Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico
Abstract
1. Introduction
2. Results
2.1. Chromatographic Analysis of the Chemical Composition of MEOE
2.2. Antioxidant Activity of MEOE
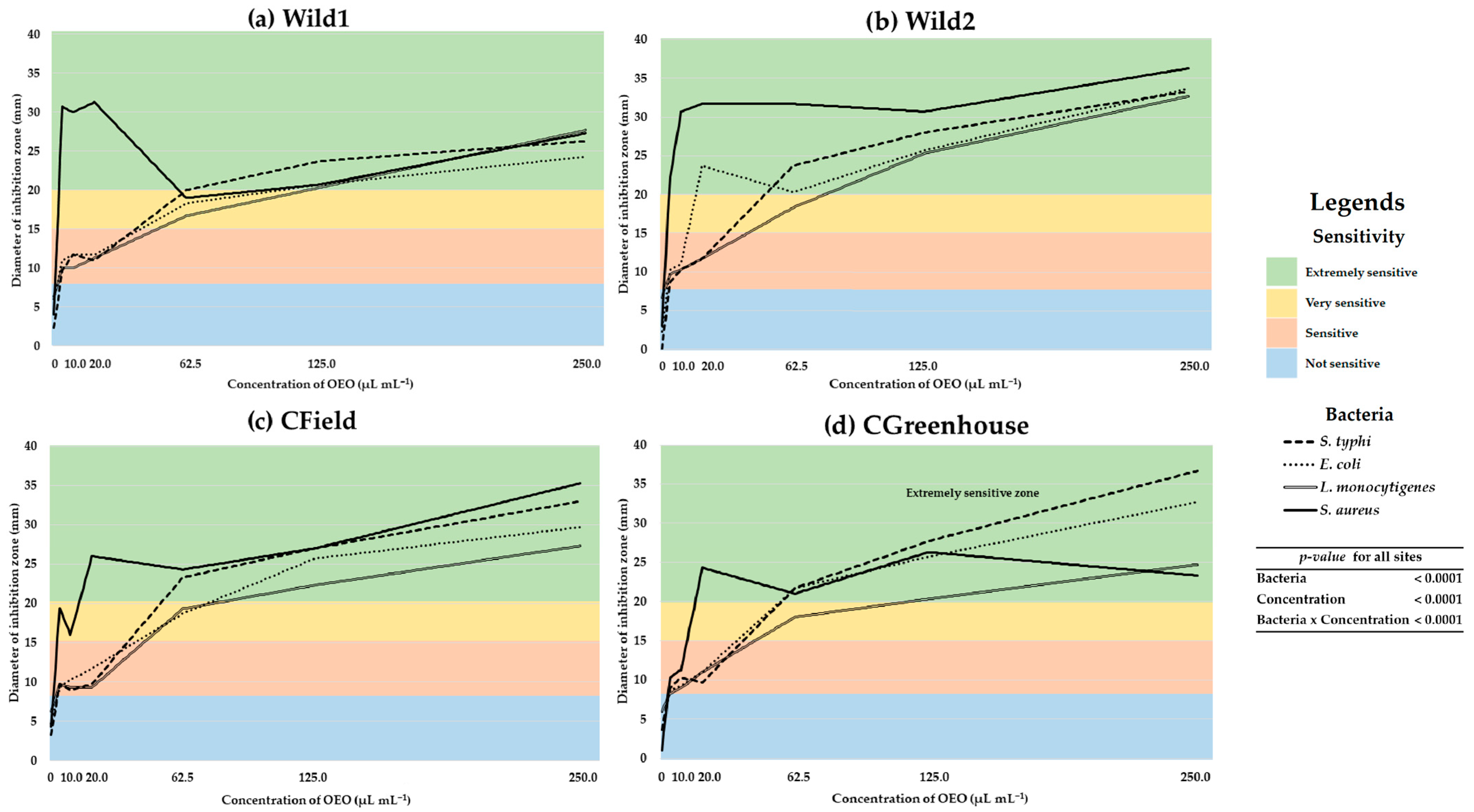
2.3. Antibacterial Activity of MEOE
3. Discussion
3.1. Chemical Composition of MEOE
3.2. Antioxidant Activity of MEOE
3.3. Antibacterial Activity of MEOE
4. Materials and Methods
4.1. Study Location and Plant Material Identification
4.2. Extraction of MOEO
4.3. Gas Chromatography Spectrometry Analysis of MOEO
4.4. Antioxidant Activity of MOEO
4.5. Antibacterial Activity of MOEO
4.5.1. Bacterial Strains
4.5.2. Agar Diffusion Method
4.5.3. Determination of Diameter of Inhibition Zone (DIZ) and Sensitivity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X. Antibacterial Activity of Oregano Essential Oils against Streptococcus mutans In Vitro and Analysis of Active Components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Maggi, F.; Craker, L. Essential Oil Profile of Oregano (Origanum vulgare L.) Populations Grown under Similar Soil and Climate Conditions. Ind. Crops Prod. 2018, 119, 183–190. [Google Scholar] [CrossRef]
- Castilho, P.C.; Savluchinske-Feio, S.; Weinhold, T.S.; Gouveia, S.C. Evaluation of the Antimicrobial and Antioxidant Activities of Essential Oils, Extracts and Their Main Components from Oregano from Madeira Island, Portugal. Food Control 2012, 23, 552–558. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Bautista-Hernandez, I.; Aguilar, C.N.; Martinez-Avila, G.C.G.; Torres-Leon, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chavez-Gonzalez, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Comision Nacional Forestal—National Forestry Commission (CONAFOR). Catalogo de Recursos Forestales Maderables y No Maderables: Arido, Tropical y Templado. Comision Nacional Forestal 2015. Available online: http://www.conafor.gob.mx/biblioteca/Catalogo_de_recursos_forestales_M_y_N.pdf (accessed on 17 March 2023). (In Spanish).
- Lawrence, B.M. The Botanical and Chemical Aspects of Oregano. Perfum. Flavorist 1984, 9, 41–51. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8965722 (accessed on 14 May 2023).
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential Oil Chemical Composition of Wild Populations of Italian Oregano Spice (Origanum vulgare ssp. hirtum (Link) Ietswaart): A Preliminary Evaluation of Their use in Chemotaxonomy by Cluster Analysis. 1. Inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents Against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Cid-Perez, T.S.; Torres-Munoz, J.V.; Nevarez-Moorillon, G.V.; Palou, E.; Lopez-Malo, A. Chemical Characterization and Antifungal Activity of Poliomintha longiflora Mexican Oregano. J. Essent. Oil Res. 2016, 28, 157–165. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhang, W.; Sun, M.; Li, H.; Xia, F.; Cui, H.; Bai, H.; Shi, L. Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars. Foods 2021, 10, 2328. [Google Scholar] [CrossRef]
- Chacon-Vargas, K.F.; Sanchez-Torres, L.E.; Chavez-Gonzalez, M.L.; Adame-Gallegos, J.R.; Nevarez-Moorillon, G.V. Mexican Oregano (Lippia berlandieri Schauer and Poliomintha longiflora Gray) Essential Oils Induce Cell Death by Apoptosis in Leishmania (Leishmania) mexicana Promastigotes. Molecules 2022, 27, 5183. [Google Scholar] [CrossRef]
- Ortega-Lozano, A.J.; Hernandez-Cruz, E.Y.; Gomez-Sierra, T.; Pedraza-Chaverri, J. Antimicrobial Activity of Spices Popularly Used in Mexico Against Urinary Tract Infections. Antibiotics 2023, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity Beyond their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Mora-Zuñiga, A.E.; Trevino-Garza, M.Z.; Amaya Guerra, C.A.; Galindo Rodriguez, S.A.; Castillo, S.; Martinez-Rojas, E.; Rodriguez-Rodriguez, J.; Baez-Gonzalez, J.G. Comparison of Chemical Composition, Physicochemical Parameters, and Antioxidant and Antibacterial Activity of the Essential Oil of Cultivated and Wild Mexican Oregano Poliomintha longiflora Gray. Plants 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, J.; Yang, C.; Chen, Y.; Yang, Y.; Zhou, C.; Wang, L.; Xia, G.; Yu, X.; Yang, H. Preparation and Characterization of Oregano Essential Oil-Loaded Dioscorea zingiberensis Starch Film with Antioxidant and Antibacterial Activity and its Application in Chicken Preservation. Int. J. Biol. Macromol. 2022, 212, 20–30. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, P.; He, Y.; Kang, S.; Shen, C.; Wang, S.; Guo, M.; Wang, L.; Shi, C. Antibacterial Effect of Oregano Essential Oil against Vibrio vulnificus and Its Mechanism. Foods 2022, 11, 403. [Google Scholar] [CrossRef]
- Li, B.; Zheng, K.; Lu, J.; Zeng, D.; Xiang, Q.; Ma, Y. Antibacterial Characteristics of Oregano Essential Oil and its Mechanisms Against Escherichia coli O157: H7. J. Food Meas. Charact. 2022, 16, 2989–2998. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Z.; Yang, J.; Zhang, M.; Cai, F.; Lu, P. ZnO Nanoparticles Stabilized Oregano Essential Oil Pickering Emulsion for Functional Cellulose Nanofibrils Packaging Films with Antimicrobial and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 190, 433–440. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Inhibitory Parameters of Essential Oils to Reduce a Foodborne Pathogen. LWT-Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Baycheva, S.K.; Dobreva, K.Z. Chemical Composition of Bulgarian White Oregano (Origanum heracleoticum L.) Essential Oils. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012107. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, X.; Chen, M.; Xie, J. Antimicrobial Activity and Mechanism of Oregano Essential Oil against Shewanella putrefaciens. J. Food Saf. 2022, 42, e12952. [Google Scholar] [CrossRef]
- Bhat, V.; Sharma, S.M.; Shetty, V.; Shastry, C.S.; Rao, C.V.; Shenoy, S.; Saha, S.; Balaji, S. Characterization of Herbal Antifungal Agent, Origanum vulgare against Oral Candida spp. Isolated from Patients with Candida-Associated Denture Stomatitis: An In Vitro Study. Contemp. Clin. Dent. 2018, 9, 3–10. [Google Scholar] [CrossRef]
- Salgado-Nava, A.A.; Hernandez-Nava, R.; Lopez-Malo, A.; Jimenez-Munguia, M.T. Antimicrobial Activity of Encapsulated Mexican Oregano (Lippia berlandieri Schauer) Essential Oil Applied on Bagels. Front. Sustain. Food Syst. 2020, 4, 537091. [Google Scholar] [CrossRef]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial Activity of Essential Oils from Different Parts of Plants against Salmonella and Listeria spp. Food Chem. 2022, 404, 134723. [Google Scholar] [CrossRef]
- Marin-Tinoco, R.I.; Camacho-Luis, A.; Silva-Marrufo, O.; Diaz-Diaz, M.; Ortega-Ramirez, A.T. Inhibition of Candida albicans by Oregano (Lippia spp.) Essential Oil from Municipality of Rodeo, Durango, Mexico. J. Microbiol. Health Educ. 2021, 3, 70–76. Available online: http://journalmhe.org/ojs3/index.php/jmhe/article/view/14 (accessed on 18 March 2023).
- Jan, S.; Rashid, M.; Abd_Allah, E.F.; Ahmad, P. Biological Efficacy of Essential Oils and Plant Extracts of Cultivated and Wild Ecotypes of Origanum vulgare L. BioMed. Res. Int. 2020, 2020, 8751718. [Google Scholar] [CrossRef]
- Goudjil, M.B.; Zighmi, S.; Hamada, D.; Mahcene, Z.; Bencheikh, S.E.; Ladjel, S. Biological Activities of Essential Oils Extracted From Thymus capitatus (Lamiaceae). S. Afr. J. Bot. 2020, 128, 274–282. [Google Scholar] [CrossRef]
- Shanaida, M.; Golembiovska, O. Identification and Component Analysis of Triterpenoids in Monarda fistulosa L. and Ocimum americanum L. (Lamiaceae) Aerial Parts. Pharm. Sci. 2018, 13, 26–31. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-LiquidMediated Extraction and Separation Processes for Bioactive Compounds: Past, Present and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Olivas, N.A.; Bejarano, C.V.; Soto, G.A.; Ortega, M.Z.; Salas, F.S.; Chávez, E.S.; Ochoa, L.H. Bioactive Compounds and Antioxidant Activity of Essential Oils of Origanum dictamnus From Mexico. AIMS Agric. Food 2020, 5, 387–394. [Google Scholar] [CrossRef]
- Cortes-Chitala, M.d.C.; Flores-Martinez, H.; Orozco-Avila, I.; Leon-Campos, C.; Suarez-Jacobo, A.; Estarron-Espinosa, M.; Lopez-Muraira, I. Identification and Quantification of Phenolic Compounds from Mexican Oregano (Lippia graveolens HBK) Hydroethanolic Extracts and Evaluation of Its Antioxidant Capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.M.; Jaleel, A.; Alyafei, M.A.M. Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes. Plants 2021, 10, 2618. [Google Scholar] [CrossRef]
- Marin-Tinoco, R.I.; Silva-Marrufo, O.; Gonzales-Güereca, M. Physical characterization-chemistry of essential oil of oregano in 6 communities of the Municipality of Rodeo, Dgo. J. Urban Rural Reg. Econ. 2019, 3, 18–23. [Google Scholar] [CrossRef]
- Manso, S.; Becerril, R.; Nerin, C.; Gomez-Lus, R. Influence of pH and Temperature Variations on Vapor Phase Action of an Antifungal Food Packaging against Five Mold Strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernandez-Munoz, P.; Gavara, R. Antioxidant and Antimicrobial Properties of Ethylene Vinyl Alcohol Copolymer Films Based on the Release of Oregano Essential Oil and Green Tea Extract Components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Camean, A.M. A Subchronic 90-day Oral Toxicity Study of Origanum vulgare Essential Oil in Rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef]
- Efrati, R.; Natan, M.; Pelah, A.; Haberer, A.; Banin, E.; Dotan, A.; Ophir, A. The Combined Effect of Additives and Processing on the Thermal Stability and Controlled Release of Essential Oils in Antimicrobial Films. J. Appl. Polym. Sci. 2014, 131, 40564. [Google Scholar] [CrossRef]
- Navarrete-Molina, C.; Meza-Herrera, C.A.; Ramirez-Flores, J.J.; Herrera-Machuca, M.A.; Lopez-Villalobos, N.; Lopez-Santiago, M.A.; Veliz-Deras, F.G. Economic Evaluation of the Environmental Impact of a Dairy Cattle Intensive Production Cluster under Arid Lands Conditions. Animal 2019, 13, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Molina, C.; Meza-Herrera, C.A.; Herrera-Machuca, M.A.; Lopez-Villalobos, N.; Lopez-Santos, A.; Veliz-Deras, F.G. To Beef or Not To Beef: Unveiling the Economic Environmental Impact Generated by the Intensive Beef Cattle Industry in an Arid Region. J. Clean. Prod. 2019, 231, 1027–1035. [Google Scholar] [CrossRef]
- Navarrete-Molina, C.; Meza-Herrera, C.A.; Herrera-Machuca, M.A.; Macias-Cruz, U.; Veliz-Deras, F.G. Not All Ruminants Were Created Equal: Environmental and Socio-Economic Sustainability of Goats Under a Marginal-Extensive Production System. J. Clean. Prod. 2020, 255, 120237. [Google Scholar] [CrossRef]
- Rios-Flores, J.L.; Rios-Arredondo, B.E.; Cantu-Brito, J.E.; Rios-Arredondo, H.E.; Armendariz-Erives, S.; Chavez-Rivero, J.A.; Navarrete-Molina, C.; Castro-Franco, R. Analisis de la Eficiencia Fisica, Economica y Social del Agua en Esparrago (Asparagus officinalis L.) y Uva (Vitis vinifera) de Mesa del DR-037 Altar-Pitiquito-Caborca, Sonora, Mexico 2014. Rev. Fac. Cienc. Agrar. 2018, 50, 101–122. (In Spanish) [Google Scholar]
- Bhatt, S.; Tewari, G.; Pande, C.; Prakash, O.; Tripathi, S. Aroma Profile and Antioxidant Potential of Origanum vulgare L.: Impact of Drying. J. Essent. Oil Bear Plants 2019, 22, 214–230. [Google Scholar] [CrossRef]
- Fikry, S.; Khalil, N.; Salama, O. Chemical Profiling, Biostatic and Biocidal Dynamics of Origanum vulgare L. Essential Oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, S.; Nitu, R.; Dahma, G.; Pilut, C.; Diaconu, M.; Neagoe, O.; Muntean, D.; Horhat, I.D.; Dragomir, A.; Lighezan, D. Chemical Composition and Antimicrobial Activity of Essential Oil of Romanian Origanum vulgare. Rev. Chim. 2019, 70, 1744–1745. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Mata-Cardenas, B.D.; Vargas-Villarreal, J.; Bazaldua-Rodriguez, A.F.; Angeles-Hernandez, I.K.; Garza-Gonzalez, J.N.; Hernandez-Garcia, M.E. Antiprotozoal Activity Against Entamoeba Histolytica of Plants Used in Northeast Mexican Traditional Medicine Bioactive Compounds from Lippia graveolens and Ruta chalepensis. Molecules 2014, 19, 21044–21065. [Google Scholar] [CrossRef]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Maricá, R.; Marco, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Cid-Perez, T.S.; Avila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevarez-Moorillon, G.V. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts from Waste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef]
- Tellez-Monzon, L.A.; Nolazco-Cama, D.M. Estudio de la Composicion Quimica del Aceite Esencial de Oregano (Origanum vulgare spp.) de Tacna. Ing. Ind. 2017, 35, 195–205. Available online: https://www.redalyc.org/articulo.oa?id=337453922010 (accessed on 5 May 2023). (In Spanish). [CrossRef][Green Version]
- Sarrazin, S.L.F.; Da Silva, L.A.; De Assunção, A.P.F.; Oliveira, R.B.; Calao, V.Y.P.; Da Silva, R.; Stashenko, E.E.; Maia, J.G.S.; Mourão, R.H.V. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warzynska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Carrasco, A.; Perez, E.; Cutillas, A.-B.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Origanum vulgare and Thymbra capitata Essential Oils from Spain: Determination of Aromatic Profile and Bioactivities. Nat. Prod. Commun. 2016, 11, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Acevedo, A.; Castañeda, M.L.; Blanco, K.M.; Cardenas, C.Y.; Reyes, J.A.; Kouznetsov, V.V.; Stashenko, E.E. Composicion y Capacidad Antioxidante de Especies Aromaticas y Medicinales con Alto Contenido de Timol y Carvacrol. Sci. Technol. 2007, 13, 125–128. Available online: https://www.redalyc.org/articulo.oa?id=84903329 (accessed on 10 May 2023). (In Spanish).
- da Costa, S.B.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Serra, A.T.; Martins, M.M.; Januário, M.I.N.; Vicente, A.A.; Delgadillo, I.; Duarte, C.; et al. Effect of the Matrix System in the Delivery and In Vitro Bioactivity of Microencapsulated Oregano Essential Oil. J. Food Eng. 2012, 110, 190–199. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Oxygen Radical Capacity (ORAC) of Selected Foods, Release 2. USDA National Nutrient Database for Standard Reference. 2010. Available online: http://www.ars.usda.gov/ARSUserFiles/80400525/Articles/AICR07_ORAC.pdf (accessed on 26 May 2023).
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant Capacity Variation in the Oregano (Origanum vulgare L.) Collection of the German National Genebank. Ind. Crops Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of Different Methods for Testing Antioxidative Activity of Oregano Essential Oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Milos, M.; Mastelic, J.; Jerkovic, I. Chemical Composition and Antioxidant Effect of Glycosidically Bound Volatile Compounds from Oregano (Origanum vulgare L. ssp. hirtum). Food Chem. 2000, 71, 79–83. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Salvatore, L.B.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular Characterization of Wild Sicilian Oregano: Phytochemical Screening of Essential Oils and Extracts, and Evaluation of their Antioxidant Activities. Chem. Biodivers. 2013, 10, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chem. 2000, 69, 167. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.M. Supplemental Antioxidants in Rabbit Nutrition: A Review. Livest. Sci. 2013, 158, 95–105. [Google Scholar] [CrossRef]
- Flores-Martinez, H.; Leon-Campos, C.; Estarron-Espinosa, M.; Orozco-Avila, I. Process Optimization for the Extraction of Antioxidants from Mexican Oregano (Lippia graveolens Hbk) by the Response Surface Methodology (RSM) Approach. Rev. Mex. Ing. Quim. 2016, 15, 773–785. Available online: https://www.redalyc.org/pdf/620/62048168009.pdf (accessed on 9 May 2023). (In Spanish). [CrossRef]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical Composition, Enantiomeric Distribution, Antimicrobial and Antioxidant Activities of Origanum majorana L. Essential Oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Antimicrobial Effect of Spices and Herbs on Shigella sonnei and Shigella flexneri. J. Food Prot. 2003, 66, 668–673. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the Volatile Composition of Essential Oils of Some Lamiaceae Spices and the Antimicrobial and Antioxidant Activities of the Entire Oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical Composition and Antimicrobial Activity of the Essential Oils from Three Chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart Growing Wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Nikmaram, N.; Esteghlal, S.; Khaneghah, A.M.; Niakousari, M.; Barba, F.J.; Roohinejad, S.; Koubaa, M. Efficiency of Ohmic Assisted Hydrodistillation for the Extraction of Essential Oil from Oregano (Origanum vulgare subsp. viride) Spices. Innov. Food Sci. Emerg. Technol. 2017, 41, 172–178. [Google Scholar] [CrossRef]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.; Raita, O.; Bischin, C.; Silaghi, R.; Vlase, L. Assessment of Rosmarinic Acid Content in Six Lamiaceae Species Extracts and their Antioxidant and Antimicrobial Potential. Pak. J. Pharm. Sci. 2015, 28, 2297–2303. [Google Scholar] [PubMed]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare Mediated Green Synthesis of Biocompatible Gold Nanoparticles Simultaneously Possessing Plasmonic, Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef] [PubMed]
- Bunghez, F.; Rotar, M.A.; Pop, R.M.; Romanciuc, F.; Csernatoni, F.; Fetea, F.; Diaconeasa, Z.; Socaciu, C. Comparative Phenolic Fingerprint and LC-ESI+QTOF-MS Composition of Oregano and Rosemary Hydrophilic Extracts in Relation to their Antibacterial Effect. Bull. UASVM Food Sci. Technol. 2015, 72, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yotova, I.; Ignatova-Ivanova, T. In Vitro Study of Antifungal Activity of Oregano (Origanum vulgare). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 321–326. Available online: https://www.ijcmas.com/vol-4-3/I.%20Yotova%20and%20Ignatova-Ivanova%20Ts.pdf (accessed on 25 March 2023).
- Ozcan, M.; Erkmen, O. Antimicrobial Activity of the Essential Oils of Turkish Plant Spice. Eur. Food Res. Technol. 2001, 212, 658–660. [Google Scholar] [CrossRef]
- Sökmen, M.; Serkedjieva, J.; Daferera, D.; Gulluce, M.; Polissiou, M.; Tepe, B.; Akpulat, A.; Sahin, F.; Sokmen, A. In Vitro Antioxidant, Antimicrobial, and Antiviral Activities of the Essential Oil and Various Extracts from Herbal Parts and Callus Cultures of Origanum acutidens. J. Agric. Food Chem. 2004, 52, 3309–3312. [Google Scholar] [CrossRef]
- Paredes-Aguilar, M.D.L.C.; Gastelum-Franco, M.G.; Silva-Vazquez, R.; Nevarez-Moorillon, G.V. Efecto Antimicrobiano del Oregano Mexicano (Lippia berlandieri Schauer) y de su Aceite Esencial Sobre Cinco Especies del Genero Vibrio. Rev. Fitotec. Mex. 2007, 30, 261–267. Available online: https://revistafitotecniamexicana.org/documentos/30-3/7r.pdf (accessed on 15 March 2023). (In Spanish). [CrossRef]
- Zweifel, C.; Stephan, R. Spices and herbs as source of Salmonella-related foodborne diseases. Food Res. Int. 2012, 45, 765–769. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.H.C.; Windisch, W. Essential Oils and Aromatic Plants in Animal Feeding—A European Perspective. A Review. Flavour Fragr. J. 2009, 25, 327–340. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeersma van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural Requirements for the Antimicrobial Activity of Carvacrol. J. Agric. Food Chem. 2006, 54, 1874. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of Action of Carvacrol on the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Kimbaris, A.; Varvatou, M.; Mantzourani, I.; Fournomiti, M. Mode of Antimicrobial Action of Origanum vulgare Essential Oil Against Clinical Pathogens. Curr. Res. Nutr. Food Sci. 2017, 5, 109–115. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and Multibeneficial Bioactivities of Carvacrol (4-isopropyl-2-methylphenol), a Component of Essential Oils Produced by Aromatic Plants and Spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Carvacrol-Rich Oregano Oil and Thymol-Rich Thyme Red Oil Inhibit Biofilm Formation and the Virulence of Uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Pubchem. Carvacrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/carvacrol#section=Solubility (accessed on 20 June 2023).
- Koraichi Saad, I.; Hassan, L.; Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and Thymol Components Inhibiting Pseudomonas aeruginosa Adherence and Biofilm Formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. Available online: https://www.internationalscholarsjournals.com/articles/carvacrol-and-thymol-components-inhibiting-pseudomonas-aeruginosa-adherence-and-biofilm-formation.pdf (accessed on 17 March 2023).
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ozkalp, B.; Sevgi, F.; Ozcan, M.; Ozcan, M.M. The Antibacterial Activity of Essential Oil of Oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. Available online: https://www.probotanic.com/pdf_istrazivanja/OriganoPDF/Antibakterijska%20aktivnost%20esencijalnog%20ulja%20divljeg%20origana.pdf (accessed on 2 May 2023).
- De Falco, E.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, Essential Oil Characterization, and Antimicrobial Activity of Three Wild Biotypes of Oregano Under Cultivation Condition in Southern Italy. Ind. Crops Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Kermani, S.; Dakhili, M.; Madani, M.; Parsa, S. Antifungal Properties of Artemisia sieberi and Origanum vulgare Essential Oils Against Candida glabrata Isolates Obtained from Patients with Vulvovaginal Candidiasis. J. Mycol. Méd. 2011, 21, 93–99. [Google Scholar] [CrossRef]
- Esen, G.; Azaz, A.D.; Kurkcuoglu, M.; Baser, K.H.C.; Tinmaz, A. Essential Oil and Antimicrobial Activity of Wild and Cultivated Origanum vulgare L. subsp. hirtum (Link) Letswaart from the Marmara Region. Turk. Flavour Fragr. J. 2007, 22, 371–376. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Zairi, A.; Nouir, S.; Khalifa, M.A.; Ouni, B.; Haddad, H.; Khelifa, A.; Trabelsi, M. Phytochemical Analysis & Assessment of Biological Properties of Essential Oils Obtained from Thyme & Rosmarinus Species. Curr. Pharm. Biotechnol. 2019, 20, 414–424. [Google Scholar] [CrossRef]
- Silva-Marrufo, O.; Marin-Tinoco, R.I. Substitute of Synthetic Chemical Fungicides Using Oregano Essential Oil for Controlling Fusarium oxysporum. Gestion Ambiente 2021, 24, 73–80. [Google Scholar] [CrossRef]
- Servicio Meteorologico Nacional—National Metereological Service (SMN). Normales Climatologicas por Estado. Informacion Climatologica. Comision Nacional del Agua. 2010. Available online: https://smn.conagua.gob.mx/es/climatologia/informacion-climatologica/normales-climatologicas-por-estado (accessed on 28 March 2023). (In Spanish).
- do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; El Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial Activity, Optical, Mechanical, and Barrier Properties of Corn Starch Films Containing Orange Essential Oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Childs, R.E.; Bardsley, W.G. The Steady-state Kinetics of Peroxidase with 2, 2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as Chromogen. Biochem. J. 1975, 145, 93–103. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1165190/pdf (accessed on 10 April 2023). [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically, 11th ed.; Approved Standard M07ed11e; National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 2019. [Google Scholar]
- Rossi, C.; Arias, G.; Lozano, N. Evaluacion Antimicrobiana y Fitoquimica de Lepechenia meyeni Walp. Salvia. Cienc. Investig. 2002, 5, 30–36. (In Spanish) [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Buzzini, P.; Pieroni, A. Antimicrobial Activity of Extracts of Clematis vitalba Towards Pathogenic Yeast and Yeast-Like Microorganisms. Fitoterapia 2003, 74, 397–400. [Google Scholar] [CrossRef]
- Jehl, F.; Bonnet, R.; Bru, J.; Caron, F.; Cattoen, C.; Cattoir, V. Comité de L’antibiogramme de la Société Française de Microbiologie; Recommandations 2016. Février 1; Societe Francaise de Microbiologie: Paris, France, 2016; Volume 117. [Google Scholar]
- Rivadeneira-Pacheco, J.L.; De La Hoz-Suarez, A.I.; Barrera-Argüello, M.V. General Analysis of the SPSS and its Usefulness in Statistics. E-IDEA J. Bus. Sci. 2020, 2, 17–25. Available online: https://revista.estudioidea.org/ojs/index.php/eidea/article/view/19 (accessed on 25 March 2023). (In Spanish).
| (a) | ||||||
| Num. | TR (min) | Compound Name | Percentage in the Sample (Relative Areas) | |||
| Wild1 | Wild2 | Cfield | CGreenhouse | |||
| 1 | 4.39 | Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)- | 0.31 | 0.32 | 0.39 | 0.41 |
| 2 | 4.70 | Tricyclo[2.2.1.0(2,6)]heptane, 1,3,3-trimethyl- | 0.43 | 0.47 | 0.42 | 0.42 |
| 3 | 5.08 | Beta-myrcene | 4.40 | 4.50 | 4.50 | 4.50 |
| 4 | 5.37 | (+)-4-Carene | 1.87 | 1.89 | 2.01 | 1.90 |
| 5 | 5.46 | o-Cymene | 13.42 | 13.24 | 11.90 | 12.50 |
| 6 | 5.51 | d-Limonene | 1.50 | 1.60 | 1.60 | 1.90 |
| 7 | 5.54 | Eucalyptol | 1.66 | 1.65 | 2.01 | 1.50 |
| 8 | 5.83 | Gamma-Terpine | 6.23 | 7.10 | 6.70 | 6.41 |
| 9 | 5.91 | Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1α,2β,5α)- | 0.20 | 0.21 | 0.31 | 0.30 |
| 10 | 6.14 | cyclohexane 1-methyl-4-(1-methylethylidene)- | 0.26 | 0.31 | 0.28 | 0.27 |
| 11 | 6.24 | Linalool | 0.95 | 0.98 | 1.02 | 1.02 |
| 12 | 7.06 | 3-cyclohexen-1-ol 4-methyl-1-(1-methylethyl)- (r)- | 1.79 | 1.80 | 2.01 | 1.84 |
| 13 | 7.22 | Alpha-terpineol | 0.27 | 0.30 | 0.29 | 0.28 |
| 14 | 7.59 | Benzene 2-methoxy-4-methyl-1-(1-methylethyl)- | 0.16 | 0.17 | 0.17 | 0.15 |
| 15 | 8.16 | Thymol | 8.90 | 6.90 | 1.90 | 13.90 |
| 16 | 8.23 | Carvacrol | 16.60 | 14.10 | 16.20 | 25.20 |
| 17 | 8.74 | Eugenol | 0.31 | 0.42 | 0.33 | 0.32 |
| 18 | 9.38 | Caryophyllene | 4.83 | 5.01 | 5.01 | 4.90 |
| 19 | 9.46 | cis-alpha-bergamotene | 1.14 | 1.10 | 2.00 | 1.20 |
| 20 | 9.66 | Humulene | 2.32 | 2.33 | 3.14 | 3.01 |
| 21 | 9.79 | Phenol, 3-(1,1-dimethylethyl)-4-methoxy | 0.41 | 0.39 | 0.51 | 0.42 |
| 22 | 10.06 | β-Bisabolene | 0.20 | 0.30 | 0.22 | 0.21 |
| 23 | 10.72 | Caryophyllene oxide | 0.76 | 0.79 | 0.79 | 0.78 |
| 24 | 10.92 | (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene | 0.28 | 0.26 | 0.30 | 0.28 |
| (b) | ||||||
| Site | Thymol (%) | Carvacrol (%) | ||||
| Wild1 | 8.4 ± 1.02 ab | 16.4 ± 1.30 ab | ||||
| Wild2 | 6.6 ± 0.99 b | 13.8 ± 1.83 ab | ||||
| CField | 1.7 ± 0.04 c | 15.7 ± 1.06 ab | ||||
| CGreenhouse | 14.1 ± 1.680 a | 25.7 ± 2.02 a | ||||
| Site/ Concentration (µL mL−1) | ABTS Antioxidant Activity (%) | |||
|---|---|---|---|---|
| 0.01 | 2.50 | 5.00 | 10.00 | |
| Wild1 | 85.74 ± 0.21 a | 90.69 ± 0.18 b | 93.44 ± 0.54 b | 95.61 ± 0.53 c |
| Wild2 | 90.80 ± 0.01 a | 93.28 ± 0.11 a | 93.28 ± 0.11 b | 95.27 ± 0.10 b |
| CField | 91.47 ± 0.05 b | 90.42 ± 0.13 b | 93.40 ± 0.20 b | 92.27 ± 0.20 b |
| CGreenhouse | 92.76 ± 0.19 b | 93.05 ± 0.01 b | 93.33 ± 0.11 b | 94.50 ± 0.28 b |
| Specie | Production System | Assay Type to Calculate the AoA 1 | % AoA | Region, Country |
|---|---|---|---|---|
| Lippia graveolens | PNO 2 | ABTS 5 | 93.4% | Durango, Mexico |
| CField 3 | 91.9% | |||
| CGH 4 | 92.3% | |||
| Poliomintha longiflora | CField | ORAC 6 | 34.7% | Nuevo Leon, Mexico [16] |
| PNO | 55.6% | |||
| Origanum vulgare | CField | DPPH 7 | 61.7–62.0% | Hubei, China [17] |
| ABTS | 91.5–95.9% | |||
| Oregano (Scientific name NS 8) | NS | DPPH | 79.0–99.8% | Collection site NS [20] |
| Origanum vulgare spp. hirtum | CField | ABTS | 8.5% | Warszawa, Poland [22] |
| Origanum vulgare spp. vulgare | 8.6% | |||
| Origanum vulgare | NS | DPPH | 12.9–47.3% | Uttarakhand, India [46] |
| Origanum vulgare | CField | ABTS | 35.0–62.5% | Murcia, Spain [55] |
| Origanum vulgare | PNO | ORAC | 52% | Alentejo, Portugal [57] |
| Poliomintha longiflora | PNO | ORAC | 92.2% | USA [58] |
| Origanum vulgare spp. vulgare | 64.7% | |||
| Oregano (Scientific name NS) | NS | ORAC | 50% | USA [59] |
| Origanum vulgare | CField | ORAC | 39.8–84.8% | Gatersleben, Germany [60] |
| Site | Latitude (N) | Longitude (W) | Altitude (m) | Soil Type | Habitat |
|---|---|---|---|---|---|
| Wild1 | 25.0233° | 104.4758° | 1471 | Phaeozem | Microphyll scrub, semiarid |
| Wild2 | 25.1786° | 104.5686° | 1446 | Chernozem | Microphyll scrub, semiarid |
| CField | 25.2386° | 104.5675° | 1338 | Fluvisol | Irrigation agriculture |
| CGreenhouse | 25.1650° | 104.5558° | 1342 | Chernozem | Controlled conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin-Tinoco, R.I.; Ortega-Ramírez, A.T.; Esteban-Mendez, M.; Silva-Marrufo, O.; Barragan-Ledesma, L.E.; Valenzuela-Núñez, L.M.; Briceño-Contreras, E.A.; Sariñana-Navarrete, M.A.; Camacho-Luis, A.; Navarrete-Molina, C. Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico. Molecules 2023, 28, 6547. https://doi.org/10.3390/molecules28186547
Marin-Tinoco RI, Ortega-Ramírez AT, Esteban-Mendez M, Silva-Marrufo O, Barragan-Ledesma LE, Valenzuela-Núñez LM, Briceño-Contreras EA, Sariñana-Navarrete MA, Camacho-Luis A, Navarrete-Molina C. Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico. Molecules. 2023; 28(18):6547. https://doi.org/10.3390/molecules28186547
Chicago/Turabian StyleMarin-Tinoco, Ruben I., Angie Tatiana Ortega-Ramírez, Maricela Esteban-Mendez, Oscar Silva-Marrufo, Laura E. Barragan-Ledesma, Luis M. Valenzuela-Núñez, Edwin A. Briceño-Contreras, Maria A. Sariñana-Navarrete, Abelardo Camacho-Luis, and Cayetano Navarrete-Molina. 2023. "Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico" Molecules 28, no. 18: 6547. https://doi.org/10.3390/molecules28186547
APA StyleMarin-Tinoco, R. I., Ortega-Ramírez, A. T., Esteban-Mendez, M., Silva-Marrufo, O., Barragan-Ledesma, L. E., Valenzuela-Núñez, L. M., Briceño-Contreras, E. A., Sariñana-Navarrete, M. A., Camacho-Luis, A., & Navarrete-Molina, C. (2023). Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico. Molecules, 28(18), 6547. https://doi.org/10.3390/molecules28186547