.A.; Ben, D.D.; et al. Pyrazolo[1,5-c]quinazoline derivatives and their simplified analogues as adenosine receptor antagonists: Synthesis, structure–affinity relationships and molecular modeling studies. Biorg. Med. Chem. 2013, 21, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.L.; Chheda, P.R.; Lentz, S.R.C.; Held, H.A.; Groves, N.P.; Hiasa, H.; Kerns, R.J. Identification of an ethyl 5,6-dihydropyrazolo[1,5-c]quinazoline-1-carboxylate as a catalytic inhibitor of DNA gyrase. Biorg. Med. Chem. 2020, 28, 115439. [Google Scholar] [CrossRef] [PubMed]
- Moro, S.; Varano, F.; Cozza, G.; Pagano, A.M.; Zagotto, G.; Chilin, A.; Guiotto, A.; Catarzi, D.; Calotta, V.; Pinna, A.L. Pyrazoloquinazoline Tricyclic System as Novel Scaffold to Design New Kinase CK2 Inhibitors. Lett. Drug Des. Discov. 2006, 3, 281–284. [Google Scholar] [CrossRef]
- Jacobson, K.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Garg, M.; Chauhan, M.; Singh, P.K.; Alex, J.M.; Kumar, R. Pyrazoloquinazolines: Synthetic strategies and bioactivities. Eur. J. Med. Chem. 2015, 97, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Hawash, M.; Ergun, S.G.; Kahraman, D.C.; Olgac, A.; Hamel, E.; Cetin-Atalay, R.; Baytas, S.N. Novel indole-pyrazole hybrids as potential tubulin-targeting agents; Synthesis, antiproliferative evaluation, and molecular modeling studies. J. Mol. Struct. 2023, 1285, 135477. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Kahraman, D.C.; Ergun, S.G.; Cetin-Atalay, R.; Baytas, S.N. Synthesis of novel indole-isoxazole hybrids and evaluation of their cytotoxic activities on hepatocellular carcinoma cell lines. BMC Chem. 2021, 15, 66. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Wang, X.; Huang, J.; Li, S.; Liu, C.; Dong, R.; Zhu, G.; Duan, C.; Jiang, F.; et al. Discovery of coumarin derivatives as potent and selective cyclin-dependent kinase 9 (CDK9) inhibitors with high antitumour activity. Eur. J. Med. Chem. 2020, 200, 112424. [Google Scholar] [CrossRef]
- Chen, Q.; Li, K.; Lu, T.; Zhou, Q. Phosphine-catalyzed domino reactions of alkynyl ketones with sulfonylhydrazones: Construction of diverse pyrazoloquinazoline derivatives. RSC Adv. 2016, 6, 24792–24796. [Google Scholar] [CrossRef]
- Ramu, G.; Krishna, N.H.; Pawar, G.; Sastry, K.N.V.; Nanubolu, J.B.; Babu, B.N. Solvent-Controlled, Tunable Domino Reaction of 3-Ylideneoxindoles with in Situ-Generated α-Aryldiazomethanes: A Facile Access to 3-Spirocyclopropyl-2-oxindole and Pyrazoloquinazolinone Scaffolds. ACS Omega 2018, 3, 12349–12360. [Google Scholar] [CrossRef] [PubMed]
- Ramu, G.; Tangella, Y.; Ambala, S.; Babu, B.N. Regioselective Ring Expansion of 3-Ylideneoxindoles with Tosyldiazomethane (TsDAM): A Metal-Free and Greener Approach for the Synthesis of Pyrazolo-[1,5-c]quinazolines. J. Org. Chem. 2020, 85, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Y.; Wang, J.-S.; Zhao, J.; Long, L.; Cui, B.-D.; Wan, N.-W.; Chen, Y.-Z. A Protocol for the Synthesis of CF2H-Containing Pyrazolo[1,5-c]quinazolines from 3-Ylideneoxindoles and in Situ Generated CF2HCHN2. J. Org. Chem. 2018, 83, 6556–6565. [Google Scholar] [CrossRef]
- Barluenga, J.; Tomás-Gamasa, M.; Aznar, F.; Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 2009, 1, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Kurma, S.H.; Sridhar, B.; Bhimapaka, C.R. Direct Access for the Regio- and Stereoselective Synthesis of N-Alkenylpyrazoles and Chromenopyrazoles. J. Org. Chem. 2021, 86, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.-Z.; Liu, Y.-F.; Cao, J.-Q.; Wang, X.-D.; Zhang, X.-S.; Zhao, C.; Zhao, Y.-Q. Novel 25-hydroxyprotopanaxadiol derivatives incorporating chloroacetyl chloride and their anti-tumor evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 5390–5394. [Google Scholar] [CrossRef]
- Shen, Z.; Yan, Y.-H.; Yang, S.; Zhu, S.; Yuan, Y.; Qiu, Z.; Jia, H.; Wang, R.; Li, G.-B.; Li, H. ProfKin: A comprehensive web server for structure-based kinase profiling. Eur. J. Med. Chem. 2021, 225, 113772. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gan, Y.C.; Li, H.Z.; Yin, J.; He, X.; Lin, L.M.; Xu, S.L.; Fang, Z.P.; Kim, B.W.; Gao, L.N.; et al. Inhibition of the CDK2 and Cyclin A complex leads to autophagic degradation of CDK2 in cancer cells. Nat. Commun. 2022, 13, 2835. [Google Scholar] [CrossRef]
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting CDK2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 2020, 25, 406–413. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Huang, J.; Liu, C.; Li, H.; Wang, C.; Hong, Q.; Lei, Y.; Xia, J.; Yu, Z.; et al. Discovery of 2H-benzo[b][1,4]oxazin-3(4H)-one derivatives as potent and selective CDK9 inhibitors that enable transient target engagement for the treatment of hematologic malignancies. Eur. J. Med. Chem. 2022, 238, 114461. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Wang, C.; Ma, Y.; Wang, T.; Li, Y.; Huang, Z.; Zhou, M.; Sun, P.; Zheng, J.; et al. Novel cyclin-dependent kinase 9 (CDK9) inhibitor with suppression of cancer stemness activity against non-small-cell lung cancer. Eur. J. Med. Chem. 2019, 181, 111535. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.; von Karstedt, S.; El Hay, M.A.; Conti, A.; Arce, F.; Montinaro, A.; Papenfuss, K.; El-Bahrawy, M.A.; Walczak, H. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2014, 21, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Sander, T. OSIRIS Property Explorer. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 20 May 2023).
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Çapan, İ.; Hawash, M.; Jaradat, N.; Sert, Y.; Servi, R.; Koca, İ. Design, synthesis, molecular docking and biological evaluation of new carbazole derivatives as anticancer, and antioxidant agents. BMC Chem. 2023, 17, 60. [Google Scholar] [CrossRef]
- Hawash, M.; Qaoud, M.T.; Jaradat, N.; Abdallah, S.; Issa, S.; Adnan, N.; Hoshya, M.; Sobuh, S.; Hawash, Z. Anticancer Activity of Thiophene Carboxamide Derivatives as CA-4 Biomimetics: Synthesis, Biological Potency, 3D Spheroid Model, and Molecular Dynamics Simulation. Biomimetics 2022, 7, 247. [Google Scholar] [CrossRef]
- Liu, J.; Mallick, S.; Xie, Y.; Grassin, C.; Lucas, B.; Scholermann, B.; Pahl, A.; Scheel, R.; Strohmann, C.; Protzel, C.; et al. Morphological Profiling Identifies the Motor Protein Eg5 as Cellular Target of Spirooxindoles. Angew. Chem. Int. Ed. 2023, 62, e202301955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).