Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays
Abstract
1. Introduction
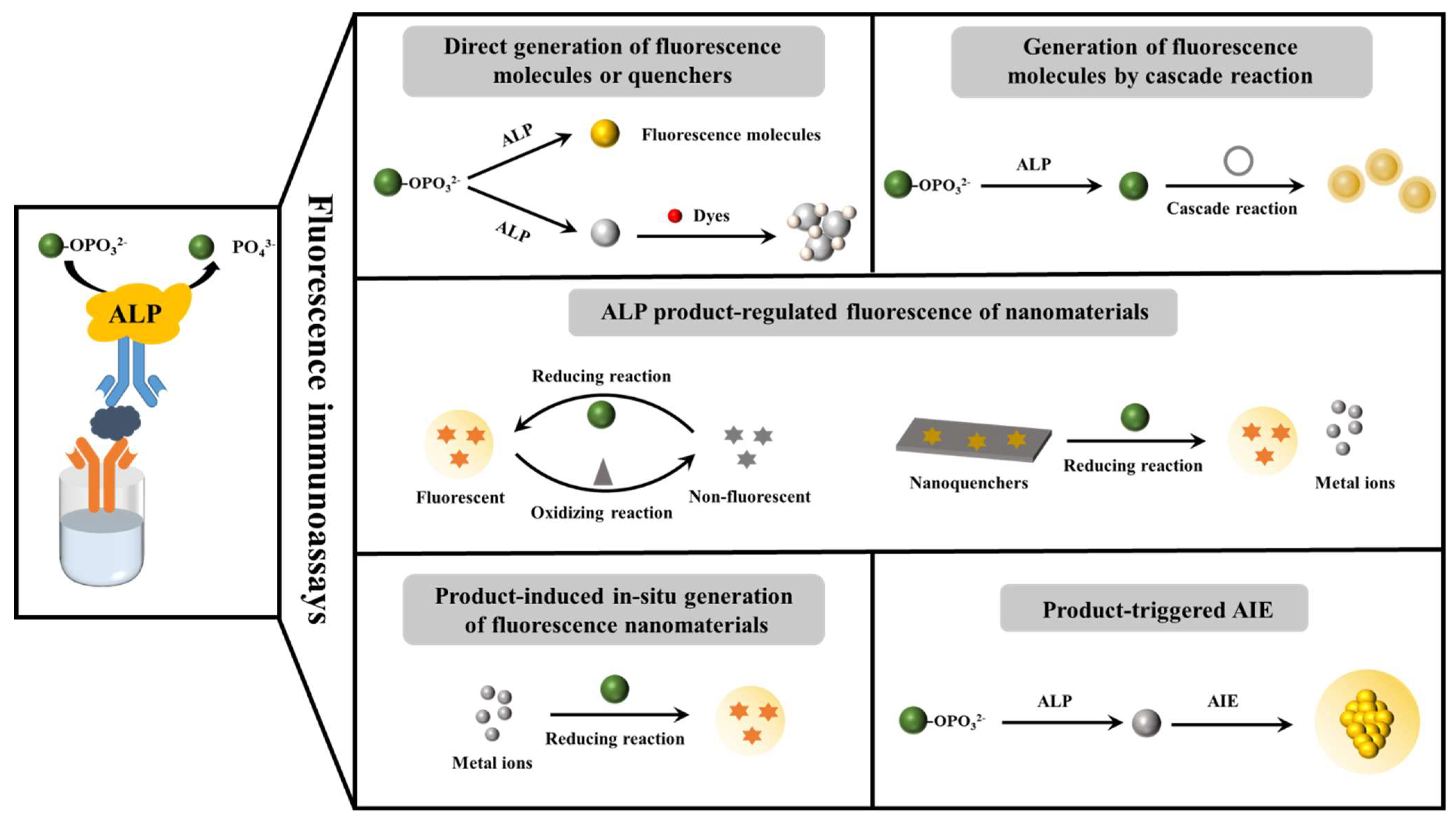
2. Fluorescence Immunoassays
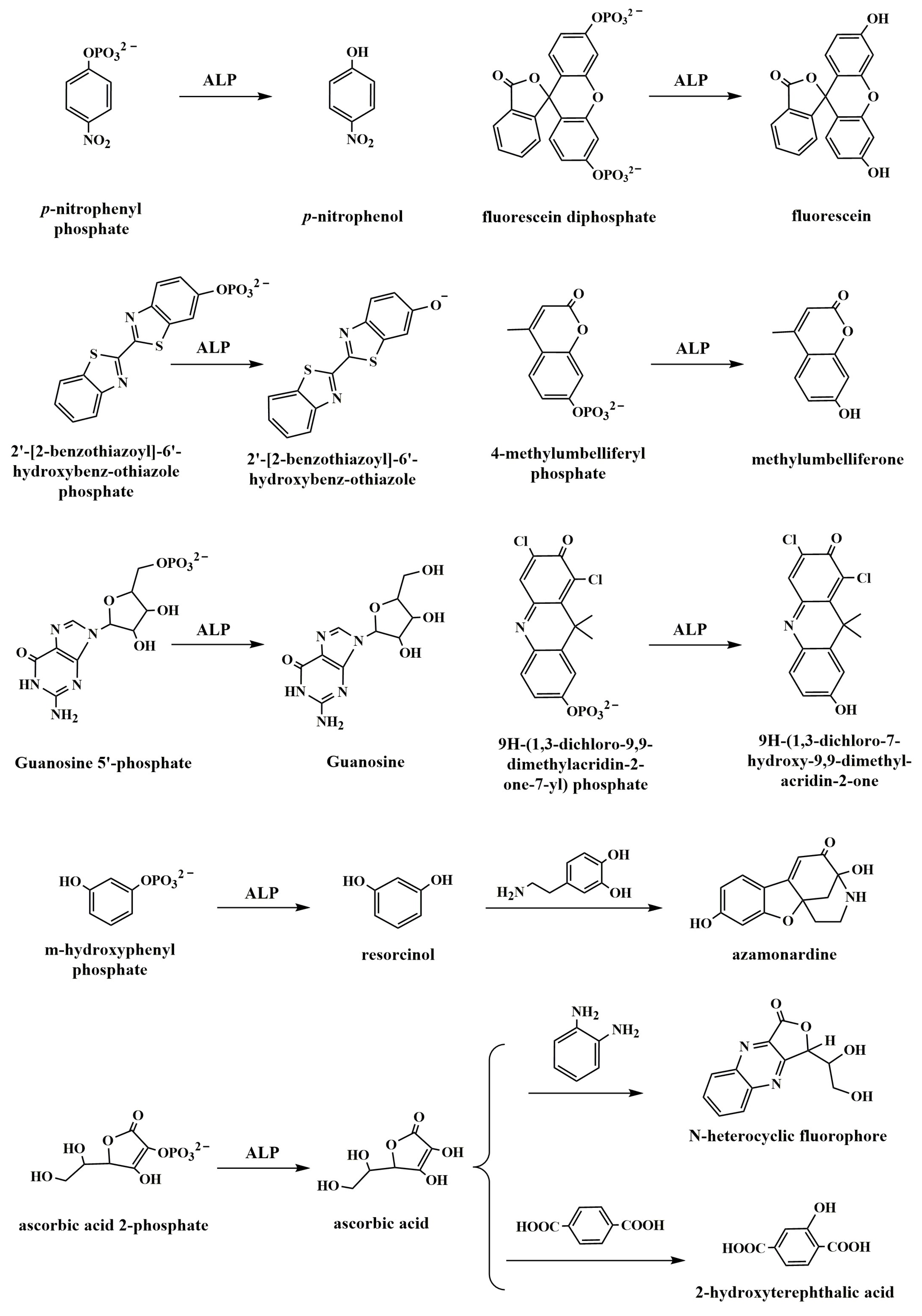
2.1. Direct Generation of Fluorescent Molecules or Quenchers
2.2. Generation of Fluorescent Molecules through Chemical Reaction or Enzymatic Cascade Reaction
2.3. Enzymatic-Product-Regulated Fluorescence of Nanomaterials
2.4. Enzymatic-Product-Induced In Situ Generation of Fluorescent Nanomaterials
2.5. Enzymatic-Product-Triggered AIE Phenomenon
| Detection Principle | ALP Substrates | Fluorescence Reporters | Target | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Direct generation of fluorescent molecules or quenchers | DDAO phosphate | DDAO | C-reactive protein | 0.1–1000 ng/mL | 58 pg/mL | [54] |
| DDAO phosphate | DDAO | AIV H5-HA | 0.23–100 ng/mL | 0.23 ng/mL | [57] | |
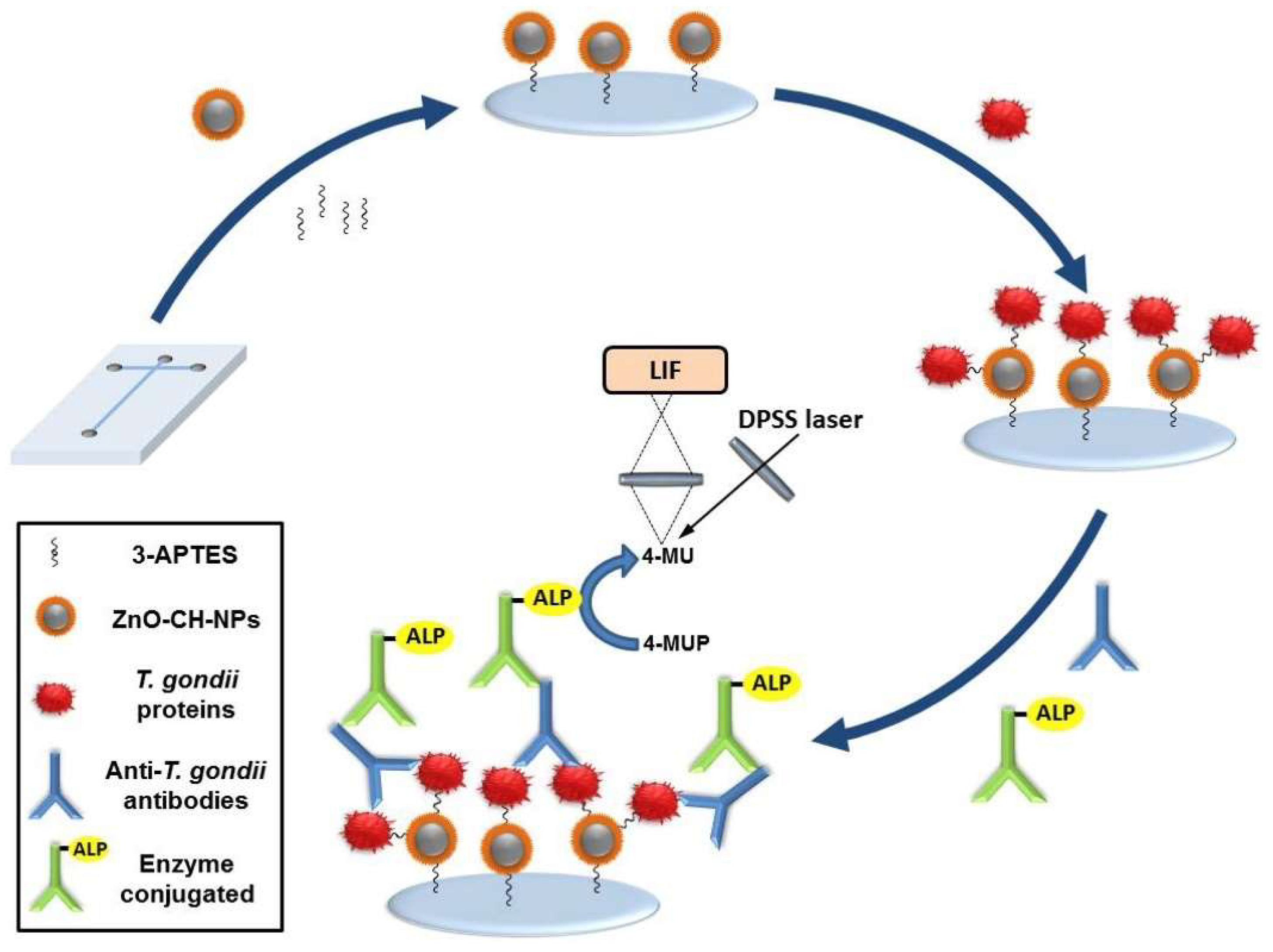
| 4-MUP | 4-MU | Anti-T-gondii IgG antibodies | 0–200 U/mL | 0.39 mU/mL | [59] | |
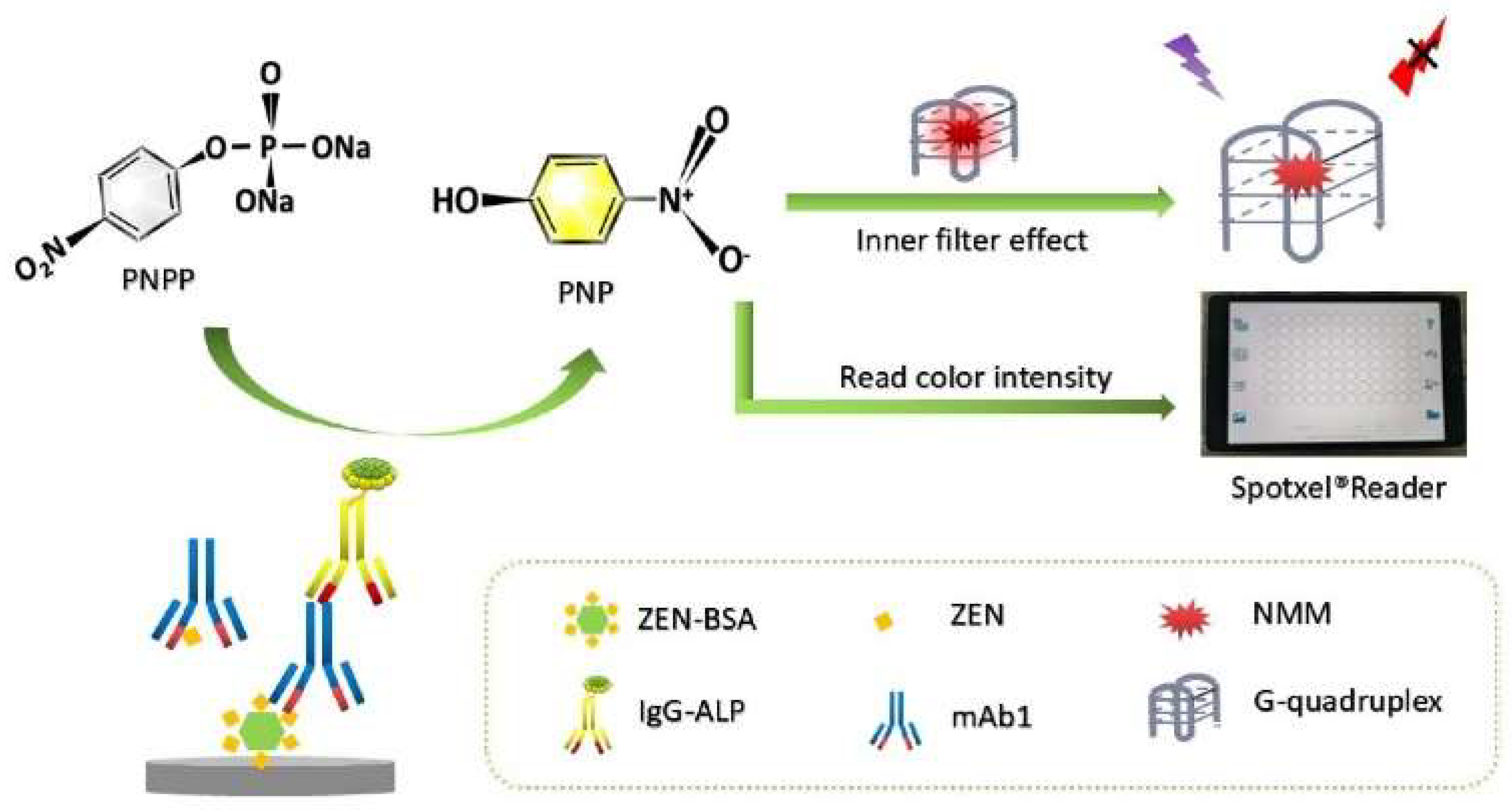
| PNPP | G4/NMM | Zearalenone | 7.5–17.5 ng/mL | 36 pg/mL | [64] | |
| Generation of fluorescent molecules through chemical reaction or enzymatic cascade reaction | AAP | N-heterocyclic fluorophore | AFP | 0.5–40 ng/mL | 0.21 ng/mL | [67] |
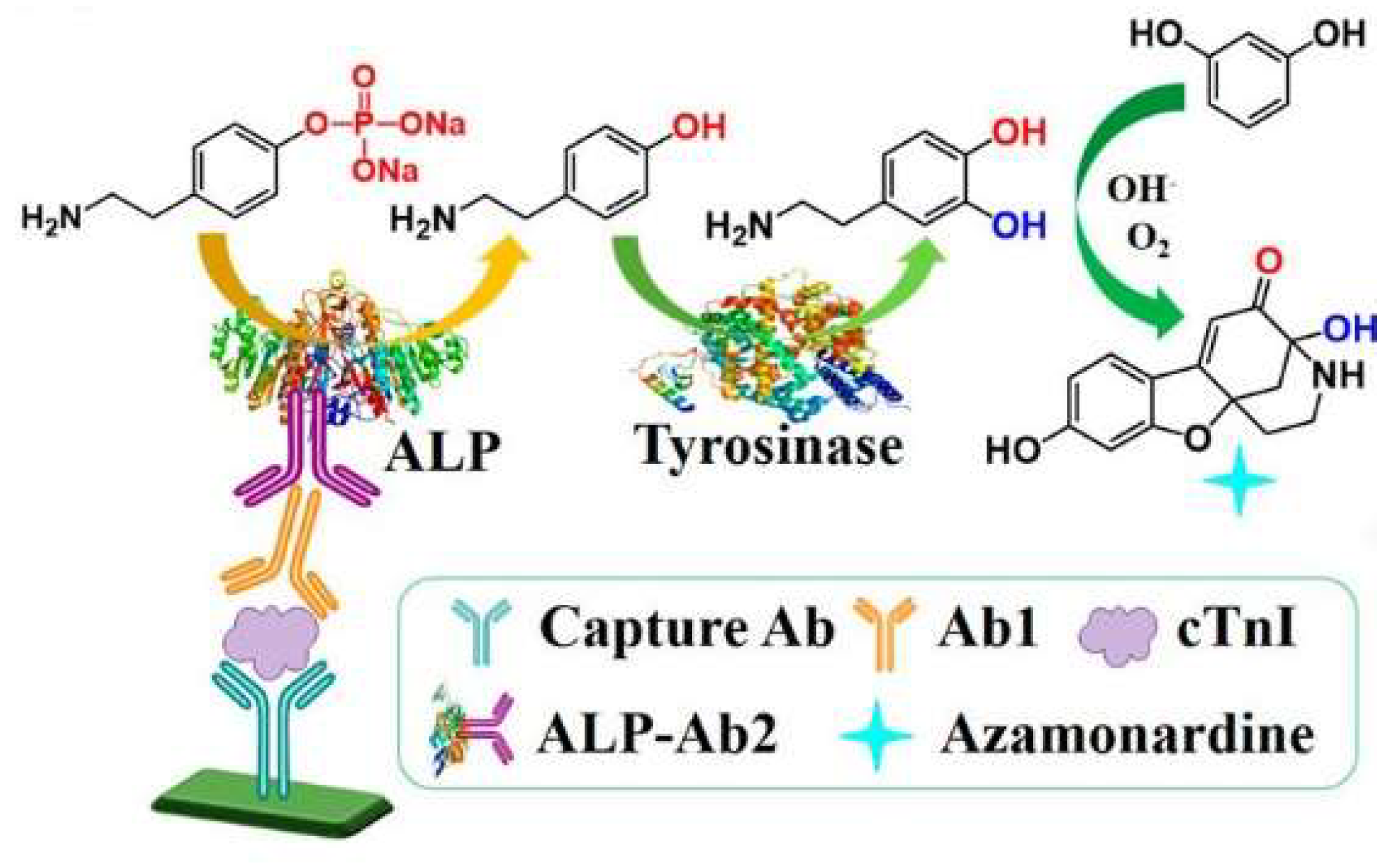
| m-HPP | Azamonardine | cTnI | 0.125–8 ng/mL | 40 pg/mL | [68] | |
| AAP | PTA-OH | CEA | 0.25–30 ng/mL | 0.08 ng/mL | [69] | |
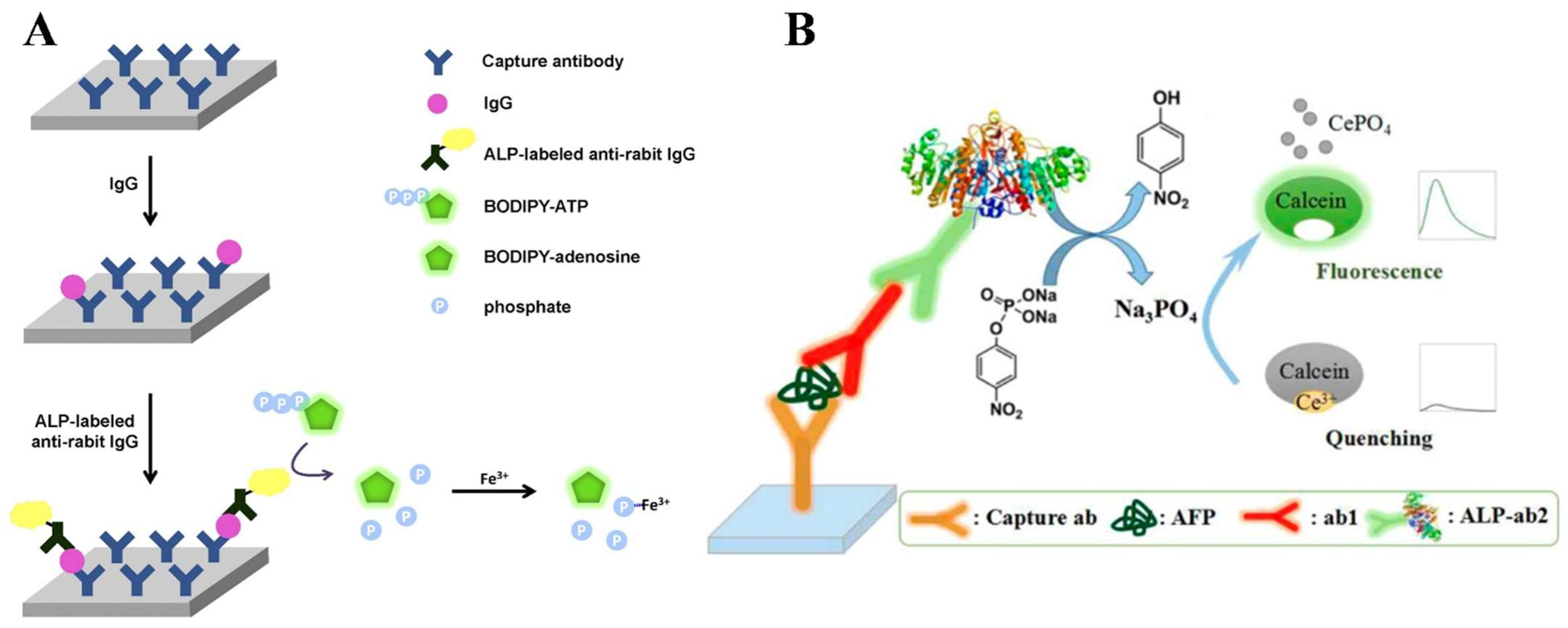
| BODIPY-ATP | BODIPY | IgG | 0–200 ng/mL | 5 ng/mL | [72] | |
| PNPP | Calcein | AFP | 0.2–1 ng/mL | 41 pg/mL | [74] | |
| PAPP | Azamonardine | cTnI | 0.05–4 ng/mL | 15 pg/mL | [75] | |
| Enzymatic-product-regulated fluorescence of nanomaterials | GMP | ThT@GMP/Eu | Mouse IgG | 0.8–100 ng/mL | 0.16 ng/mL | [82] |
| PNPP | CDs | Aflatoxin M1 | 0.003–0.81 ng/mL | 18.6 pg/mL | [86] | |
| AAP | AuNCs | Escherichia coli O157:H7 | 3.3 × 103–3.3 × 106 cfu/mL | 920 cfu/mL | [87] | |
| AAP | CdTe QDs | HIV-1 p24 antigen | 1–100 pg/mL | 0.2 pg/mL | [93] | |
| AAP | CDs | Human IgG | 40 ng/mL–4 μg/mL | 150 pg/mL | [94] | |
| AAP | CdSe QDs | Ethyl carbamate | 100 ng/mL–10 μg/mL | 24.3 ng/mL | [95] | |
| AAP | AuNCs | Mouse IgG | 0.005–50 ng/mL | 1.5 pg/mL | [97] | |
| AAP | CDs | Aflatoxin B1 | 1 ng/kg–1 μg/kg | 0.69 ng/kg | [109] | |
| Enzymatic-product-induced in situ generation of fluorescent nanomaterials | PAPP | Si CNPs | PSA | 0.02–20 ng/mL | 4.1 pg/mL | [112] |
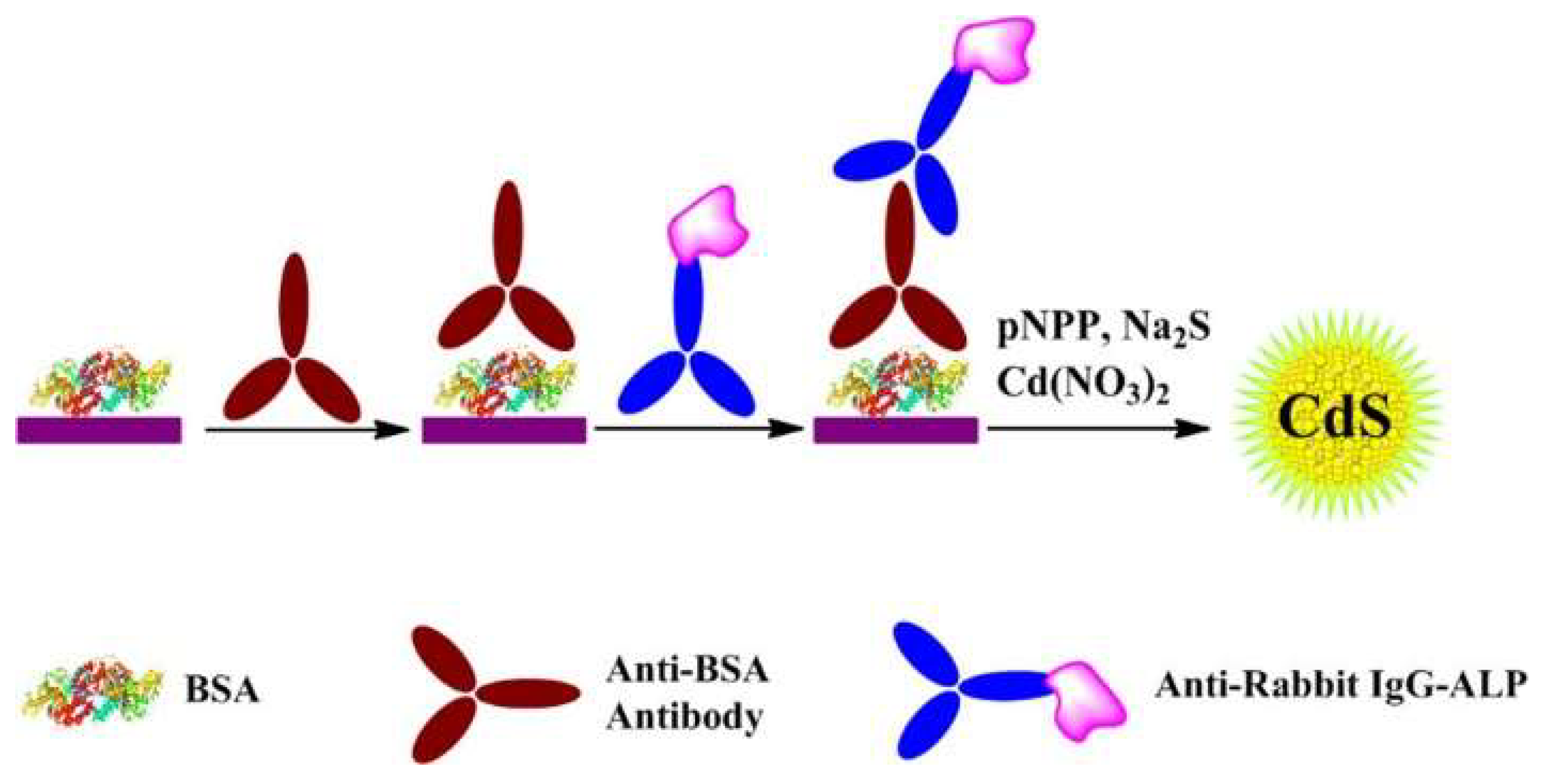
| PNPP | CdS QDs | Anti-BSA Antibody | 0–500 ng/mL | 0.4 ng/mL | [115] | |
| Enzymatic-product-triggered AIE phenomenon | AAP | Self-clickable TPE-based AIEgens | Rabbit anti-human IgG | 0–50 ng/mL | 1.2 ng/mL | [121] |
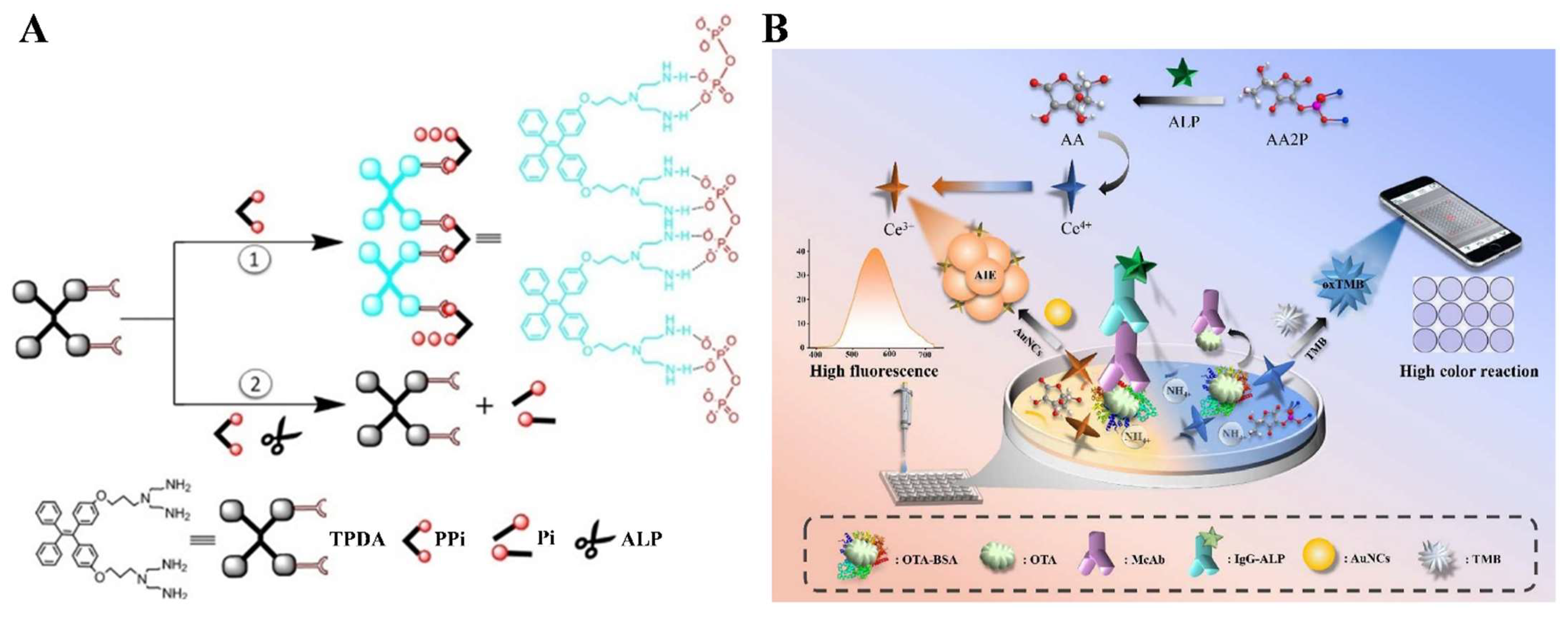
| AAP | AuNCs | Ochratoxin A | 0–500 ng/mL | 0.62 ng/mL | [122] |
3. Colorimetric Immunoassays
3.1. ALP-Catalyzed Production of Chromogenic Product
3.2. Enzymatic-Product-Triggered Chromogenic Reaction
3.3. Enzymatic-Product-Triggered Plasmonic Phenomenon
3.3.1. Enzymatic-Product-Induced Aggregation of Plasmonic NPs
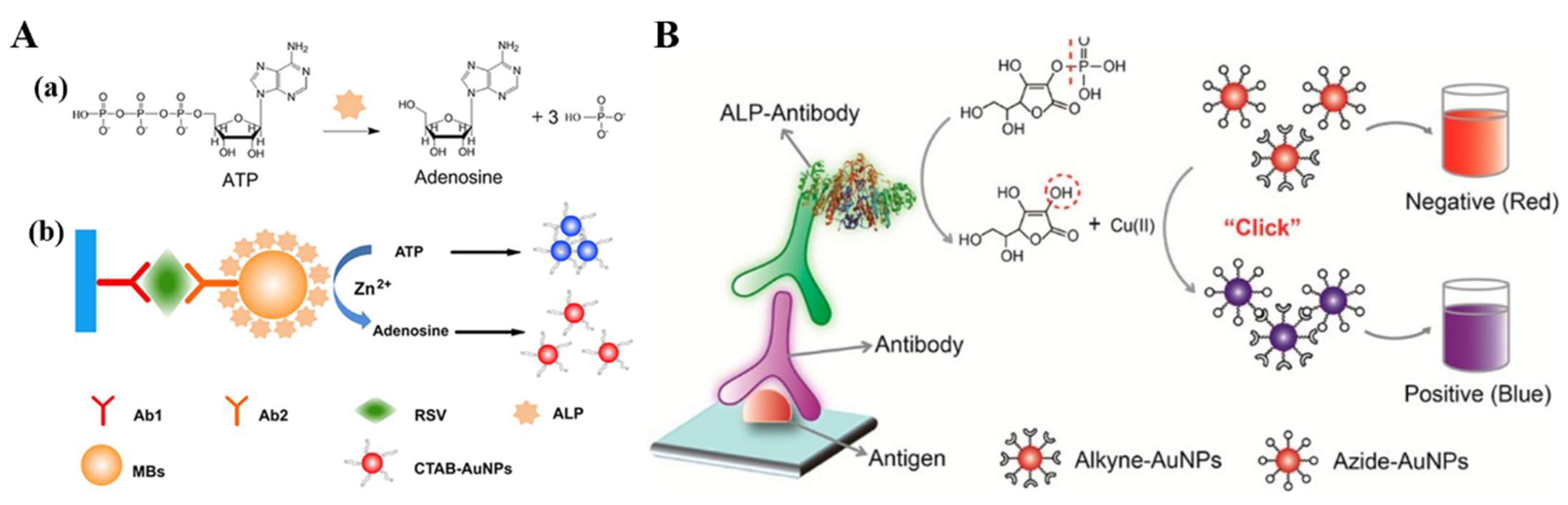
3.3.2. Enzymatic-Product-Induced In Situ Metallization or Bioetching of Plasmonic NPs
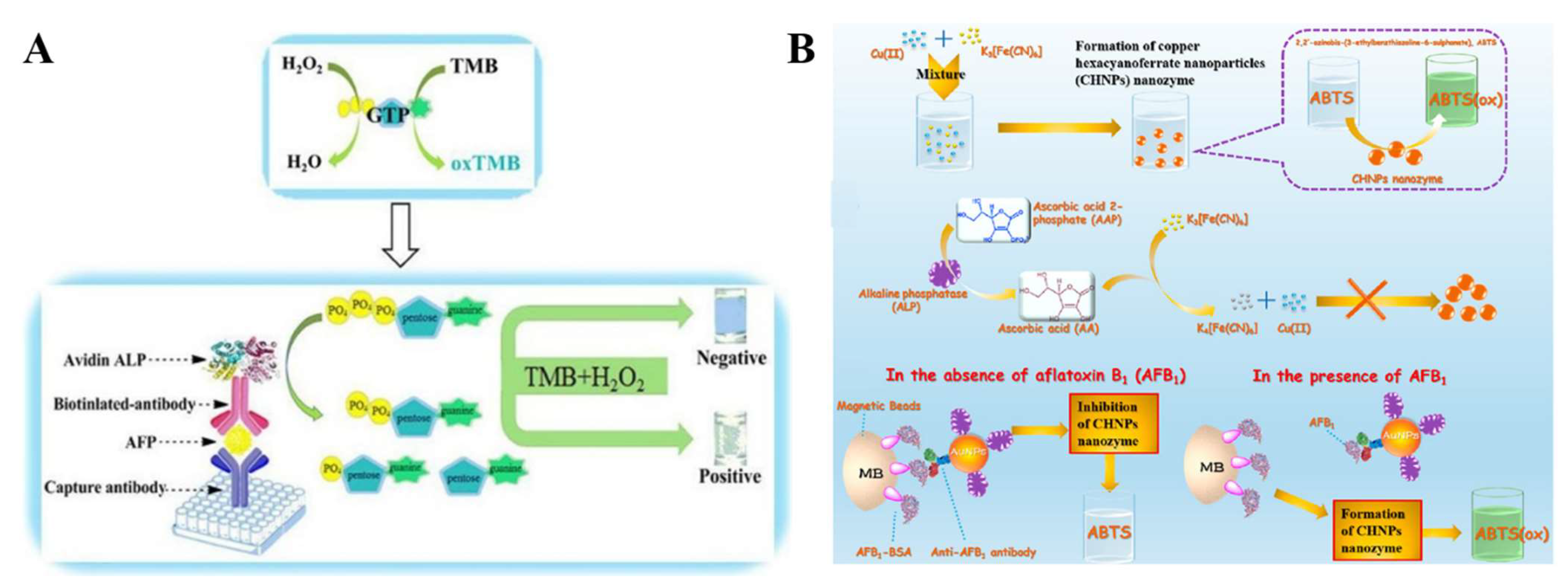
3.4. Enzymatic-Product-Mediated Activity Change of Artificial Enzymes or Nanozymes
| Detection Principle | ALP Substrates | Chromogenic Substrates/Reactions | Target | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| ALP-catalyzed production of chromogenic product | PNPP | PNPP | IgG | 0.5–400 ng/mL | 62.5 pg/mL | [126] |
| PNPP | PNPP | TNF-α | 0–10 ng/mL | 120 pg/mL | [127] | |
| 3-IP | 3-IP | Mouse IgG | 0.3–250 ng/mL | 0.3 ng/mL | [128] | |
| PNPP | PNPP | 2-Deoxycytidine | 10–1000 μM | Not reported | [129] | |
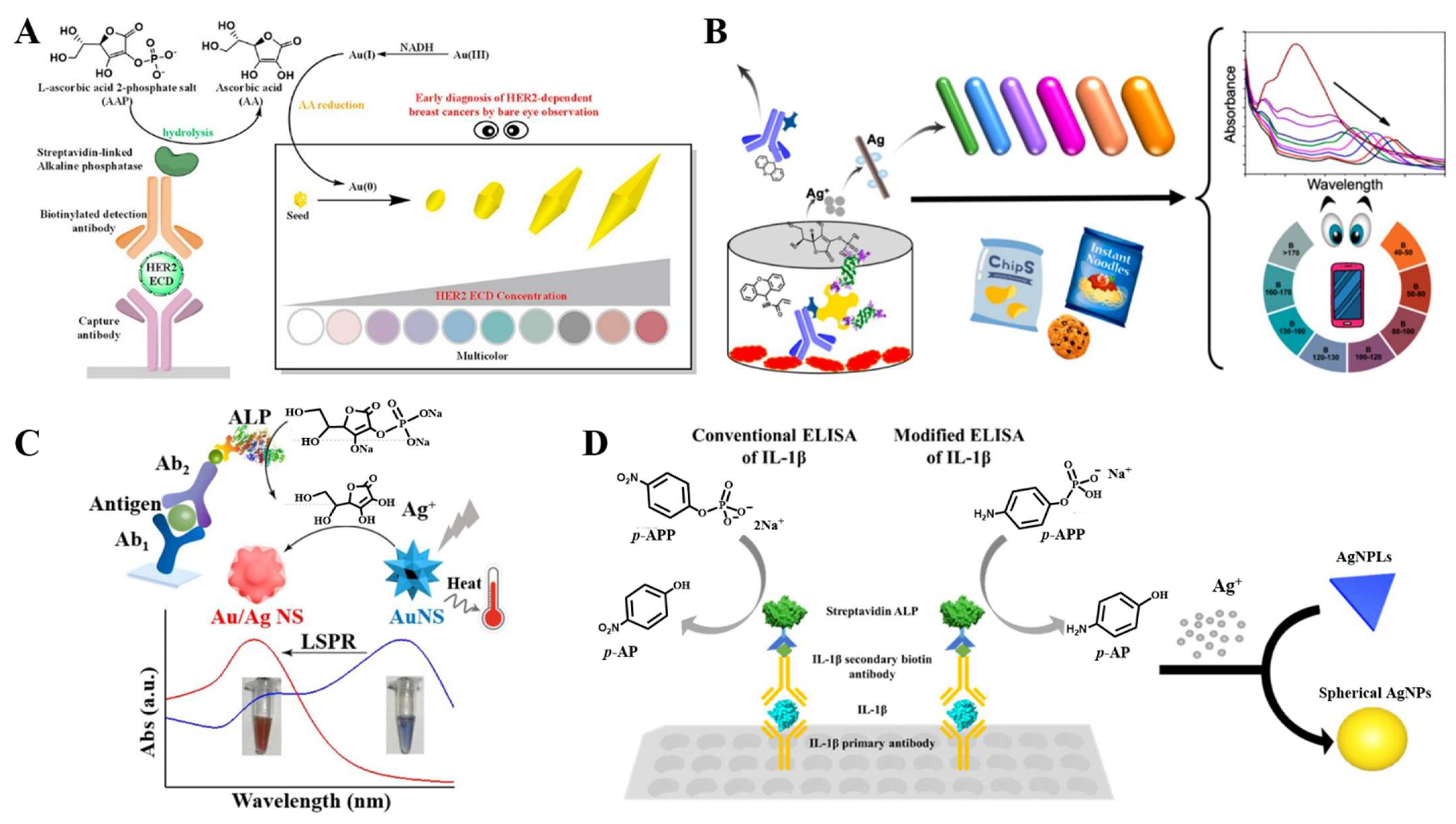
| Enzymatic-product-triggered chromogenic reaction | PAPP | The reaction between diethanolamine and PAP | AFP | 0.1–20 ng/mL | 0.1 ng/mL | [131] |
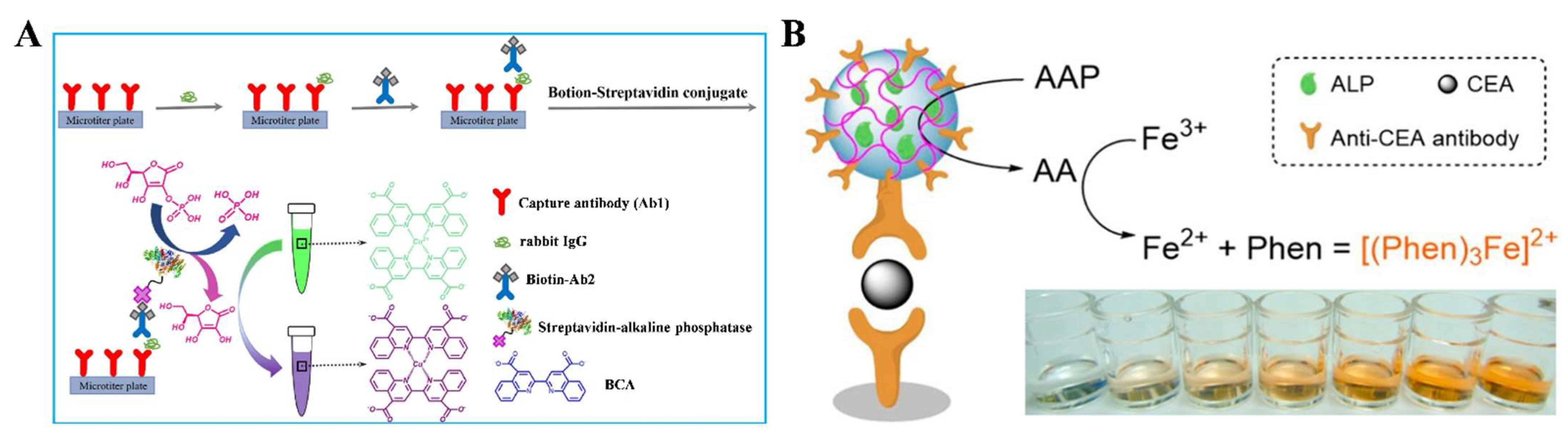
| AAP | Cu(I)-bicinchoninic complex | Rabbit IgG | 0.1–25 ng/mL | 0.05 ng/mL | [133] | |
| AAP | Fe(III)-phenanthroline complex | CEA | 0.05–100 ng/mL | 21.1 pg/mL | [134] | |
| AAP | Fe(III)- tris-(bathophenanthroline) complex | AFP | 0.01–5 ng/mL | 5 pg/mL | [135] | |
| APP | In situ formation of Prussian blue | PSA | 1–800 ng/mL | 1.2 ng/mL | [136] | |
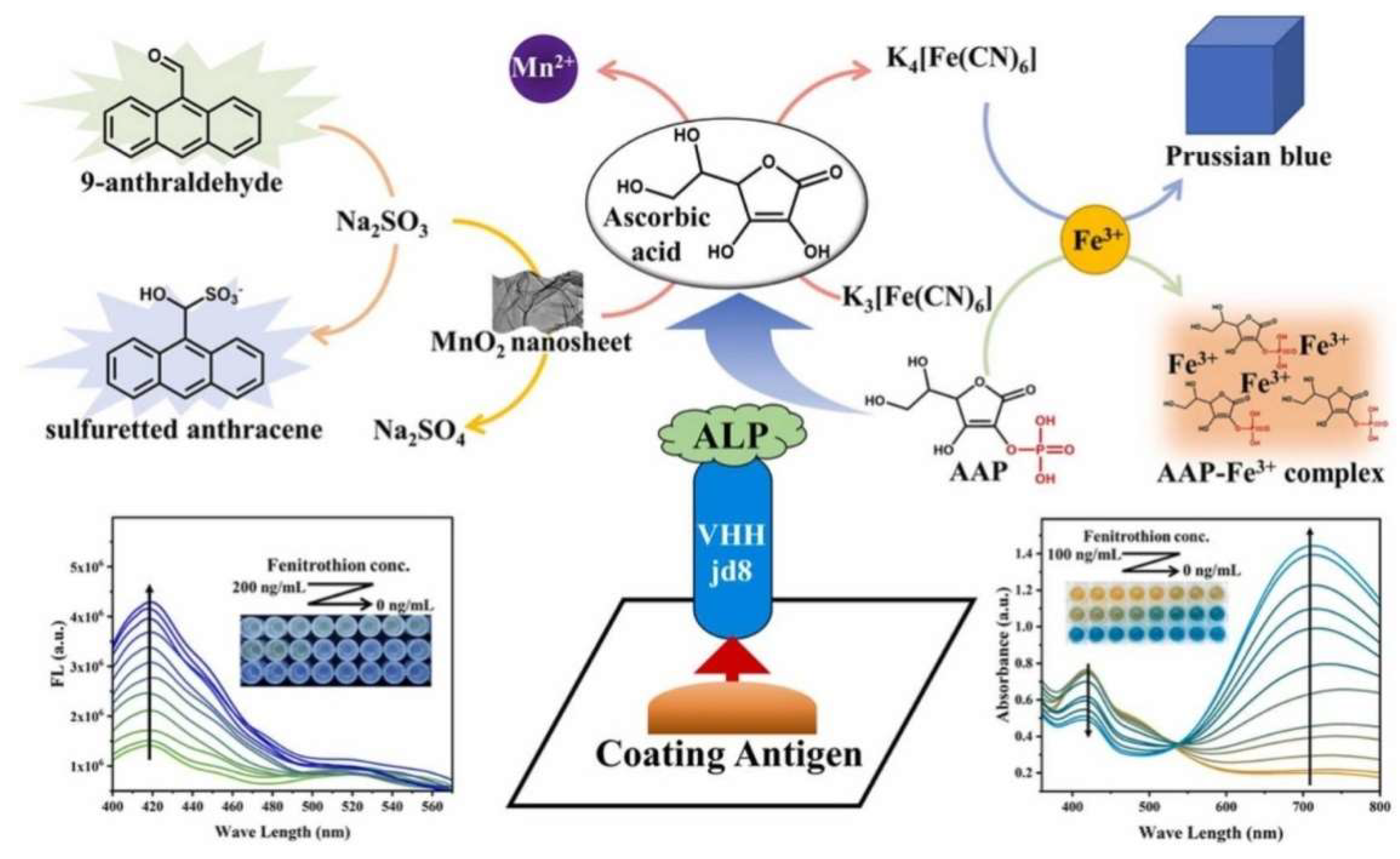
| APP | In situ formation of Prussian blue | Fenitrothion | 4.7–11.6 ng/mL | 3 ng/mL | [137] | |
| Enzymatic-product-induced aggregation of plasmonic NPs | AAP | Mn2+-mediated aggregation of AuNPs | Fumonisin B1 | 6.25–200 ng/mL | 0.15 ng/mL | [142] |
| ATP | Zn2+-mediated aggregation of AuNPs | Respiratory syncytial virus | 0.1–30 pg/mL | 21 fg/mL | [143] | |
| AAP | AuNPs-based click reaction | Norfloxacin | 3.18 × 10−2–6.88 × 103 pg/mL | 10fg/mL | [144] | |
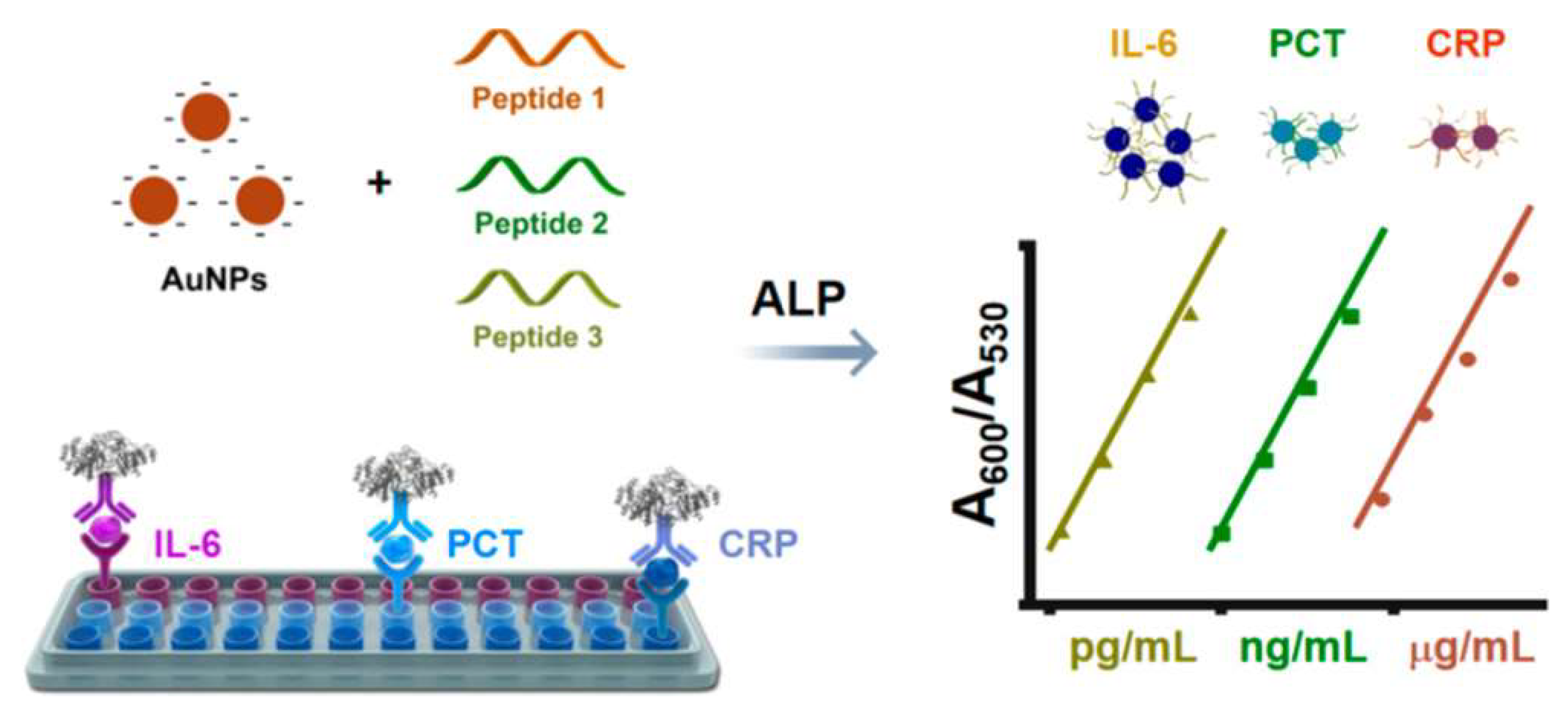
| Peptide | AuNPs | PCT, IL-6, CRP | 0.2–25 ng/mL, 50–1600 pg/mL, 3.15–100 μg/mL | 0.24 ng/mL, 12.5 pg/mL, 1.15 μg/mL | [146] | |
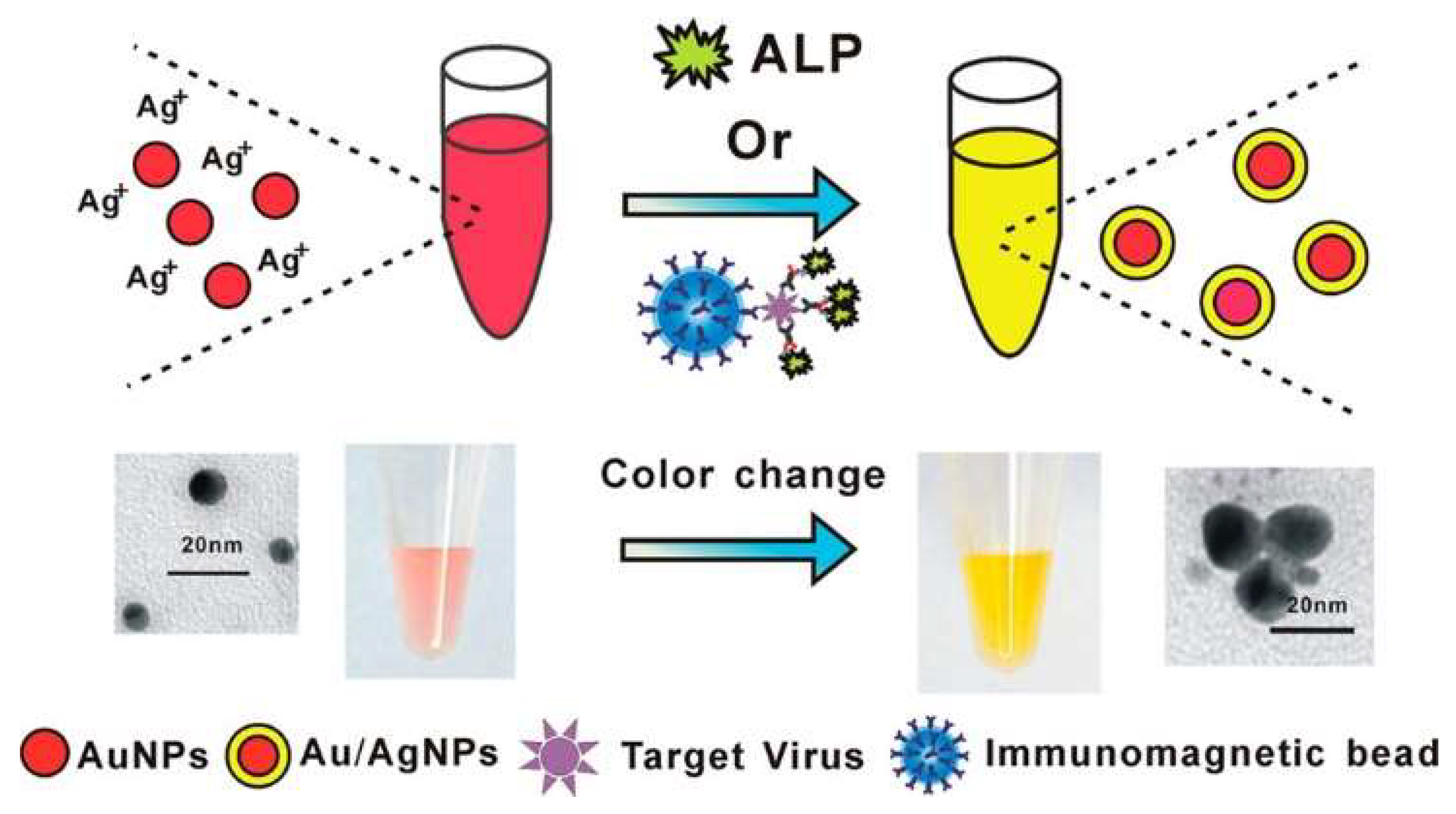
| Enzymatic-product-induced in situ metallization or bioetching of plasmonic NPs | AAP | Ag growth on SiO2@AuNPs | IgG | 0.7–70 pM | 0.14 pM | [147] |
| PAPP | Ag growth on AuNPs | H9N2 AIV | 0.02–1 ng/mL | 17.5 pg/mL | [148] | |
| AAP | Growth of AuNPs | Tyramine | 0.313–20 mg/L | 0.246 mg/L | [149] | |
| AAP | Growth of AuNPs | HER2 ECD | 1–7 ng/mL | 0.05 ng/mL | [151] | |
| AAP | Ag growth on AuNRs | Xanthylacrylamide | 0.3–17.2 ng/mL | 0.06 ng/mL | [152] | |
| AAP | Iodine-mediated etching of AuNRs | Human IgG | 0.1–10 ng/mL | 100 pg/mL | [162] | |
| Enzymatic-product-mediated activity change of artificial enzymes or nanozymes | GTP | GTP-accelerated TMB oxidation | AFP | 1–100 ng/mL | 0.5 ng/mL | [164] |
| AAP | In situ generated CHNPs to catalyze ABTS oxidation | Aflatoxin B1 | 1 pg/mL–20 ng/mL | 0.73 pg/mL | [170] | |
| AAP | In situ generated PdNPs to catalyze TMB oxidation | PSA | 5–50 ng/mL | 1 ng/mL | [168] |
4. Chemiluminescence Immunoassays
5. SERS Immunoassays
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, e12–e14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC-Trend. Anal. Chem. 2020, 126, 115861–115879. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Zhao, Y.; Wang, F.; Liu, L.; Sun, Z. Electrochemical biosensors by in situ dissolution of self-assembled nanolabels into small monomers on electrode surface. Sens. ActuatorsB Chem. 2020, 325, 128777. [Google Scholar] [CrossRef]
- Yi, Z.; Ren, Y.; Li, Y.; Li, Y.; Long, F.; Zhu, A. Optical biosensors for microbial toxin detection: Recent advances and future trends. Microchem. J. 2023, 191, 108894–108911. [Google Scholar] [CrossRef]
- Fu, X.; Chen, L.; Choo, J. Optical nanoprobes for ultrasensitive immunoassay. Anal. Chem. 2017, 89, 124–137. [Google Scholar] [CrossRef]
- Rizzo, F. Optical immunoassays methods in protein analysis: An overview. Chemosensors 2022, 10, 326. [Google Scholar] [CrossRef]
- Lee, D.; Hwang, J.; Seo, Y.; Gilad, A.A.; Choi, J. Optical immunosensors for the efficient detection of target biomolecules. Biotechnol. Bioprocess Eng. 2018, 23, 123–133. [Google Scholar] [CrossRef]
- Wang, Y.; Xianyu, Y. Nanobody and nanozyme-enabled immunoassays with enhanced specificity and sensitivity. Small Methods 2022, 6, e2101576–e2101599. [Google Scholar] [CrossRef]
- Banno, Y.; Nomiyama, T.; Okuno, S.; Ide, S.; Kaji, N. Quantitative evaluation of interleukin-4 by immunowall devices made of gelatin methacryloyl hydrogel. Molecules 2023, 28, 4635. [Google Scholar] [CrossRef]
- Radha, R.; Shahzadi, S.K.; Al-Sayah, M.H. Fluorescent immunoassays for detection and quantification of cardiac troponin I: A short review. Molecules 2021, 26, 4812. [Google Scholar] [CrossRef]
- Chang, J.F.; Yu, L.; Hou, T.; Hu, R.X.; Li, F. Direct and specific detection of glyphosate using a phosphatase-like nanozyme-mediated chemiluminescence strategy. Anal. Chem. 2023, 95, 4479–4485. [Google Scholar] [CrossRef]
- Lee, J.; Takemura, K.; Park, E.Y. Plasmonic nanomaterial-based optical biosensing platforms for Virus detection. Sensors 2017, 17, 2332. [Google Scholar] [CrossRef]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review—Nanozyme-based immunosensors and immunoassays: Recent developments and future trends. J. Electrochem. Soc. 2019, 167, 037508–037518. [Google Scholar] [CrossRef]
- Pei, X.; Tao, G.; Wu, X.; Ma, Y.; Li, R.; Li, N. Nanomaterial-based multiplex optical sensors. Analyst 2020, 145, 4111–4123. [Google Scholar] [CrossRef] [PubMed]
- Sang, P.; Hu, Z.; Cheng, Y.; Yu, H.; Yang, F.; Xie, Y.; Yao, W.; Guo, Y.; Qian, H. Exonuclease III-assisted nucleic acid amplification fluorescence immunoassay for the ultrasensitive detection of chloramphenicol in milk. Sens. Actuators B Chem. 2021, 347, 130564–130569. [Google Scholar] [CrossRef]
- Yang, W.; Shen, Y.; Zhang, D.; Xu, W. Protein-responsive rolling circle amplification as a tandem template to drive amplified transduction of fluorescence signal probes for highly sensitive immunoassay. Chem. Commun. 2018, 54, 10195–10198. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Cui, T. Applications of fluorescent materials in the detection of alkaline phosphatase activity. J. Biomed. Mater. Res. Part B 2021, 109, 214–226. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Li, Z.; Chen, H.; Qiu, H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens. Bioelectron. 2020, 148, 111811–111821. [Google Scholar] [CrossRef]
- Huang, J.; Wei, F.; Cui, Y.; Hou, L.; Lin, T. Fluorescence immunosensor based on functional nanomaterials and its application in tumor biomarker detection. RSC Adv. 2022, 12, 31369–31379. [Google Scholar] [CrossRef]
- Sadiq, Z.; Safiabadi Tali, S.H.; Hajimiri, H.; Al-Kassawneh, M.; Jahanshahi-Anbuhi, S. Gold nanoparticles-based colorimetric assays for environmental monitoring and food safety evaluation. Crit. Rev. Anal. Chem. 2023, 53, 1–36. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, D.; Wu, D.; Hou, W.; Wang, L.; Li, N.; Sun, Z. Duplex-specific nuclease-based electrochemical biosensor for the detection of microRNAs by conversion of homogeneous assay into surface-tethered electrochemical analysis. Anal. Chim. Acta 2021, 1149, 338199. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wu, D.; Sun, T.; Wang, Y.; Ren, X.; Zhao, F.; Liu, L.; Yi, X. Magnetic bead-based electrochemical and colorimetric methods for the detection of poly(ADP-ribose) polymerase-1 with boronic acid derivatives as the signal probes. Sens. Actuators B Chem. 2021, 327, 128913. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.; Yu, H.; Sun, W.; Yi, X.; Liu, L. Magnetic bead-based electrochemical and colorimetric assays of circulating tumor cells with boronic acid derivatives as the recognition elements and signal probes. Talanta 2021, 221, 121640. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H.; Wu, Q.; Xiong, Y.; Wang, J.; Ding, Y. Recent advances in enzyme-enhanced immunosensors. Biotechnol. Adv. 2021, 53, 107867–107883. [Google Scholar] [CrossRef] [PubMed]
- Noji, H.; Minagawa, Y.; Ueno, H. Enzyme-based digital bioassay technology-key strategies and future perspectives. Lab Chip 2022, 22, 3092–3109. [Google Scholar] [CrossRef]
- Sun, J.; Ning, X.; Cui, L.; Ling, M.; Xu, X.; He, S. Assembly of “carrier free” enzymatic nano-reporters for improved ELISA. Analyst 2020, 145, 6541–6548. [Google Scholar] [CrossRef]
- Xia, N.; Sun, T.; Liu, L.; Tian, L.; Sun, Z. Heterogeneous sensing of post-translational modification enzymes by integrating the advantage of homogeneous analysis. Talanta 2022, 237, 122949. [Google Scholar] [CrossRef]
- Grigorenko, V.G.; Andreeva, I.P.; Rubtsova, M.Y.; Egorov, A.M. Recombinant horseradish peroxidase: Production and analytical applications. Biochemistry 2015, 80, 408–416. [Google Scholar] [CrossRef]
- Shi, D.; Sun, Y.; Lin, L.; Shi, C.; Wang, G.; Zhang, X. Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(II). Analyst 2016, 141, 5549–5554. [Google Scholar] [CrossRef]
- Xianyu, Y.; Zhu, K.; Chen, W.; Wang, X.; Zhao, H.; Sun, J.; Wang, Z.; Jiang, X. Enzymatic assay for Cu(II) with horseradish peroxidase and its application in colorimetric logic gate. Anal. Chem. 2013, 85, 7029–7032. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, L.; Lv, F.; Wang, S. Bioluminescence as a light source for photosynthesis. Chem. Commun. 2013, 49, 10685–10687. [Google Scholar] [CrossRef] [PubMed]
- Abucayon, E.; Ke, N.; Cornut, R.; Patelunas, A.; Miller, D.; Nishiguchi, M.K.; Zoski, C.G. Investigating catalase activity through hydrogen peroxide decomposition by bacteria biofilms in real time using scanning electrochemical microscopy. Anal. Chem. 2014, 86, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhuo, Y.; Liao, N.; Chai, Y.; Yuan, R. Ultrasensitive immunoassay based on a pseudobienzyme amplifying system of choline oxidase and luminol-reduced Pt@Au hybrid nanoflowers. Chem. Commun. 2014, 50, 14627–14630. [Google Scholar] [CrossRef] [PubMed]
- Manes, T.; Hoylaerts, M.F.; Müller, R.; Lottspeich, F.; Holke, W.; Millán, J.L. Genetic complexity, structure, and characterization of highly active bovine intestinal alkaline phosphatases. J. Biol. Chem. 1998, 273, 23353–23360. [Google Scholar] [CrossRef]
- Li, S.-J.; Li, C.-Y.; Li, Y.-F.; Fei, J.; Wu, P.; Yang, B.; Ou-Yang, J.; Nie, S.-X. Facile and sensitive near-infrared fluorescence probe for the detection of endogenous akaline phosphatase activity in vivo. Anal. Chem. 2017, 89, 6854–6860. [Google Scholar] [CrossRef]
- Zhang, S.; Garcia-D’Angeli, A.; Brennan, J.P.; Huo, Q. Predicting detection limits of enzyme-linked immunosorbent assay (ELISA) and bioanalytical techniques in general. Analyst 2014, 139, 439–445. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC-Trend. Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Zhang, X.-Y.; Jiang, A.-Q.; Yang, Y.-S.; Zhu, H.-L. Fluorescent probes for the detection of alkaline phosphatase in biological systems: Recent advances and future prospects. TrAC-Trend. Anal. Chem. 2021, 136, 116189–116217. [Google Scholar] [CrossRef]
- Shaban, S.M.; Byeok Jo, S.; Hafez, E.; Ho Cho, J.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord. Chem. Rev. 2022, 465, 214567–214604. [Google Scholar] [CrossRef]
- Zherdev, A.V.; Dzantiev, B.B. Detection limits of immunoanalytical systems: Limiting factors and methods of reduction. J. Anal. Chem. 2022, 77, 391–401. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722–121737. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Zhang, M.; Hua, L.; Zhan, S. Plasmonic gold nanostructures for biosensing and bioimaging. Microchim. Acta 2021, 188, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wei, Q. Plasmonic molecular assays: Recent advances and applications for mobile health. Nano Res. 2018, 11, 5439–5473. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Feng, K.; Kang, Y.; Zhao, J.J.; Liu, Y.L.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Electrochemical immunosensor with aptamer-based enzymatic amplification. Anal. Biochem. 2008, 378, 38–42. [Google Scholar] [CrossRef]
- Jones, A.; Dhanapala, L.; Kankanamage, R.N.T.; Kumar, C.V.; Rusling, J.F. Multiplexed immunosensors and immunoarrays. Anal. Chem. 2020, 92, 345–362. [Google Scholar] [CrossRef]
- Gil Rosa, B.; Akingbade, O.E.; Guo, X.; Gonzalez-Macia, L.; Crone, M.A.; Cameron, L.P.; Freemont, P.; Choy, K.L.; Guder, F.; Yeatman, E.; et al. Multiplexed immunosensors for point-of-care diagnostic applications. Biosens. Bioelectron. 2022, 203, 114050–114066. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Sato, T.; Hirama, M.; Fujii, I. Highly sensitive and practical fluorescent sandwich ELISA for ciguatoxins. Anal. Chem. 2018, 90, 7318–7324. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Mohammadi, S.; Salimi, A. Current advances of carbon dots based biosensors for tumor marker detection, cancer cells analysis and bioimaging. TrAC-Trend. Anal. Chem. 2019, 115, 83–99. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, Y.; Lv, X.; He, J.; Xie, F.; Li, J.; Cai, J. Nanomaterial-based fluorescent biosensor for food safety analysis. Biosensors 2022, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ju, Q.; Pang, S.; Wei, N.; Zhang, Y. Recent progress of fluorescent probes for the detection of alkaline phosphatase (ALP): A review. Dyes. Pigments 2021, 194, 109569–109582. [Google Scholar] [CrossRef]
- Nishiyama, K.; Kasama, T.; Nakamata, S.; Ishikawa, K.; Onoshima, D.; Yukawa, H.; Maeki, M.; Ishida, A.; Tani, H.; Baba, Y.; et al. Ultrasensitive detection of disease biomarkers using an immuno-wall device with enzymatic amplification. Analyst 2019, 144, 4589–4595. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, Z.L.; Wang, F.; Cai, J.; Dong, J.X.; Zhang, J.R.; Si, R.; Wang, C.L.; Wang, Y.; Shen, Y.D.; et al. Isolation of bactrian camel single domain antibody for parathion and development of one-step dc-FEIA method using VHH-alkaline phosphatase Fusion protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef]
- Huo, J.; Li, Z.; Wan, D.; Li, D.; Qi, M.; Barnych, B.; Vasylieva, N.; Zhang, J.; Hammock, B.D. Development of a highly sensitive direct competitive fluorescence enzyme immunoassay based on a nanobody-alkaline phosphatase Fusion protein for detection of 3-phenoxybenzoic acid in urine. J. Agric. Food. Chem. 2018, 66, 11284–11290. [Google Scholar] [CrossRef]
- Chavez Ramos, K.; Nishiyama, K.; Maeki, M.; Ishida, A.; Tani, H.; Kasama, T.; Baba, Y.; Tokeshi, M. Rapid, sensitive, and selective detection of H5 hemagglutinin from avian influenza virus using an immunowall device. ACS Omega 2019, 4, 16683–16688. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wan, D.B.; Xiong, Y.H.; He, Z.Y.; Wang, X.X.; Gee, S.J.; Ryu, D.; Hammock, B.D. Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin A in cereal. Anal. Chem. 2015, 87, 1387–1394. [Google Scholar] [CrossRef]
- Medawar-Aguilar, V.; Jofre, C.F.; Fernandez-Baldo, M.A.; Alonso, A.; Angel, S.; Raba, J.; Pereira, S.V.; Messina, G.A. Serological diagnosis of Toxoplasmosis disease using a fluorescent immunosensor with chitosan-ZnO-nanoparticles. Anal. Biochem. 2019, 564–565, 116–122. [Google Scholar] [CrossRef]
- Obayashi, Y.; Iinobc, R.; Noji, H. A single-molecule digital enzyme assay using alkaline phosphatase with a cumarin-based fluorogenic substrate. Analyst 2015, 140, 5065–5073. [Google Scholar] [CrossRef]
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2020, 128, 9–16. [Google Scholar] [CrossRef]
- Tsaloglou, M.-N.; Jacobs, A.; Morgan, H. A fluorogenic heterogeneous immunoassay for cardiac muscle troponin cTnI on a digital microfluidic device. Anal. Bioanal. Chem. 2014, 406, 5967–5976. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Martinez-Tome, M.J.; Mallavia, R.; Mateo, C.R. Fluorescent biosensor for phosphate determination based on immobilized polyfluorene-liposomal nanoparticles coupled with alkaline phosphatase. ACS Appl. Mater. Interfaces 2017, 9, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, X.; Xiao, Y.; Fang, H.; Zhang, G.; Yang, H.; Zhou, Y. Fluorescence and colorimetric dual-mode immunoassay based on G-quadruplex/N-methylmesoporphyrin IX and p-nitrophenol for detection of zearalenone. Food Chem. 2023, 401, 134190–134195. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Fenol, J. Fluorimetric determination of total ascorbic acid by a stopped-flow mixing technique. Analyst 2001, 126, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Diao, Y.; Sun, C.; Yang, J.; Wang, Y.; Sun, S. Fluorimetric determination of ascorbic acid with ophenylenediamine. Talanta 2003, 59, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Peng, C.; Zhu, S.; Sun, J.; Yang, X. Fluorescence immunoassay based on the alkaline phosphatase triggered in situ fluorogenic reaction of o-phenylenediamine and ascorbic acid. Anal. Chem. 2019, 91, 2978–2984. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Lu, S.; Liu, G.; Sun, J.; Yang, X. Fluorometric and colorimetric dual-readout immunoassay based on an alkaline phosphatase-triggered reaction. Anal. Chem. 2019, 91, 7828–7834. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, M.; Xue, Y.; Li, J.; Wang, E. In situ fluorogenic reaction generated via ascorbic acid for the construction of universal sensing platform. Anal. Chem. 2021, 93, 6873–6880. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Deng, X.; Cong, Y.; Jiang, X. Peptidic β-sheet binding with Congo Red allows both reduction of error variance and signal amplification for immunoassays. Biosens. Bioelectron. 2016, 86, 211–218. [Google Scholar] [CrossRef]
- Geng, F.; Liu, X.; Wei, T.; Wang, Z.; Liu, J.; Shao, C.; Liu, G.; Xu, M.; Feng, L. An alkaline phosphatase-induced immunosensor for SARS-CoV-2 N protein and cardiac troponin I based on the in situ fluorogenic self-assembly between N-heterocyclic boronic acids and alizarin red S. Sens. Actuators B Chem. 2023, 378, 133121–133129. [Google Scholar] [CrossRef]
- Lin, J.H.; Yang, Y.C.; Shih, Y.C.; Hung, S.Y.; Lu, C.Y.; Tseng, W.L. Photoinduced electron transfer between Fe(III) and adenosine triphosphate-BODIPY conjugates: Application to alkaline-phosphatase-linked immunoassay. Biosens. Bioelectron. 2016, 77, 242–248. [Google Scholar] [CrossRef]
- Mu, X.; Jiang, X.; Zhang, Y.; Liu, X.; Zhang, S.; Wang, W.; Huang, Y.; Ma, P.; Song, D. Sensitive ratiometric fluorescence probe based on chitosan carbon dots and calcein for alkaline phosphatase detection and bioimaging in cancer cells. Anal. Chim. Acta 2021, 1188, 339163–339170. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, J.; Lu, Y.; Sun, J.; Yang, X. Fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase. Anal. Chem. 2018, 90, 3505–3511. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Lu, S.; Bao, X.; Sun, J.; Yang, X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal. Chem. 2018, 90, 7754–7760. [Google Scholar] [CrossRef]
- Sun, C.; Shi, Y.; Tang, M.; Hu, X.; Long, Y.; Zheng, H. A signal amplification strategy for prostate specific antigen detection via releasing oxidase-mimics from coordination nanoparticles by alkaline phosphatase. Talanta 2020, 213, 120827–120833. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, Z.; Tang, M.; Long, Y.; Zheng, H. Reducing background absorbance via a double-lock strategy for detection of alkaline phosphatase and α-fetoprotein. Microchim. Acta 2020, 187, 489–497. [Google Scholar] [CrossRef]
- Sund, H.; Blomberg, K.; Meltola, N.; Takalo, H. Design of novel, water soluble and highly luminescent Europium labels with potential to enhance immunoassay sensitivities. Molecules 2017, 22, 1807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Huang, L.; Jia, C.; Gao, X.; Liu, S.; Wang, S.; Zhao, P.; Sun, J.; Zhang, D.; et al. Schiff-base chemistry-coupled catechol oxidase-like nanozyme reaction as a universal sensing mode for ultrasensitive biosensing. Anal. Chem. 2023, 95, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-H.; Liu, F.; Peng, Z.-Q.; Yu, K.; Rong, L.-Q.; Wang, Y.; Wu, P.; Liang, R.-P.; Qiu, J.-D. Lanthanide phosphate nanoparticle-based one-step optical discrimination of alkaline phosphatase activity. ACS Appl. Nano Mater. 2020, 3, 2336–2345. [Google Scholar] [CrossRef]
- Wang, F.; Hu, X.; Hu, J.; Peng, Q.; Zheng, B.; Du, J.; Xiao, D. Fluorescence assay for alkaline phosphatase activity based on energy transfer from terbium to europium in lanthanide coordination polymer nanoparticles. J. Mater. Chem. B 2018, 6, 6008–6015. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Li, Y.; Tan, H. Fluorescent enzyme-linked immunosorbent assay based on alkaline phosphatase-responsive coordination polymer composite. Microchim. Acta 2021, 188, 263–272. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Li, L.; Wang, G.; Su, X. β-Cyclodextrin modified silver nanoclusters for highly sensitive fluorescence sensing and bioimaging of intracellular alkaline phosphatase. Talanta 2020, 207, 120315–120322. [Google Scholar] [CrossRef]
- Luo, L.; Jia, B.-Z.; Wei, X.-Q.; Xiao, Z.-L.; Wang, H.; Sun, Y.-M.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L. Development of an inner filter effect-based fluorescence immunoassay for the detection of acrylamide using 9-xanthydrol derivatization. Sens. Actuators B Chem. 2021, 332, 129561–129568. [Google Scholar] [CrossRef]
- Tang, C.; Qian, Z.; Huang, Y.; Xu, J.; Ao, H.; Zhao, M.; Zhou, J.; Chen, J.; Feng, H. A fluorometric assay for alkaline phosphatase activity based on β-cyclodextrin-modified carbon quantum dots through host-guest recognition. Biosens. Bioelectron. 2016, 83, 274–280. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Zhang, X.; Luo, P.; Lin, G.; Jiang, W. Highly photoluminescent carbon dots-based immunosensors for ultrasensitive detection of aflatoxin M1 residues in milk. Food Chem. 2021, 355, 129443–129450. [Google Scholar] [CrossRef]
- Fang, B.; Peng, J.; Zhang, G.; Xing, K.; Chen, W.; Liu, D.; Shan, S.; Xiong, Y.; Lai, W. I2/I--mediated fluorescence quenching of an Ag+-doped gold nanocluster-based immunoassay for sensitive detection of Escherichia coli O157:H7 in milk. J. Dairy Sci. 2022, 105, 2922–2930. [Google Scholar] [CrossRef]
- Jie, Z.; Qi, G.; Xu, C.; Jin, Y. Enzymatic preparation of plasmonic-fluorescent quantum dot-gold hybrid nanoprobes for sensitive detection of glucose and alkaline phosphatase and dual-modality cell imaging. Anal. Chem. 2019, 91, 14074–14079. [Google Scholar]
- Lu, H.-F.; Zhang, M.-M.; Wu, D.; Huang, J.-L.; Zhu, L.-L.; Wang, C.-M.; Zhang, Q.-L. Colorimetric and fluorescent dual-mode sensing of alkaline phosphatase activity in L-02 cells and its application in living cell imaging based on in-situ growth of silver nanoparticles on graphene quantum dots. Sens. Actuators B Chem. 2018, 258, 461–469. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Wang, J.; Gyimah, E.; Hu, X.; Zhang, Z. A novel fluorescence immunoassay based on AgNCs and ALP for ultrasensitive detection of sulfamethazine (SMZ) in environmental and biological samples. Talanta 2019, 199, 72–79. [Google Scholar] [CrossRef]
- Ni, P.; Chen, C.; Jiang, Y.; Zhang, C.; Wang, B.; Cao, B.; Li, C.; Lu, Y. Gold nanoclusters-based dual-channel assay for colorimetric and turn-on fluorescent sensing of alkaline phosphatase. Sens. Actuators B Chem. 2019, 301, 127080–127086. [Google Scholar] [CrossRef]
- Chen, P.; Yan, S.; Sawyer, E.; Ying, B.; Wei, X.; Wu, Z.; Geng, J. Rapid and simple detection of ascorbic acid and alkaline phosphatase via controlled generation of silver nanoparticles and selective recognition. Analyst 2019, 144, 1147–1152. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, Z.; Huang, K.; Wei, Y.; Li, D.; Yan, S.; Huang, J.; Geng, J.; Tao, C.; Chen, P.; et al. Fluorescence and visual immunoassay of HIV-1 p24 antigen in clinical samples via multiple selective recognitions of CdTe QDs. Microchim. Acta 2021, 188, 422–430. [Google Scholar] [CrossRef]
- Song, P.; Liu, Q.; Zhang, Y.; Liu, W.; Meng, M.; Yin, Y.; Xi, R. The chemical redox modulated switch-on fluorescence of carbon dots for probing alkaline phosphatase and its application in an immunoassay. RSC Adv. 2018, 8, 162–169. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Z.L.; Luo, L.; Dong, Y.Z.; Yang, J.Y.; Lei, H.T.; Wang, H.; Shen, Y.D.; Xu, Z.L. Development of Cu(II)/Cu(I)-induced quantum dot-mediated fluorescence immunoassay for the sensitive determination of ethyl carbamate. Microchim. Acta 2020, 187, 533–542. [Google Scholar] [CrossRef]
- Xie, W.; Tian, M.; Luo, X.; Jiang, Y.; He, N.; Liao, X.; Liu, Y. A dual-mode fluorescent and colorimetric immunoassay based on in situ ascorbic acid-induced signal generation from metal-organic frameworks. Sens. Actuators B Chem. 2020, 302, 127180–127186. [Google Scholar] [CrossRef]
- Hu, X.L.; Wu, X.M.; Fang, X.; Li, Z.J.; Wang, G.L. Switchable fluorescence of gold nanoclusters for probing the activity of alkaline phosphatase and its application in immunoassay. Biosens. Bioelectron. 2016, 77, 666–672. [Google Scholar] [CrossRef]
- Fang, X.; Li, X.-Q.; Wang, H.; Wu, X.-M.; Wang, G.-L. Tuning surface states to achieve the modulated fluorescence of carbon dots for probing the activity of alkaline phosphatase and immunoassay of α-fetoprotein. Sens. Actuators B Chem. 2018, 257, 620–628. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, W.; Ma, X.; Zhang, Y.; Yue, C.; Fang, W.; Hu, Y.; Wang, J.; Dang, J.; Zhao, H.; et al. Nitrogen-doped carbon dots-V2O5 nanobelts sensing platform for sensitive detection of ascorbic acid and alkaline phosphatase activity. Anal. Chim. Acta 2019, 1089, 131–143. [Google Scholar] [CrossRef]
- Deng, R.; Xie, X.; Vendrell, M.; Chang, Y.T.; Liu, X. Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles. J. Am. Chem. Soc. 2011, 133, 20168–20171. [Google Scholar] [CrossRef]
- Xiao, T.; Sun, J.; Zhao, J.; Wang, S.; Liu, G.; Yang, X. FRET effect between fluorescent polydopamine nanoparticles and MnO2 nanosheets and its application for sensitive sensing of alkaline phosphatase. ACS Appl. Mater. Interfaces 2018, 10, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, B.; Chen, M.; Liu, M.; Leng, Y.; Liu, X.; Li, Y.; Liu, Z. Fluorescence turn-on sensing of ascorbic acid and alkaline phosphatase activity based on graphene quantum dots. Sens. Actuators B Chem. 2016, 235, 356–361. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Han, Y.; Pan, W.; Zhang, T.; Tang, B. A highly selective and instantaneous nanoprobe for detection and imaging of ascorbic acid in living cells and in vivo. Anal. Chem. 2014, 86, 3924–3930. [Google Scholar] [CrossRef]
- Liang, M.Y.; Zhao, B.; Xiong, Y.; Chen, W.X.; Huo, J.Z.; Zhang, F.; Wang, L.; Li, Y. A “turn-on” sensor based on MnO2 coated UCNPs for detection of alkaline phosphatase and ascorbic acid. Dalton Trans. 2019, 48, 16199–16210. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.D.; Banerjee, M.; Chatterjee, A. Review of 2D MnO2 nanosheets as FRET-based nanodot fluorescence quenchers in chemosensing applications. ACS Appl. Nano Mater. 2022, 5, 17373–17412. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S. CDs-MnO2-TPPS ternary system for ratiometric fluorescence detection of ascorbic acid and alkaline phosphatase. ACS Omega 2021, 6, 16565–16572. [Google Scholar] [CrossRef]
- Luo, L.; Lin, S.Q.; Wu, Z.Y.; Wang, H.; Chen, Z.J.; Deng, H.; Shen, Y.D.; Zhang, W.F.; Lei, H.T.; Xu, Z.L. Nanobody-based fluorescent immunoassay using carbon dots anchored cobalt oxyhydroxide composite for the sensitive detection of fenitrothion. J. Hazard. Mater. 2022, 439, 129701. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, R.; Kong, D.; Zhao, X.; Liu, F.; Yan, X.; Lin, Y.; Lu, G. Switchable fluorescence immunoassay using gold nanoclusters anchored cobalt oxyhydroxide composite for sensitive detection of imidacloprid. Sens. Actuators B Chem. 2019, 283, 207–214. [Google Scholar] [CrossRef]
- Tang, D.; Lin, Y.; Zhou, Q. Carbon dots prepared from Litchi chinensis and modified with manganese dioxide nanosheets for use in a competitive fluorometric immunoassay for aflatoxin B1. Microchim. Acta 2018, 185, 476–484. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Sun, J.; Mari, G.M.; Yu, X.; Ke, Y.; Li, J.; Wang, Z.; Yu, W.; Wen, K.; et al. Development of a fluorescence immunoassay for highly sensitive detection of amantadine using the nanoassembly of carbon dots and MnO2 nanosheets as the signal probe. Sens. Actuators B Chem. 2019, 286, 214–221. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q.; Jin, Y.; Li, B. Fluorescent enzyme-linked immunoassay strategy based on enzyme-triggered in-situ synthesis of fluorescent copper nanoclusters. Sens. Actuators B Chem. 2019, 281, 28–33. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, D.; Wang, B.; Ni, P.; Jiang, Y.; Zhang, C.; Yang, F.; Lu, Y.; Sun, J. Alkaline phosphatase-triggered in situ formation of silicon-containing nanoparticles for a fluorometric and colorimetric dual-channel immunoassay. Anal. Chem. 2020, 92, 4639–4646. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Wang, S.; Lu, S.; Sun, J.; Yang, X. Enzyme-induced in situ generation of polymer carbon dots for fluorescence immunoassay. Sens. Actuators B Chem. 2020, 306, 127583–127590. [Google Scholar] [CrossRef]
- Sun, J.; Hu, T.; Chen, C.; Zhao, D.; Yang, F.; Yang, X. Fluorescence immunoassay system via enzyme-enabled in situ synthesis of fluorescent silicon nanoparticles. Anal. Chem. 2016, 88, 9789–9795. [Google Scholar] [CrossRef]
- Malashikhina, N.; Garai-Ibabe, G.; Pavlov, V. Unconventional application of conventional enzymatic substrate: First fluorogenic immunoassay based on enzymatic formation of quantum dots. Anal. Chem. 2013, 85, 6866–6870. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, L.; Zeng, F.; Wu, S. Biomarker-activatable probes based on smart AIEgens for fluorescence and optoacoustic imaging. Coord. Chem. Rev. 2022, 458, 214438–214459. [Google Scholar] [CrossRef]
- Li, H.Y.; Lin, H.Y.; Lv, W.X.; Gai, P.P.; Li, F. Equipment-free and visual detection of multiple biomarkers via an AIE luminogen-based paper biosensor. Biosens. Bioelectron. 2020, 165, 112336. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, C.F.; Hou, T.; Li, F. Amphiphile-mediated ultrasmall AIE dots for ultrasensitive fluorescence biosensing. Anal. Chem. 2017, 89, 9100–9107. [Google Scholar] [CrossRef]
- Dou, L.; Li, Q.; Wang, Z.; Shen, J.; Yu, W. AIEgens: Next generation signaling source for immunoassays? ACS Sens. 2022, 7, 3243–3257. [Google Scholar] [CrossRef]
- Liu, W.; Yu, W.; Li, X.; Zhao, X.; Zhang, Y.; Song, P.; Yin, Y.; Xi, R.; Meng, M. Pyrophosphate-triggered intermolecular cross-linking of tetraphenylethylene molecules for multianalyte detection. Sens. Actuators B Chem. 2018, 266, 170–177. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, W.; Xu, S.; Liu, B. A biosensor based on self-clickable AIEgen: A signal amplification strategy for ultrasensitive immunoassays. Chem. Commun. 2017, 53, 5287–5290. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Zhang, Q.; Zhang, G.; Wu, S.; Yang, H.; Zhou, Y. Cerium ions triggered dual-readout immunoassay based on aggregation induced emission effect and 3,3’,5,5’-tetramethylbenzidine for fluorescent and colorimetric detection of ochratoxin A. Anal. Chim. Acta 2022, 1231, 340445–340453. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Deng, D.; Mu, X.; Liu, A.; Xie, J.; Zhou, D.; Yang, P.; Xing, Y.; Liu, L. Colorimetric immunoassays based on pyrroloquinoline quinone-catalyzed generation of Fe(II)-ferrozine with tris(2-carboxyethyl)phosphine as the reducing reagent. Sens. Actuators B Chem. 2020, 306, 127571. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, X. Integration of nanomaterials for colorimetric immunoassays with improved performance: A functional perspective. Analyst 2016, 141, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, L.; Dang, J.; Xie, Y.; Zhang, Y.; Pu, J.; Long, G.; Li, Y.; Yuan, Y.; et al. Spectrophotometric-dual-enzyme-simultaneous assay in one reaction solution: Chemometrics and experimental models. Anal. Chem. 2013, 85, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.B.; Tang, Y.; Yang, H.M. Development of an efficient signal amplification strategy for label-free enzyme immunoassay using two site-specific biotinylated recombinant proteins. Anal. Chim. Acta 2015, 859, 66–71. [Google Scholar] [CrossRef]
- Kim, D.; Seo, H.D.; Ryu, Y.; Kim, H.S. Functionalized gold nanoparticles with zinc finger-fused proteins as a colorimetric immunoassay platform. Anal. Chim. Acta 2020, 1126, 154–162. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Enhancement of lateral flow immunoassay by alkaline phosphatase: A simple and highly sensitive test for potato virus X. Microchim. Acta 2017, 185, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.; Emara, S.; Askal, H.; El-Rabbat, N.; Akizawa, T.; Yoshiokab, M. Enzyme-linked immunosorbent assay for 2-deoxycytidine. Anal. Chim. Acta 2000, 404, 179–186. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Z.; Beier, R.C.; Jiang, H.; Wu, Y.; Shen, J. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal. Chem. 2013, 85, 1995–1999. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Bao, X.; Wang, Q.; Yang, X. Alkaline phosphatase assay based on the chromogenic interaction of diethanolamine with 4-aminophenol. Anal. Chem. 2018, 90, 6339–6345. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, B.; Dang, P.; Li, L.; Kong, J.; Zhang, X. Facile colorimetric assay of alkaline phosphatase activity using Fe(II)-phenanthroline reporter. Anal. Chim. Acta 2017, 950, 170–177. [Google Scholar] [CrossRef]
- Lei, L.; Xie, W.; Chen, Z.; Jiang, Y.; Liu, Y. Metal ion chelation-based color generation for alkaline phosphatase-linked high-performance visual immunoassays. Sens. Actuators B Chem. 2018, 273, 35–40. [Google Scholar] [CrossRef]
- Wu, S.; Tan, H.; Wang, C.; Wang, J.; Sheng, S. A Colorimetric immunoassay based on coordination polymer composite for the detection of carcinoembryonic antigen. ACS Appl. Mater. Interfaces 2019, 11, 43031–43038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Zhang, Z.; Chen, L. Chemical redox-cycling for improving the sensitivity of colorimetric enzyme-linked immunosorbent assay. Anal. Chem. 2019, 91, 1254–1259. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Zhang, Y.Z.; Song, D.; Li, J.; Xu, Z.R. Alkaline phosphatase-regulated in situ formation of chromogenic probes for multicolor visual sensing of biomarkers. Talanta 2021, 228, 122222. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zeng, X.; Deng, H.; Wang, Y.; Zhang, Y.-F.; Shen, Y.-D.; Luo, L.; Wang, H.; Chen, Z.-J.; Xu, Z.-L. Phosphate-triggered ratiometric multicolor immunosensor based on nanobody-alkaline phosphatase fusion protein for sensitive detection of fenitrothion. Sens. Actuators B Chem. 2022, 373, 132734–132742. [Google Scholar] [CrossRef]
- Shang, C.; Li, Y.; Zhang, Q.; Tang, S.; Tang, X.; Ren, H.; Hu, P.; Lu, S.; Li, P.; Zhou, Y. Alkaline phosphatase-triggered dual-signal immunoassay for colorimetric and electrochemical detection of zearalenone in cornmeal. Sens. Actuators B Chem. 2022, 358, 131525–131532. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, W.; Zhang, J.; Tang, D. Competitive photometric and visual ELISA for aflatoxin B1 based on the inhibition of the oxidation of ABTS. Microchim. Acta 2017, 184, 2387–2394. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Li, J. Gold nanoparticles with special shapes: Controlled synthesis, surface-enhanced Raman scattering, and the application in biodetection. Sensors 2007, 7, 3299–3311. [Google Scholar] [CrossRef]
- Li, C.M.; Zhen, S.J.; Wang, J.; Li, Y.F.; Huang, C.Z. A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens. Bioelectron. 2013, 43, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Zhang, Q.; Zha, Y.; Lu, S.; Yang, Y.; Li, P.; Zhou, Y. Colorimetric immunoassay via smartphone based on Mn2+-mediated aggregation of AuNPs for convenient detection of fumonisin B1. Food Control 2022, 132, 108481–108488. [Google Scholar] [CrossRef]
- Zhan, L.; Wu, W.B.; Yang, L.; Huang, C.Z. Sensitive detection of respiratory syncytial virus based on a dual signal amplified plasmonic enzyme-linked immunosorbent assay. Anal. Chim. Acta 2017, 962, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xia, C.; Ning, B.A.; Xu, Z.; Liu, X.; Zuo, H.; Cai, L.; Sun, T.; Liu, Y. Fluorescent and colorimetric detection of Norfloxacin with a bifunctional ligand and enzymatic signal amplification system. Microchem. J. 2022, 179, 107660–107666. [Google Scholar] [CrossRef]
- Xianyu, Y.; Wang, Z.; Jiang, X. A plasmonic nanosensor for immunoassay via enzyme-triggered click chemistry. ACS Nano 2014, 8, 12741–12747. [Google Scholar] [CrossRef]
- Ran, B.; Zheng, W.; Dong, M.; Xianyu, Y.; Chen, Y.; Wu, J.; Qian, Z.; Jiang, X. Peptide-Mediated Controllable Cross-Linking of Gold Nanoparticles for Immunoassays with Tunable Detection Range. Anal. Chem. 2018, 90, 8234–8240. [Google Scholar] [CrossRef]
- Pham, X.H.; Hahm, E.; Kim, T.H.; Kim, H.M.; Lee, S.H.; Lee, Y.S.; Jeong, D.H.; Jun, B.H. Enzyme-catalyzed Ag growth on Au nanoparticle-assembled structure for highly sensitive colorimetric immunoassay. Sci. Rep. 2018, 8, 6290–6296. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhao, J.Y.; Pang, D.W.; Zhang, Z.L. Enzyme-induced metallization as a signal amplification strategy for highly sensitive colorimetric detection of avian influenza virus particles. Anal. Chem. 2014, 86, 2752–2759. [Google Scholar] [CrossRef]
- Luo, L.; Luo, S.Z.; Jia, B.Z.; Zhang, W.F.; Wang, H.; Wei, X.Q.; Shen, Y.D.; Lei, H.T.; Xu, Z.L.; Yang, J.Y. A high-resolution colorimetric immunoassay for tyramine detection based on enzyme-enabled growth of gold nanostar coupled with smartphone readout. Food Chem. 2022, 396, 133729–133736. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, F.; Gao, Y.; Cheng, J.; Fu, F. Regulating the growth rate of gold nanobipyramids via a HClNADH-ascorbic acid system toward a dual-channel multicolor colorimetric immunoassay for simultaneously screening and detecting multiple sulfonamides. Anal. Chem. 2023, 95, 10438–10447. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Q.; Zhong, Y.; Yu, X.; Wu, Y.; Fu, F. A multicolor immunosensor for sensitive visual detection of breast cancer biomarker based on sensitive NADH-ascorbic-acid-mediated growth of gold nanobipyramids. Anal. Chem. 2020, 92, 1534–1540. [Google Scholar] [CrossRef]
- Fu, H.-J.; Luo, L.; Wang, Y.; Wang, C.-L.; Wang, H.; Shen, Y.-D.; Lei, H.-T.; Hildebrandt, N.; Xu, Z.-L. Enzyme-induced silver deposition on gold nanorods for naked-eye and Smartphone detection of acrylamide in food. ACS Appl. Nano Mater. 2022, 5, 12915–12925. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, M.; Wang, W.; Jiang, Q.; Wang, F.; Pang, D.W.; Liu, X. Plasmonic and photothermal immunoassay via enzyme-triggered crystal growth on gold nanostars. Anal. Chem. 2019, 91, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Shaban, S.M.; Cho, S.Y.; Kim, D.H. Detection of periodontal disease marker with geometrical transformation of Ag nanoplates. Anal. Chem. 2023, 95, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, J.; Li, J.; Ju, H. A plasmonic colorimetric strategy for biosensing through enzyme guided growth of silver nanoparticles on gold nanostars. Biosens. Bioelectron. 2016, 78, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-Z.; Yang, J.-Y.; Jia, B.-Z.; Wang, H.; Chen, Z.-J.; Wei, X.-Q.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L.; Luo, L. Multicolorimetric and fluorometric dual-modal immunosensor for histamine via enzyme-enabled metallization of gold nanorods and inner filter effect of carbon dots. Food Control 2022, 137, 108941–108949. [Google Scholar] [CrossRef]
- He, S.; Huang, Q.; Zhang, Y.; Zhang, H.; Xu, H.; Li, X.; Ma, X. Magnetic beads-based multicolor colorimetric immunoassay for ultrasensitive detection of aflatoxin B1. Chin. Chem. Lett. 2021, 32, 1462–1465. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Z. Enzyme-catalysed deposition of ultrathin silver shells on gold nanorods: A universal and highly efficient signal amplification strategy for translating immunoassay into a litmus-type test. Chem. Commun. 2015, 51, 6928–6931. [Google Scholar] [CrossRef]
- Zha, Y.; Lu, S.; Hu, P.; Ren, H.; Liu, Z.; Gao, W.; Zhao, C.; Li, Y.; Zhou, Y. Dual-modal immunosensor with functionalized gold nanoparticles for ultrasensitive detection of chloroacetamide herbicides. ACS Appl. Mater. Interfaces 2021, 13, 6091–6098. [Google Scholar] [CrossRef]
- Xianyu, Y.; Lin, Y.; Chen, Q.; Belessiotis-Richards, A.; Stevens, M.M.; Thomas, M.R. Iodide-mediated rapid and sensitive surface etching of gold nanostars for biosensing. Angew. Chem. Int. Ed. 2021, 60, 9891–9896. [Google Scholar] [CrossRef]
- Singh, M.M.; Satija, J. Enzyme-assisted metal nanoparticles etching based plasmonic ELISA: Progress and insights. Anal. Biochem. 2022, 654, 114820–114828. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, S.; Cheng, F.; Chen, L. Iodine-mediated etching of gold nanorods for plasmonic ELISA based on colorimetric detection of alkaline phosphatase. ACS Appl. Mater. Interfaces 2015, 7, 27639–27645. [Google Scholar] [CrossRef] [PubMed]
- Gai, P.P.; Pu, L.; Wang, C.; Zhu, D.Q.; Li, F. CeO2@NC nanozyme with robust dephosphorylation ability of phosphotriester: A simple colorimetric assay for rapid and selective detection of paraoxon. Biosens. Bioelectron. 2023, 220, 114841. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, M.; Liu, L.; Pang, Y.; Long, Y.; Zheng, H. GTP as a peroxidase-mimic to mediate enzymatic cascade reaction for alkaline phosphatase detection and alkaline phosphatase-linked immunoassay. Sens. Actuators B Chem. 2018, 275, 43–49. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, F.; Fu, R.; Yang, Y.; Jiao, B.; He, Y. Enzyme–nanozyme cascade reaction-mediated etching of gold nanorods for the detection of Escherichia coli. ACS Appl. Nano Mater. 2020, 3, 9016–9025. [Google Scholar] [CrossRef]
- Chen, W.; Li, M.; Chen, Z.; Yan, Z.; Li, J.; Guo, L.; Ding, C.; Huang, Y. Dual enzyme induced colorimetric sensor for simultaneous identifying multiple pathogens. Biosens. Bioelectron. 2023, 234, 115344–115351. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.N.; Li, Y.; Yang, F.Q. Enzyme-regulated in situ formation of copper hexacyanoferrate nanoparticles with oxidase-mimetic behaviour for colorimetric detection of ascorbate oxidase. Biosensors 2023, 13, 344. [Google Scholar] [CrossRef]
- Gao, Z.; Hou, L.; Xu, M.; Tang, D. Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci. Rep. 2014, 4, 3966–3973. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, B.; Xu, H.; He, Z.; Zhou, Y.; Chen, Q.; Sun, Z.; Cao, H.; Liu, X. Enzyme cascade-amplified immunoassay based on the nanobody-alkaline phosphatase fusion and MnO2 nanosheets for the detection of ochratoxin A in coffee. RSC Adv. 2021, 11, 21760–21766. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Guo, J.; Wang, Y.; Lin, Y.; Ye, S.; Zhuang, J.; Tang, D. Enzyme-controllable just-in-time production system of copper hexacyanoferrate nanoparticles with oxidase-mimicking activity for highly sensitive colorimetric immunoassay. Talanta 2022, 247, 123546–123553. [Google Scholar] [CrossRef]
- Jin, L.Y.; Dong, Y.M.; Wu, X.M.; Cao, G.X.; Wang, G.L. Versatile and amplified biosensing through enzymatic cascade reaction by coupling alkaline phosphatase in situ generation of photoresponsive nanozyme. Anal. Chem. 2015, 87, 10429–10436. [Google Scholar] [CrossRef]
- Xie, W.; Lei, L.; Tian, M.; Zhang, Z.; Liu, Y. A high-resolution colorimetric immunoassay platform realized by coupling enzymatic multicolor generation with smartphone readout. Analyst 2018, 143, 2901–2907. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.M.; Ying, X. Evaluation of carbohydrate antigen 50 in human serum using magnetic particle-based chemiluminescence enzyme immunoassay. Anal. Chim. Acta 2007, 598, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Meng, M.; Zhang, Y.; Yin, Y.; Zhang, X.; Xi, R. Chemiluminescence enzyme immunoassay using magnetic nanoparticles for detection of neuron specific enolase in human serum. Anal. Chim. Acta 2012, 722, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Fu, J.; Gee, S.J.; Hammock, B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.S.; Lee, J.H. Chemiluminescent dual-enzyme immunoassays capable of simultaneously quantifying carbohydrate antigen 19-9 and carcinoma embryonic antigen in a sample. Anal. Chim. Acta 2019, 1060, 88–96. [Google Scholar] [CrossRef]
- Liu, R.; Wang, C.; Jiang, Q.; Zhang, W.; Yue, Z.; Liu, G. Magnetic-particle-based, ultrasensitive chemiluminescence enzyme immunoassay for free prostate-specific antigen. Anal. Chim. Acta 2013, 801, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Huang, J.; Xu, X.; Yang, L. Immunoassays using optical-fiber sensor with all-directional chemiluminescent collection. Anal. Chem. 2020, 92, 6257–6262. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, D.; Shi, G.; Lin, L. Dual-labeled chemiluminescence enzyme immunoassay for simultaneous measurement of total prostate specific antigen (TPSA) and free prostate specific antigen (FPSA). Luminescence 2017, 32, 1547–1553. [Google Scholar] [CrossRef]
- Sasamoto, H.; Maeda, M.; Tsuji, A. Chemiluminescent assay of alkaline phosphatase using phenacyl phosphate. Anal. Chim. Acta 1995, 306, 161–166. [Google Scholar] [CrossRef]
- Kokado, A.; Tsuji, A.; Maeda, M. Chemiluminescence assay of alkaline phosphatase using cortisol-21-phosphate as substrate and its application to enzyme immunoassays. Anal. Chim. Acta 1997, 337, 335–340. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Zhang, Y.; Lau, C.; Lu, J. Combination of quantum dot fluorescence with enzyme chemiluminescence for multiplexed detection of lung cancer biomarkers. Anal. Methods 2010, 2, 1236–1243. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, H.; Ju, H. Flow-through multianalyte chemiluminescent immunosensing system with designed substrate zone-resolved technique for sequential detection of tumor markers. Anal. Chem. 2006, 78, 6999–7005. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yang, L.; Deng, S.; Hao, Y.; Zhang, K.; Wang, X.; Liu, Y.; Liu, H.; Chen, Y.; Xie, M. Development of nanosensor by bioorthogonal reaction for multi-detection of the biomarkers of hepatocellular carcinoma. Sens. Actuators B Chem. 2021, 334, 129653–129661. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Dong, M.; Zheng, W.; Sun, J.; Jiang, X. Double-enzymes-mediated bioluminescent sensor for quantitative and ultrasensitive point-of-care testing. Anal. Chem. 2017, 89, 5422–5427. [Google Scholar] [CrossRef]
- Granger, J.H.; Schlotter, N.E.; Crawford, A.C.; Porter, M.D. Prospects for point-of-care pathogen diagnostics using surface-enhanced Raman scattering (SERS). Chem. Soc. Rev. 2016, 45, 3865–3882. [Google Scholar] [CrossRef]
- Siddhanta, S.; Kuzmin, A.N.; Pliss, A.; Baev, A.S.; Khare, S.K.; Chowdhury, P.K.; Ganguli, A.K.; Prasad, P.N. Advances in Raman spectroscopy and imaging for biomedical research. Adv. Opt. Photonics 2023, 15, 318–384. [Google Scholar] [CrossRef]
- Harper, M.M.; McKeating, K.S.; Faulds, K. Recent developments and future directions in SERS for bioanalysis. Phys. Chem. Chem. Phys. 2013, 15, 5312–5328. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Hua, J.; Chen, D.; Meng, H.; Li, Z.; Xiao, L. Enzyme activity-modulated etching of gold nanobipyramids@MnO2 nanoparticles for ALP assay using surface-enhanced Raman spectroscopy. Nanoscale 2020, 12, 10390–10398. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, W.; Gu, B. Detection of alkaline phosphatase using surface-enhanced Raman spectroscopy. Anal. Chem. 2006, 78, 3379–3384. [Google Scholar] [CrossRef]
- Campbell, F.M.; Ingram, A.; Monaghan, P.; Cooper, J.; Sattar, N.; Eckersall, P.D.; Graham, D. SERRS immunoassay for quantitative human CRP analysis. Analyst 2008, 133, 1355–1357. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, H.; Tram, K.; Zhang, S.; Zhao, Y.; Han, L.; Chen, Z.; Huan, S. A paper-based surface-enhanced resonance Raman spectroscopic (SERRS) immunoassay using magnetic separation and enzyme-catalyzed reaction. Analyst 2013, 138, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, M.; Yi, X.; Sun, D. Enzyme-triggered click chemistry combined with surface-enhanced Raman spectroscopy for the simple and sensitive detection of alkaline phosphatase activity from complex biological samples. Analyst 2022, 147, 2494–2499. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Liang, Y.; Jiang, J.; Shen, G.; Yu, R. Surface-enhanced Raman scattering for immunoassay based on the biocatalytic production of silver nanoparticles. Anal. Sci. 2009, 25, 347–352. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Gao, M.X.; Zhan, L.; Gong, M.; Zhen, S.J.; Huang, C.Z. An enzyme-induced Au@Ag core-shell nanoStructure used for an ultrasensitive surface-enhanced Raman scattering immunoassay of cancer biomarkers. Nanoscale 2017, 9, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
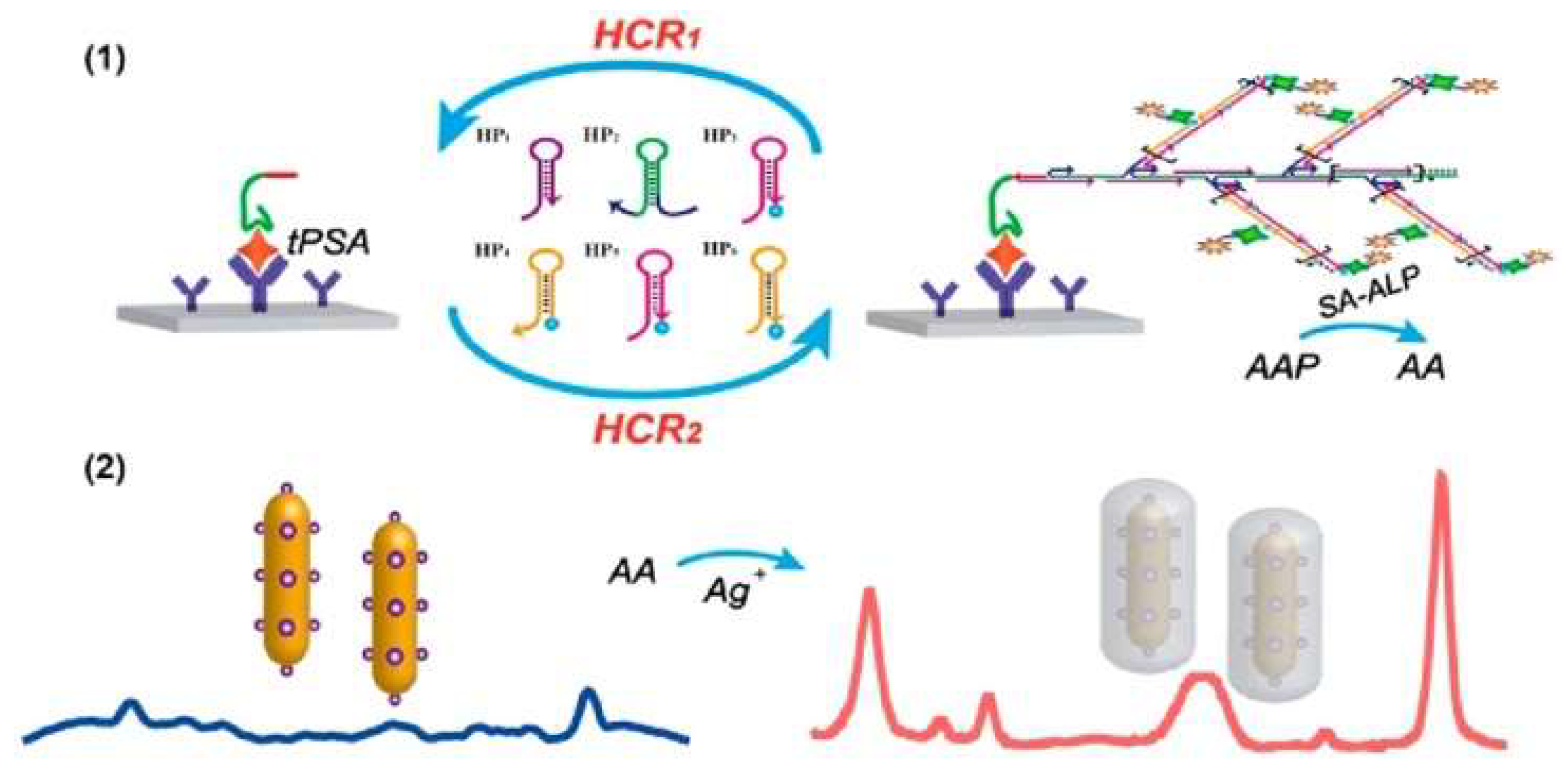
- Cun, F.; Huang, Z.; Lin, Q.; Yu, G.; Chen, H.; Kong, J.; Weng, W. Hybridized chain reaction-amplified alkaline phosphatase-induced Ag-shell nanostructure for the sensitive and rapid surface-enhanced Raman scattering immunoassay of exosomes. Anal. Chem. 2023, 95, 10025–10033. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Xia, C.; Yang, L.; Li, Y.F.; Li, C.M.; Huang, C.Z. DNA nanofirecrackers assembled through hybridization chain reaction for ultrasensitive SERS immunoassay of prostate specific antigen. Anal. Chem. 2020, 92, 4046–4052. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Chang, Y.; Lou, J.; Zhang, S.; Yi, X. Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays. Molecules 2023, 28, 6565. https://doi.org/10.3390/molecules28186565
Liu L, Chang Y, Lou J, Zhang S, Yi X. Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays. Molecules. 2023; 28(18):6565. https://doi.org/10.3390/molecules28186565
Chicago/Turabian StyleLiu, Lin, Yong Chang, Jiaxin Lou, Shuo Zhang, and Xinyao Yi. 2023. "Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays" Molecules 28, no. 18: 6565. https://doi.org/10.3390/molecules28186565
APA StyleLiu, L., Chang, Y., Lou, J., Zhang, S., & Yi, X. (2023). Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays. Molecules, 28(18), 6565. https://doi.org/10.3390/molecules28186565







