The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Variation in VFC Profile
2.3. Screening of the KACs
2.3.1. Sulfides
2.3.2. Aldehydes
2.3.3. Ketones
2.3.4. Acids
2.3.5. Esters
2.3.6. Alcohols
2.3.7. Others
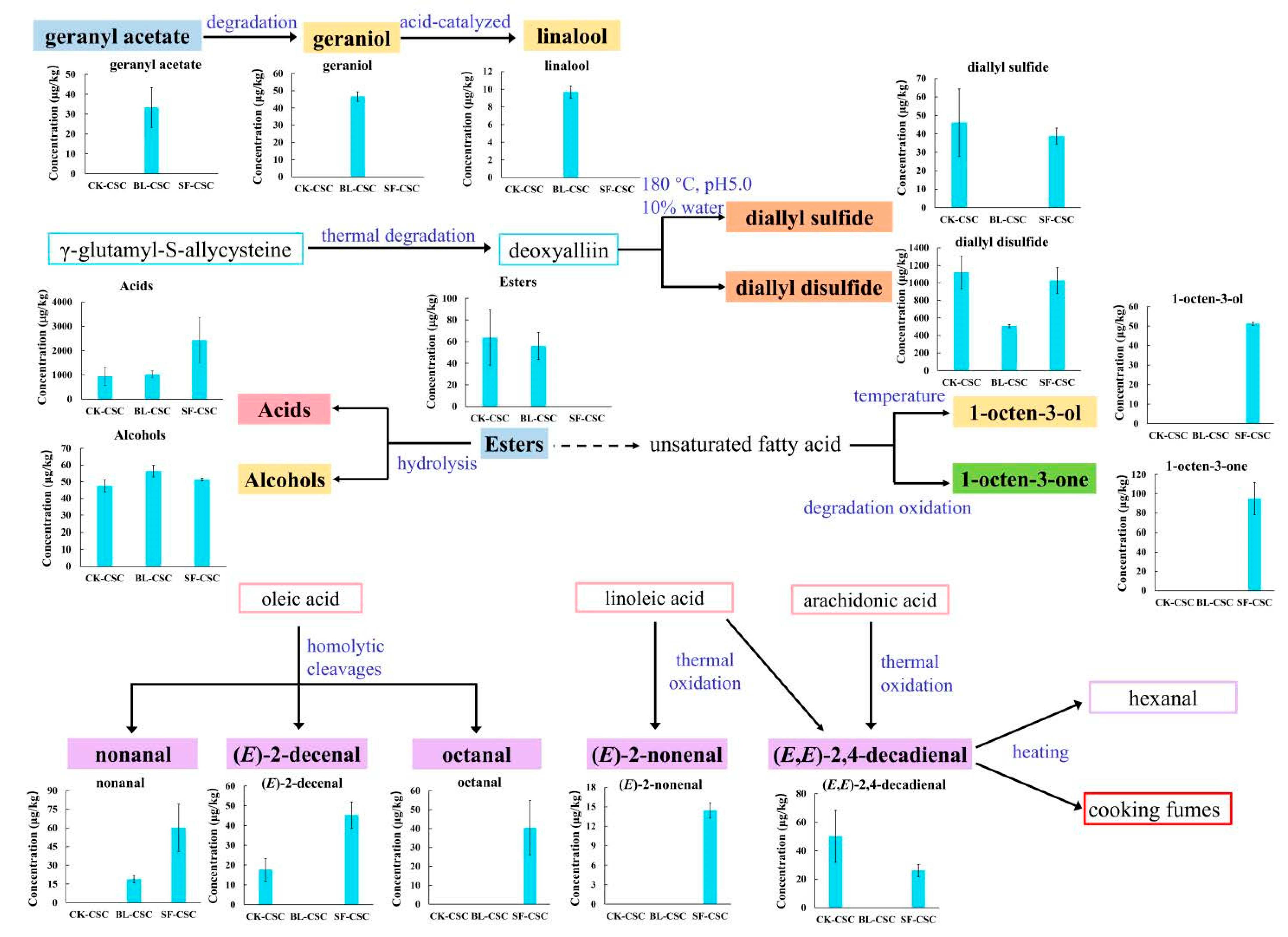
2.4. Comparison of the KACs and Possible Pathways
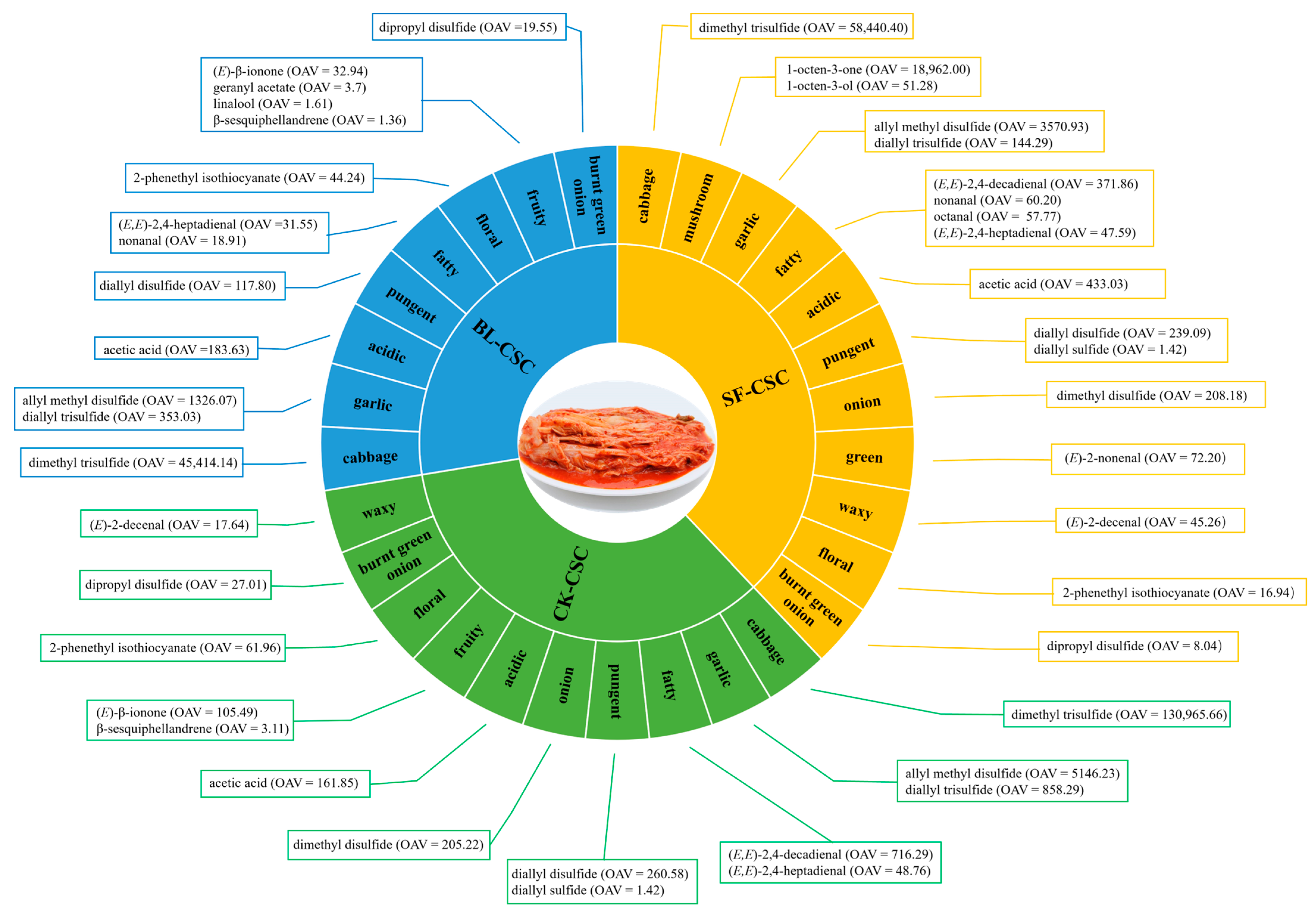
2.5. Odor Profiles
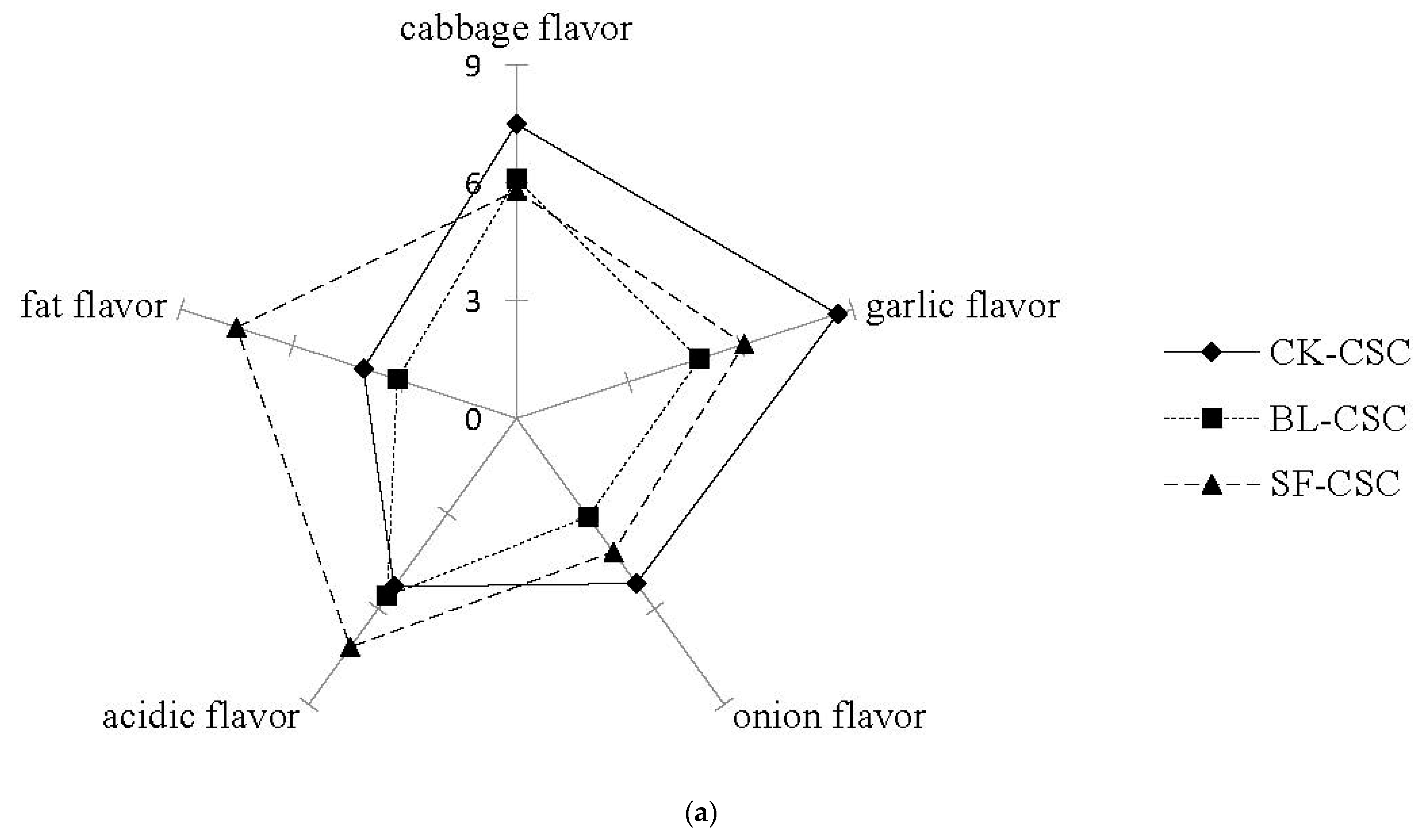
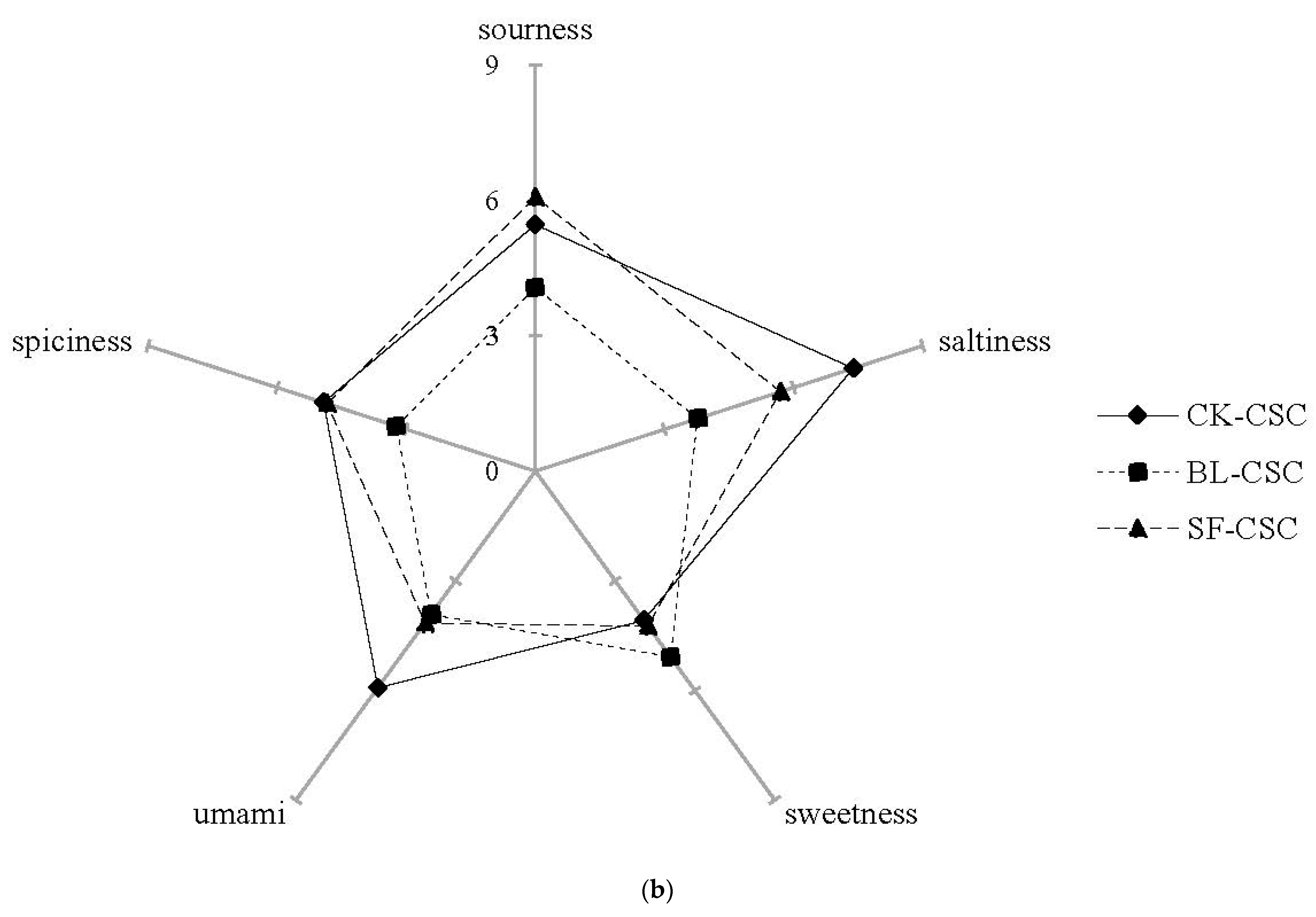
2.6. Sensory Evaluation
3. Materials and Methods
3.1. Preparation of Chinese Spicy Cabbage
3.2. Cooking Process
3.3. TTA Analysis
3.4. Total Sugar
3.5. Sensory Evaluation
3.6. GC-MS Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, L.; Mu, G.; Wang, M.; Zhao, T.; Tuo, Y.; Zhu, X.; Qian, F. Microbial Diversity and Quality-Related Physicochemical Properties of Spicy Cabbage in Northeastern China and Their Correlation Analysis. Foods 2022, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Liu, L.; Guan, H.; Zhang, X.; Sun, X.; Li, H.; Liu, W. Dynamic prediction model of ripening degree of Chinese spicy cabbage under fluctuation temperatures. Food Packag. Shelf Life 2021, 30, 100744. [Google Scholar] [CrossRef]
- Lee, H.-G.; Jeong, S.; Yoo, S. Development of a calcium hydroxide–dye kimchi ripening indicator and its application in kimchi packaging. Food Chem. 2023, 400, 134039. [Google Scholar] [CrossRef]
- Oun, A.A.; Roy, S.; Shin, G.H.; Yoo, S.; Kim, J.T. pH-sensitive smart indicators based on cellulose and different natural pigments for tracing kimchi ripening stages. Int. J. Biol. Macromol. 2023, 242, 124905. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, S.; Park, J.Y.; Yoo, S. Effect of pasteurization on delayed kimchi ripening and regression analysis for shelf life estimation of kimchi. Food Sci. Nutr. 2019, 7, 711–720. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-G.; Lee, J.-W.; Kim, W.-G.; Chung, Y.-J.; Byun, M.-W. The combined effects of N2-packaging, heating and gamma irradiation on the shelf-stability of Kimchi, Korean fermented vegetable. Food Control 2008, 19, 56–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, R.; Song, H.; Zheng, S.; Chen, Y.; Zhou, N. Effect of Stir Frying on the Antioxidant Capacity and Aroma Components of Bok Choy (Chinese Cabbage). Int. J. Food Prop. 2016, 19, 2536–2549. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014, 161, 162–167. [Google Scholar] [CrossRef]
- Akdaş, Z.Z.; Bakkalbaşı, E. Influence of different cooking methods on color, bioactive compounds, and antioxidant activity of kale. Int. J. Food Prop. 2017, 20, 877–887. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Özcan, M.M.; Alkaltham, M.S.; Uslu, N.; Hayat, K. Influence of boiling on total phenol, antioxidant activity, and phenolic compounds of celery (Apium graveolens L.) root. J. Food Process. Preserv. 2020, 45, e15171. [Google Scholar]
- Reis, G.C.L.; Guidi, L.R.; Fernandes, C.; Godoy, H.T.; Gloria, M.B.A. UPLC-UV Method for the Quantification of Free Amino Acids, Bioactive Amines, and Ammonia in Fresh, Cooked, and Canned Mushrooms. Food Anal. Methods 2020, 13, 1613–1626. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Sun, B.; Yuan, J.; Wang, Q.-M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Cappa, C.; Benedetti, S.; Giovanelli, G. Influence of Cooking Conditions on Nutritional Properties and Sensory Characteristics Interpreted by E-Senses: Case-Study on Selected Vegetables. Foods 2020, 9, 607. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Influence of Different Frying Processes on the Flavor Characteristics and Sensory Profile of Garlic Oil. Molecules 2019, 24, 4456. [Google Scholar] [CrossRef]
- Bi, S.; Wang, A.; Lao, F.; Shen, Q.; Liao, X.; Zhang, P.; Wu, J. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. 2021, 339, 127878. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Y.; Luo, R.; Shen, F.; Bai, H.; Ding, D. Formation of flavor volatile compounds at different processing stages of household stir-frying mutton sao zi in the northwest of China. LWT 2021, 139, 110735. [Google Scholar] [CrossRef]
- Lu, K.; Liu, L.; Zi, J.; Song, L.; Xie, W. New insights from flavoromics on different heating methods of traditional fermented shrimp paste: The volatile components and metabolic pathways. LWT 2022, 168, 113880. [Google Scholar] [CrossRef]
- Lynda, A.D.; Lila, B.M.; Sabrina, Z.H.; Salima, B.; Farid, B.; Khodir, M. Influence of the thermal processing on the physico-chemical properties and the antioxidant activity of a solanaceae vegetable: Eggplant. J. Food Qual. 2016, 39, 181–191. [Google Scholar]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Park, S.H.; Lee, M.-A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Odour and Flavour Threshold Values in Air, Water and Other Media. Available online: http://www.thresholdcompilation.com/ (accessed on 1 November 2022).
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.H.; Lee, M.-A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric characterization of aroma active compounds in fermented fish paste in comparison with fish sauce, fermented soy paste and sauce products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-Active Compounds in Kimchi during Fermentation. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Characterization of key aroma compounds in aged garlic extract. Food Chem. 2020, 312, 126081. [Google Scholar] [CrossRef]
- Boelens, M.; Gemert, L.J. Volatile character-impact sulfur compounds and their sensory properties. Perfum. Flavorist 1993, 18, 29–39. [Google Scholar]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors. In Volatile Sulfur Compounds in Food; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. [Google Scholar]
- Li, K.; Zhan, P.; Tian, H.; Wang, P.; Ji, Y. Effects of drying time on the aroma of garlic (Allium sativum L.) seasoning powder. Flavour Fragr. J. 2021, 36, 99–110. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Jeleń, H.H. Determination of the odor threshold concentrations and partition coefficients of isothiocyanates from Brassica vegetables in aqueous solution. LWT 2020, 131, 109793. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, H.; Ou, C.; Xie, C.; Cao, J.; Zhang, X. A comparative study of volatile flavor components in four types of zaoyu using comprehensive two-dimensional gas chromatography in combination with time-of-flight mass spectrometry. J. Food Process. Preserv. 2021, 45, e15230. [Google Scholar] [CrossRef]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Liu, Y.; Li, J.; Song, H.L. Identification of key off-flavor compounds in thermally treated watermelon juice via gas chromatography-olfactometry-mass spectrometry, aroma recombination, and omission experiments. Foods 2020, 9, 227. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Mao, B.; Cui, S.; Tang, X.; Zhang, H.; Zhao, J.; Chen, W. Gas chromatography–mass spectrometry-based metabolomics analysis of metabolites in commercial and inoculated pickles. J. Sci. Food Agric. 2021, 101, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ho, C.-T.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Tu, Z.-C.; Zhang, L.; Lin, D.-R.; Sha, X.-M.; Zeng, K.; Wang, H.; Pang, J.-J.; Tang, P.-P. Characterization of Volatile Compounds in Grass Carp (Ctenopharyngodon idellus) Soup Cooked Using a Traditional Chinese Method by GC-MS. J. Food Process. Preserv. 2017, 41, e12995. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; de Sousa Galvâo, M.; Sandes, R.D.D.; Neta, M.T.S.L.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Yao, H.; Su, H.; Ma, J.; Zheng, J.; He, W.; Wu, C.; Hou, Z.; Zhao, R.; Zhou, Q. Widely targeted volatileomics analysis reveals the typical aroma formation of Xinyang black tea during fermentation. Food Res. Int. 2023, 164, 112387. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, K.H.; Han, D.M.; Lee, S.H.; Jeon, C.O. Effects of glutinous rice paste and fish sauce on kimchi fermentation. LWT 2023, 173, 114253. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of Fermentation Temperature on the Volatile Composition of Kimchi. J. Food Sci. 2016, 81, C2623–C2629. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhao, Y.; Guan, H.; Jin, C.; Gong, H.; Sun, X.; Wang, P.; Li, H.; Liu, W. Effect of Levilactobacillus brevis as a starter on the flavor quality of radish paocai. Food Res. Int. 2023, 168, 112780. [Google Scholar] [CrossRef]
- Mottram, D.S.; Elmore, J.S. Control of the Maillard Reaction during the Cooking of Food. Control Mail. Pathw. Gener. Flavors 2010, 14, 143–155. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Guan, Y.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Fan, X.; Liu, G.; Qiao, Y.; Zhang, Y.; Leng, C.; Chen, H.; Sun, J.; Feng, Z. Characterization of volatile compounds by SPME-GC-MS during the ripening of Kedong sufu, a typical Chinese traditional bacteria-fermented soybean product. J. Food Sci. 2019, 84, 2441–2448. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS characterization of volatile compounds of Chinese traditional-chopped pepper during fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. [Google Scholar] [CrossRef]
- Pueschel, V.A.; Schieberle, P. Changes in the key aroma compounds of matsutake mushroom (Tricholoma matsutake Sing.) from Canada during pan-frying elucidated by application of the sensomics approach. Eur. Food Res. Technol. 2021, 247, 51–65. [Google Scholar] [CrossRef]
- Chetschik, I.; Granvogl, M.; Schieberle, P. Comparison of the Key Aroma Compounds in Organically Grown, Raw West-African Peanuts (Arachis hypogaea) and in Ground, Pan-Roasted Meal Produced Thereof. J. Agric. Food Chem. 2008, 56, 10237–10243. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Gong, C.; Tan, X.; Ren, Y.; Yao, K.; Zhang, Q.; Chi, Y. Physicochemical, microbial, and aroma characteristics of Chinese pickled red peppers (Capsicum annuum) with and without biofilm. RSC Adv. 2020, 10, 6609–6617. [Google Scholar] [CrossRef]
- Luo, S.; Li, Q.; Chen, A.; Liu, X.; Pu, B. The Aroma Composition of Baby Ginger Paocai. J. Food Qual. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile compounds identified in headspace samples of peanut oil heated under temperatures ranging from 50 to 200 °C. J. Agric. Food Chem. 1993, 41, 1467–1470. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.-H.; Chang, P.-S. Headspace-solid phase microextraction (HS-SPME) analysis of oxidized volatiles from free fatty acids (FFA) and application for measuring hydrogen donating antioxidant activity. Food Chem. 2007, 105, 414–420. [Google Scholar] [CrossRef]
- Ohta, T.; Morimitsu, Y.; Sameshima, Y.; Samuta, T.; Ohba, T. Transformation from geraniol, nerol and their glucosides into linalool and α-terpineol during shochu distillation. J. Ferment. Bioeng. 1991, 72, 347–351. [Google Scholar] [CrossRef]
- Marcinkowska, M.A.; Jeleń, H.H. Role of Sulfur Compounds in Vegetable and Mushroom Aroma. Molecules 2022, 27, 6116. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Rosen, R.T.; Hartman, T.G.; Ho, C.T. Volatile compounds generated from thermal degradation of alliin and deoxyalliin in an aqueous solution. J. Agric. Food Chem. 1994, 42, 146–153. [Google Scholar] [CrossRef]
- Zhao, Q.; Xi, J.; Xu, D.; Jin, Y.; Wu, F.; Tong, Q.; Xu, X. Effect of optimal-water boiling cooking on the volatile compounds in 26 Japonica rice varieties from China. Food Res. Int. 2022, 155, 111078. [Google Scholar] [CrossRef]
- Boskou, G.; Salta, F.N.; Chiou, A.; Troullidou, E.; Andrikopoulos, N.K. Content of trans,trans-2,4-decadienal in deep-fried and pan-fried potatoes. Eur. J. Lipid Sci. Technol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Pérez, M.B.; Maniero, M.Á.; Londonio, A.; Smichowski, P.; Wuilloud, R.G. Effects of common cooking heat treatments on selenium content and speciation in garlic. J. Food Compos. Anal. 2018, 70, 54–62. [Google Scholar] [CrossRef]
- AOAC. Acidity (Titratable) of Fruit Products. 942.15. Official Method of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
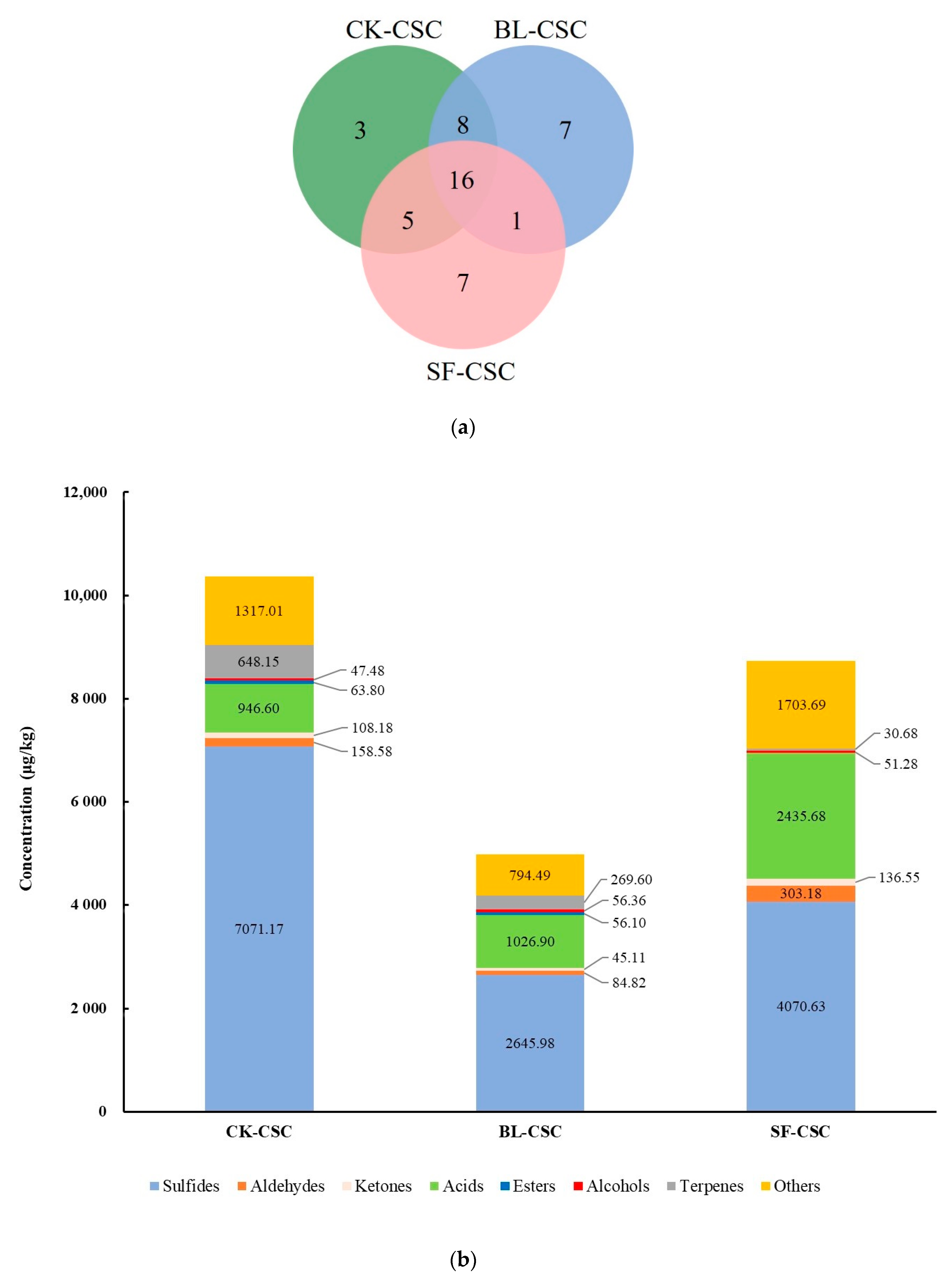


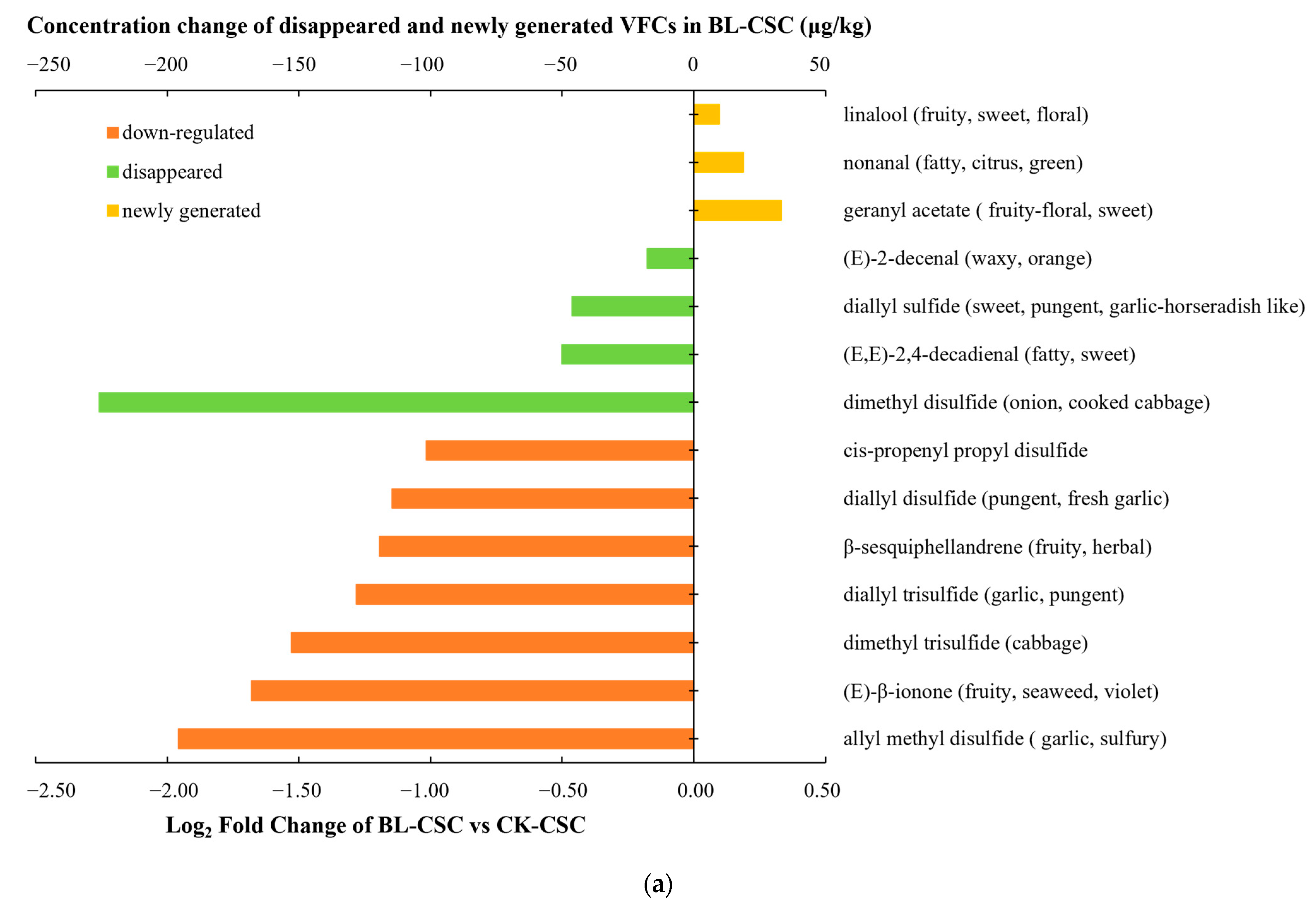
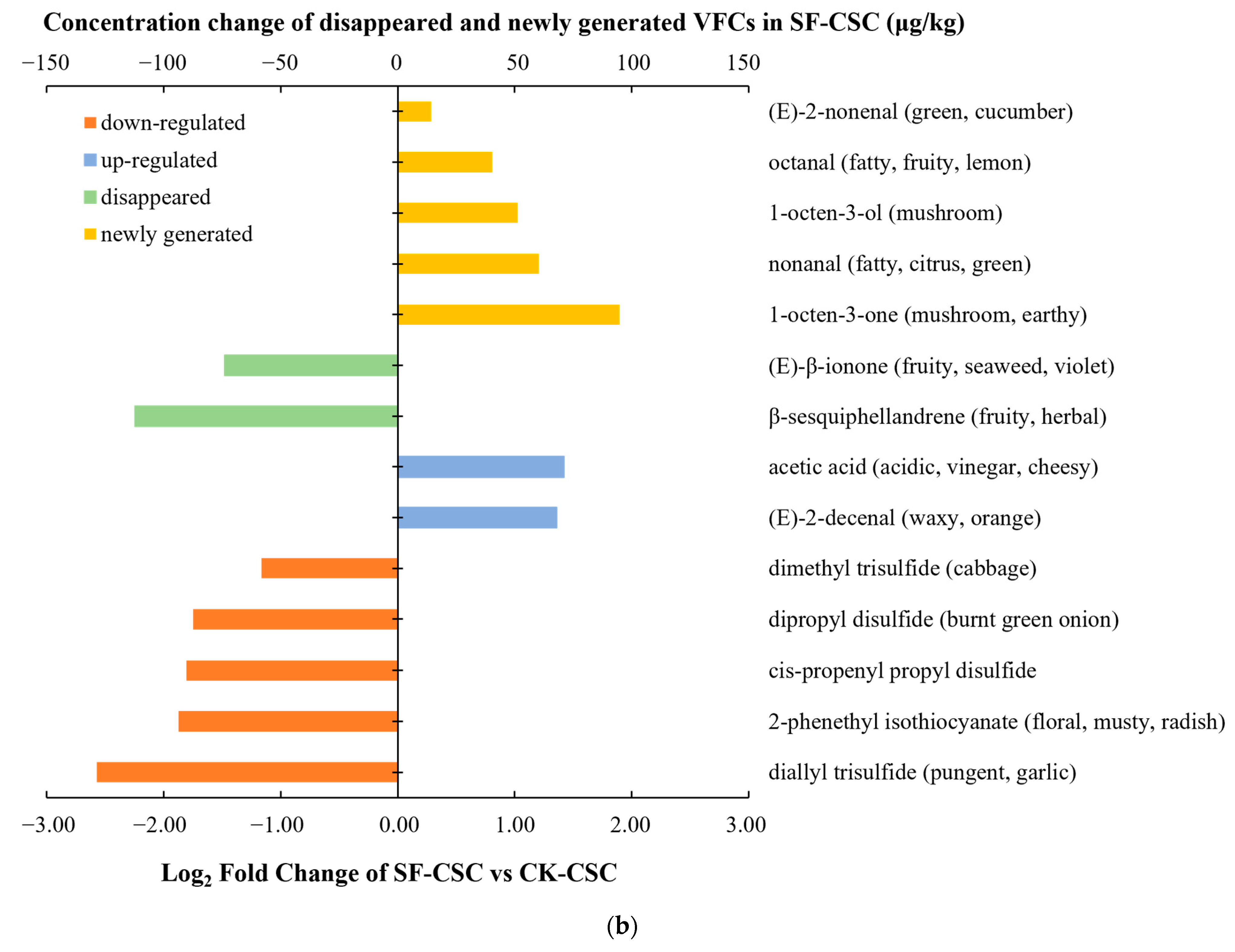
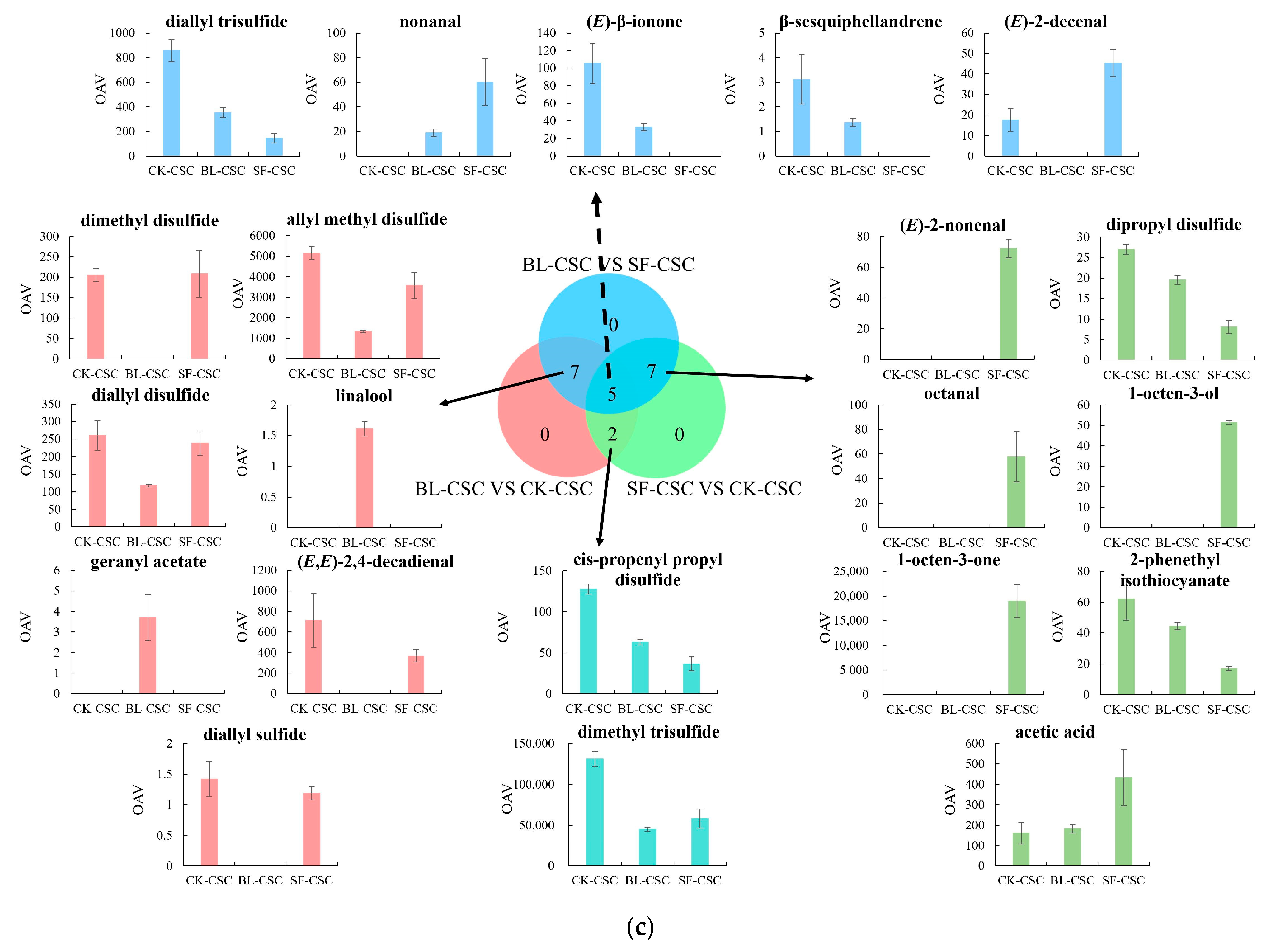
| Number | Compounds | LRI a | LRI b | Odor Threshold (μg/kg) | Concentration (μg/kg, CK-CSC) | OAV (CK-CSC) | Concentration (μg/kg, BL-CSC) | OAV (BL-CSC) | Concentration (μg/kg, SF-CSC) | OAV (SF-CSC) | Odor Description |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfides (15) | |||||||||||
| 1 | Diallyl sulfide | 1146 | 1148 ± 3 | 32.5 [21] | 46.11 ± 9.36 a | 1.42 | n.d. | 38.80 ± 3.45 a | 1.19 | Pungent, sweet, Garlic–horseradish-like [22] | |
| 2 | Dimethyl disulfide | 1078 | 1077 ± 8 | 1.1 [23] | 225.74 ± 17.44 a | 205.22 | n.d. | 229 ± 62.44 a | 208.18 | Onion, cooked cabbage [23] | |
| 3 | Methyl propyl disulfide | 1233 | 1239 ± 13 | 66.71 ± 5.48 a | n.d. | 38.18 ± 3.93 b | Garlic, sour [24] | ||||
| 4 | Allyl methyl disulfide | 1285 | 1281 ± 10 | 0.3 [25] | 1543.87 ± 96.53 a | 5146.23 | 397.82 ± 20.65 c | 1326.07 | 1071.28 ± 198.03 b | 3570.93 | Garlic, sulfury [22] |
| 5 | Methyl cis-propenyl disulfide | 1299 | 1303 ± 5 | 207.70 ± 25.44 a | 50.83 ± 5.95 c | 92.14 ± 12.22 b | Garlic [24] | ||||
| 6 | Dipropyl disulfide | 1383 | 1378 ± 11 | 3.2 [26] | 86.43 ± 3.98 a | 27.01 | 62.57 ± 3.59 b | 19.55 | 25.74 ± 5.13 c | 8.04 | Burnt green onion [27] |
| 7 | cis-Propenyl propyl disulfide | 1436 | 1421 ± 15 | 2 [26] | 255.34 ± 12.34 a | 127.67 | 126.40 ± 6.18 b | 63.20 | 73.15 ± 17.12 c | 36.58 | |
| 8 | Diallyl disulfide | 1488 | 1475 ± 14 | 4.3 [25] | 1120.50 ± 184.75 a | 260.58 | 506.52 ± 16.65 b | 117.8 | 1028.07 ± 147.04 a | 239.09 | Pungent, fresh garlic [25] |
| 9 | 3-Vinyl-1,2-dithi-4-ene | 1750 | 1750 ± N/A | 66.09 ± 12.70 a | n.d. | n.d. | Garlic, pungent [28] | ||||
| 10 | Dimethyl trisulfide | 1388 | 1377 ± 11 | 0.0099 [25] | 1296.56 ± 92.64 a | 130,965.66 | 449.60 ± 22.99 b | 45,414.14 | 578.56 ± 115.07 b | 58,440.40 | Cabbage [25] |
| 11 | Allyl methyl trisulfide | 1594 | 1593 ± 1 | 1131.95 ± 186.52 a | 423.38 ± 22.42 b | 641.48 ± 99.38 b | Sulfury, eggy [25] | ||||
| 12 | Allyl propyl trisulfide | 1737 | 1760 ± 37 | 73.72 ± 3.43 a | 52.15 ± 6.09 b | n.d. | |||||
| 13 | Diallyl trisulfide | 1794 | 1805 ± 16 | 0.34 [25] | 291.82 ± 30.88 a | 858.29 | 120.03 ± 13.32 b | 353.03 | 49.06 ± 12.95 c | 144.29 | Garlic, pungent [25] |
| 14 | 2-Butyl isothiocyanate | 1287 | 1287 ± N/A | 39.05 ± 1.61 a | 14.25 ± 1.00 b | 35.81 ± 6.63 a | |||||
| 15 | 2-Phenethyl isothiocyanate | 2234 | 2234 ± 0 | 10 [29] | 619.58 ± 137.41 a | 61.96 | 442.43 ± 22.35 a | 44.24 | 169.36 ± 15.40 b | 16.94 | Floral, musty, radish [24] |
| Aldehydes (10) | |||||||||||
| 16 | (E)-2-Heptenal | 1328 | 1323 ± 11 | 13 [21] | n.d. | 7.77 ± 1.13 a | <1 | n.d. | Fatty, almond, green [14] | ||
| 17 | (E,E)-2,4-Heptadienal | 1503 | 1495 ± 11 | 1 [30] | 48.76 ± 17.36 a | 48.76 | 31.55 ± 3.53 a | 31.55 | 47.59 ± 10.18 a | 47.59 | Fatty, nutty, green [30] |
| 18 | Octanal | 1289 | 1289 ± 9 | 0.7 [21] | n.d. | n.d. | 40.44 ± 14.32 a | 57.77 | Fatty, fruity, lemon [14] | ||
| 19 | Nonanal | 1395 | 1391 ± 8 | 1 [21] | n.d. | 18.91 ± 3.02 b | 18.91 | 60.20 ± 19.00 a | 60.20 | Fatty, citrus, green [14] | |
| 20 | (E)-2-Nonenal | 1542 | 1534 ± 10 | 0.2 [14] | n.d. | n.d. | 14.44 ± 1.19 a | 72.20 | Green, cucumber [14] | ||
| 21 | (E)-2-Decenal | 1649 | 1644 ± 11 | 1 [30] | 17.64 ± 5.67 b | 17.64 | n.d. | 45.26 ± 6.65 a | 45.26 | Waxy, orange [30] | |
| 22 | (E,E)-2,4-Decadienal | 1818 | 1811 ± 16 | 0.07 [21] | 50.14 ± 18.26 a | 716.29 | n.d. | 26.03 ± 4.30 b | 371.86 | Fatty, sweet [24] | |
| 23 | 1-Pentadecanal | 2041 | 2041 ± 0 | 430 [31] | 13.66 ± 3.46 a | <1 | 5.35 ± 1.05 b | <1 | n.d. | Metal, plastic [31] | |
| 24 | Benzaldehyde | 1534 | 1520 ± 14 | 350 [21] | n.d. | n.d. | 55.69 ± 8.76 a | <1 | Cherry, nutty, almond [14] | ||
| 25 | 2,4-Dimethylbenzaldehyde | 1725 | 1726 ± 16 | 1000 [14] | 28.38 ± 5.63 a | <1 | 21.24 ± 8.48 a | <1 | 13.53 ± 6.62 a | <1 | Bitter almond [14] |
| Ketones (4) | |||||||||||
| 26 | 1-Octen-3-one | 1302 | 1300 ± 8 | 0.005 [21] | n.d. | n.d. | 94.81 ± 16.81 a | 18,962.00 | Mushroom, earthy [24] | ||
| 27 | Geranyl acetone | 1861 | 1859 ± 9 | 60 [21] | 34.34 ± 9.04 a | <1 | 22.05 ± 7.54 a | <1 | n.d. | Floral, green [32] | |
| 28 | (E)-β-Ionone | 1947 | 1940 ± 17 | 0.7 [14] | 73.84 ± 16.22 a | 105.49 | 23.06 ± 2.73 b | 32.94 | n.d. | Fruity, seaweed, violet [14] | |
| 29 | 4-Hydroxy-3-methylacetophenone | 2213 | 2210 ± 31 | n.d. | n.d. | 41.74 ± 8.38 a | |||||
| Acids (3) | |||||||||||
| 30 | Acetic acid | 1456 | 1449 ± 13 | 5.5 [23] | 890.15 ± 290.45 b | 161.85 | 1010.19 ± 118.51 b | 183.67 | 2381.65 ± 752.05 a | 433.03 | Acidic, vinegar, cheesy [23] |
| 31 | Hexanoic acid | 1856 | 1846 ± 12 | 3000 [21] | n.d. | n.d. | 29.11 ± 4.89 a | <1 | Sweaty, cheesy, sharp, goaty, bad breath [33] | ||
| 32 | Octanoic acid | 2065 | 2060 ± 15 | 3000 [21] | 56.45 ± 14.16 a | <1 | 16.71 ± 2.48 b | <1 | 24.92 ± 4.15 b | <1 | Sweat, cheese [33] |
| Esters (4) | |||||||||||
| 33 | Geranyl acetate | 1755 | 1752 ± 11 | 9 [21] | n.d. | 33.27 ± 10.00 a | 3.70 | n.d. | Fruity–floral, sweet [34] | ||
| 34 | Methyl palmitate | 2218 | 2208 ± 10 | 4 × 106 [35] | 34.52 ± 10.94 a | <1 | n.d. | n.d. | Oily, waxy, fatty [35] | ||
| 35 | Ethyl palmitate | 2257 | 2251 ± 9 | 2000 [35] | 29.28 ± 10.04 a | <1 | 16.85 ± 2.07 a | <1 | n.d. | Fatty, fruity, waxy [35] | |
| 36 | Ethyl linolenate | 2604 | 2591 ± 13 | 450 [35] | n.d. | 5.98 ± 0.61 a | <1 | n.d. | Fatty, waxy [35] | ||
| Alcohols (4) | |||||||||||
| 37 | 1-Octen-3-ol | 1148 | 1450 ± 7 | 1 [21] | n.d. | n.d. | 51.28 ± 0.86 a | 51.28 | Mushroom [14] | ||
| 38 | (E)-2-Octen-1-ol | 1615 | 1614 ± 7 | 100 [14] | 47.48 ± 3.53 a | <1 | n.d. | n.d. | Green, soap, plastic [14] | ||
| 39 | Linalool | 1544 | 1547 ± 7 | 6 [21] | n.d. | 9.68 ± 0.71 a | 1.61 | n.d. | Fruity, sweet, floral [33] | ||
| 40 | Geraniol | 1852 | 1847 ± 10 | 40–75 [21] | n.d. | 46.68 ± 2.78 a | <1 | n.d. | Sweet rose, pleasant, floral [34] | ||
| Terpenes (5) | |||||||||||
| 41 | β-Sesquiphellandrene | 1773 | 1772 ± 8 | 36 [36] | 112.02 ± 35.92 a | 3.11 | 48.98 ± 5.53 b | 1.36 | n.d. | Fruity, herbal [36] | |
| 42 | Zingiberene | 1723 | 1724 ± 9 | 170.58 ± 48.77 a | 63.60 ± 12.44 b | 17.68 ± 4.48 b | Spice, fresh, sharp [36] | ||||
| 43 | β-Bisabolene | 1729 | 1727 ± 11 | 80.63 ± 20.22 a | 33.43 ± 3.09 b | n.d. | Balsamic, woody [36] | ||||
| 44 | Farnesene | 1744 | 1746 ± 9 | n.d. | 12.34 ± 0.23 a | n.d. | Citrus, herbal, lavender, bergamot [36] | ||||
| 45 | Curcumene | 1778 | 1777 ± 9 | 284.92 ± 111.48 a | 111.25 ± 15.00 b | 13.00 ± 2.42 b | Herbal [36] | ||||
| Nitriles (1) | |||||||||||
| 46 | Benzenepropanenitrile | 2047 | 2041 ± 7 | 1317.01 ± 207.72 a | 763.87 ± 33.90 b | 1703.69 ± 321.51 a | Aldehydic, spicy [37] | ||||
| Phenols (1) | |||||||||||
| 47 | 2,4-Di-tert-butylphenol | 2316 | 2318 ± 10 | n.d. | 30.62 ± 10.89 a | n.d. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guan, H.; Zhang, X.; Xing, S.; Liu, W.; Kim, I.-C.; Gong, H. The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage. Molecules 2023, 28, 6539. https://doi.org/10.3390/molecules28186539
Li H, Guan H, Zhang X, Xing S, Liu W, Kim I-C, Gong H. The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage. Molecules. 2023; 28(18):6539. https://doi.org/10.3390/molecules28186539
Chicago/Turabian StyleLi, Huamin, Hui Guan, Xiru Zhang, Shaohua Xing, Wenli Liu, In-Cheol Kim, and Hansheng Gong. 2023. "The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage" Molecules 28, no. 18: 6539. https://doi.org/10.3390/molecules28186539
APA StyleLi, H., Guan, H., Zhang, X., Xing, S., Liu, W., Kim, I.-C., & Gong, H. (2023). The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage. Molecules, 28(18), 6539. https://doi.org/10.3390/molecules28186539






