Thermal Investigations of Annelated Triazinones—Potential Analgesic and Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Characterisation of Annelated Triazinones in Air (1–9)
2.2. Thermal Characterisation of Annelated Triazinones in Nitrogen (1–9)
2.3. Evaluation of the Effect of the Tested Compounds (1–9) on Erythrocytes
2.4. Assessment of the Risk of Side Effects of All the Compounds (1–9)
2.5. Predicting Molecular Targets for the Investigated Compounds (1–9)
| Compound | Bioactivity Score | |||||
|---|---|---|---|---|---|---|
| GPCR Ligand | Kinase Inhibitor | Enzyme Inhibitor | Ion Channel Modulator | Nuclear Receptor Ligand | Protease Inhibitor | |
| 1 | −0.16 | −0.34 | −0.35 | −0.62 | −0.80 | −0.82 |
| 2 | −0.20 | −0.31 | −0.37 | −0.67 | −0.79 | −0.85 |
| 3 | −0.19 | −0.37 | −0.42 | −0.69 | −0.78 | −0.83 |
| 4 | −0.18 | −0.37 | −0.40 | −0.68 | −0.78 | −0.82 |
| 5 | −0.20 | −0.31 | −0.35 | −0.75 | −0.76 | −0.86 |
| 6 | −0.07 | −0.20 | −0.39 | −0.61 | −0.81 | −0.82 |
| 7 | −0.15 | −0.33 | −0.40 | −0.61 | −0.78 | −0.84 |
| 8 | −0.14 | −0.34 | −0.37 | −0.60 | −0.77 | −0.81 |
| 9 | −0.13 | −0.31 | −0.38 | −0.58 | −0.73 | −0.79 |
3. Materials and Methods
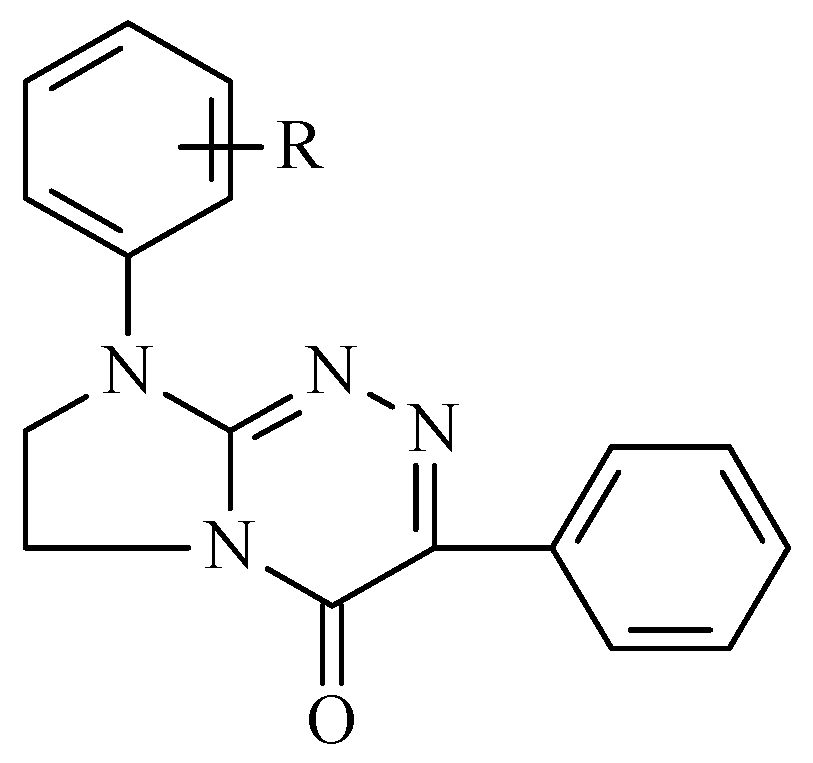
3.1. A Set of the Investigated Disubstituted Annelated Triazinones (1–9)
3.2. Thermal Analysis Methods
3.3. Investigation of the Effect of Compounds 1–9 on Erythrocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sztanke, K. Inventor, Medical University of Lublin, Assignee. New 8-Aryl-3-phenyl-6,7-dihydro-4H-imidazo[2,1-c][1,2,4]triazine-4-ones and Methods for Their Manufacture. Polish Patent PL 199750, 31 October 2008. [Google Scholar]
- Sztanke, K.; Pasternak, K.; Sztanke, M.; Kandefer-Szerszeń, M.; Kozioł, A.E.; Dybała, I. Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1,2,4-triazinones. Bioorg. Med. Chem. Lett. 2009, 19, 5095–5100. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Reversed-phase liquid chromatography with octadecylsilyl, immobilized artificial membrane and cholesterol columns in correlation studies with in silico biological descriptors of newly synthesized antiproliferative and analgesic active compounds. J. Chromatogr. A 2013, 1318, 92–101. [Google Scholar]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Kozak, J.; Tyszczuk-Rotko, K.; Sadok, I.; Sztanke, K.; Sztanke, M. Application of a Screen-Printed Sensor Modified with Carbon Nanofibers for the Voltammetric Analysis of an Anticancer Disubstituted Fused Triazinone. Int. J. Mol. Sci. 2022, 23, 2429. [Google Scholar] [CrossRef]
- Giron, D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J. Therm. Anal. Calorim. 2002, 68, 335–357. [Google Scholar] [CrossRef]
- Hsieh, W.H.; Cheng, W.T.; Chen, L.C.; Lin, S.Y. Non-isothermal dehydration kinetic study of aspartame hemihydrate using DSC, TGA and DSC-FTIR microspectroscopy. Asian J. Pharm. Sci. 2018, 13, 212–219. [Google Scholar]
- Sangeetha Margreat, S.; Ramalingam, S.; Sebastian, S.; Xavier, S.; Periandy, S.; Daniel, J.C.; Maria Julie, M. DFT, spectroscopic, DSC/TGA, electronic, biological and molecular docking investigation of 2,5-thiophenedicarboxylic acid: A promising anticancer agent. J. Mol. Struct. 2020, 1200, 127099. [Google Scholar]
- Sikorska-Iwan, M.; Modzelewska-Banachiewicz, B. Thermal behaviour of 1,2,4-triazole and 1,2,4-triazine derivatives. J. Therm. Anal. Calorim. 2005, 81, 119–123. [Google Scholar] [CrossRef]
- Alamro, F.S.; Tolan, D.A.; El-Nahas, A.M.; Ahmed, H.A.; El-Atawy, M.A.; Al-Kadhi, N.S.; Aziz, S.G.; Shibl, M.F. Wide. Nematogenic azomethine/ester liquid crystals based on new biphenyl derivatives: Mesomorphic and computational studies. Molecules 2022, 27, 4150. [Google Scholar] [CrossRef]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and numerical analysis of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage. J. Energy Storage 2022, 56, 105975. [Google Scholar]
- Bianco, N.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Multi-objective optimization of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage: Experiments and numerical modeling. Appl. Therm. Eng. 2022, 215, 119047. [Google Scholar] [CrossRef]
- Łyszczek, R.; Bartyzel, A.; Głuchowska, H.; Mazur, L.; Sztanke, M.; Sztanke, K. Thermal investigations of biologically important fused azaisocytosine-containing congeners and the crystal structure of one representative. J. Anal. Appl. Pyrolysis 2018, 155, 141–151. [Google Scholar] [CrossRef]
- Bartyzel, A.; Sztanke, M.; Sztanke, K. Thermal behaviour of antiproliferative active 3-(2-furanyl)-8-aryl-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-ones. J. Therm. Anal. Calorim. 2017, 130, 1541–1551. [Google Scholar] [CrossRef][Green Version]
- Shamsipur, M.; Pourmortazavi, S.M.; Beigi, A.A.M.; Heydari, R.; Khatibi, M. Thermal stability and decomposition kinetic studies of acyclovir and zidovudine drug compounds. AAPS PharmSciTech 2013, 14, 287–293. [Google Scholar] [CrossRef]
- Yoshida, M.I.; Gomes, E.C.; Soares, C.D.; Oliveira, M.A. Thermal behavior study and decomposition kinetics of amiodarone hydrochloride under isothermal conditions. Drug Dev. Ind. Pharm. 2011, 37, 638–647. [Google Scholar] [CrossRef]
- Materazzi, S. Thermogravimetry—Infrared Spectroscopy (TG-FTIR) Coupled Analysis. Appl. Spectrosc. Rev. 1997, 32, 385–404. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Brown, R.F.C. Melting point and molecular symmetry. J. Chem. Educ. 2000, 77, 724–731. [Google Scholar] [CrossRef]
- Prahlada Rao, S.; Sunkada, S. Making sense of boiling points and melting points. Resonance 2007, 12, 43–57. [Google Scholar] [CrossRef]
- Ostasz, A.; Łyszczek, R.; Sztanke, K.; Sztanke, M. TG-DSC and TG-FTIR Studies of Annelated Triazinylacetic Acid Ethyl Esters—Potential Anticancer Agents. Molecules 2023, 28, 1735. [Google Scholar]
- Plis, A.; Kotyczka-Morańska, M.; Kopczyński, M.; Łabojko, G. Furniture wood waste as a potential renewable energy surce. A thermogravimetric and kinetic analysis. J. Therm. Anal. Calorim. 2016, 125, 1357–1371. [Google Scholar] [CrossRef]
- Pettersson, M.; Khriachtchev, L.; Jolkkonen, S.; Räsänen, M. Photochemistry of HNCO in Solid Xe: Channels of UV Photolysis and Creation of H2NCO Radicals. J. Phys. Chem. A 1999, 103, 9154–9162. [Google Scholar] [CrossRef]
- Raveendra, M.; Chandrasekhar, M.; Chandrasekhar Reddy, K.; Venkatesulu, A.; Sivakumar, K.; Dayananda Reddy, K. Study on thermo physical properties of binary mixture containing aromatic alcohol with aromatic, substituted aromatic amines at different temperatures interms of FT-IR, 1H NMR spectroscopic and DFT method. Fluid Phase Equilibria 2018, 462, 85–99. [Google Scholar]
- Bernstein, M.P.; Sandford, S.A.; Allamandola, L.J. The Infrared Spectra of Nitriles and Related Compounds Frozen in Ar and H2O. Astrophys. J. 1997, 476, 932–942. [Google Scholar] [PubMed]
- Liu, W.; Tang, Y.; Li, F.; Ge, X.G.; Zhang, Z.J. TG-FTIR characterization of flame retardant polyurethane foams materials. IOP Conf. Series Mater. Sci. Eng. 2016, 137, 012033. [Google Scholar] [CrossRef]
- Ju, W.; Lu, C.; Liu, C.; Jiang, W.; Zhang, Y.; Hong, F. Rapid Identification of Atmospheric Gaseous Pollutants Using Fourier-Transform Infrared Spectroscopy Combined with Independent Component Analysis. J. Spectrosc. 2020, 2020, 8920732. [Google Scholar]
- Aapro, M.S. Innovative Metabolites in Solid Tumours; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Taliani, S.; Pugliesi, I.; Barresi, E.; Simorini, F.; Salerno, S.; La Motta, C.; Marini, A.M.; Cosimelli, B.; Cosconati, S.; Di Maro, S.; et al. 3-Aryl-[1,2,4]triazino[4,3-a]benzimidazol-4(10H)-one: A novel template for the design of highly selective A2B adenosine receptor antagonists. J. Med. Chem. 2012, 55, 1490–1499. [Google Scholar] [CrossRef]
- Jeelani, A.; Muthu, S.; Narayana, B. Molecular structure determination, bioactivity score, spectroscopic and quantum computational studies on (E)-N′-(4-chlorobenzylidene)-2-(napthalen-2-yloxy)acetohydrazide. J. Mol. Struct. 2021, 1241, 130558. [Google Scholar]
- Hussein, Y.T.; Azeez, Y.H. DFT analysis and in silico exploration of drug-likeness, toxicity prediction, bioactivity score, and chemical reactivity properties of the urolithins. J. Biomol. Struct. Dyn. 2023, 41, 1168–1177. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Chemical Reactivity Properties and Bioactivity Scores of the Angiotensin II Vasoconstrictor Octapeptide. In Cheminformatics and Its Applications; IntechOpen: London, UK, 2020. [Google Scholar]
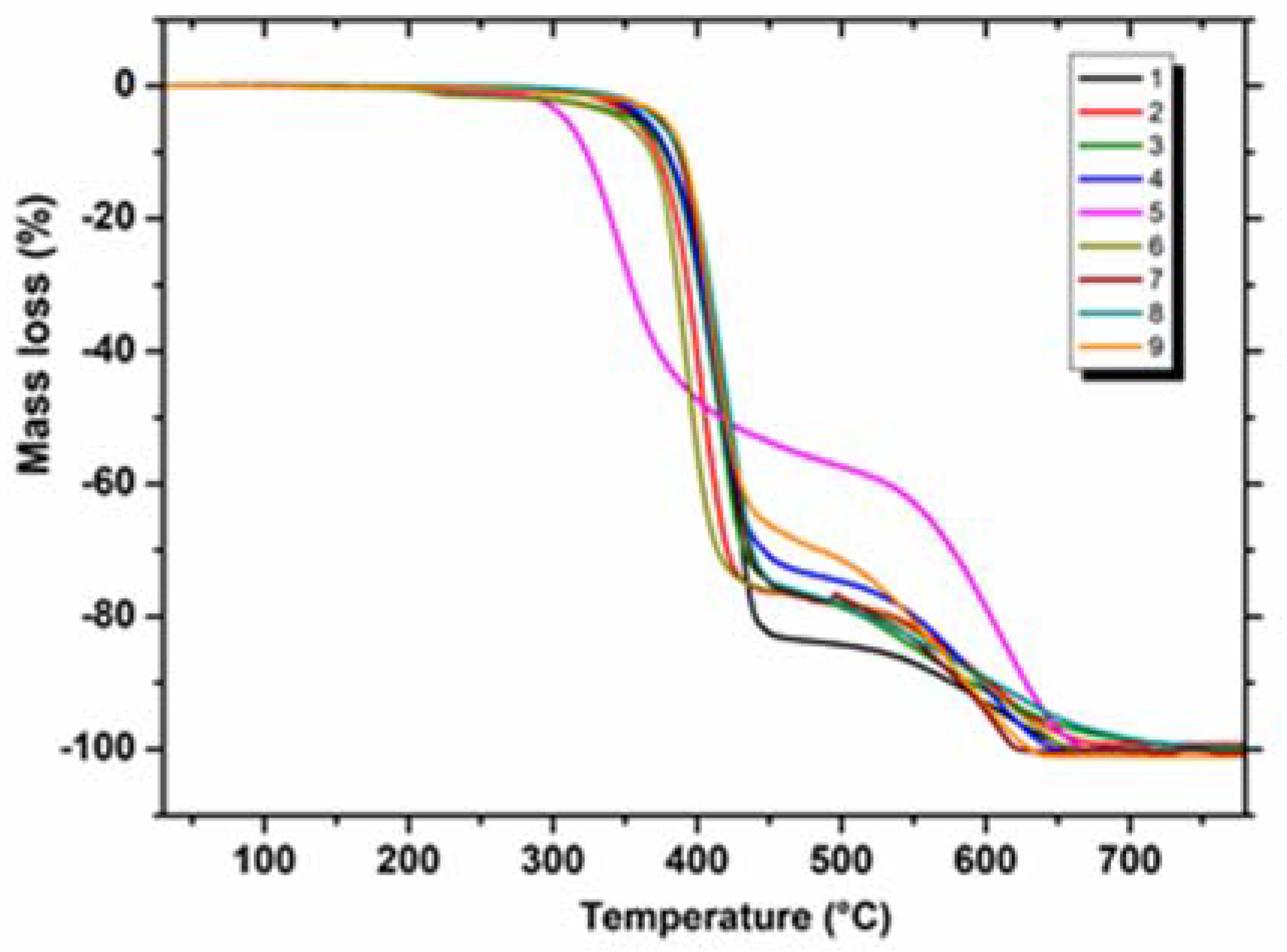

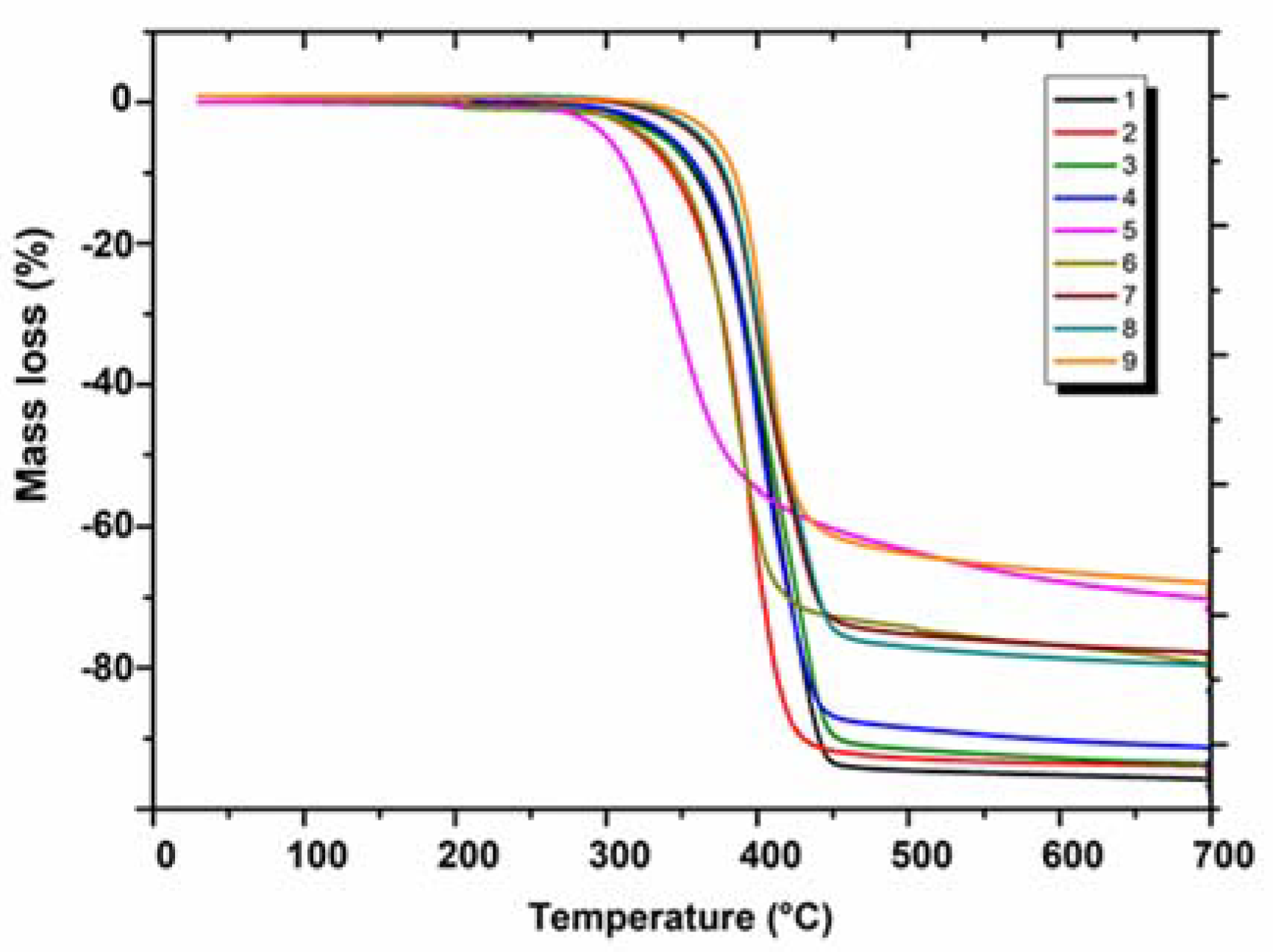

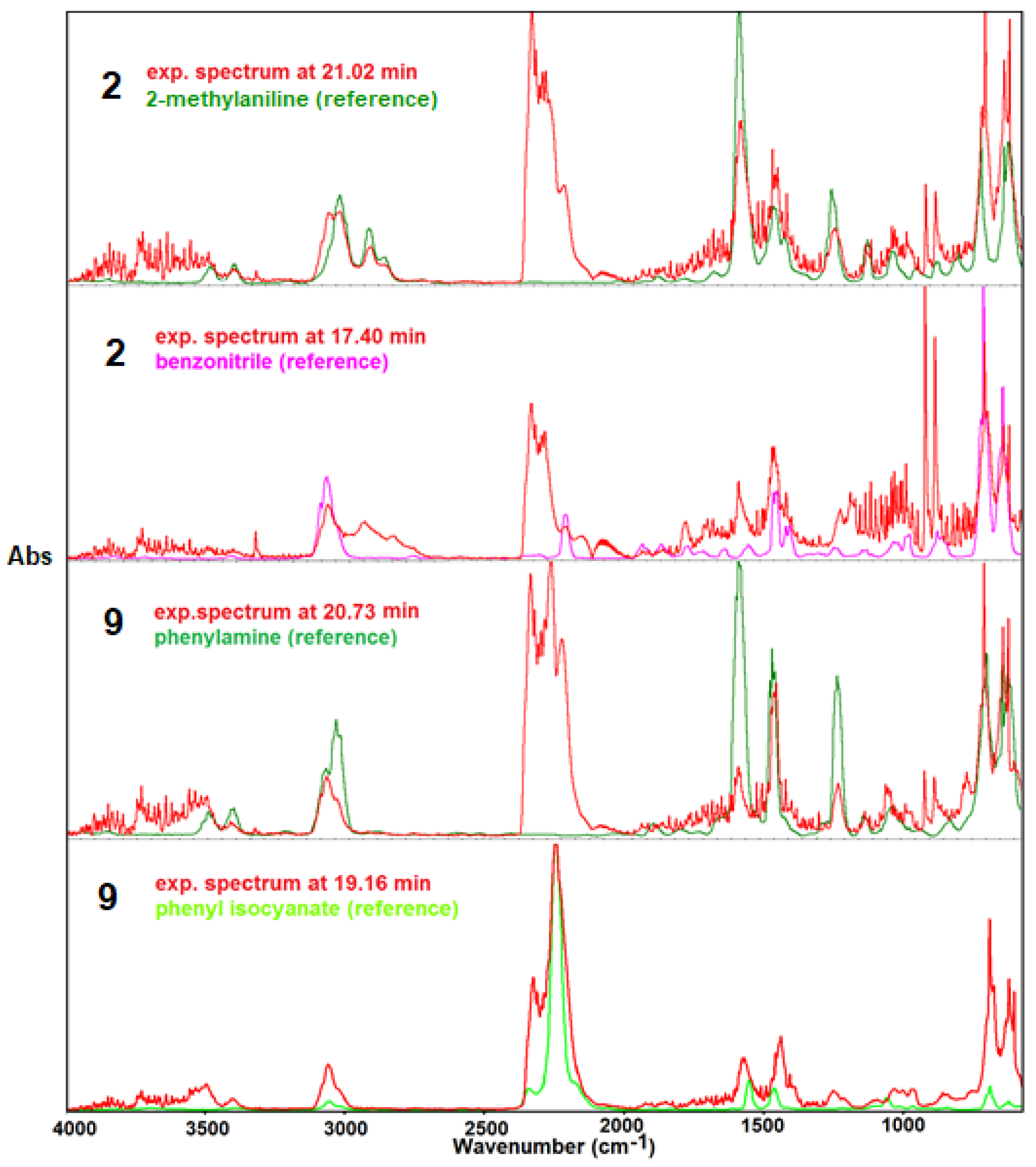
| Sample | Melting Process | Decomposition Process | ||||
|---|---|---|---|---|---|---|
| Tonset [°C] | Tpeak [°C] | ΔHm[kJ·mol−1] | Step 1 | Step 2 | ||
| ΔT1 [°C] | Δm1 [%] | ΔT2 [°C] | ||||
| 1 | 212 | 216 | 29.18 | 288–471 | 83.56 | 471–670 |
| 2 | 238 | 241 | 34.12 | 279–454 | 76.44 | 454–676 |
| 3 | 215 | 218 | 32.58 | 253–460 | 75.56 | 460–768 |
| 4 | 237 | 241 | 31.04 | 283–473 | 73.31 | 473–670 |
| 5 | 216 | 221 | 36.99 | 241–453 | 53.95 | 453–685 |
| 6 | 223 | 227 | 26.77 | 247–464 | 76.69 | 464–668 |
| 7 | 210 | 213 | 31.42 | 284–491 | 78.00 | 491–632 |
| 8 | 262 | 266 | 28.85 | 296–460 | 75.55 | 460–745 |
| 9 | 267 | 270 | 39.10 | 290–455 | 67.00 | 455–650 |
| Compound/Control | Haemolytic Activity (%) A | Inhibition (%) of Oxidative Haemolysis | |
|---|---|---|---|
| Induced by AAPH B | Induced by H2O2 C | ||
| 1 | 2.47 ± 0.12 | 55 ± 4.4 | 59 ± 6.1 |
| 2 | 4.04 ± 0.13 | 42 ± 4.5 | 45 ± 4.0 |
| 3 | 2.14 ± 0.17 | 81 ± 9.3 | 77 ± 7.0 |
| 4 | 3.79 ± 0.15 | 60 ± 5.7 | 58 ± 4.4 |
| 5 | 4.12 ± 0.23 | 48 ± 3.2 | 34 ± 5.1 |
| 6 | 2.39 ± 0.11 | 80 ± 6.6 | 76 ± 8.8 |
| 7 | 4.78 ± 0.14 | 39 ± 4.5 | 60 ± 5.5 |
| 8 | 3.46 ± 0.28 | 69 ± 7.0 | 57 ± 4.2 |
| 9 | 2.39 ± 0.17 | 74 ± 5.4 | 83 ± 7.1 |
| Triton X-100 | 100 | - | - |
| Ascorbic acid | - | 100 | - |
| Trolox | - | - | 100 |
| Compound | Mutagenicity | Tumorigenicity | Irritating Effects | Reproductive Effects |
|---|---|---|---|---|
| 1 |  |  |  |  |
| 2 |  |  |  |  |
| 3 |  |  |  |  |
| 4 |  |  |  |  |
| 5 |  |  |  |  |
| 6 |  |  |  |  |
| 7 |  |  |  |  |
| 8 a |  |  |  |  |
| 9 |  |  |  |  |
 —no risk, score: 1.0; a—data published in Ref. [5].
—no risk, score: 1.0; a—data published in Ref. [5].Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztanke, M.; Sztanke, K.; Ostasz, A.; Głuchowska, H.; Łyszczek, R. Thermal Investigations of Annelated Triazinones—Potential Analgesic and Anticancer Agents. Molecules 2023, 28, 6542. https://doi.org/10.3390/molecules28186542
Sztanke M, Sztanke K, Ostasz A, Głuchowska H, Łyszczek R. Thermal Investigations of Annelated Triazinones—Potential Analgesic and Anticancer Agents. Molecules. 2023; 28(18):6542. https://doi.org/10.3390/molecules28186542
Chicago/Turabian StyleSztanke, Małgorzata, Krzysztof Sztanke, Agnieszka Ostasz, Halina Głuchowska, and Renata Łyszczek. 2023. "Thermal Investigations of Annelated Triazinones—Potential Analgesic and Anticancer Agents" Molecules 28, no. 18: 6542. https://doi.org/10.3390/molecules28186542
APA StyleSztanke, M., Sztanke, K., Ostasz, A., Głuchowska, H., & Łyszczek, R. (2023). Thermal Investigations of Annelated Triazinones—Potential Analgesic and Anticancer Agents. Molecules, 28(18), 6542. https://doi.org/10.3390/molecules28186542