Development and Assessment of 1,5–Diarylpyrazole/Oxime Hybrids Targeting EGFR and JNK–2 as Antiproliferative Agents: A Comprehensive Study through Synthesis, Molecular Docking, and Evaluation
Abstract
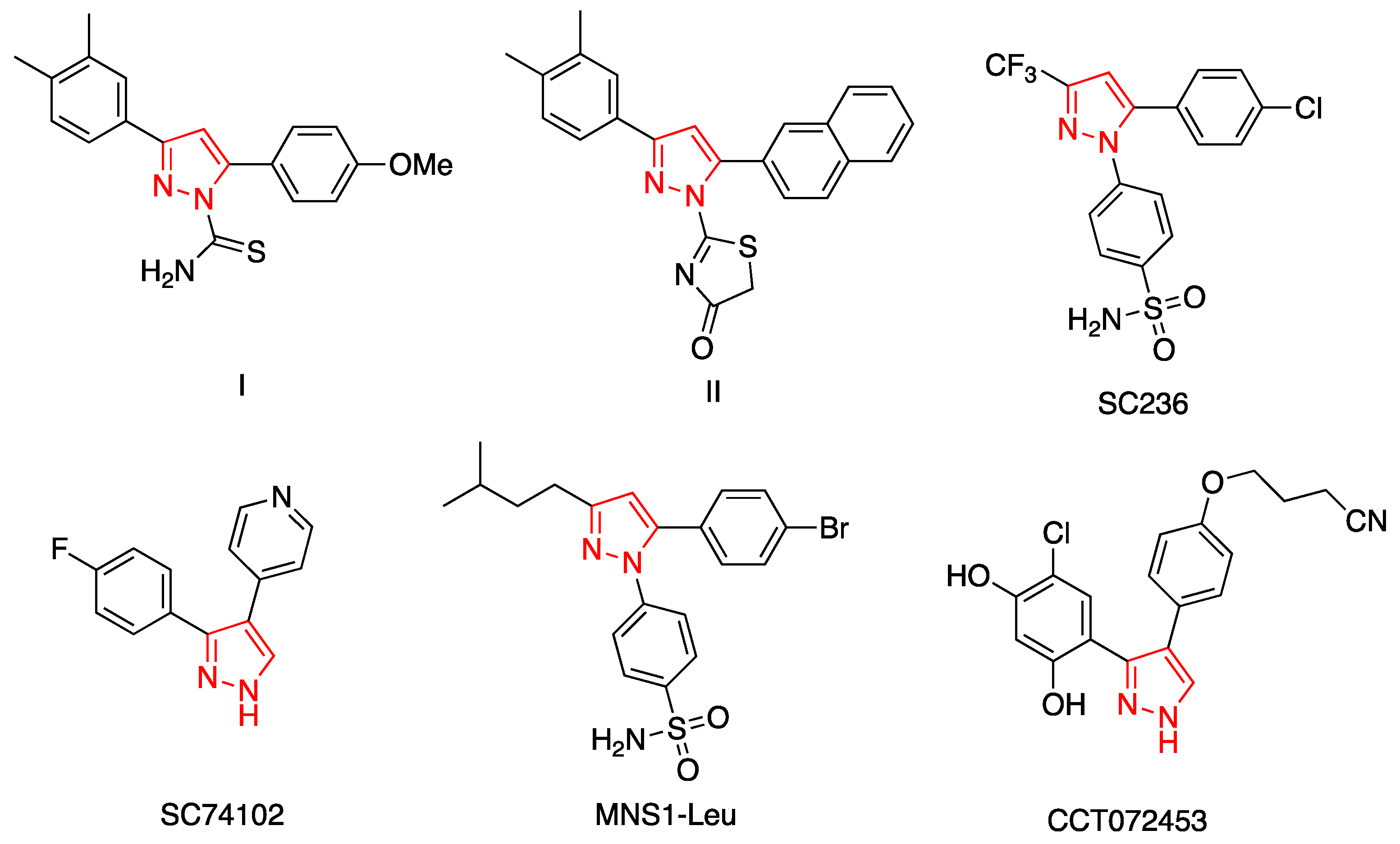
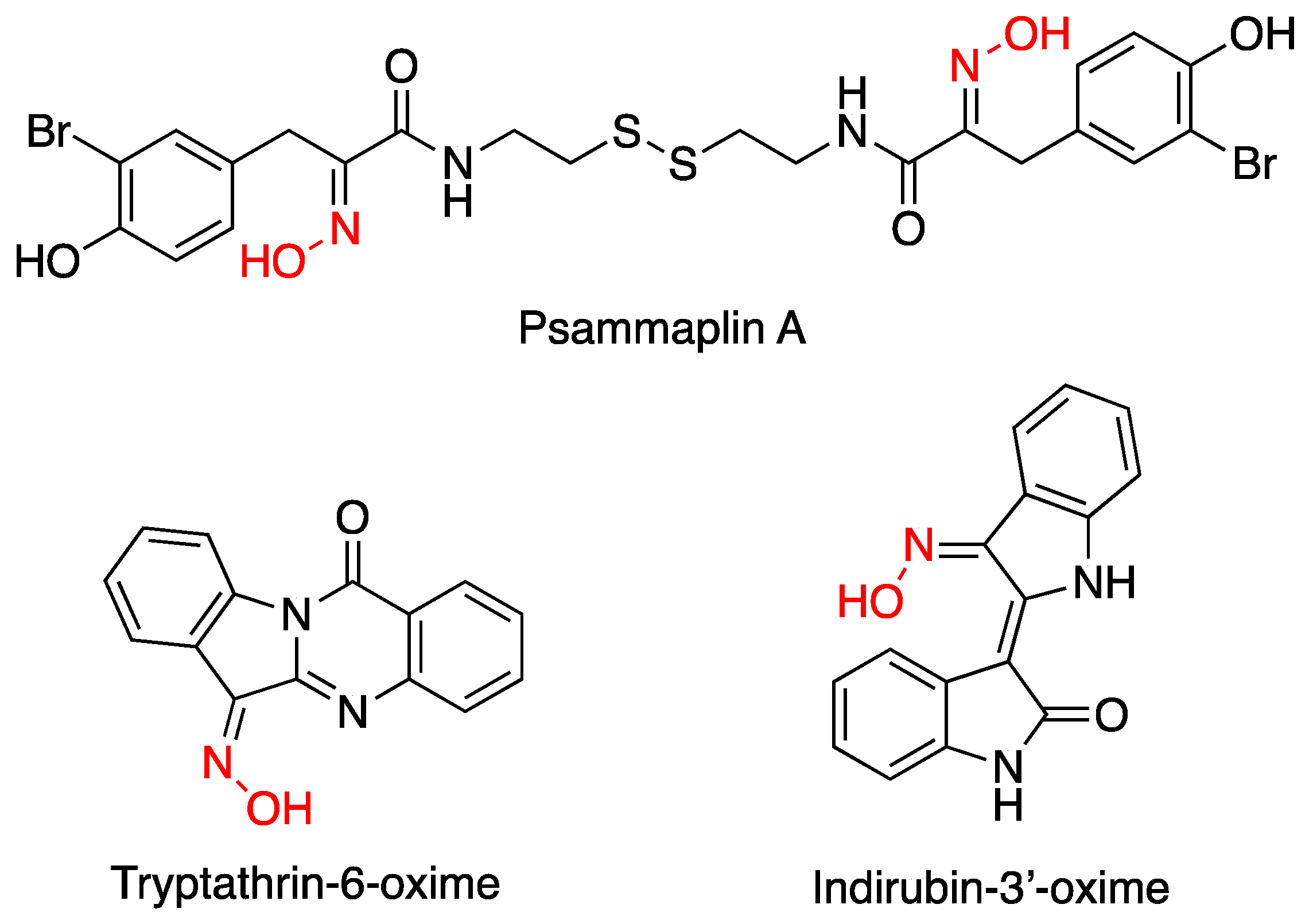
1. Introduction
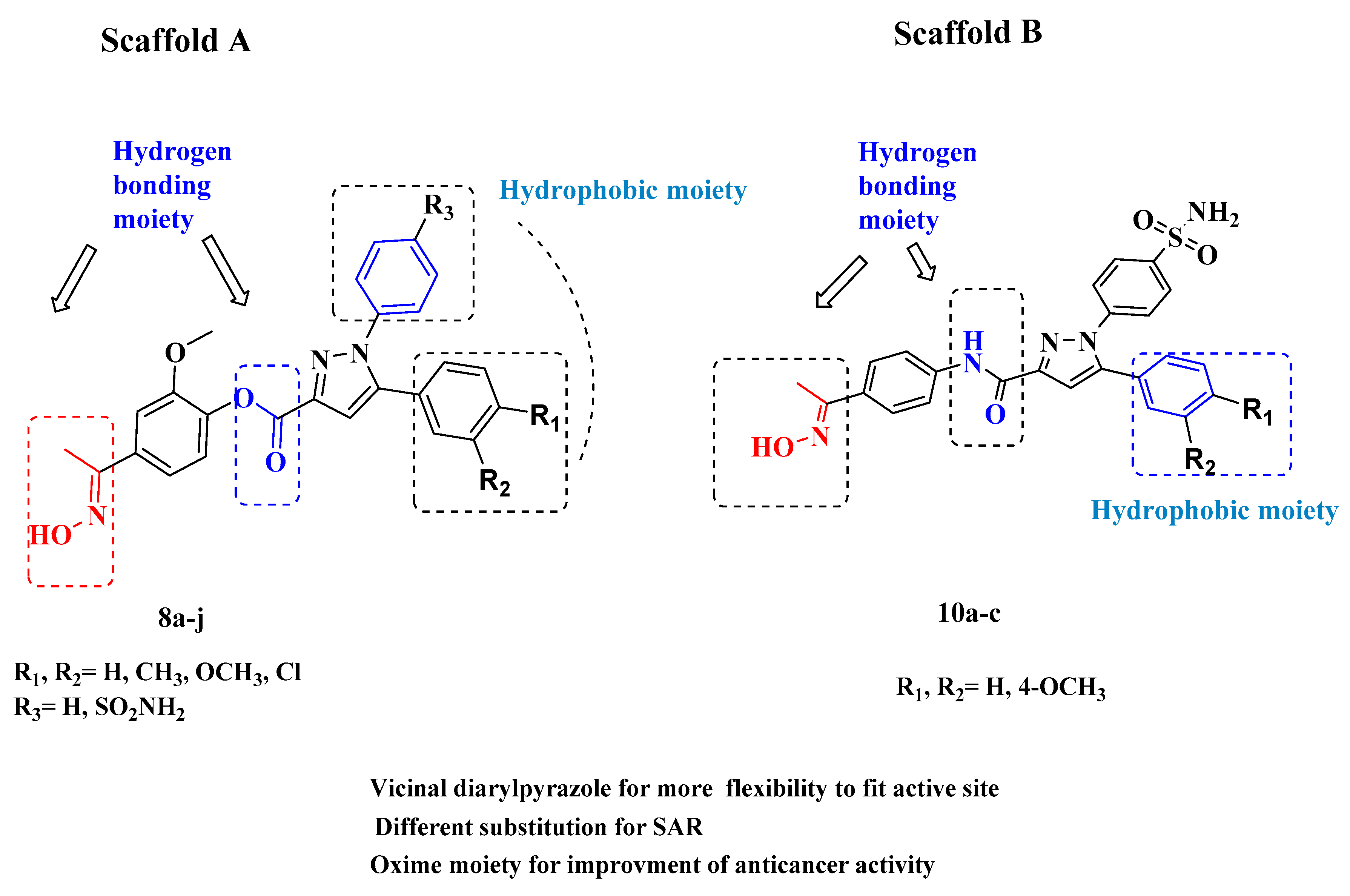
2. Results and Discussion
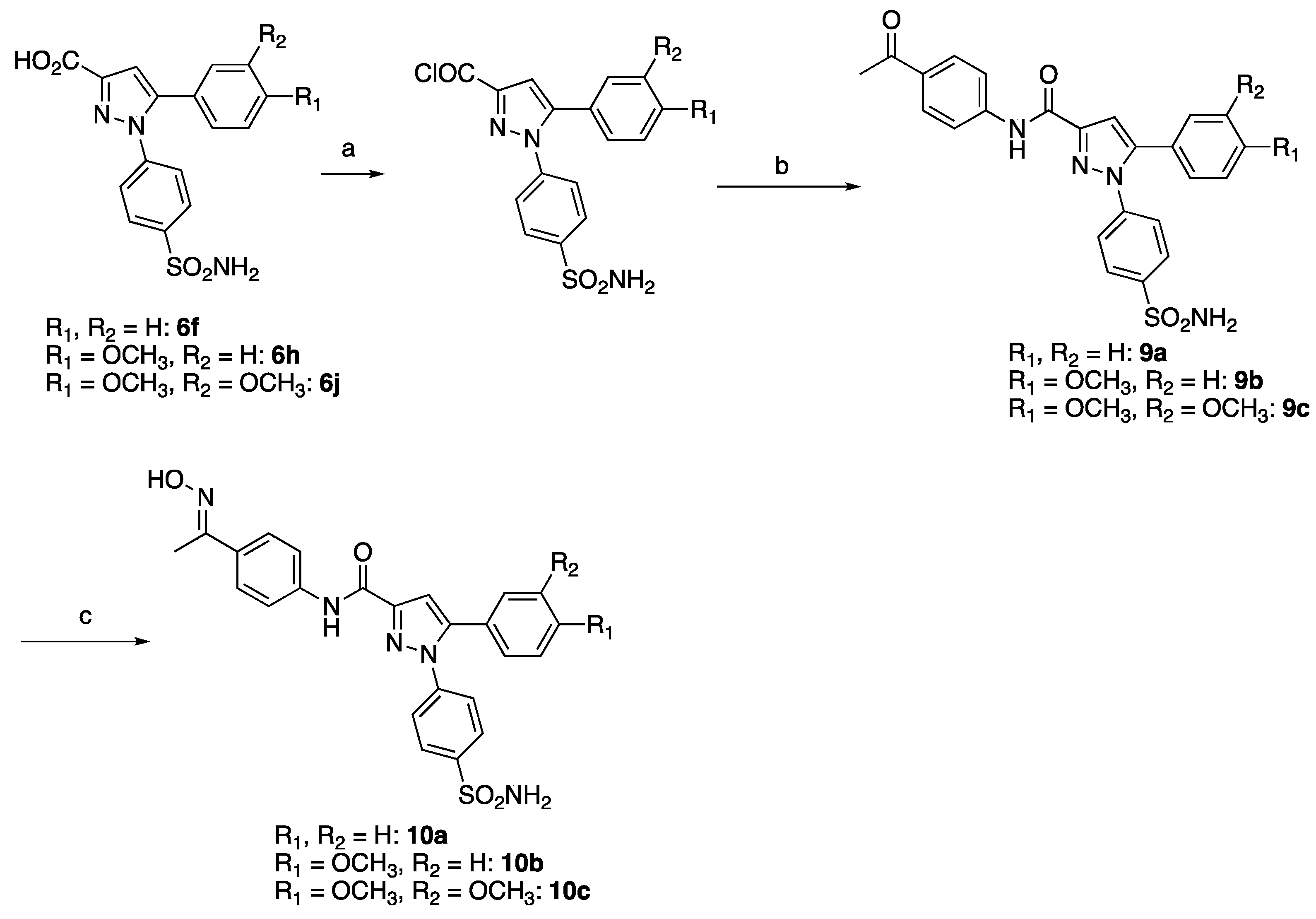
2.1. Chemistry
2.2. Biology
2.2.1. In Vitro Antiproliferative Screening Activities
2.2.2. In Vitro Cytotoxicity Measurements (IC50) against Five Cancer Cell Lines
2.2.3. Evaluation of EGFR and JNK-2 Inhibitory Activity
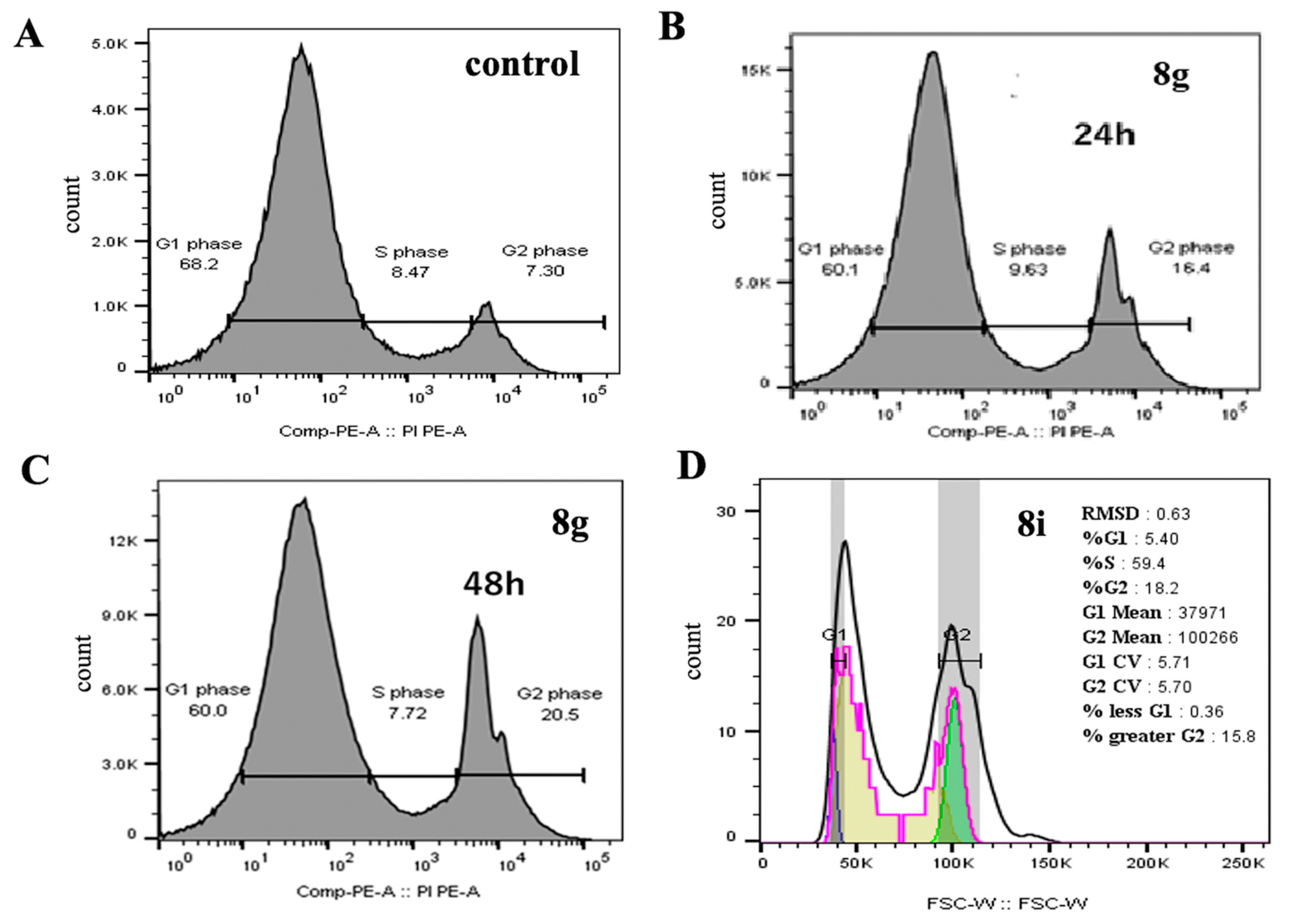
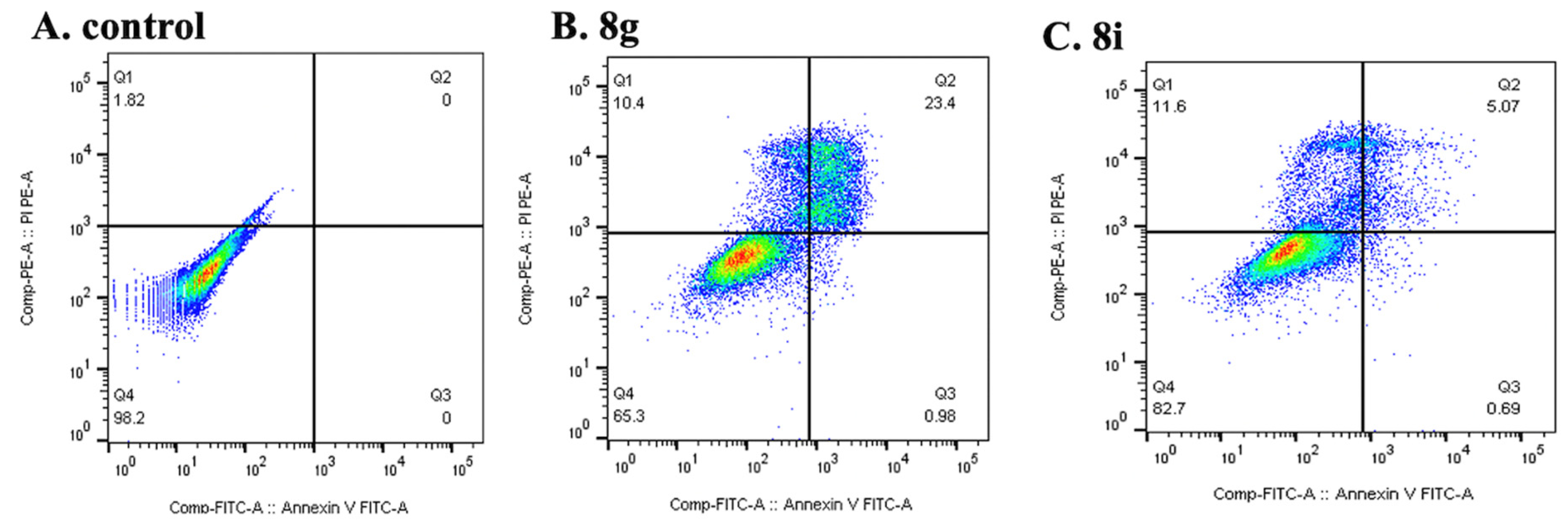
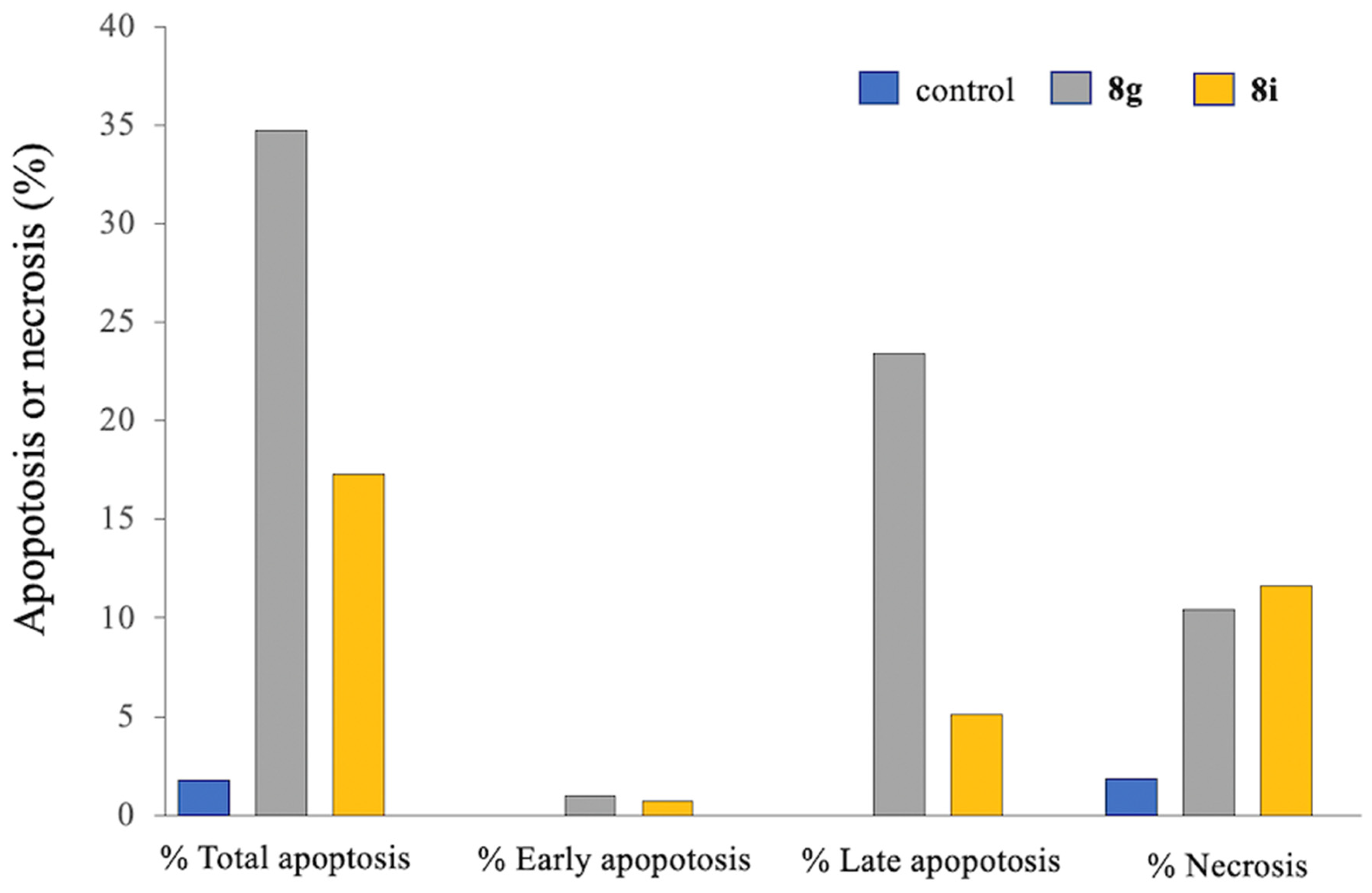
2.2.4. Cell Cycle Analysis and Apoptosis Detection
Cell Cycle Analysis
Apoptosis Assay
2.2.5. Evaluation of Cytotoxicity towards the Normal Cell Line PC12
2.3. Measurement of Nitric Oxide Release
2.4. Docking
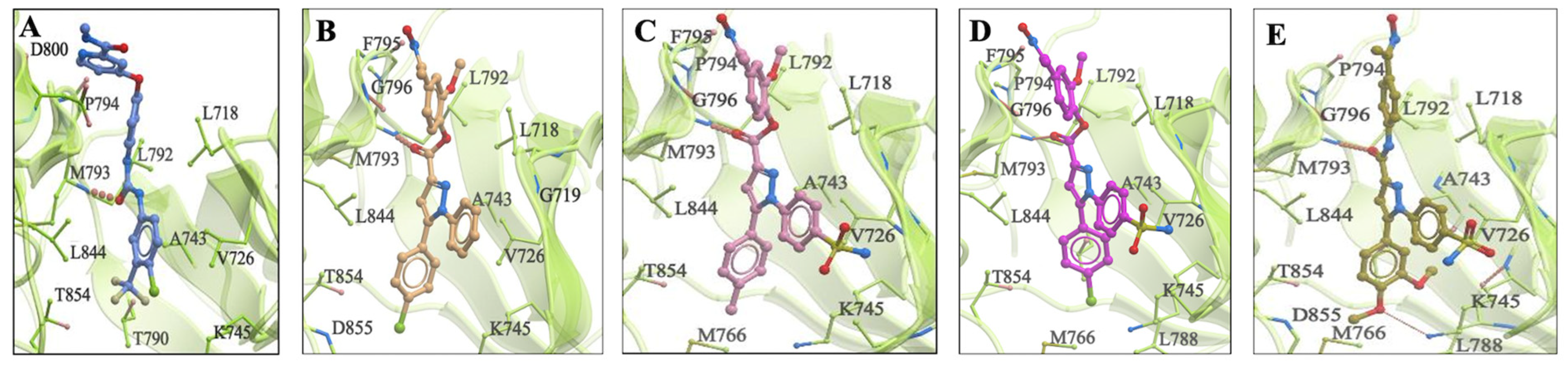
2.4.1. In Silico Molecular Docking Study into EGFR
2.4.2. In Silico Molecular Docking Study on JNK-2
3. Conclusions
4. Experimental Section
4.1. Chemistry
4.1.1. Material and Equipment
4.1.2. General Procedure for the Synthesis of Ethyl 4-(Substituted Phenyl)-2-Hydroxy-4-Oxobut-2-Enoates (2a–e)
4.1.3. General Procedure for Synthesis of 4-Hydrazinylbenzenesulfonamide Hydrochloride 4b
4.1.4. General Procedure for the Synthesis of Ethyl 1,5 Diarypyarzole-3-Carboxylate (5a–j)
- Ethyl 1,5–diphenyl–1H–pyrazole–3–carboxylate (5a): Reddish brown solid; yield (75%); mp: 85–87 °C (lit. 86 °C) [58].Ethyl 1–phenyl–5–(p–tolyl)–1H–pyrazole–3–carboxylate (5b): Reddish solid; yield (80%), mp: 87–88 °C (lit. 84–86 °C) [59].Ethyl 5–(4–methoxyphenyl)–1–phenyl–1H–pyrazole–3–carboxylate (5c): Reddish brown solid; yield (81%); mp: 97–99 °C (lit. 97 °C) [55].Ethyl 5–(4–chlorophenyl)–1–phenyl–1H–pyrazole–3–carboxylate (5d): Reddish brown solid; yield (89%); mp: 92–94 °C (lit. 95–97 °C) [55].Ethyl 5–(3,4–dimethoxyphenyl)–1–phenyl–1H–pyrazole–3–carboxylate (5e): Brownish solid, yield (63%); mp: 174–176 °C (lit. 177 °C) [60].Ethyl 5–phenyl–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (5f): Reddish powder; yield (66%), mp: 192–194 °C (lit. 192) [61].Ethyl 1–(4–sulfamoylphenyl)–5–(p–tolyl)–1H–pyrazole–3–carboxylate (5g): Reddish brown; yield (75%), mp: 227–228 °C (lit. 227 °C) [62].Ethyl 5–(4–methoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (5h): Reddish brown powder; yield (71%), mp: 207–209 °C (lit. 205–207 °C) [63].Ethyl 5–(4–chlorophenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (5i): Reddish brown powder; yield (80%), mp: 107–109 °C (lit. 108 °C) [63].Ethyl 5–(3,4–dimethoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (5j): Reddish brown powder; yield (64%); mp: 214–215 °C [64].
4.1.5. General Procedure for the Synthesis of 1,5-Diarypyrazole Carboxylic Acids (6a–j)
- 1,5–Diphenyl–1H–pyrazole–3–carboxylic acid (6a): Brown powder; yield (84%); mp: 180–182 °C (lit. 182–183) [56].1–Phenyl–5–(p–tolyl)–1H–pyrazole–3–carboxylic acid (6b): Reddish powder; yield (87%); mp: 171–172 °C [66].5–(4–Methoxyphenyl)–1–phenyl–1H–pyrazole–3–carboxylic acid (6c): Reddish brown powder; yield (79%); mp: 192–195 °C (lit. 196–197 °C) [64].5–(4–Chlorophenyl)–1–phenyl–1H–pyrazole–3–carboxylic acid (6d): Yellowish brown powder; yield (78%); mp: > 300 °C [62].5–(3,4–Dimethoxyphenyl)–1–phenyl–1H–pyrazole–3–carboxylic acid (6e): Brown powder; yield (84%); mp: 213–214 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 7.94 7.52 (m, 5H, Ar-H), 7.39 (s, 1H, pyrazole-H), 6.94–6.68 (m, 3H, Ar-H), 3.79 (s, 3H, OCH3), 3.77 (s, 3H, OCH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 163.36, 160.36, 145.81, 144.09, 142.55, 130.71, 128.76, 127.45, 126.47, 125.40, 122.05, 120.40, 115.26, 110.45, 56.23, 56.12; ESI-MS (LR) m/z [M+H]+ for C18H17N2O4 calculated: 325.1, found: 325.3.5–Phenyl–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylica acid (6f): Yellowish brown powder; yield (78%), mp: 184–186 °C (lit. 188 °C) [65].1–(4–Sulfamoylphenyl)–5–(p–tolyl)–1H–pyrazole–3–carboxylic acid (6g): Reddish brown powder; yield (88%); mp: 194–195 °C [56].5–(4–Methoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylic acid (6h): Brownish powder; yield (73%), mp: 197–198 °C [67].5–(4–Chlorophenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylic acid (6i): Yellowish brown powder; yield (84%); mp: 212–214 °C [68].5–(3,4–Dimethoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylic acid (6j): Reddish brown powder; yield (77%); mp: 206–208 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.76 (s, 1H, OH), 7.99 (s, 1H, Ar-H), 7.91 (d, J= 8.00 Hz, 2H, Ar-H), 7.79 (d, J= 8.00 Hz, 2H, Ar-H), 7.65–7.60 (m, 4H, 2Ar-H, SO2NH2), 7.43 (s, 1H, pyrazole-H), 3.89 (s, 6H, 2 OCH3);13C-NMR (100 MHz, DMSO-d6) δ (ppm): 163.36, 159.98, 147.36, 145.23, 141.21, 131.47, 130.17, 128.82, 126.87, 125.54, 122.87, 119.99, 115.14, 107.53, 56.38, 56.21; ESI-MS (LR) m/z [M+H]+ for C18H18N3O6S calculated: 404.1, found: 404.0.
4.1.6. General Procedure for Synthesis of 4-Acetyl-2-Methoxyphenyl 5-(4-Subistituted-phenyl) 1-(4-Substituted-Phenyl)-1H-Pyrazole-3-Carboxylate (7a–j)
- 4–Acetyl–2–methoxyphenyl 1,5–diphenyl–1H–pyrazole–3–carboxylate (7a): Yellowish brown solid; yield (75%); mp: 98–100 °C; IR (ATR) cm−1; 1748 (COO-Ph), 1725 (Co-CH3), 1574 (C=C); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.65 (d, J = 8.50 Hz, 1H, Ar-H), 7.46 (d, J = 8.00 Hz, 2H, Ar-H), 7.43–7.39 (m, 3H, Ar-H), 7.38 (s, 1H, Ar-H), 7.34–7.32 (m, 3H, Ar-H), 7.30 (s, 1H, pyrazole-H), 7.28–7.27 (m, 2H, Ar-H), 6.84 (d, J = 8.50 Hz, 1H, Ar-H), 3.75 (s, 3H, OCH3), 2.57 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.35, 160.07, 151.86, 147.61, 146.33, 145.04, 143.48, 142.86, 141.18, 139.68, 136.44, 129.91, 129.45, 129.11, 126.55, 123.97, 122.69, 114.98, 111.62, 56.74, 27.17; ESI-MS m/z [M+Na]+ for C25H20N2NaO4 calculated: 435.1320, found: 435.1309.4–Acetyl–2–methoxyphenyl–1–phenyl 5–p–tolyl–1H–pyrazole–3–carboxylate (7b): Yellowish solid; yield (81%); mp: 110–112 °C; IR (ATR) cm−1; 1743 (COO-Ph), 1710 (CO-CH3), 1575 (C=C); 1H-NMR (500 MHz, CDCl3) δ (ppm): 7.65 (s, 1H, Ar-H), 7.53–7.50 (m, 6H, Ar-H), 7.36–7.35 (m, 2H, Ar-H), 7.15 (s, 1H, pyrazole-H), 6.92–6.94 (m, 3H, Ar-H), 3.88 (s, 3H, OCH3), 2.62 (s, 3H, CH3), 2.33 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 197.40, 159.67, 150.10, 146.64, 143.95, 142.51, 139.46, 138.90, 136.03, 130.16, 129.85, 128.80, 125.35, 124.00, 122.97, 121.94, 111.97, 109.93, 56.48, 26.65, 21.49; ESI-MS m/z [M+Na]+ for C26H22N2NaO4 calculated: 449.1477, found: 449.1483.4–Acetyl–2–methoxyphenyl–5–(4–methoxyphenyl) 1–phenyl–1H–pyrazole–3–carboxylate (7c): Yellowish brown solid; yield (85%); mp: 69–71 °C; IR (ATR) cm−1; 1743 (COO-Ph), 1725 (CO-CH3), 1577 (C=C); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.64 (d, J = 7.00 Hz, 1H, Ar-H), 7.46 (d, J = 8.50 Hz, 2H, Ar-H), 7.42 (s, 1H, Ar-H), 7.38 (d, J = 7.00 Hz, 1H, Ar-H), 7.34 (d, J = 7.50 Hz, 2H, Ar-H), 7.22 (s, 1H, pyrazole-H), 7.17 (d, J = 8.50 Hz, 2H, Ar-H), 6.88–6.85 (m, 3H, Ar-H), 3.84 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 2.58 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.73, 159.99, 151.77, 148.69, 145.56, 143.22, 142.20, 139.78, 136.74, 130.95, 130.16, 129.55, 125.76, 124.02, 122.28, 116.09, 115.07, 111.96, 110.36, 57.17, 55.74, 26.65; ESI-MS m/z [M+Na]+ for C26H22N2NaO5 calculated: 465.1426, found: 465.1428.4–Acetyl–2–methoxyphenyl–5–(4–chlorophenyl) 1–phenyl–1H–pyrazole–3–carboxylate (7d): Yellowish brown solid; yield (73%); mp: 85–87 °C; IR (ATR) cm−1; 1739 (COO-Ph), 1728 (CO-CH3), 1575 (C=C); 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.60 (s, 1H, Ar-H), 7.56 (d, J = 6.00 Hz, 1H, Ar-H), 7.50–7.46 (m, 4H, Ar-H), 7.32–7.35 (m, 2H, Ar-H), 7.17–7.15 (m, 2H, Ar-H), 7.14 (s, 1H, pyrazole-H), 6.90 (d, J = 6.50 Hz, 2H, Ar-H), 3.88 (s, 3H, OCH3), 2.51 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 197.11, 160.00, 150.43, 147.01, 146.31, 143.53, 143.23, 138.32, 135.31, 133.65, 129.23, 128.88, 127.17, 126.08, 125.74, 123.01, 113.97, 111.69, 109.80, 56.14, 27.26; ESI-MS m/z [M+H]+ for C25H20ClN2O4 calculated: 447.1106, found: 447.1085.4–Acetyl–2–methoxyphenyl–5–(3,4–dimethoxyphenyl) 1–phenyl–1H–pyrazole–3–carboxylate (7e): Reddish yellow solid; yield (71%); mp: 74–76 °C; IR (ATR) cm−1; 1740 (COO-Ph), 1715 (CO-CH3), 1589 (C=C); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.65 (s, 1H, Ar-H), 7.63 (s, 1H, Ar-H), 7.56 (d, J = 8.50 Hz, 1H, Ar-H), 7.46 (d, J = 8.50 Hz, 2H, Ar-H), 7.38–7.41 (m, 3H, Ar-H), 7.30 (s, 1H, pyrazole-H), 6.97 (d, J = 8.50 Hz, 1H, Ar-H), 6.85–6.83 (m, 2H, Ar-H), 3.84 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 2.59 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.38, 160.00, 153.54, 151.71, 149.03, 147.99, 147.03, 142.92, 140.14, 136.74, 130.54, 130.34, 129.55, 127.07, 123.73, 122.65, 121.95, 115.07, 112.67, 112.01, 110.57, 57.15, 56.48, 55.44, 26.95; ESI-MS m/z [M+H]+ for C27H25N2O6 calculated: 473.1707, found: 473.1714.4–Acetyl–2–methoxyphenyl–5–phenyl 1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (7f): Yellowish solid; yield (65%); mp: 69–72 °C; IR (ATR) cm−1; 1735 (COO-Ph), 1724 (CO-CH3), 1589 (C=C aromatic), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.91 (s, 1H, Ar-H), 7.87 (d, J = 8.50 Hz, 2H, Ar-H), 7.80 (d, J = 8.00 Hz, 1H, Ar-H), 7.68–7.64 (m, 3H, Ar-H), 7.55 (d, J = 8.50 Hz, 2H), 7.53 (s, 2H, SO2NH2), 7.41–7.39 (m, 2H, Ar-H), 7.35 (s, 1H, pyrazole-H), 7.31 (d, J = 8.00 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3), 2.57 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.75, 167.77, 159.18, 151.90, 144.65, 144.14, 142.86, 141.18, 135.65, 132.33,129.48, 129.40, 127.04, 125.27, 124.50, 123.58, 122.00, 112.02, 111.12, 56.74, 28.85; ESI-MS m/z [M+Na]+ for C25H21N3 Na O6S calculated: 514.1049, found: 514.1044.4–Acetyl–2–methoxyphenyl–1–(4–sulfamoylphenyl) 5–p–tolyl–1H–pyrazole–3–carboxylate (7g): Yellowish solid; yield (78%), mp: 83–85 °C; IR (ATR) cm−1; 1746 (COO-Ph), 1718 (CO-CH3), 1595 (C=C), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.95 (d, J = 8.50 Hz, 1H, Ar-H), 7.86 (d, J = 8.50 Hz, 2H, Ar-H), 7.69–7.63 (m, 2H, Ar-H), 7.55 (d, J = 8.50 Hz, 2H, Ar-H), 7.52 (s, 2H, SO2NH2), 7.39 (d, J = 8.50 Hz, 2H, Ar-H), 7.29 (s, 1H, pyrazole-H), 7.19 (d, J = 8.50 Hz, 2H, Ar-H), 3.84 (s, 3H, OCH3), 2.60 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.74, 163.64, 151.71, 146.50, 144.25, 143.93, 142.21, 139.63, 130.19, 129.12, 128.82, 128.45, 127.77, 126.14, 125.04, 123.75, 122.31, 112.64, 110.31, 56.44, 27.25, 21.47; ESI-MS m/z [M+Na]+ for C26H23N3NaO6S calculated: 528.1205, found: 528.1205.4–Acetyl–2–methoxyphenyl 5–(4–methoxyphenyl) 1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (7h): Yellowish solid; yield (55%), mp: 80–83 °C; IR (ATR) cm−1; 1750 (COO-Ph), 1720 (CO-CH3), 1585 (C=C), 1164 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.86 (d, J = 9.00 Hz, 2H, Ar-H), 7.66 (s, 1H, Ar-H), 7.64 (d, J = 7.50 Hz, 2H, Ar-H), 7.55 (d, J = 9.00 Hz, 2H, Ar-H), 7.51 (s, 2H, SO2NH2), 7.39 (d, J = 8.00 Hz, 1H, Ar-H), 7.13 (s, 1H, pyrazole-H), 7.26–7.23 (m, 3H, Ar-H), 3.84 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 2.60 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 197.35, 160.08, 151.86, 148.90, 144.65, 143.35, 141.58, 136.44, 130.40, 129.61, 127.43, 126.55, 123.98, 123.58, 121.01, 115.47, 113.69, 111.12, 57.53, 55.85, 26.68; ESI-MS m/z [M+Na]+ for C26H23N3 Na O7S calculated: 544.1154, found: 544.1153.4–Acetyl–2–methoxyphenyl–5–(4–chlorophenyl) 1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (7i): Yellowish brown solid; yield (76%); mp: 71–74 °C; IR (ATR) cm−1; 1744 (COO-Ph), 1726 (CO-CH3), 1591 (C=C), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm):, 7.89 (d, J = 8.00 Hz, 2H, Ar-H), 7.86 (s, 1H, Ar-H), 7.80 (d, J = 9.00 Hz, 1H, Ar-H), 7.65 (d, J = 9.00 Hz, 1H, Ar-H), 7.57 (d, J = 10.00 Hz, 2H, Ar-H), 7.52 (s, 2H, SO2NH2), 7.47 (d, J = 8.00 Hz, 2H, Ar-H), 7.38 (s, 1H, pyrazole-H), 7.33 (d, J = 10.00 Hz, 2H, Ar-H), 3.84 (s, 3H, OCH3), 2.69 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.80, 162.85, 159.53, 144.43, 143.25, 143.15, 141.69, 136.56, 132.33, 129.46, 127.90, 127.43, 126.56, 126.09, 123.83, 122.31, 120.09, 112.31, 111.90, 57.14, 27.14; ESI-MS m/z [M-H]− for C25H20ClN3NaO6S calculated: 524.0689, found: 524.0688.4–Acetyl–2–methoxyphenyl–5–(3,4–dimethoxyphenyl) 1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (7j): Yellowish brown solid; yield (52%); mp: 75–78 °C; IR (ATR) cm−1; 1749 (COO-Ph), 1727 (CO-CH3), 1579 (C=C), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.05 (d, J = 8.50 Hz, 2H, Ar-H), 7.96 (d, J = 7.50 Hz, 1H, Ar-H), 7.80 (s, 1H, Ar-H), 7.74 (d, J = 8.50 Hz, 2H, Ar-H), 7.69 (s, 2H, SO2NH2), 7.56 (d, J = 7.50 Hz, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.10 (d, J = 7.50 Hz, 1H, Ar-H), 7.05 (s, 1H, pyrazole-H), 6.95 (d, J = 7.50 Hz, 1H, Ar-H), 3.99 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 2.75 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.75, 163.93, 151.47, 149.29, 148.89, 145.80, 144.68, 143.33, 142.86, 142.08, 136.04, 129.13, 127.41, 126.55, 125.27, 123.58, 122.29, 121.00, 112.90, 111.12, 109.84, 57.13, 56.74, 54.96, 27.17; ESI-MS m/z [M+Na]+ for C27H25N3NaO8S calculated: 574.1260, found: 574.1261.
4.1.7. General Procedure for Synthesis of (E)-4-(1-(Hydroxyimino)Ethyl)-2-Methoxyphenyl 5-(4-Substituted Phenyl)-1-(4-Substituted Phenyl)-1H-Pyrazole-3-Carboxylate (8a–j)
- (E)–4–(1–(Hydroxyimino)ethyl)2–methoxyphenyl 1,5–diphenyl–1H–pyrazole–3–carboxylate (8a): Yellowish brown solid; yield (67%); mp: 167–170 °C; IR (ATR) cm−1; 3191 (OH), 1756 (C=O), 1594 (C=C aromatic); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.30 (s, 1H, OH), 7.47 (s, 1H, Ar-H), 7.41–7.37 (m, 4H, Ar-H), 7.33–7.30 (m, 3H, Ar-H), 7.28–7.23 (m, 3H, Ar-H), 7.20 (s, 1H, pyrazole-H), 7.00 (d, J = 8.00 Hz, 1H, Ar-H), 6.78 (d, J = 8.00 Hz, 1H, Ar-H), 3.73 (s, 3H, OCH3), 2.06 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 160.07, 152.75, 148.50, 148.01, 144.05, 142.67, 139.72, 136.71, 132.58, 130.41, 129.62, 128.72, 127.84, 126.55, 123.37, 119.35, 118.92, 116.26, 109.46, 56.24, 12.14; ESI-MS m/z [M+Na]+ for C25H21N3NaO4 calculated: 450.1430, found: 450.1423.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 1–phenyl–5–p–tolyl–1H–pyrazole–3–carboxylate (8b): Yellowish green powder; yield (75%); mp: 123–125 °C; IR (ATR) cm−1; 3138 (OH), 1736 (C=O), 1593 (C=C aromatic); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.36 (s, 1H, OH), 7.40–7.35 (m, 3H, Ar-H), 7.29 (s, 1H, Ar-H), 7.15–7.05 (m, 5H, 4 Ar-H, pyrazole-H), 6.96 (d, J = 7.50 Hz, 2H, Ar-H), 6.75 (d, J = 7.50 Hz, 2H, Ar-H), 3.74 (s, 3H, OCH3), 2.21 (s, 3H, Ph-CH3), 2.02 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 160.73, 153.20, 152.18, 151.13, 147.97, 147.97, 142.01, 139.43, 136.85, 129.81, 129.06, 128.64, 126.07, 124.01, 119.19, 116.09, 115.42, 111.97, 108.89, 56.78, 21.85, 11.83; ESI-MS m/z [M+H]+ for C26H24N3O4 calculated: 442.1761, found: 442.1743.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(4–methoxyphenyl) 1–phenyl–1H–pyrazole–3–carboxylate (8c): Yellowish brown powder; yield (75%); mp: 132–135 °C; IR (ATR) cm−1; 3400 (OH), 1739 (C=O), 1590 (C=C aromatic); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.43 (s, 1H, OH), 7.47 (d, J = 8.50 Hz, 1H, Ar-H), 7.34 (d, J = 10.00 Hz, 2H, Ar-H), 7.25 (s, 1H, Ar-H), 7.18 (d, J = 10.00 Hz, 2H, Ar-H), 7.11–7.08 (m, 2H, Ar-H), 7.04 (s, 1H, pyrazole-H), 7.95 (d, J = 8.50 Hz, 1H, Ar-H), 6.80–6.77 (m, 3H, Ar-H), 3.68 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 2.02 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 162.15, 159.58, 150.58, 147.22, 145.43, 143.76, 142.47, 140.80, 136.88, 130.90, 129.61, 127.83, 126.55, 123.18, 121.16, 120.61, 115.87, 114.57, 109.43, 56.74, 54.96, 14.71; ESI-MS m/z [M+H]+ for C26H24N3O5 calculated: 458.1716, found: 458.1715.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(4–chlorophenyl)–1–phenyl–1H–pyrazole–3–carboxylate (8d): Yellowish brown powder; yield (65%); mp: 106–109 °C; IR (ATR) cm−1; 3228 (OH), 1744 (C=O), 1593 (C=C aromatic); 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.90 (s, 1H, OH), 7.60 (d, J = 8.40 Hz, 1H, Ar-H), 7.42 (d, J = 10.00 Hz, 2H, Ar-H), 7.39 (d, J = 10 Hz, 2H, Ar-H), 7.36 (s, 1H, Ar-H), 7.26–7.21 (m, 3H, 2Ar-H, pyrazole-H), 7.00 (d, J = 8.00 Hz, 2H, Ar-H), 6.75 (d, J = 8.00 Hz, 2H, Ar-H), 3.74 (s, 3H, OCH3), 2.07 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 160.00, 153.68, 151.87, 147.38, 143.64, 142.89, 139.83, 139.48, 136.84, 134.19, 132.02, 131.01, 129.71, 128.38, 126.38, 123.19, 119.48, 115.55, 109.63, 55.38, 12.45; ESI-MS m/z [M+H]+ for C25H21ClN3O4 calculated: 462.1188, found: 462.1184.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(3,4–dimethoxy phenyl)–1–phenyl–1H–pyrazole–3–carboxylate (8e): Yellowish white powder; yield (77%); mp: 115–118 °C; IR (ATR) cm−1; 3300 (OH), 1740 (C=O), 1593 (C=C aromatic); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.43 (s, 1H, OH), 7.56 (s, 1H, Ar-H), 7.46 (s, 1H, Ar-H), 7.40 (d, J = 6.50 Hz, 1H, Ar-H), 7.25–7.19 (m, 4H, Ar-H), 7.01–6.98 (m, 2H, Ar-H), 7.16 (s, 1H, pyrazole-H), 6.79 (d, J = 8.50, 2H, Ar-H), 3.73 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 2.06 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 161.75, 159.58, 153.64, 150.58, 150.19, 149.29, 148.01, 145.05, 144.15, 142.86, 139.50, 130.41, 127.84, 125.76, 121.67, 121.62, 121.41, 119.71, 115.86, 112.90, 109.84, 56.74, 55.85, 54.56, 11.65; ESI-MS m/z [M+H]+ for C27H24N2NaO6 calculated: 488.1816, found: 488.1808.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–phenyl–1–(4–sulfamoyl phenyl)–1H–pyrazole–3–carboxylate (8f): Yellowish powder; yield (53%); mp: 122–124 °C; IR (ATR) cm−1; 3681 (OH), 1744 (C=O), 1590 (C=C aromatic), 1165 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.17 (s, 1H, OH), 7.86 (d, J = 6.50 Hz, 2H, Ar-H), 7.55 (d, J = 6.5 Hz, 2H, Ar-H), 7.51 (s, 2H, SO2NH2),7.40–7.35 (m, 4H, Ar-H), 7.32–7.30 (m, 3H, Ar-H), 7.24 (s, 1H, Ar-H) 7.19 (s, 1H, pyrazole-H), 3.79 (s, 3H, OCH3), 2.06 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 165.53, 159.99, 153.18, 151.12, 145.63, 143.53, 142.52, 141.49, 139.80, 136.74, 130.18, 129.18, 127.77, 126.80, 123.77, 123.30, 118.83, 111.65, 109.60, 55.71, 12.56; ESI-MS m/z [M+Na]+for C25H22N4 Na O6S calculated: 529.1158, found: 529.1158.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 1–(4–sulfamoylphenyl)–5–p–tolyl–1H–pyrazole–3–carboxylate (8g): Yellowish white powder; yield (69%); mp: 195–197 °C; IR (ATR) cm−1; 3255 (OH), 1740 (C=O), 1594 (C=C aromatic), 1164 (SO2NH2), 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.34 (s, 1H, OH), 7.95 (d, J = 7.50, 1H, Ar-H), 7.86 (d, J = 7.5 Hz, 2H, Ar-H), 7.68 (d, J = 7.50 Hz, 1H, Ar-H), 7.54 (d, J = 8.50 Hz, 2H, Ar-H), 7.52 (s, 2H, SO2NH2), 7.40 (s, 1H, Ar-H), 7.27 (s, 1H, pyrazole-H), 7.24–7.19 (m, 4H, Ar-H), 3.78 (s, 3H, OCH3), 2.28 (s, 3H, CH3), 2.16 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 160.42, 153.19, 151.27, 145.80, 144.61, 142.05, 139.88, 139.41, 136.67, 130.21, 129.17, 127.98, 127.33, 126.51, 126.10, 123.30, 118.84, 111.67, 110.12, 56.45, 21.50, 12.56; ESI-MS m/z [M+Na]+ for C26H24N4NaO6S calculated: 543.1314, found: 543.1304.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(4–methoxyphenyl)–1–(4–sulfamoyl phenyl)–1H–pyrazole–3–carboxylate (8h): Yellowish white powder; yield (49%), mp: 125–127 °C; IR (ATR) cm−1; 3400 (OH), 1742 (C=O), 1596 (C=C aromatic), 1165 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.27 (s, 1H, OH), 7.87 (d, J = 8.50 Hz, 2H, Ar-H), 7.55 (d, J = 9.50 Hz, 2H, Ar-H), 7.51 (s, 2H, SO2NH2), 7.40 (s, 1H, Ar-H), 7.24–7.20 (m, 4H, Ar-H), 7.19 (s, 1H, pyrazole-H), 6.95 (d, J = 9.50 Hz, 2H, Ar-H), 3.79 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 2.16 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 161.75, 159.18, 153.97, 151.07, 145.93, 144.64, 143.75, 142.47, 139.90, 136.44, 130.41, 129.62, 127.78, 123.18, 122.03, 121.41, 118.82, 115.47, 111.11, 56.74, 55.85, 12.14; ESI-MS m/z [M+Na]+ for C26H24N4NaO7S calculated: 559.1263, found: 559.1274.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(4–chlorophenyl)–1–(4–sulfamoyl phenyl)–1H–pyrazole–3–carboxylate (8i): Yellowish brown powder; yield (67%); mp: 110–112 °C; IR (ATR) cm−1; 3371 (OH), 1743 (C=O), 1595 (C=C aromatic), 1161 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.44 (s, 1H, OH), 7.85 (d, J = 10.00 Hz, 2H, Ar-H), 7.76 (d, J = 6.50 Hz, 1H, Ar-H), 7.61 (s, 1H, Ar-H), 7.49 (s, 2H, SO2NH2), 7.40–7.33 (m, 4H, 3Ar-H, pyrazole-H), 7.26 (d, J = 10.00 Hz, 2H, Ar-H), 7.19 (d, J = 10.00 Hz, 2H, Ar-H), 3.71 (s, 3H, OCH3), 1.90 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 159.57, 150.82, 144.50, 144.38, 143.30, 141.53, 136.59, 134.61, 132.25,, 129.43, 128.25, 127.46, 126.50, 124.90, 123.26, 120.17, 119.66, 118.84, 109.89, 56.38, 11.76; ESI-MS m/z [M+1]+ for C25H22ClN4O6S calculated: 541.0943, found: 541.0948.(E)–4–(1–(Hydroxyimino)ethyl)–2–methoxyphenyl 5–(3,4–dimethoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxylate (8j): Yellowish powder; yield (48%); mp: 129–131 °C; IR (ATR) cm−1; 3300 (OH), 1731 (C=O), 1595 (C=C aromatic); 1164 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.26 (s, 1H, OH), 7.87 (d, J = 9.00 Hz, 2H, Ar-H), 7.85 (d, J = 9.00 Hz, 2H, Ar-H), 7.52 (s, 2H, SO2NH2), 7.41 (s, 1H, Ar-H), 7.32 (s, 1H, Ar-H), 7.25–7.24 (m, 1H, Ar-H), 6.93 (d, J = 8.50 Hz, 1H, Ar-H), 6.88 (s, 1H, pyrazole-H), 6.81–6.75 (m, 2H, Ar-H), 3.79 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 2.17 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 167.78, 159.18, 152.75, 149.29, 148.50, 145.44, 144.65, 143.36, 141.58, 140.30, 136.44, 132.58, 129.12, 127.83, 126.15, 122.30, 121.40, 119.33, 112.91, 110.73, 109.84, 56.28, 56.06, 55.89, 11.75; ESI-MS m/z [M+Na]+ for C27H26N4NaO8S calculated: 589.1369, found: 589.1380.
4.1.8. General Procedure for Synthesis of N-(4-Acetylphenyl)-5-(4-Subistitutedphenyl)-1-(4-Sulfamoylphenyl)-1H-Pyrazole-3-Carboxamide (9a–c)
- N–(4–Acetylphenyl)–5–phenyl–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxamide (9a): Yellowish brown solid; yield (88%); mp: 81–83 °C; IR (ATR) cm−1; 1725 (CO-CH3), 1675 (CONH), 1591 (C=C aromatic), 1161 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.53 (s, 1H, NH), 7.96 (d, J = 9.00 Hz, 2H, Ar-H), 7.86 (d, J = 9.00 Hz, 2H, Ar-H), 7.68–7.62 (m, 3H, Ar-H), 7.57 (d, J = 9.00 Hz, 2H, Ar-H), 7.49 (s, 2H, SO2NH2), 7.40 (d, J = 9.00 Hz, 2H, Ar-H), 7.30–7.32 (m, 2H, Ar-H), 7.20 (s, 1H, pyrazole-H), 2.52 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.09, 167.91, 154.18, 145.63, 144.56, 143.96, 142.53, 133.27, 132.23, 131.21, 129.83, 127.84, 125.96, 125.26, 120.60, 119.04, 109.61, 26.95; ESI-MS m/z [M+Na]+ for C24H20N4 NaO4S calculated: 483.1103, found: 483.1109.N–(4–Acetylphenyl)–5–(4–methoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxamide (9b): Yellowish brown solid; yield (66%); mp: 74–76 °C; IR (ATR) cm−1; 1715 (CO-CH3),1681 (CONH), 1594 (C=C aromatic), 1160 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 11.18 (s, 1H, NH), 7.87 (d, J = 7.60 Hz, 2H, Ar-H), 7.84 (d, J = 7.6.0 Hz, 2H, Ar-H), 7.80 (d, J = 9.00 Hz, 2H, Ar-H), 7.62 (d, J = 9.00 Hz, 2H, Ar-H), 7.54 (s, 2H, SO2NH2), 7.33 (s, 1H, pyrazole-H), 6.98 (d, J = 7.60 Hz, 2H, Ar-H), 6.96 (d, J = 7.60 Hz, 2H, Ar-H), 3.82 (s, 3H, OCH3), 2.47 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 195.96, 167.20, 154.55, 146.06, 144.59, 142.88, 133.27, 132.59, 131.09, 130.12, 129.18, 126.18, 125.30, 123.46, 120.64, 118.98, 113.06, 55.42, 26.61; ESI-MS m/z [M-H]− for C25H21N4O5S calculated: 489.1238, found: 489.1254.N–(4–Acetylphenyl)–5–(3,4–dimethoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxamide (9c): Brownish solid; yield (60%); mp: 81–83 °C; IR (ATR) cm−1; 1720 (CO-CH3), 1669 (CONH), 1590 (C=C aromatic), 1160 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.55 (s, 1H, NH), 7.95 (s, 1H, Ar-H), 7.92 (d, J = 9.00 Hz, 2H, Ar-H), 7.84 (d, J = 7.50 Hz, 2H, Ar-H), 7.76 (d, J = 8.50 Hz, 1H, Ar-H), 7.72 (d, J = 9.00 Hz, 2H, Ar-H), 7.62–7.56 (m, 4H, 2 Ar-H, SO2NH2), 7.37 (s, 1H, pyrazole-H), 7.01 (d, J = 8.50 Hz, 1H, Ar-H), 3.82 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 2.53 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.06, 164.12, 153.21, 152.80, 150.42, 148.06, 145.60, 143.23, 142.71, 132.93, 130.56, 129.86, 129.15, 126.10, 124.03, 123.54, 119.19,116.49, 110.95, 56.76, 55.71, 26.62; ESI-MS m/z [M-H]− for C26H23N4O6S calculated: 519.1344, found: 519.1345.
4.1.9. General Procedure for Synthesis of (E)-N-(4-(1-(Hydroxyimino)Ethyl) Phenyl)-5-(4-Subistituted Phenyl)-1-(4-Sulfamoylphenyl)-1H-Pyrazole-3-Carboxamide (10a–c)
- (E)–N–(4–(1–(Hydroxyimino)ethyl)phenyl)–5–phenyl–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxamide (10a): Yellowish powder; yield (55%); mp: 168–170 °C; IR (ATR) cm−1; 3681 (OH), 1680 (CONH), 1598 (C=C aromatic), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.30 (s, 1H, NH), 8.75 (s, 1H, OH), 7.79 (d, J = 8.00 Hz, 2H, Ar-H), 7.63 (d, J = 8.00 Hz, 2H, Ar-H), 7.50–7.56 (m, 3H, Ar-H), 7.47–7.44 (m, 2H, Ar-H), 7.40 (s, 2H, SO2NH2), 7.35 (d, J = 10.00 Hz, 2H, Ar-H), 7.29 (s, 1H, pyrazole-H), 7.21 (d, J = 10.00 Hz, 2H, Ar-H), 2.06 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 160.05, 153.24, 151.71, 148.09, 143.55, 141.92, 133.69, 129.88, 129.39, 129.30, 127.44, 126.52, 126.42, 121.34, 120.78, 120.33, 109.58, 12.14; ESI-MS m/z [M+H]+ for C24H22N5O4S calculated: 476.1387, found: 476.1397.(E)–N–(4–(1–(Hydroxyimino)ethyl)phenyl)–5–(4–methoxyphenyl)–1–(4–sulfamoylphenyl)–1H–pyrazole–3–carboxamide (10b): Yellowish brown powder; yield (51%); mp: 110–112 °C; IR (ATR) cm−1; 3350 (OH), 1677 (CONH), 1596 (C=C aromatic), 1162 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.31 (s, 1H, NH), 10.02 (s, 1H, OH), 7.84 (d, J = 8.00 Hz, 2H, Ar-H), 7.78 (d, J = 8.50 Hz, 2H, Ar-H), 7.52 (d, J = 8.50 Hz, 2H, Ar-H), 7.46 (d, J = 9.00 Hz, 2H, Ar-H), 7.41 (s, 2H, SO2NH2), 7.30 (s, 1H, pyrazole-H), 6.97 (d, J = 8.00 Hz, 2H, Ar-H), 6.91 (d, J = 9.00 Hz, 2H, Ar-H), 3.80 (s, 3H, CH3), 2.07 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 167.18, 155.50, 144.08, 142.51, 133.80, 132.35, 131.38, 130.65, 129.47, 127.11, 126.41, 126.09, 125.97, 123.46, 121.51, 114.33, 111.98, 56.12, 17.34; ESI-MS m/z [M-H]− for C25H22N5O5S calculated: 504.1347, found: 504.1377.(E)–5–(3,4–Dimethoxyphenyl)–N–(4–(1–(hydroxyimino)ethyl)phenyl)–1–(4–sulfamoyl phenyl)–1H–pyrazole–3–carboxamide (10c): Brownish powder; yield (44%), mp: 105–107 °C; IR (ATR) cm−1; 3220 (OH), 1680 (CONH), 1591 (C=C aromatic), 1161 (SO2NH2); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 10.36 (s, 1H, NH), 10.09 (s, 1H, OH), 7.92 (s, 1H, Ar-H), 7.79 (d, J = 7.50 Hz, 1H, Ar-H), 7.63 (d, J = 8.50 Hz, 2H, Ar-H), 7.52 (d, J = 7.50 Hz, 2H, Ar-H), 7.50–7.44 (m, 4H, 2ArH, SO2NH2), 7.36 (d, J = 8.50 Hz, 2H, Ar-H), 7.27 (s, 1H, pyrazole-H), 6.99 (d, J = 7.50 Hz, 1H, Ar-H), 3.77 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 2.08 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 167.78, 159.18, 152.75, 149.29, 148.50, 145.44, 144.65, 143.36, 140.30, 136.44, 132.58, 129.12, 127.83, 126.15, 122.30, 121.40, 119.33, 112.91, 109.84, 56.19, 55.96, 12.14; ESI-MS m/z [M-H]− for C26H24N5O6S calculated: 534.1447, found: 534.1430.
4.2. Measurement of Nitric Oxide Release
4.2.1. Materials and Methods
4.2.2. Preparation of Nitrite Standard Curve
4.2.3. NO Release Assay
4.2.4. Procedure
4.3. Biology
4.3.1. Materials and Methods
4.3.2. Evaluation of Anticancer Activity
4.3.3. EGFR Inhibitory Assay
4.3.4. JNK-2 Inhibitory Assay
4.3.5. Apoptosis Analysis
4.3.6. Cell Cycle Analysis
4.3.7. Evaluation of Cytotoxicity against PC12 Cells
4.3.8. Molecular Docking on EGFR and JNK-2
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gallorini, M.; Cataldi, A.; di Giacomo, V. Cyclin-dependent kinase modulators and cancer therapy. BioDrugs 2012, 26, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Shoman, M.; Abdel-Aziz, S.; Narumi, A.; Konno, H.; Abdelaziz, M. Design, Synthesis, Molecular Modeling and Biological Evaluation of Novel 1,5-Diarylpyrazole Carboxamide Derivatives as Antiproliferative Agents. J. Adv. Biomed. Pharm. Sci. 2021, 4, 152–159. [Google Scholar] [CrossRef]
- Kibria, G.; Hatakeyama, H.; Harashima, H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch. Pharmacal Res. 2014, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Abdelrahman, K.; Abdel-Aziz, S.; Marzouk, A.; Konno, H.; Tajiri, M.; Osman, M. Design, synthesis, molecular docking and biological evaluation of novel 1,5-diarylpyrazole-N,O-dimethyl hydroxamate derivatives as antiproliferative agents. J. Adv. Biomed. Pharm. Sci. 2021, 4, 214–225. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; Alkhoja, O.A.; Mohamed, W.R. Design, synthesis and antitumor activity of novel pyrazolo [3,4-d] pyrimidine derivatives as EGFR-TK inhibitors. Bioorganic Chem. 2016, 66, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rego, R.; Foster, N.; Smyrk, T.; Le, M.; O’connell, M.; Sargent, D.; Windschitl, H.; Sinicrope, F. Prognostic effect of activated EGFR expression in human colon carcinomas: Comparison with EGFR status. Br. J. Cancer 2010, 102, 165–172. [Google Scholar] [CrossRef]
- Cao, C.; Lu, S.; Sowa, A.; Kivlin, R.; Amaral, A.; Chu, W.; Yang, H.; Di, W.; Wan, Y. Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs. Cancer Lett. 2008, 266, 249–262. [Google Scholar] [CrossRef]
- Bridges, A. The rationale and strategy used to develop a series of highly potent, irreversible, inhibitors of the epidermal growth factor receptor family of tyrosine kinases. Curr. Med. Chem. 1999, 6, 825–844. [Google Scholar] [CrossRef]
- Iida, K.; Nakayama, K.; Rahman, M.; Rahman, M.; Ishikawa, M.; Katagiri, A.; Yeasmin, S.; Otsuki, Y.; Kobayashi, H.; Nakayama, S. EGFR gene amplification is related to adverse clinical outcomes in cervical squamous cell carcinoma, making the EGFR pathway a novel therapeutic target. Br. J. Cancer 2011, 105, 420–427. [Google Scholar] [CrossRef]
- Huang, P.; Xu, X.; Wang, L.; Zhu, B.; Wang, X.; Xia, J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell. Mol. Med. 2014, 18, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.; Hsuan, J.; Clark, S.; Maziarz, R.; Waterfield, M.; Flavell, R.; Haley, J. Expression of epidermal-growth-factor receptor in the K562 cell line by transfection. Altered receptor biochemistry. Biochem. J. 1990, 271, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.P.; Tan, E.; Saeui, C.T.; Bovonratwet, P.; Sklar, S.; Bhattacharya, R.; Yarema, K.J. Metabolic flux-driven sialylation alters internalization, recycling, and drug sensitivity of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic cancer cells. Oncotarget 2016, 7, 66491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butterworth, S.; Cross, D.A.; Finlay, M.R.V.; Ward, R.A.; Waring, M.J. The structure-guided discovery of osimertinib: The first US FDA approved mutant selective inhibitor of EGFR T790M. Medchemcomm 2017, 8, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Wang, S.M.; Villaseñor, A.G.; Tsing, S.; Walter, D.; Browner, M.F.; Barnett, J.; Kuglstatter, A. The crystal structure of JNK2 reveals conformational flexibility in the MAP kinase insert and indicates its involvement in the regulation of catalytic activity. J. Mol. Biol. 2008, 383, 885–893. [Google Scholar] [CrossRef]
- Kuglstatter, A.; Ghate, M.; Tsing, S.; Villaseñor, A.G.; Shaw, D.; Barnett, J.W.; Browner, M.F. X-ray crystal structure of JNK2 complexed with the p38α inhibitor BIRB796: Insights into the rational design of DFG-out binding MAP kinase inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 5217–5220. [Google Scholar] [CrossRef]
- Abdelrahman, K.S.; Hassan, H.A.; Abdel-Aziz, S.A.; Marzouk, A.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. JNK signaling as a target for anticancer therapy. Pharmacol. Rep. 2021, 73, 405–434. [Google Scholar] [CrossRef]
- Ozgur, E.; Kayhan, H.; Kismali, G.; Senturk, F.; Sensoz, M.; Ozturk, G.G.; Sel, T. Effects of radiofrequency radiation on colorectal cancer cell proliferation and inflammation. Turk. J. Biochem. 2021, 46, 525–532. [Google Scholar] [CrossRef]
- Yue, Y.; Yan, L.; Yan-Ping, G.; Li, Z.; Lu-Bo, G. GL-V9 reverses adriamycin resistance in hepatocellular carcinoma cells by affecting JNK2-related autophagy. Chin. J. Nat. Med. 2020, 18, 491–499. [Google Scholar] [CrossRef]
- Tian, X.; Traub, B.; Shi, J.; Huber, N.; Schreiner, S.; Chen, G.; Zhou, S.; Henne-Bruns, D.; Knippschild, U.; Kornmann, M. c-Jun N-terminal kinase 2 suppresses pancreatic cancer growth and invasion and is opposed by c-Jun N-terminal kinase 1. Cancer Gene Ther. 2022, 29, 73–86. [Google Scholar] [CrossRef]
- Merkerova, M.; Bruchova, H.; Brdicka, R. JNK2 and p38 MAPK over-expressions do not represent key events in chronic myeloid leukemia transformation. Neoplasma 2007, 54, 503–510. [Google Scholar] [PubMed]
- Lin, C.S.; Lin, C.L.; Ying, T.H.; Chiou, H.L.; Hung, C.H.; Liao, W.S.; Hsieh, Y.H.; Kao, S.H. β-Mangostin inhibits the metastatic power of cervical cancer cells attributing to suppression of JNK2/AP-1/Snail cascade. J. Cell. Physiol. 2020, 235, 8446–8460. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, S.; Momose, H.; Shimizu, N.; Kitagawa, D.; Seo, J.; Yamasaki, T.; Nakagawa, K.; Kajiho, H.; Penninger, J.M.; Katada, T.; et al. Blockage by SP600125 of Fcε receptor-induced degranulation and cytokine gene expression in mast cells is mediated through inhibition of phosphatidylinositol 3-kinase signaling pathway. J. Biochem. 2009, 145, 345–354. [Google Scholar] [CrossRef]
- Kardosh, A.; Wang, W.; Uddin, J.; Petasis, N.A.; Hofman, F.M.; Chen, T.C.; Schonthal, A.H. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt’s lymphoma in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bhatia, P.; Alam, O.; Naim, M.J.; Nawaz, F.; Sheikh, A.A.; Jha, M. Recent advancement in the discovery and development of COX-2 inhibitors: Insight into biological activities and SAR studies (2008–2019). Bioorganic Chem. 2019, 89, 103007. [Google Scholar] [CrossRef]
- Chandrakantha, B.; Isloor, A.M.; Shetty, P.; Isloor, S.; Malladi, S.; Fun, H.K. Synthesis, characterization and antimicrobial activity of novel ethyl 1-(N-substituted)-5-phenyl-1 H-pyrazole-4-carboxylate derivatives. Med. Chem. Res. 2012, 21, 2702–2708. [Google Scholar] [CrossRef]
- Manvar, D.; Pelliccia, S.; La Regina, G.; Famiglini, V.; Coluccia, A.; Ruggieri, A.; Anticoli, S.; Lee, J.-C.; Basu, A.; Cevik, O. New 1-phenyl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides inhibit hepatitis C virus replication via suppression of cyclooxygenase-2. Eur. J. Med. Chem. 2015, 90, 497–506. [Google Scholar] [CrossRef]
- Pathak, R.B.; Chovatia, P.; Parekh, H. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorganic Med. Chem. Lett. 2012, 22, 5129–5133. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Wang, S.-F.; Luo, Z.-L.; Qiu, H.-Y.; Wang, P.-F.; Zhang, X.; Yang, Y.-A.; Yin, Y.; Zhang, F.; Zhu, H.-L. Synthesis and biological evaluation of compounds which contain pyrazole, thiazole and naphthalene ring as antitumor agents. Bioorganic Med. Chem. Lett. 2014, 24, 2324–2328. [Google Scholar] [CrossRef]
- Yu, L.; Wu, W.K.K.; Li, Z.J.; Liu, Q.C.; Li, H.T.; Wu, Y.C.; Cho, C.H. Enhancement of doxorubicin cytotoxicity on human esophageal squamous cell carcinoma cells by indomethacin and 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide (SC236) via inhibiting P-glycoprotein activity. Mol. Pharmacol. 2009, 75, 1364–1373. [Google Scholar] [CrossRef]
- Zhang, L.; Peterson, T.E.; Lu, V.M.; Parney, I.F.; Daniels, D.J. Antitumor activity of novel pyrazole-based small molecular inhibitors of the STAT3 pathway in patient derived high grade glioma cells. PLoS ONE 2019, 14, e0220569. [Google Scholar] [CrossRef]
- Sharp, S.Y.; Boxall, K.; Rowlands, M.; Prodromou, C.; Roe, S.M.; Maloney, A.; Powers, M.; Clarke, P.A.; Box, G.; Sanderson, S. Correction: In Vitro Biological Characterization of a Novel, Synthetic Diaryl Pyrazole Resorcinol Class of Heat Shock Protein 90 Inhibitors. Cancer Res. 2019, 79, 287. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, A.A.; Abdel-Aziz, S.A.; Abdelrahman, K.S.; Wanas, A.S.; Gouda, A.M.; Youssif, B.G.; Abdel-Aziz, M. Design and synthesis of new 1,6-dihydropyrimidin-2-thio derivatives targeting VEGFR-2: Molecular docking and antiproliferative evaluation. Bioorganic Chem. 2020, 102, 104090. [Google Scholar] [CrossRef]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Abuo-Rahma, G.E.D.A.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G. Novel quinoline derivatives carrying nitrones/oximes nitric oxide donors: Design, synthesis, antiproliferative and caspase-3 activation activities. Arch. Der Pharm. 2019, 352, 1800270. [Google Scholar] [CrossRef] [PubMed]
- Abuo-Rahma, G.E.-D.A.; Abdel-Aziz, M.; Beshr, E.A.; Ali, T.F. 1,2,4-Triazole/oxime hybrids as new strategy for nitric oxide donors: Synthesis, anti-inflammatory, ulceroginicity and antiproliferative activities. Eur. J. Med. Chem. 2014, 71, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xu, N.; Klamer, G.; Ko, K.-H.; Khoo, M.; Ma, D.; Moore, J.; O’Brien, T.A.; Dolnikov, A. Small-molecule inhibitor of glycogen synthase kinase 3β 6-bromoindirubin-3-oxime inhibits hematopoietic regeneration in stem cell recipient mice. Stem Cells Dev. 2015, 24, 724–736. [Google Scholar] [CrossRef]
- Zhang, X.; Castanotto, D.; Nam, S.; Horne, D.; Stein, C. 6BIO enhances oligonucleotide activity in cells: A potential combinatorial anti-androgen receptor therapy in prostate cancer cells. Mol. Ther. 2017, 25, 79–91. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, S.; Zhu, P.; Huang, M.; Gao, W.; Zhu, R.; Qian, J.; Peng, Y.; Zhang, Y.; Dai, H. Design, synthesis, and biological evaluation of novel thiazolyl substituted bis-pyrazole oxime derivatives with potent antitumor activities by selectively inducing apoptosis and ROS in cancer cells. Med. Chem. 2019, 15, 743–754. [Google Scholar] [CrossRef]
- Hong, S.; Shin, Y.; Jung, M.; Ha, M.W.; Park, Y.; Lee, Y.-J.; Shin, J.; Oh, K.B.; Lee, S.K.; Park, H.-G. Efficient synthesis and biological activity of Psammaplin A and its analogues as antitumor agents. Eur. J. Med. Chem. 2015, 96, 218–230. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Zaprutko, L. Recent advances in synthesis and biological activity of triterpenic acylated oximes. Phytochem. Rev. 2015, 14, 203–231. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Atochin, D.N.; Zanoza, S.O.; Gaidarzhy, N.M.; Lyakhov, S.A. Synthesis, biological evaluation, and molecular modeling of 11H-indeno [1,2-b] quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Hanks, T.S.; Kochetkova, I.; Pascual, D.W.; Jutila, M.A.; Quinn, M.T. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol. Pharmacol. 2012, 81, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sha, S.; Wang, K.; Zhang, Y.-H.; Liu, Y.-D.; Ju, G.-D.; Wang, B.; Zhu, H.-L. Discovery of chromeno [4,3-c] pyrazol-4(2H)-one containing carbonyl or oxime derivatives as potential, selective inhibitors PI3Kα. Chem. Pharm. Bull. 2016, 64, 1576–1581. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Plotnikov, M.B.; Khlebnikov, A.I.; Plotnikova, T.M.; Quinn, M.T. Oximes: Novel therapeutics with anticancer and anti-inflammatory potential. Biomolecules 2021, 11, 777. [Google Scholar] [CrossRef]
- Soliman, R. Preparation and antidiabetic activity of some sulfonylurea derivatives of 3,5-disubstituted pyrazoles. J. Med. Chem. 1979, 22, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Ruggiero, E.; De Stefano, L.; Rizzolio, F.; Di Cesare Mannelli, L.; Ghelardini, C.; Chicca, A.; Lapillo, M. Discovery of 1,5-diphenylpyrazole-3-carboxamide derivatives as potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J. Med. Chem. 2018, 61, 1340–1354. [Google Scholar] [CrossRef]
- Shin, U.C.; Choi, J.S.; Beak, Y.J.; Lee, M.W.; Kim, H.S.; Choi, D.W.; Kim, D.G.; Kim, S.W. Development of a 68Ga-labelled PET tracer for carbonic anhydrase IX-overexpressed tumors using the artificial sweetener saccharin. J. Label. Compd. Radiopharm. 2020, 64, 129–139. [Google Scholar] [CrossRef]
- Gonzalez, R.M.; Seurynck-Servoss, S.L.; Crowley, S.A.; Brown, M.; Omenn, G.S.; Hayes, D.F.; Zangar, R.C. Development and validation of sandwich ELISA microarrays with minimal assay interference. J. Proteome Res. 2008, 7, 2406–2414. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; El-Sheakh, A.R. Hepatoprotective effect of celecoxib against tamoxifen-induced liver injury via inhibiting ASK-1/JNK pathway in female rats. Life Sci. 2019, 231, 116573. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Ito, H.; Hiramitsu, T.; Nishitani, K.; Akiyoshi, M.; Kitaori, T.; Yasuda, T.; Nakamura, T. Celecoxib inhibits production of MMP and NO via down-regulation of NF-κB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol. Int. 2008, 28, 727–736. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Levasseur, J.; Piragasam, R.S.; Abdelhamid, G.; Dyck, J.R.; Fahlman, R.P.; Siraki, A.G.; El-Kadi, A.O. 2-Methoxyestradiol protects against pressure overload-induced left ventricular hypertrophy. Sci. Rep. 2018, 8, 2780. [Google Scholar] [CrossRef]
- Pozarowski, P.; Grabarek, J.; Darzynkiewicz, Z. Flow cytometry of apoptosis. Curr. Protoc. Cell Biol. 2003, 21, 18.18.11–18.18.33. [Google Scholar] [CrossRef]
- Yosaatmadja, Y.; Silva, S.; Dickson, J.M.; Patterson, A.V.; Smaill, J.B.; Flanagan, J.U.; McKeage, M.J.; Squire, C.J. Binding mode of the breakthrough inhibitor AZD9291 to epidermal growth factor receptor revealed. J. Struct. Biol. 2015, 192, 539–544. [Google Scholar] [CrossRef]
- Jousserandot, A.; Boucher, J.-L.; Henry, Y.; Niklaus, B.; Clement, B.; Mansuy, D. Microsomal cytochrome P450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: Mechanism of the oxidative cleavage of their CN (OH) bond with formation of nitrogen oxides. Biochemistry 1998, 37, 17179–17191. [Google Scholar] [CrossRef]
- Radwan, A.A.; Ghorab, M.M.; Alsaid, M.S.; Alanazi, F.K. Novel ethyl 1,5-disubstituted-1H-pyrazole-3-carboxylates as a new class of antimicrobial agents. Acta Pharm. 2014, 64, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z.-C.; Li, X.; Abbas, M.; Wu, S.-Y.; Ren, S.-Z.; Liu, Q.-X.; Liu, Y.; Chen, P.-W.; Duan, Y.-T. Design, synthesis and evaluation of novel diaryl-1,5-diazoles derivatives bearing morpholine as potent dual COX-2/5-LOX inhibitors and antitumor agents. Eur. J. Med. Chem. 2019, 169, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1 H-pyrazol-1-yl] benzenesulfonamide (SC-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef]
- Batchu, H.; Bhattacharyya, S.; Kant, R.; Batra, S. Palladium-catalyzed chelation-assisted regioselective oxidative dehydrogenative homocoupling/ortho-hydroxylation in N-phenylpyrazoles. J. Org. Chem. 2015, 80, 7360–7374. [Google Scholar] [CrossRef] [PubMed]
- Murray, W.V.; Wachter, M.P. A simple regioselective synthesis of ethyl 1,5-diarylpyrazole-3-carboxylates. J. Heterocycl. Chem. 1989, 26, 1389–1392. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, H.I.; Ece, A.; Bua, S.; Angeli, A.; Sakagami, H.; Sahin, E.; Supuran, C.T. Synthesis, biological evaluation and in silico modelling studies of 1,3,5-trisubstituted pyrazoles carrying benzenesulfonamide as potential anticancer agents and selective cancer-associated hCA IX isoenzyme inhibitors. Bioorganic Chem. 2019, 92, 103222. [Google Scholar] [CrossRef]
- Rogez-Florent, T.; Meignan, S.; Foulon, C.; Six, P.; Gros, A.; Bal-Mahieu, C.; Supuran, C.T.; Scozzafava, A.; Frédérick, R.; Masereel, B. New selective carbonic anhydrase IX inhibitors: Synthesis and pharmacological evaluation of diarylpyrazole-benzenesulfonamides. Bioorganic Med. Chem. 2013, 21, 1451–1464. [Google Scholar] [CrossRef]
- Harrison, C. 18 months sliced off Celebrex’s patent protection. Nat. Rev. Drug Discov. 2014, 13, 326. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Wang, Z.-C.; Ren, S.-Z.; Shen, F.-Q.; Man, R.-J.; Zhu, H.-L. Coumarin sulfonamides derivatives as potent and selective COX-2 inhibitors with efficacy in suppressing cancer proliferation and metastasis. Bioorganic Med. Chem. Lett. 2016, 26, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Raiford, L.C.; Hill, E.L. Effect of Constitution on the Rearrangement of the Phenylhydrazones of Some Unsymmetrically Substituted Dibenzalacetones1. J. Am. Chem. Soc. 1934, 56, 174–176. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wagner, K.M.; Morisseau, C.; Liu, J.-Y.; Dong, H.; Wecksler, A.T.; Hammock, B.D. Synthesis and structure—Activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J. Med. Chem. 2011, 54, 3037–3050. [Google Scholar] [CrossRef]
- Uddin, M.J.; Crews, B.C.; Ghebreselasie, K.; Marnett, L.J. Design, synthesis, and structure–activity relationship studies of fluorescent inhibitors of cycloxygenase-2 as targeted optical imaging agents. Bioconjugate Chem. 2013, 24, 712–723. [Google Scholar] [CrossRef]
- Chen, L.W.; Wang, P.F.; Tang, D.J.; Tao, X.X.; Man, R.J.; Qiu, H.Y.; Wang, Z.C.; Xu, C.; Zhu, H.L. Metronidazole containing pyrazole derivatives potently inhibit tyrosyl-tRNA synthetase: Design, synthesis, and biological evaluation. Chem. Biol. Drug Des. 2016, 88, 592–598. [Google Scholar] [CrossRef]
- Abdelazeem, A.H.; El-Din, A.G.S.; Abdel-Fattah, M.M.; Amin, N.H.; El-Moghazy, S.M.; El-Saadi, M.T. Discovery of novel urea-diarylpyrazole hybrids as dual COX-2/sEH inhibitors with improved anti-inflammatory activity and highly reduced cardiovascular risks. Eur. J. Med. Chem. 2020, 205, 112662. [Google Scholar] [CrossRef] [PubMed]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Goła̧bek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef]
- Hotsumi, M.; Tajiri, M.; Nikaido, Y.; Sato, T.; Makabe, K.; Konno, H. Design, synthesis, and evaluation of a water soluble C5-monoketone type curcumin analogue as a potent amyloid β aggregation inhibitor. Bioorganic Med. Chem. Lett. 2019, 29, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Franchina, T.; Minciullo, P.L.; Profita, M.; Zanghì, M.; David, A.; Kennez, I.; Adamo, V. IL-33/IL-31 axis: A new pathological mechanisms for EGFR tyrosine kinase inhibitors-associated skin toxicity. J. Cell. Biochem. 2013, 114, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Masago, K.; Fujita, S.; Hatachi, Y.; Fukuhara, A.; Sakuma, K.; Ichikawa, M.; Kim, Y.H.; Mio, T.; Mishima, M. Clinical significance of pretreatment serum amphiregulin and transforming growth factor-α, and an epidermal growth factor receptor somatic mutation in patients with advanced non-squamous, non-small cell lung cancer. Cancer Sci. 2008, 99, 2295–2301. [Google Scholar] [CrossRef]
- Gil-Martínez, A.L.; Cuenca, L.; Estrada, C.; Sánchez-Rodrigo, C.; Fernández-Villalba, E.; Herrero, M.T. Unexpected exacerbation of neuroinflammatory response after a combined therapy in old Parkinsonian mice. Front. Cell. Neurosci. 2018, 12, 451. [Google Scholar] [CrossRef]
- Cenariu, D.; Fischer-Fodor, E.; Țigu, A.B.; Bunea, A.; Virág, P.; Perde-Schrepler, M.; Toma, V.-A.; Mocan, A.; Berindan-Neagoe, I.; Pintea, A. Zeaxanthin-Rich Extract from Superfood Lycium barbarum Selectively Modulates the Cellular Adhesion and MAPK Signaling in Melanoma versus Normal Skin Cells In Vitro. Molecules 2021, 26, 333. [Google Scholar] [CrossRef]
- Neves, M.A.; Totrov, M.; Abagyan, R. Docking and scoring with ICM: The benchmarking results and strategies for improvement. J. Comput.-Aided Mol. Des. 2012, 26, 675–686. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Totrov, M.; Abagyan, R. Pocketome via comprehensive identification and classification of ligand binding envelopes. Mol. Cell. Proteom. 2005, 4, 752–761. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]


| Compound | Growth Inhibition (GI%) | ||||
|---|---|---|---|---|---|
| DLD–1 | Hela | K562 | SUIT–2 | HepG2 | |
| 7a | 0 | 55 | 0 | 101 | 5 |
| 7b | 81 | 83 | 70 | 103 | 85 |
| 7c | 0 | 52 | 48 | 68 | 0 |
| 7d | 72 | 104 | 50 | 104 | 31 |
| 7e | 59.40 | 73 | 0 | 101 | 14 |
| 7f | 18.65 | 16 | 24 | 75 | 10 |
| 7g | 96.70 | 30 | 77 | 92 | 66 |
| 7h | 0 | 0 | 0 | 73 | 0 |
| 7i | 46.00 | 101 | 101 | 101 | 63 |
| 7j | 0.10 | 10 | 11 | 81 | 0 |
| 8a | 25.70 | 33 | 94 | 77 | 0 |
| 8b | 92.00 | 60 | 99 | 102 | 107 |
| 8c | 0 | 78 | 67 | 61 | 51 |
| 8d | 72.00 | 48 | 95 | 77 | 98 |
| 8e | 29.20 | 77 | 93 | 83 | 99 |
| 8f | 72.90 | 107 | 76 | 95 | 80 |
| 8g | 85.80 | 78 | 87 | 96 | 93 |
| 8h | 52.30 | 7 | 78 | 73 | 63 |
| 8i | 34.00 | 100 | 97 | 98 | 90 |
| 8j | 9.60 | 53 | 34 | 79 | 60 |
| 9a | 60.70 | 9 | 66 | 60 | 36 |
| 9b | 42.15 | 0 | 31 | 45 | 19 |
| 9c | 16.80 | 9 | 57 | 42 | 22 |
| 10a | 99.00 | 0 | 84 | 88 | 72 |
| 10b | 72.50 | 2 | 84 | 50 | 2 |
| 10c | 92.40 | 75 | 87 | 103 | 52 |
| Daunorubicin | 82.45 | 100 | 100 | 92 | 100 |
| Compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| DLD–1 | Hela | K562 | SUIT–2 | HepG2 | |
| 7b | 13 | 55 | NT | 45 | 45 |
| 7d | 81 | 15 | NT | 43 | NT |
| 7g | 13 | NT | NT | 31 | NT |
| 7i | ND | 25 | 93 | 92 | NT |
| 8a | NT | NT | 22 | NT | NT |
| 8b | 10 | 32 | 13 | 27 | 35.7 |
| 8d | 14.4 | 57 | 9 | NT | 23.3 |
| 8e | NT | 22 | 20 | NT | 4.7 |
| 8f | NT | 22 | 15.6 | NT | 22.3 |
| 8g | 32.3 | 8 | 7.6 | 19 | 12.3 |
| 8h | NT | 74 | 21 | 26 | NT |
| 8i | ND | 13 | 71 | 62 | ND |
| 10a | 26 | NT | 16 | NT | ND |
| 10b | 36 | NT | ND | NT | NT |
| 10c | NT | 5 | 29 | 13 | NT |
| Daunorubicin | 30 | 0.097 | 13.30 | 9 | 22 |
| Compound | IC50 (µM) against EGFR–TK | IC50 (µM) against JNK–2 |
|---|---|---|
| 8b | >1000 | - |
| 8d | 8 | 49 |
| 8g | 18 | >200 |
| 8i | 21 | 1 |
| 10c | 12 | - |
| Sorafenib | 3.5 | 1 |
| Compound | Amount of NO Released (mol/mol) | |||
|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | |
| 8a | 0.095 ± 0.036 | 0.121 ± 0.047 | 0.108 ± 0.042 | 0.056 ± 0.022 |
| 8b | 0.102 ± 0.039 | 0.159 ± 0.019 | 0.105 ± 0.025 | 0.095 ± 0.030 |
| 8d | 0.096 ± 0.036 | 0.106 ± 0.040 | 0.101 ± 0.038 | 0.079 ± 0.030 |
| 8e | 0.064 ± 0.022 | 0.120 ± 0.046 | 0.081 ± 0.030 | 0.048 ± 0.018 |
| 8f | 0.096 ± 0.036 | 0.116 ± 0.045 | 0.079 ± 0.030 | 0.048 ± 0.018 |
| 8g | 0.083 ± 0.035 | 0.154 ± 0.044 | 0.141 ± 0.039 | 0.089 ± 0.019 |
| 8h | 0.099 ± 0.029 | 0.158 ± 0.014 | 0.104 ± 0.033 | 0.094 ± 0.0022 |
| 8i | 0.105 ± 0.029 | 0.115 ± 0.018 | 0.109 ± 0.046 | 0.087 ± 0.035 |
| 10a | 0.054 ± 0.023 | 0.108 ± 0.043 | 0.069 ± 0.024 | 0.036 ± 0.012 |
| 10b | 0.095 ± 0.035 | 0.114 ± 0.046 | 0.079 ± 0.031 | 0.031 ± 0.018 |
| 10c | 0.075 ± 0.036 | 0.151 ± 0.049 | 0.114 ± 0.030 | 0.081 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, K.S.; Hassan, H.A.; Abdel-Aziz, S.A.; Marzouk, A.A.; Shams, R.; Osawa, K.; Abdel-Aziz, M.; Konno, H. Development and Assessment of 1,5–Diarylpyrazole/Oxime Hybrids Targeting EGFR and JNK–2 as Antiproliferative Agents: A Comprehensive Study through Synthesis, Molecular Docking, and Evaluation. Molecules 2023, 28, 6521. https://doi.org/10.3390/molecules28186521
Abdelrahman KS, Hassan HA, Abdel-Aziz SA, Marzouk AA, Shams R, Osawa K, Abdel-Aziz M, Konno H. Development and Assessment of 1,5–Diarylpyrazole/Oxime Hybrids Targeting EGFR and JNK–2 as Antiproliferative Agents: A Comprehensive Study through Synthesis, Molecular Docking, and Evaluation. Molecules. 2023; 28(18):6521. https://doi.org/10.3390/molecules28186521
Chicago/Turabian StyleAbdelrahman, Kamal S., Heba A. Hassan, Salah A. Abdel-Aziz, Adel A. Marzouk, Raef Shams, Keima Osawa, Mohamed Abdel-Aziz, and Hiroyuki Konno. 2023. "Development and Assessment of 1,5–Diarylpyrazole/Oxime Hybrids Targeting EGFR and JNK–2 as Antiproliferative Agents: A Comprehensive Study through Synthesis, Molecular Docking, and Evaluation" Molecules 28, no. 18: 6521. https://doi.org/10.3390/molecules28186521
APA StyleAbdelrahman, K. S., Hassan, H. A., Abdel-Aziz, S. A., Marzouk, A. A., Shams, R., Osawa, K., Abdel-Aziz, M., & Konno, H. (2023). Development and Assessment of 1,5–Diarylpyrazole/Oxime Hybrids Targeting EGFR and JNK–2 as Antiproliferative Agents: A Comprehensive Study through Synthesis, Molecular Docking, and Evaluation. Molecules, 28(18), 6521. https://doi.org/10.3390/molecules28186521






