Electrophysical Properties and Heat Capacity of Activated Carbon Obtained from Coke Fines
Abstract
:1. Introduction
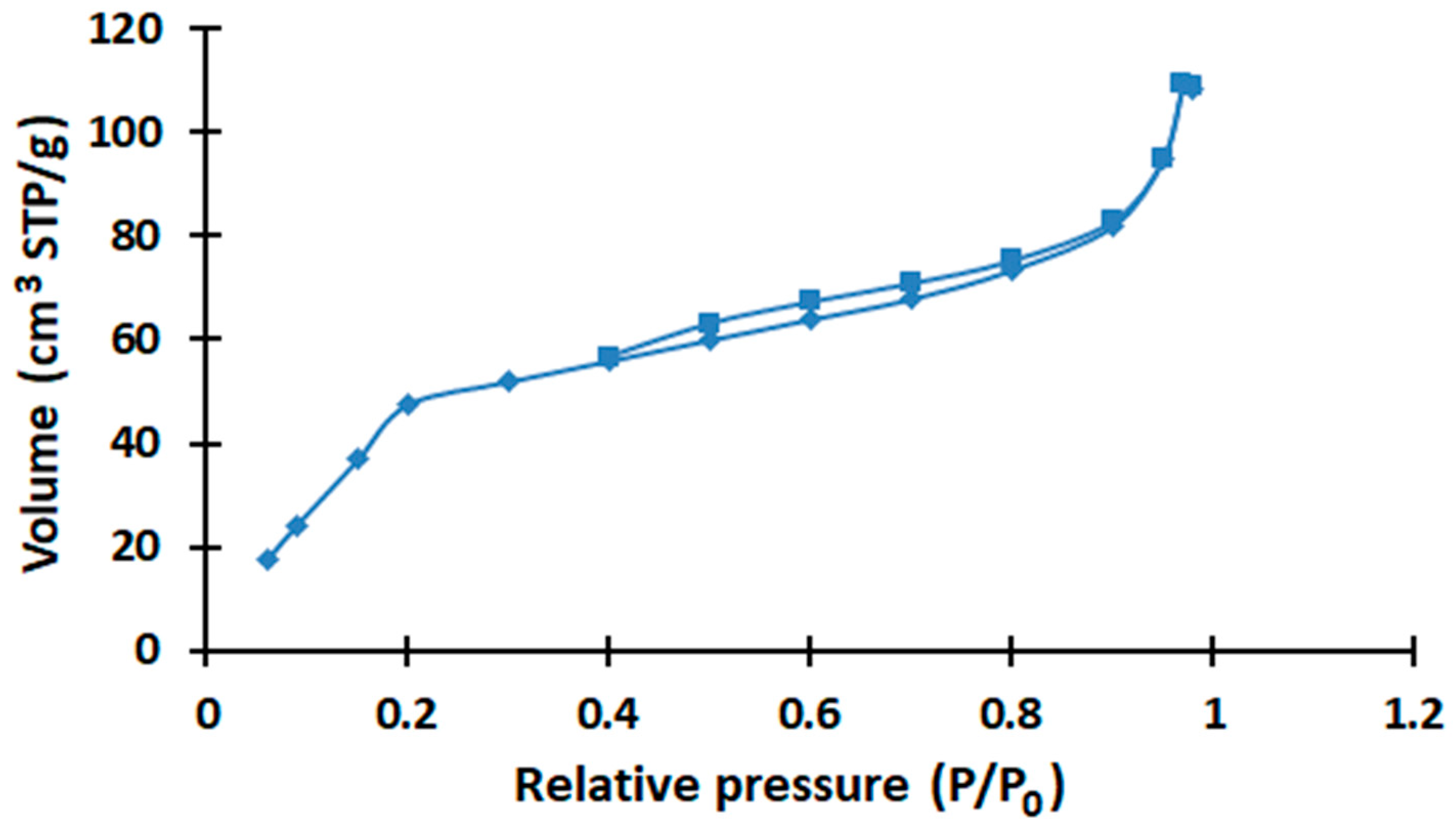
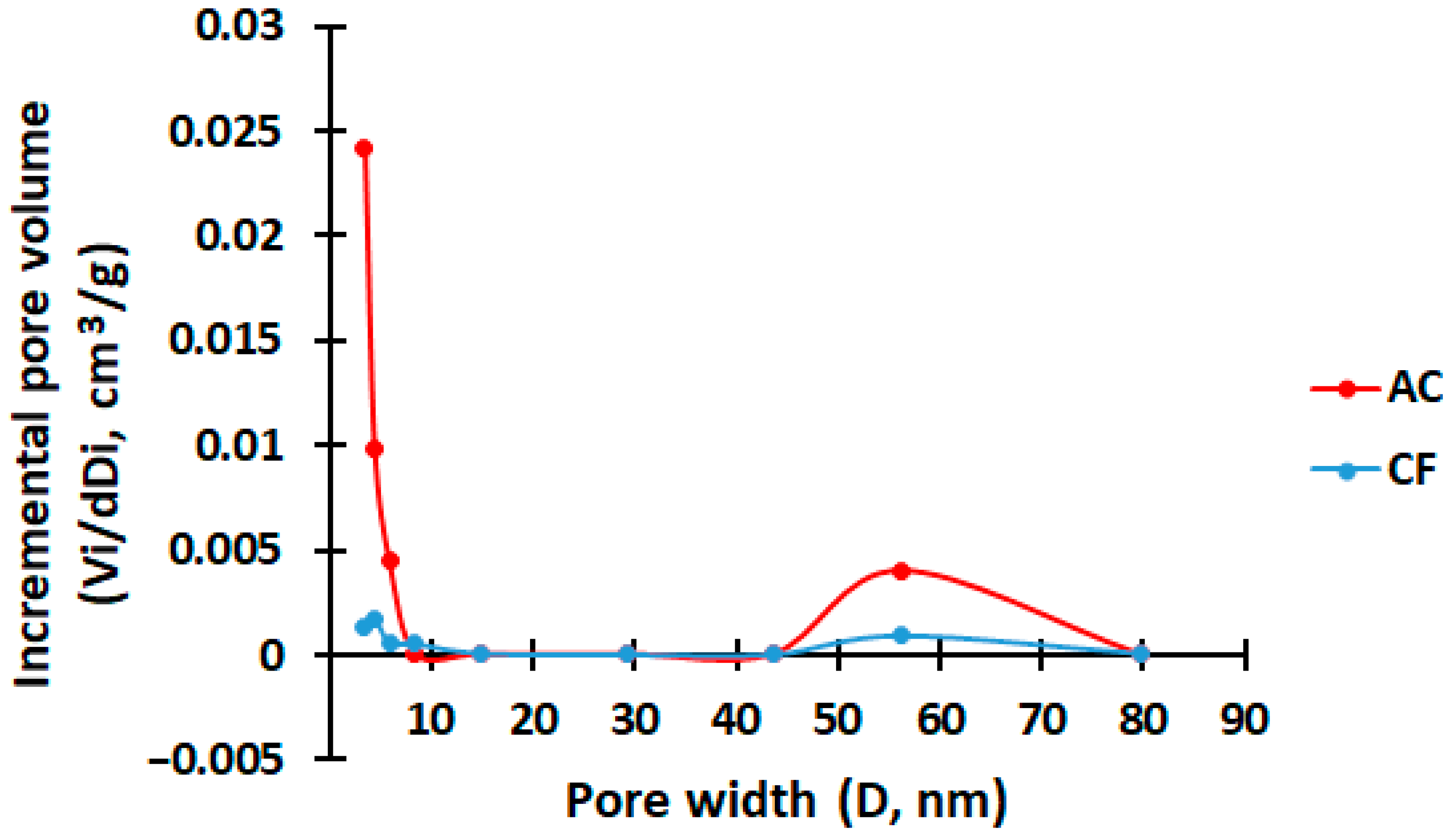
2. Results and Discussion
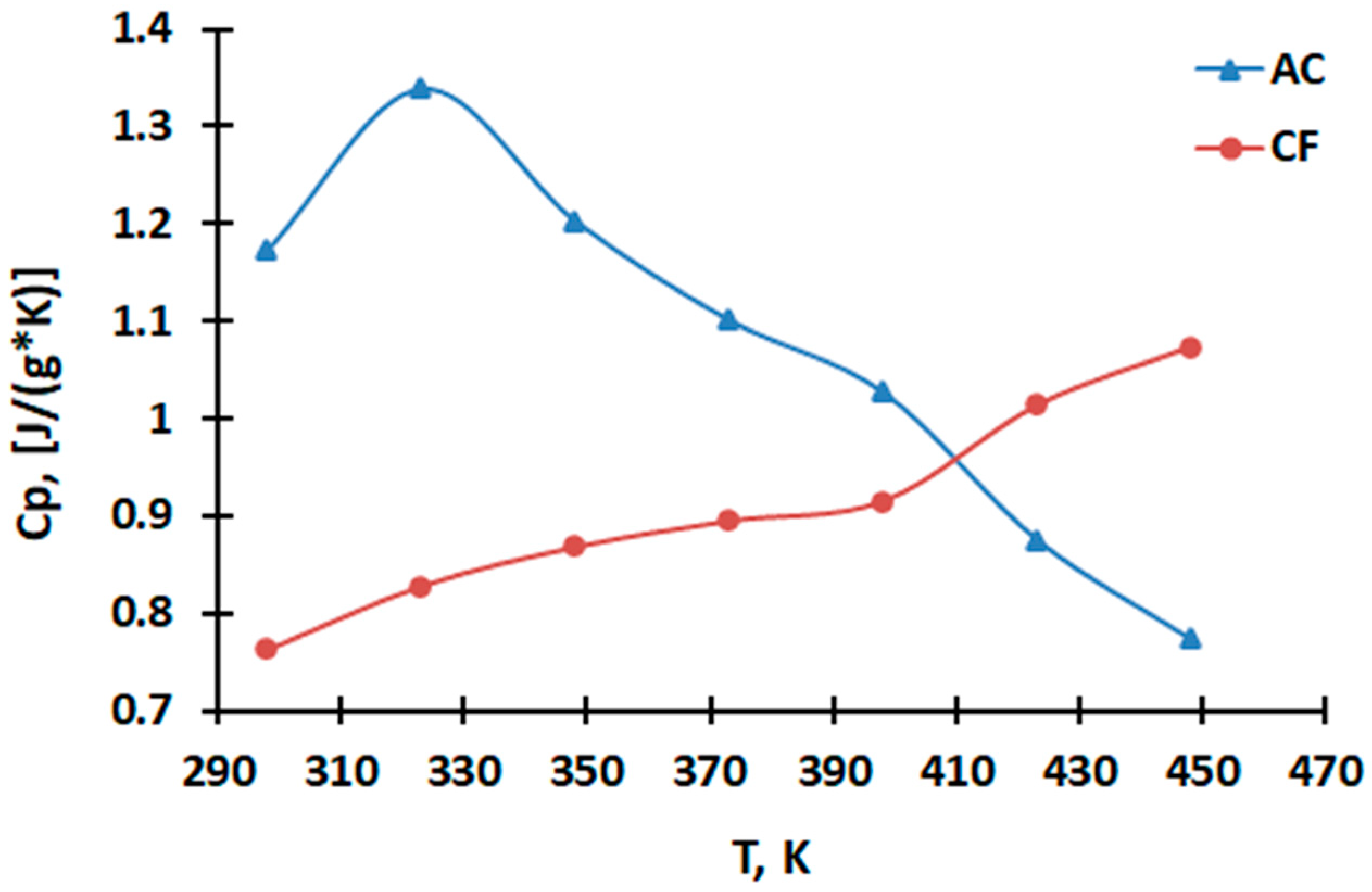
2.1. Heat Capacity Measurement
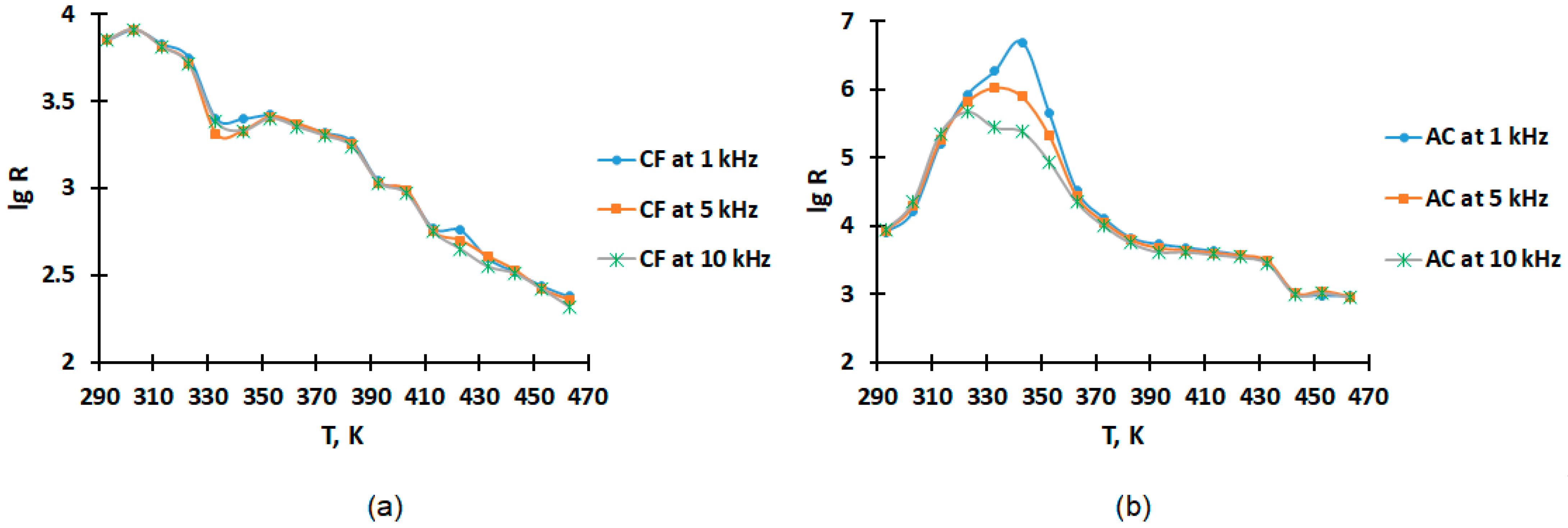
2.2. Electrophysical Properties Measurement
3. Materials and Methods
3.1. Results of Heat Capacity Measurement
3.2. Results of Electrophysical Properties Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, L.L. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Liu, J.; Mirri, F.; Notarianni, M.; Pasquali, M.; Motta, N. High performance all-carbon thin film supercapacitors. J. Power Sources 2015, 274, 823–830. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Orugba, Y.O.; Arias, D.M.; Cuentas-Gallegos, A.K.; Okolie, J.A.; Okoye, P.U. Sponge-like nanoporous activated carbon from corn husk as a sustainable and highly stable supercapacitor electrode for energy storage. Diam. Relat. Mater. 2023, 138, 110176. [Google Scholar] [CrossRef]
- Tu, J.; Qiao, Z.; Wang, Y.; Li, G.; Zhang, X.; Li, G.; Ruan, D. American ginseng biowaste-derived activated carbon for high-performance supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 16–24. [Google Scholar] [CrossRef]
- Ayinla, R.T.; Dennis, J.O.; Zaid, H.B.M.; Usman, F.; Yar, A. Effect of particle size on the physical properties of activated palm kernel shell for supercapacitor application. Key Eng. Mater. 2020, 833, 129–133. [Google Scholar] [CrossRef]
- Hu, S.-C.; Cheng, J.; Wang, W.-P.; Sun, G.-T.; Hu, L.-L.; Zhu, M.-Q.; Huang, X.-H. Structural changes and electrochemical properties of lacquer wood activated carbon prepared by phosphoric acid-chemical activation for supercapacitor applications. Renew. Energy 2021, 177, 82–94. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Yeleuov, M.; Sultakhan, S.T.; Maltay, A.B.; Zhaparova, A.A.; Assylkhanova, D.D.; Nemkayeva, R.R. Electrochemical performance of chemically activated carbons from sawdust as supercapacitor electrodes. Nanomaterials 2022, 12, 3391. [Google Scholar] [CrossRef]
- Piñeiro-Prado, I.; Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Design of activated carbon/activated carbon asymmetric capacitors. Front. Mater. 2016, 3, 16. [Google Scholar] [CrossRef]
- Boujibar, Q.; Ghamouss, F.; Ghosh, A.; Ouafae Achak, Q.; Chafik, T. Activated carbon with exceptionally high surface area and tailored nanoporosity obtained from natural anthracite and its use in supercapacitors. J. Power Sources 2019, 436, 4255. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, T.; Dong, D.; Zhang, Y. Using biochar and coal as the electrode material for supercapacitor applications. Front. Energy Res. 2020, 7, 159. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, N.; Feng, M. Activated carbon derived from waste oil shale semi-coke for supercapacitor application. Molecules 2023, 28, 4804. [Google Scholar] [CrossRef]
- Kierzek, K.; Gryglewicz, G. Activated carbons and their evaluation in electric double layer capacitors. Molecules 2020, 25, 4255. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.H.; Lee, T. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef] [PubMed]
- Bláha, M.; Bouša, M.; Valeš, V.; Frank, O.; Kalbáč, M. Two-Dimensional CVD-graphene/polyaniline supercapacitors: Synthesis strategy and electrochemical operation. ACS Appl. Mater. Interfaces 2021, 13, 34686–34695. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, T.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Masarapu, C.; Zeng, H.F.; Hung, K.H.; Wei, B. Effect of temperature on the capacitance of carbon nanotube supercapacitors. ACS Nano 2009, 3, 2199–2206. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Nie, P.; Xu, G.; Shi, M.; Wang, J.; Wu, Y.; Fu, R.; Dou, H.; Zhang, X. Progress of nanostructured electrode materials for supercapacitors. Adv. Sustain. Syst. 2017, 2, 1700110. [Google Scholar] [CrossRef]
- Wang, Z.; Melvin, G.J.M. Carbon Nanomaterials for Energy Storage Devices. In Nanotechnology: Applications in Energy, Drug and Food; Springer: Cham, Switzerland, 2019; pp. 1–29. [Google Scholar]
- De, B.; Banerjee, S.; Verma, K.D.; Pal, T.; Manna, P.K.; Kar, K.K. Carbon nanotube as electrode materials for supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II; Springer: Cham, Switzerland, 2020; pp. 229–243. [Google Scholar]
- Bavio, M.A.; Acosta, G.G.; Kessler, T. Synthesis and characterization of polyaniline and polyaniline—Carbon nanotubes nanostructures for electrochemical supercapacitors. J. Power Sources 2014, 245, 475–481. [Google Scholar] [CrossRef]
- Xu, M.-W.; Bao, S.-J.; Li, H.-L. Synthesis and characterization of mesoporous nickel oxide for electrochemical capacitor. J. Solid State Electrochem. 2007, 11, 372–377. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Lin, L.-Y. Material effects on the electrocapacitive performance for the Energy-storage electrode with nickel cobalt oxide core/shell nanostructures. Electrochim. Acta 2017, 250, 335–347. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chena, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Malik, S.; Gul, I.H.; Baig, M.M. Hierarchical MnNiCo ternary metal oxide/graphene nanoplatelets composites as high rated electrode material for supercapacitors. Ceram. Int. 2021, 47, 17008–17014. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, C.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Bazaluk, O.; Hrubiak, A.; Moklyak, V.; Moklyak, M.; Kieush, L.; Rachiy, B.; Gasyuk, I.; Yavorskyi, Y.; Koveria, A.; Lozynskyi, V.; et al. Structurally Dependent Electrochemical Properties of Ultrafine Superparamagnetic ’Core/Shell’ γ-Fe2O3/Defective α-Fe2O3 Composites in Hybrid Supercapacitors. Materials 2021, 14, 6977. [Google Scholar] [CrossRef] [PubMed]
- Ordabaeva, A.T.; Muldakhmetov, Z.M.; Gazaliev, A.M.; Kim, S.V.; Shaikenova, Z.S.; Meiramov, M.G. Production of activated carbon from sifted coke and determination of its physicochemical characteristics. Molecules 2023, 28, 5661. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.A.; Loh, W.S.; Ng, K. Heat of adsorption and adsorbed phase specific heat capacity of methane/activated carbon system. Procedia Eng. 2013, 56, 118–125. [Google Scholar] [CrossRef]
- Strizhenov, E.M.; Chugaev, S.S.; Men’shchikov, I.E.; Shkolin, A.V.; Zherdev, A.A. Heat and mass transfer in an adsorbed natural gas storage system filled with monolithic carbon adsorbent during circulating gas charging. Nanomaterials 2021, 11, 3274. [Google Scholar] [CrossRef]
- Querejeta, N.; García, S.; Álvarez-Gutiérrez, N.; Rubieraa, F.; Pevida, C. Measuring heat capacity of activated carbons for CO2 capture. J. CO2 Util. 2019, 33, 148–156. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P.; Dey, T.K. Effect of temperature on dielectric properties and penetration depth of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS. Fuel 2015, 153, 257–266. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; 472p. [Google Scholar]
- Muthmann, J.; Blaker, C.; Pasel, C.; Luckas, M.; Schledorn, C.; Bathen, D. Characterization of structural and chemical modifications during the steam activation of activated carbons. Microporous Mesoporous Mater. 2020, 309, 110549. [Google Scholar] [CrossRef]
- Xie, X.; Wu, D.; Wu, H.; Hou, C.; Sun, X.; Zhang, Y.; Yu, R.; Zhang, S.; Wang, B.; Du, W. Dielectric parameters of activated carbon derived from rosewood and corncob. J. Mater. Sci. Mater. Electron. 2020, 31, 18077–18084. [Google Scholar] [CrossRef]
- Technical Description and Operating Instructions of the IT-S-400; (In Russian). Aktobe Plant “Etalon”: Aktyubinsk, Kazakhstan, 1986.
- Platunov, E.S.; Buravoy, S.E.; Kurepin, V.V.; Petrov, G.S. Thermophysical Measurements and Devices; Mechanical Engineering: Leningrad, Russia, 1986; p. 256. (In Russian) [Google Scholar]
- Spiridonov, V.P.; Lopatkin, A.A. Mathematical Processing of Experimental Data; MSU: Moscow, Russia, 1970; p. 221. (In Russian) [Google Scholar]
- Robie, R.A.; Hewingway, B.S.; Fisher, I.K. Thermodynamic Properties of Minerals and Related Substances at 298.15 and (105 Paskals) Pressure and at Higher Temperature; United States Government Printing Office: Washington, DC, USA, 1978; p. 456.
- Okazaki, K. Technology of Ceramic Dielectrics; Energiya: Moscow, Russia, 1976; p. 256. (In Russian) [Google Scholar]
- Zhumadilov, E.K.; Davrenbekov, S.Z.; Mustafin, E.S.; Kasenov, B.K.; Edilbaeva, S.T. Investigation of electrophysical properties of chromite GdSrCr2O5,5. Bull. NAS RK 2004, 5, 114–118. [Google Scholar]

| T, K | Coke Fines | Activated Carbon |
|---|---|---|
| Cp ± δ | Cp ± δ | |
| 298 | 0.7629 ± 0.0180 | 1.1730 ± 0.0221 |
| 323 | 0.8280 ± 0.0215 | 1.3383 ± 0.0290 |
| 348 | 0.8681 ± 0.0180 | 1.2027 ± 0.0178 |
| 373 | 0.8949 ± 0.0251 | 1.1008 ± 0.0410 |
| 398 | 0.9152 ± 0.0159 | 1.0268 ± 0.0303 |
| 423 | 1.0133 ± 0.0170 | 0.8752 ± 0.0210 |
| 448 | 1.0729 ± 0.0297 | 0.7732 ± 0.0163 |
| T, K | CF | AC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C, nF | R, OM | ε | lgε | lgR | C, nF | R, OM | ε | lgε | lgR | |
| 1 kHz | ||||||||||
| 293 | 5729.7 | 7105 | 57,732,052 | 7.76 | 3.85 | 2267 | 7865 | 16,315,808 | 7.21 | 3.90 |
| 303 | 4425.3 | 8210 | 44,589,010 | 7.65 | 3.91 | 1299.3 | 15,870 | 9,351,182 | 6.97 | 4.20 |
| 313 | 3605.4 | 6702 | 36,327,756 | 7.56 | 3.83 | 16.189 | 157,300 | 116,514 | 5.07 | 5.20 |
| 323 | 2917.7 | 5618 | 29,398,539 | 7.47 | 3.75 | 3.6397 | 818,000 | 26,195 | 4.42 | 5.91 |
| 333 | 5162.6 | 2534 | 52,017,993 | 7.72 | 3.40 | 0.40742 | 1,836,000 | 2932 | 3.47 | 6.26 |
| 343 | 6548.3 | 2520 | 65,980,208 | 7.82 | 3.40 | 0.04409 | 4,917,000 | 317 | 2.50 | 6.69 |
| 353 | 2036.7 | 2658 | 20,521,645 | 7.31 | 3.42 | 8.1254 | 451,700 | 58,479 | 4.77 | 5.65 |
| 363 | 2321.3 | 2337 | 23,389,255 | 7.37 | 3.37 | 76.22 | 32,820 | 548,562 | 5.74 | 4.52 |
| 373 | 3697.1 | 2088 | 37,251,718 | 7.57 | 3.32 | 163.73 | 12,920 | 1,178,380 | 6.07 | 4.11 |
| 383 | 6777.9 | 1879 | 68,293,641 | 7.83 | 3.27 | 270.29 | 6681 | 1,945,302 | 6.29 | 3.82 |
| 393 | 27,544 | 1087 | 277,531,397 | 8.44 | 3.04 | 337.89 | 5388 | 2,431,826 | 6.39 | 3.73 |
| 403 | 43,275 | 966.6 | 436,035,842 | 8.64 | 2.99 | 449.5 | 4815 | 3,235,093 | 6.51 | 3.68 |
| 413 | 72,480 | 589.1 | 730,303,357 | 8.86 | 2.77 | 810.33 | 4260 | 5,832,020 | 6.77 | 3.63 |
| 423 | >99,999 | 568.9 | 1,007,582,857˂ | 9.00˂ | 2.76 | 2917.5 | 3692 | 20,997,517 | 7.32 | 3.57 |
| 433 | >99,999 | 397.4 | 1,007,582,857˂ | 9.00˂ | 2.60 | 6636.5 | 2864 | 47,763,504 | 7.68 | 3.46 |
| 443 | >99,999 | 332.9 | 1,007,582,857˂ | 9.00˂ | 2.52 | 45,213 | 1047 | 325,402,138 | 8.51 | 3.02 |
| 453 | >99,999 | 273.4 | 1,007,582,857˂ | 9.00˂ | 2.44 | 81,282 | 958.8 | 584,994,062 | 8.77 | 2.98 |
| 463 | >99,999 | 241.1 | 1,007,582,857˂ | 9.00˂ | 2.38 | 72,280 | 938.5 | 520,205,837 | 8.72 | 2.97 |
| T, K | 5 kHz | |||||||||
| CF | AC | |||||||||
| C, nF | R, OM | ε | lgε | lgR | C, nF | R, OM | ε | lgε | lgR | |
| 293 | 287.04 | 7001 | 2,892,195 | 6.46 | 3.85 | 100.63 | 8377 | 724,243 | 5.86 | 3.92 |
| 303 | 263.37 | 8187 | 2,653,698 | 6.42 | 3.91 | 38.961 | 19,330 | 280,406 | 5.45 | 4.29 |
| 313 | 280.69 | 6494 | 2,828,213 | 6.45 | 3.81 | 0.93929 | 179,000 | 6760 | 3.83 | 5.25 |
| 323 | 366.19 | 5136 | 3,689,705 | 6.57 | 3.71 | 0.12581 | 642,000 | 905 | 2.96 | 5.81 |
| 333 | 1016.6 | 2033 | 10,243,190 | 7.01 | 3.31 | 0.03235 | 1,036,000 | 233 | 2.37 | 6.02 |
| 343 | 636.39 | 2158 | 6,412,221 | 6.81 | 3.33 | 0.01649 | 782,300 | 119 | 2.07 | 5.89 |
| 353 | 222.7 | 2555 | 2,243,909 | 6.35 | 3.41 | 0.7858 | 207,400 | 5655 | 3.75 | 5.32 |
| 363 | 285.97 | 2341 | 2,881,414 | 6.46 | 3.37 | 7.6724 | 27,130 | 55,219 | 4.74 | 4.43 |
| 373 | 471.15 | 2032 | 4,747,274 | 6.68 | 3.31 | 22.825 | 11,130 | 164,274 | 5.22 | 4.05 |
| 383 | 737.33 | 1769 | 7,429,285 | 6.87 | 3.25 | 42.547 | 6159 | 306,215 | 5.49 | 3.79 |
| 393 | 3122.9 | 1079 | 31,466,120 | 7.50 | 3.03 | 61.784 | 4649 | 444,665 | 5.65 | 3.67 |
| 403 | 4685.8 | 972.6 | 47,213,790 | 7.67 | 2.99 | 63.882 | 4355 | 459,765 | 5.66 | 3.64 |
| 413 | 14,462 | 557.7 | 145,718,090 | 8.16 | 2.75 | 97.663 | 3965 | 702,890 | 5.85 | 3.60 |
| 423 | 42,585 | 506 | 429,083,450 | 8.63 | 2.70 | 270.05 | 3593 | 1,943,575 | 6.29 | 3.56 |
| 433 | 64,673 | 405.5 | 651,640,577 | 8.81 | 2.61 | 537.41 | 2999 | 3,867,789 | 6.59 | 3.48 |
| 443 | 78,170 | 335.7 | 787,635,395 | 8.90 | 2.53 | 3755.8 | 1033 | 27,030,840 | 7.43 | 3.01 |
| 453 | 41,549 | 265.9 | 418,644,788 | 8.62 | 2.42 | 12,019 | 1103 | 86,501,853 | 7.94 | 3.04 |
| 463 | 48,901 | 231.7 | 492,723,020 | 8.69 | 2.36 | 6025.4 | 916.2 | 43,365,360 | 7.64 | 2.96 |
| T, K | 10 kHz | |||||||||
| CF | AC | |||||||||
| C, nF | R, OM | ε | lgε | lgR | C, nF | R, OM | ε | lgε | lgR | |
| 293 | 87.024 | 7032 | 876,848 | 5.94 | 3.85 | 26.717 | 8657 | 192,285 | 5.28 | 3.94 |
| 303 | 62.159 | 8057 | 626,310 | 5.80 | 3.91 | 12.159 | 22,400 | 87,509 | 4.94 | 4.35 |
| 313 | 87.164 | 6433 | 878,258 | 5.94 | 3.81 | 0.15926 | 224,000 | 1146 | 3.06 | 5.35 |
| 323 | 82.933 | 5166 | 835,627 | 5.92 | 3.71 | 0.0239 | 464,800 | 172 | 2.24 | 5.67 |
| 333 | 332.41 | 2401 | 3,349,340 | 6.52 | 3.38 | 0.0165 | 275,400 | 119 | 2.07 | 5.44 |
| 343 | 209.15 | 2160 | 2,107,381 | 6.32 | 3.33 | 0.01581 | 238,000 | 114 | 2.06 | 5.38 |
| 353 | 81.966 | 2511 | 825,884 | 5.92 | 3.40 | 0.35065 | 87,410 | 2524 | 3.40 | 4.94 |
| 363 | 113.54 | 2258 | 1,144,021 | 6.06 | 3.35 | 3.3075 | 22,460 | 23,804 | 4.38 | 4.35 |
| 373 | 182.13 | 2011 | 1,835,129 | 6.26 | 3.30 | 9.291 | 9992 | 66,868 | 4.83 | 4.00 |
| 383 | 302 | 1718 | 3,042,931 | 6.48 | 3.24 | 17.338 | 5607 | 124,783 | 5.10 | 3.75 |
| 393 | 1086 | 1080 | 10,942,459 | 7.04 | 3.03 | 28.703 | 4123 | 206,578 | 5.32 | 3.62 |
| 403 | 1606.6 | 935.3 | 16,187,988 | 7.21 | 2.97 | 26.412 | 4205 | 190,090 | 5.28 | 3.62 |
| 413 | 3506.4 | 563.1 | 35,330,239 | 7.55 | 2.75 | 42.101 | 3829 | 303,005 | 5.48 | 3.58 |
| 423 | 6223.9 | 450.9 | 62,711,577 | 7.80 | 2.65 | 87.57 | 3433 | 630,249 | 5.80 | 3.54 |
| 433 | 17,710 | 355.2 | 178,444,708 | 8.25 | 2.55 | 158.55 | 2803 | 1,141,099 | 6.06 | 3.45 |
| 443 | 11,488 | 324.3 | 115,752,276 | 8.06 | 2.51 | 1156.7 | 1008 | 8,324,877 | 6.92 | 3.00 |
| 453 | 22,214 | 260.2 | 223,826,694 | 8.35 | 2.42 | 3174.7 | 1038 | 22,848,609 | 7.36 | 3.02 |
| 463 | 28,432 | 211.1 | 286,478,823 | 8.46 | 2.32 | 1676.4 | 902.1 | 12,065,206 | 7.08 | 2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordabaeva, A.T.; Muldakhmetov, Z.M.; Kim, S.V.; Kasenova, S.B.; Sagintaeva, Z.I.; Gazaliev, A.M. Electrophysical Properties and Heat Capacity of Activated Carbon Obtained from Coke Fines. Molecules 2023, 28, 6545. https://doi.org/10.3390/molecules28186545
Ordabaeva AT, Muldakhmetov ZM, Kim SV, Kasenova SB, Sagintaeva ZI, Gazaliev AM. Electrophysical Properties and Heat Capacity of Activated Carbon Obtained from Coke Fines. Molecules. 2023; 28(18):6545. https://doi.org/10.3390/molecules28186545
Chicago/Turabian StyleOrdabaeva, Aigul T., Zainulla M. Muldakhmetov, Sergey V. Kim, Shuga B. Kasenova, Zhenisgul I. Sagintaeva, and Arstan M. Gazaliev. 2023. "Electrophysical Properties and Heat Capacity of Activated Carbon Obtained from Coke Fines" Molecules 28, no. 18: 6545. https://doi.org/10.3390/molecules28186545
APA StyleOrdabaeva, A. T., Muldakhmetov, Z. M., Kim, S. V., Kasenova, S. B., Sagintaeva, Z. I., & Gazaliev, A. M. (2023). Electrophysical Properties and Heat Capacity of Activated Carbon Obtained from Coke Fines. Molecules, 28(18), 6545. https://doi.org/10.3390/molecules28186545






