Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products
Abstract
:1. Introduction
2. Results and Discussion
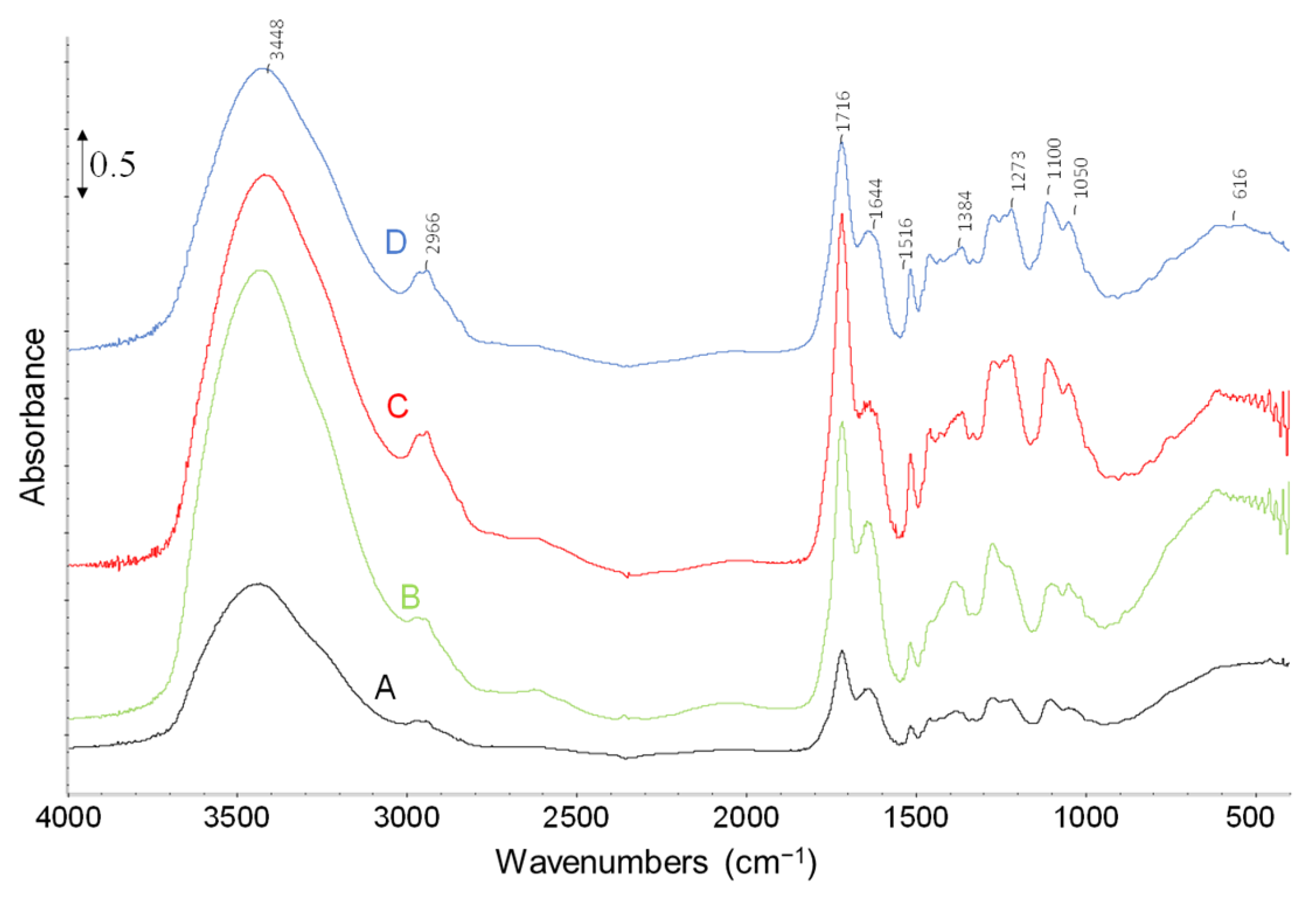
2.1. Characterization of Biomass
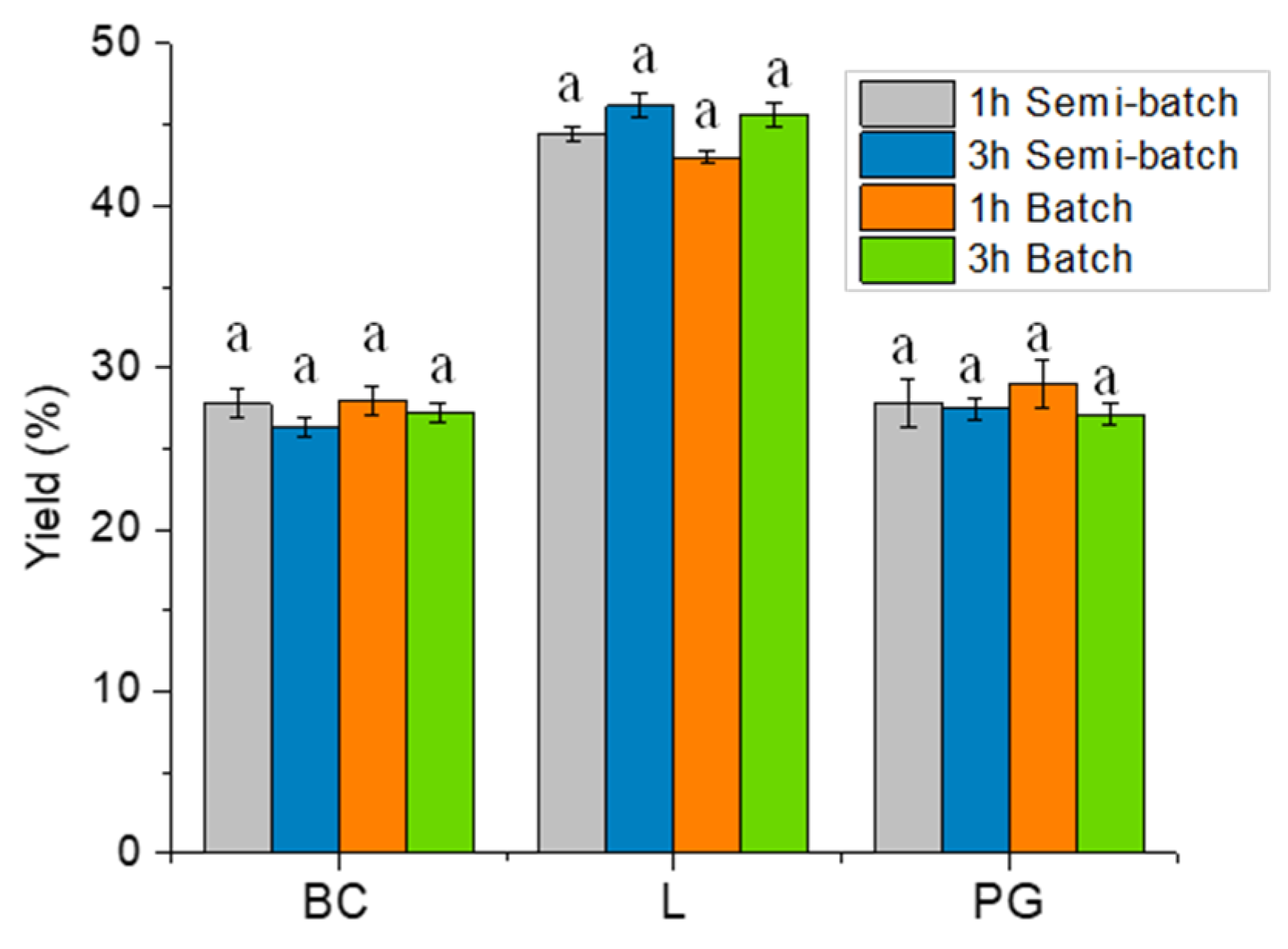
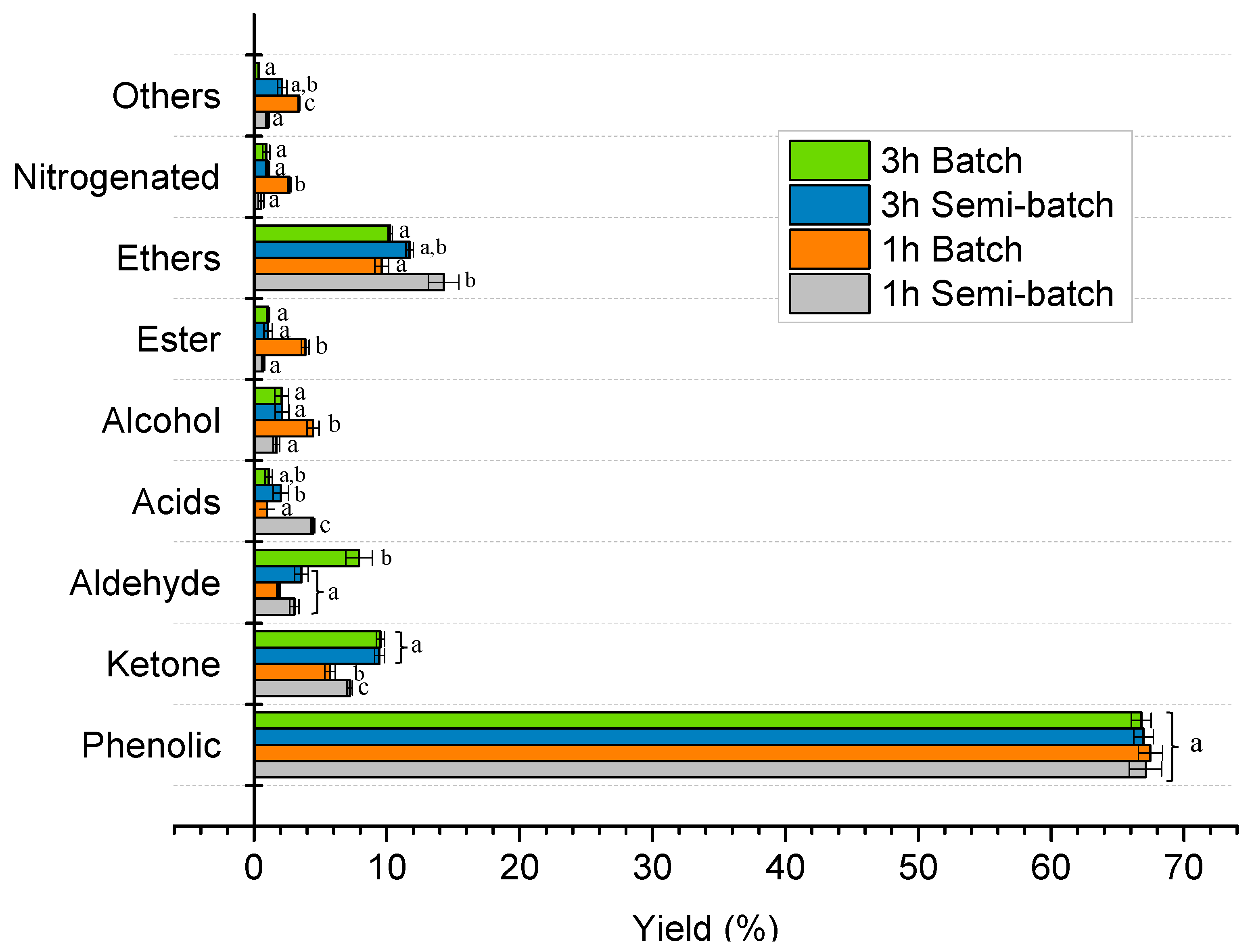
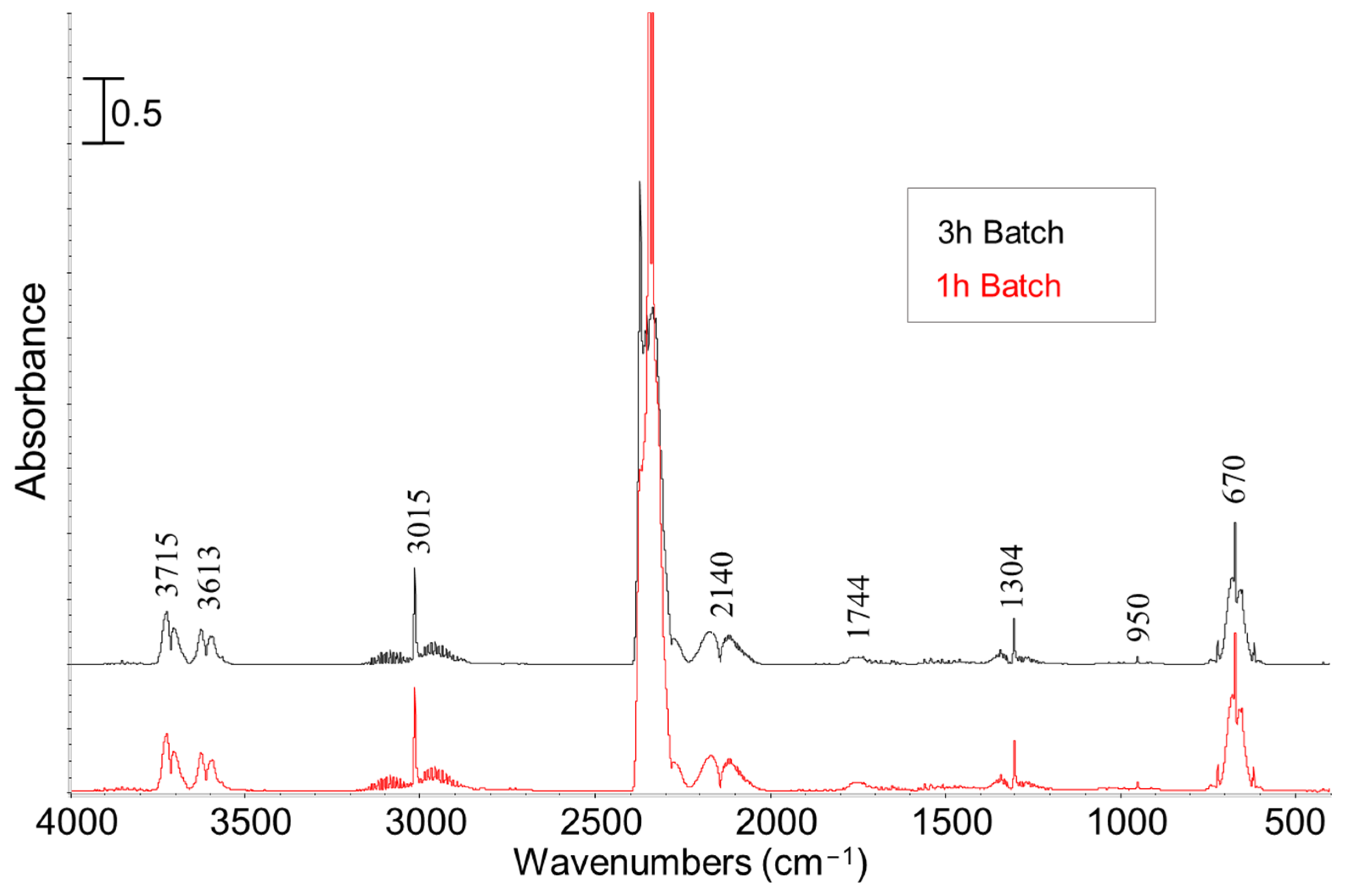
2.2. Thermal Pyrolysis Results
Effect of Reaction Time and Nitrogen Flow Condition
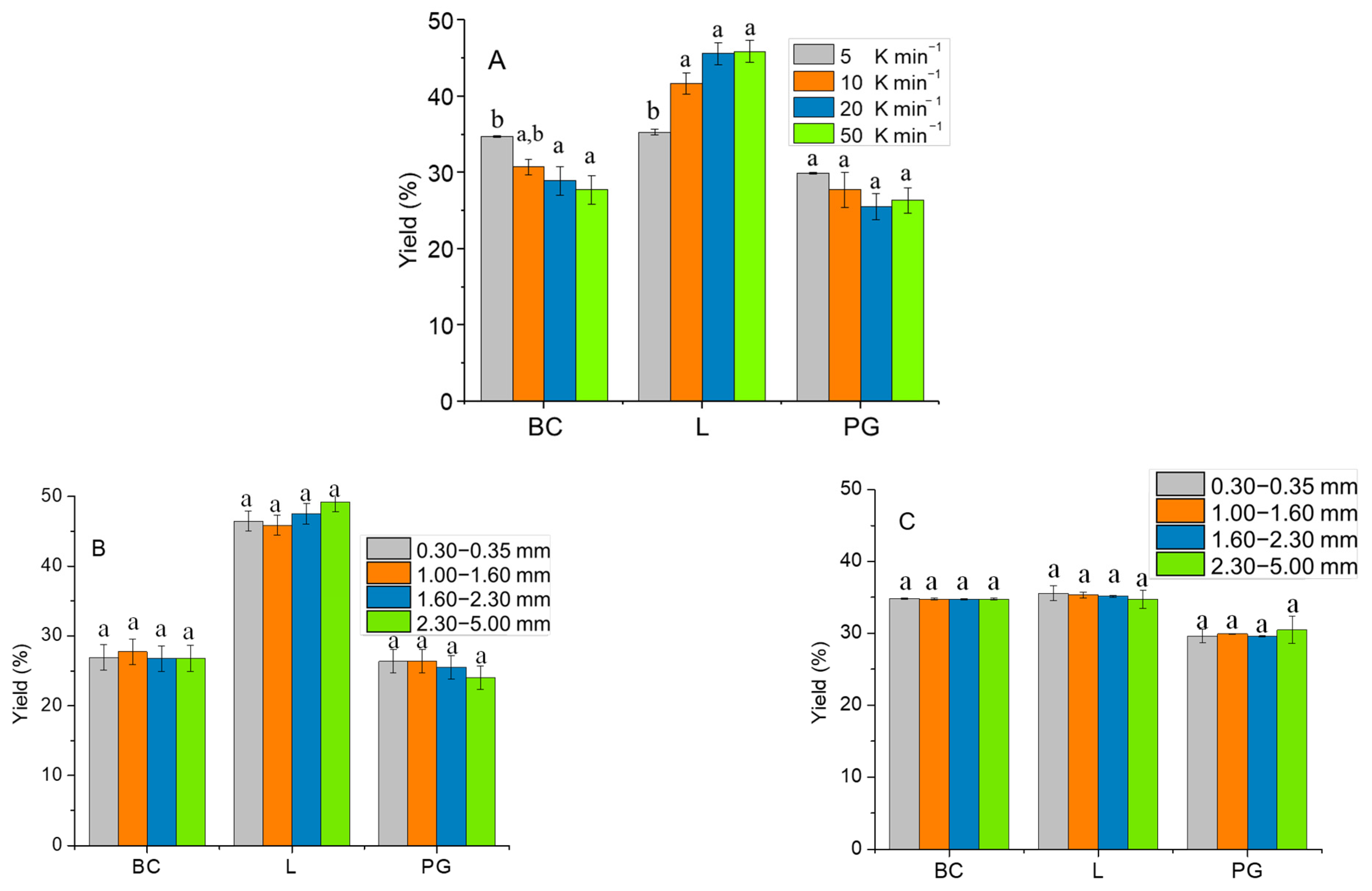
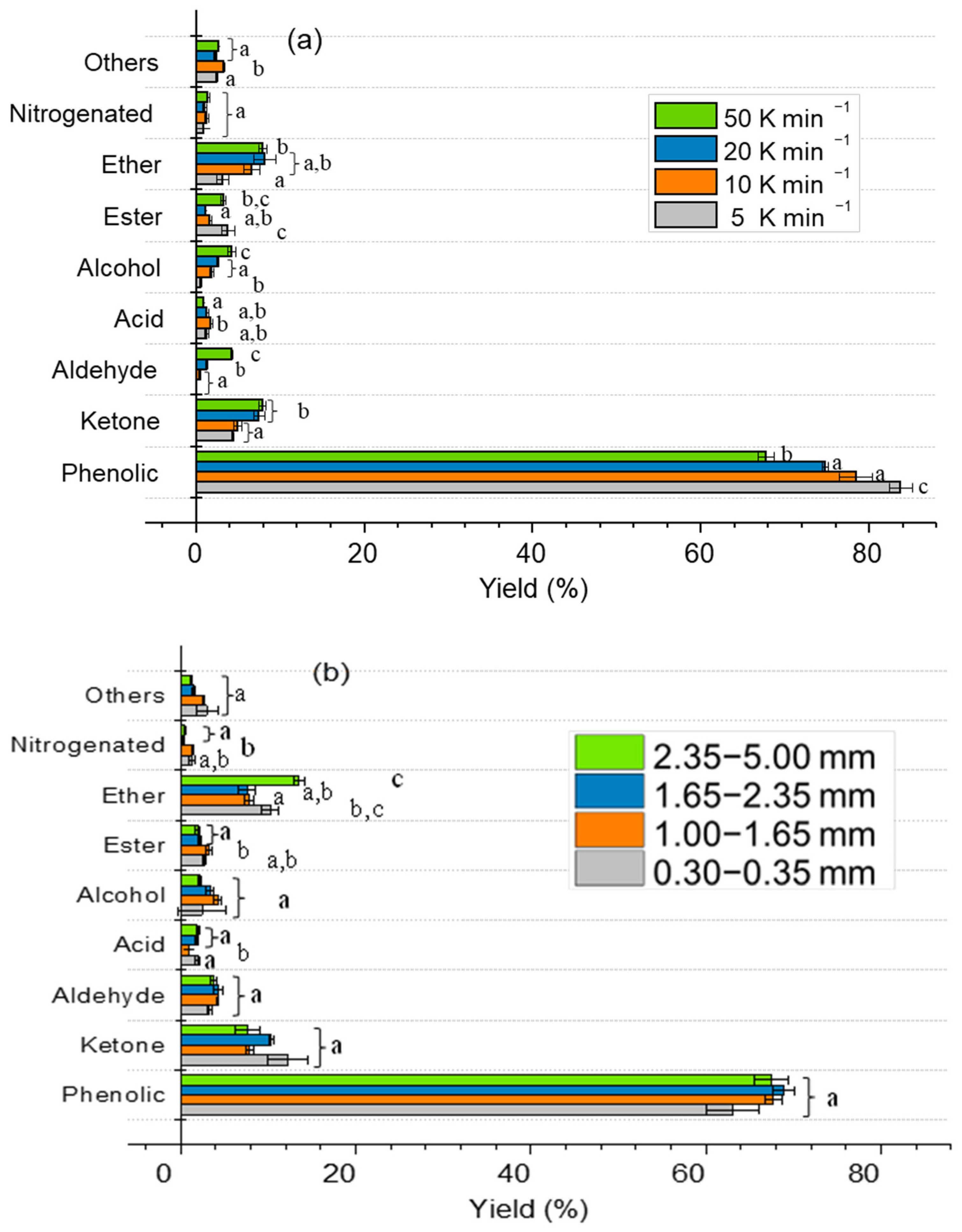
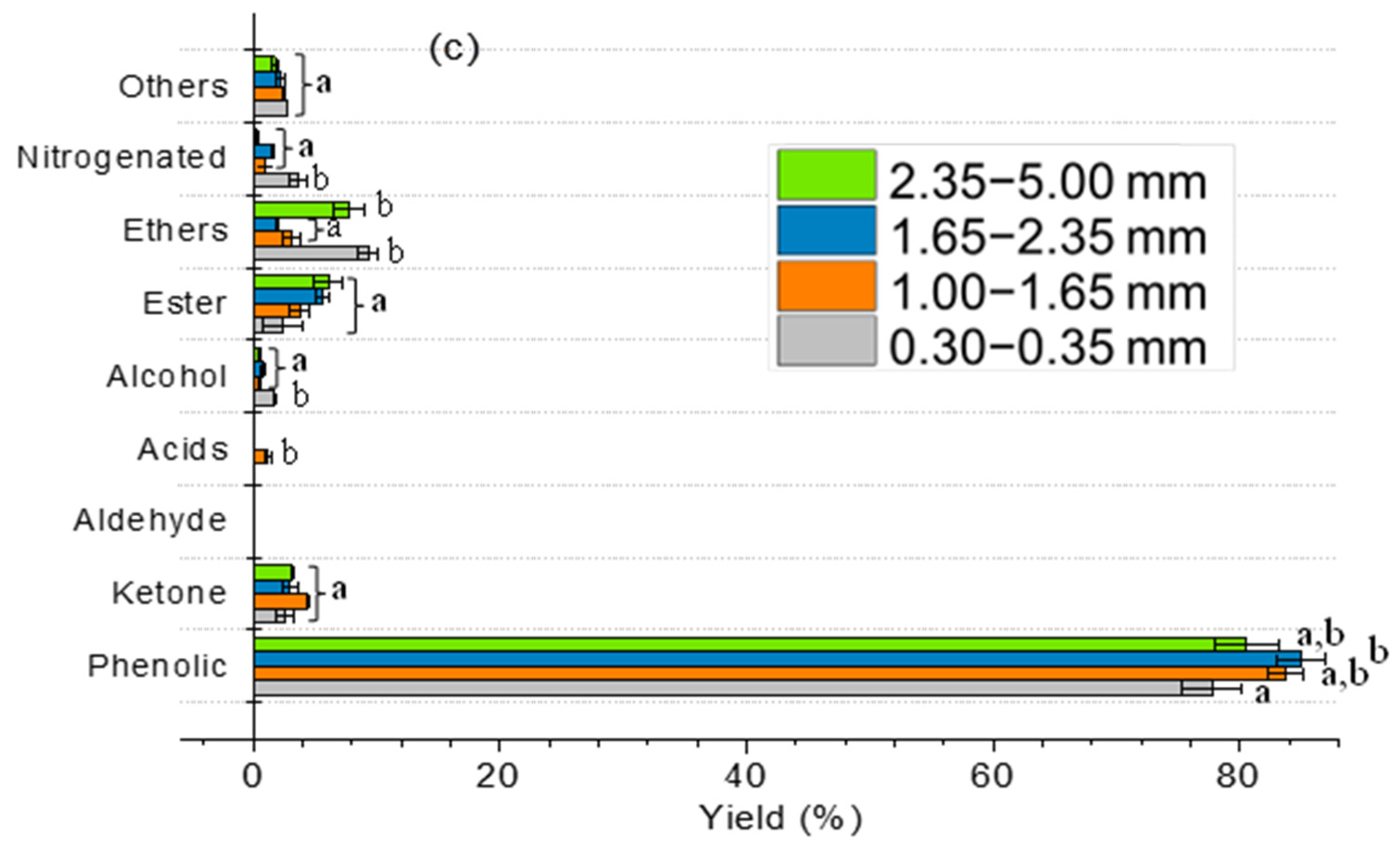
2.3. Effect of Particle Size and Heating Rate
3. Materials and Methods
3.1. Materials
3.2. Characterization of Biomass
3.3. Pyrolysis Plant and Experimental Set-Up
3.3.1. Investigation of the Effect of Reaction Time and Nitrogen Purging Conditions
3.3.2. Investigation of the Effect of Biomass Particle Size and Heating Rate
3.4. Characterizations of Pyrolysis Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Casazza, A.A.; Spennati, E.; Converti, A.; Busca, G. Production of Carbon-Based Biofuels by Pyrolysis of Exhausted Arthrospira Platensis Biomass after Protein or Lipid Recovery. Fuel Process. Technol. 2020, 201, 106336. [Google Scholar] [CrossRef]
- Nahak, B.K.; Preetam, S.; Sharma, D.; Shukla, S.K.; Syväjärvi, M.; Toncu, D.C.; Tiwari, A. Advancements in Net-Zero Pertinency of Lignocellulosic Biomass for Climate Neutral Energy Production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Spennati, E.; Casazza, A.A.; Converti, A.; Busca, G. Thermocatalytic Pyrolysis of Exhausted Arthrospira platensis Biomass after Protein or Lipid Recovery. Energies 2020, 13, 5246. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Cheela, V.R.S.; D’Adamo, I.; Iacovidou, E.; Islam, M.R.; Johnson, M.; Miller, T.R.; Parajuly, K.; Parchomenko, A.; Radhakrishan, L.; et al. Zero Waste Approach towards a Sustainable Waste Management. Resour. Environ. Sustain. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Hossain, A.; Saelee, T.; Tulaphol, S. Catalytic Hydrogenolysis of Lignin into Phenolics by Internal Hydrogen over Ru Catalyst. ChemCatChem 2022, 14, e202200549. [Google Scholar] [CrossRef]
- Ghalibaf, M.; Al, R.; Hita, I.; Deuss, P.J.; Jan, H.; De Wild, P. Valorization Potential of Technical Lignins from Norway Spruce (Picea abies) via Pyrolysis. J. Anal. Appl. Pyrolysis 2022, 165, 105549. [Google Scholar] [CrossRef]
- Borella, M.; Casazza, A.A.; Garbarino, G.; Riani, P.; Busca, G. A Study of the Pyrolysis Products of Kraft Lignin. Energies 2022, 15, 991. [Google Scholar] [CrossRef]
- García Martín, J.F.; Cuevas, M.; Feng, C.H.; Mateos, P.Á.; García, M.T.; Sánchez, S. Energetic Valorisation of Olive Biomass: Olive-Tree Pruning, Olive Stones and Pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Marquina, J.; Colinet, M.J.; Pablo-Romero, M. del P. The Economic Value of Olive Sector Biomass for Thermal and Electrical Uses in Andalusia (Spain). Renew. Sustain. Energy Rev. 2021, 148, 111278. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive Stone an Attractive Source of Bioactive and Valuable Compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Bader, N.; Sager, U.; Schneiderwind, U.; Ouederni, A. Foam and Granular Olive Stone-Derived Activated Carbons for NO2 Filtration from Indoor Air. J. Environ. Chem. Eng. 2019, 7, 103005. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2018, 9, 465–477. [Google Scholar] [CrossRef]
- Greco, G.; Di Stasi, C.; Rego, F.; González, B.; Manyà, J.J. Effects of Slow-Pyrolysis Conditions on the Products Yields and Properties and on Exergy Efficiency: A Comprehensive Assessment for Wheat Straw. Appl. Energy 2020, 279, 115842. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, W.; Guo, L.; Zhai, M.; Dong, P.; Qi, G. Energy and Exergy Analysis of Rice Husk High-Temperature Pyrolysis. Int. J. Hydrogen Energy 2016, 41, 21121–21130. [Google Scholar] [CrossRef]
- Engamba Esso, S.B.; Xiong, Z.; Chaiwat, W.; Kamara, M.F.; Longfei, X.; Xu, J.; Ebako, J.; Jiang, L.; Su, S.; Hu, S.; et al. Review on Synergistic Effects during Co-Pyrolysis of Biomass and Plastic Waste: Significance of Operating Conditions and Interaction Mechanism. Biomass Bioenergy 2022, 159, 106415. [Google Scholar] [CrossRef]
- Duman, G.; Yanik, J. Journal of Analytical and Applied Pyrolysis Effects of Pyrolysis Temperature and Pressure on Agronomic Properties of Biochar. J. Anal. Appl. Pyrolysis 2023, 169, 105858. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The Slow and Fast Pyrolysis of Cherry Seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Di Blasi, C.; Signorelli, G.; Di Russo, C.; Rea, G. Product Distribution from Pyrolysis of Wood and Agricultural Residues. Ind. Eng. Chem. Res. 1999, 38, 2216–2224. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of Temperature and Particle Size on Bio-Char Yield from Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Dai, X.; Shimizu, B.; Tam, M.S.; Grønli, M. Attainment of the Theoretical Yield of Carbon from Biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Liu, Q.; Li, X. Preparation of Porous Carbon Materials from Biomass Pyrolysis Vapors for Hydrogen Storage. Appl. Energy 2022, 306, 118131. [Google Scholar] [CrossRef]
- Manyà, J.J.; Roca, F.X.; Perales, J.F. TGA Study Examining the Effect of Pressure and Peak Temperature on Biochar Yield during Pyrolysis of Two-Phase Olive Mill Waste. J. Anal. Appl. Pyrolysis 2013, 103, 86–95. [Google Scholar] [CrossRef]
- Azuara, M.; Sáiz, E.; Manso, J.A.; García-Ramos, F.J.; Manyà, J.J. Study on the Effects of Using a Carbon Dioxide Atmosphere on the Properties of Vine Shoots-Derived Biochar. J. Anal. Appl. Pyrolysis 2017, 124, 719–725. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated Carbon as Catalyst Support: Precursors, Preparation, Modification and Characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Norizan, A.; Uemura, Y.; Afif, H.A.; Osman, N.; Omar, W.N.; Abdullah, B.; Tsutsui, T. Fast Pyrolysis of Oil Palm Kernel Shell in a Fluidized Bed Reactor: The Effect of Pyrolysis Temperature on the Yields of Pyrolysis Products. Appl. Mech. Mater. 2014, 625, 616–619. [Google Scholar] [CrossRef]
- Onay, O.; Kockar, O.M. Slow, Fast and Flash Pyrolysis of Rapeseed. Renew. Energy 2003, 28, 2417–2433. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A Review on Operating Parameters for Optimum Liquid Oil Yield in Biomass Pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Babu, B. V Biomass Pyrolysis: A State-of-the-Art Review. Bioprod. Bioref. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of Cellulose and Lignin Content on Pyrolysis and Combustion Characteristics for Several Types of Biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Tatterson, D.F.; Robinson, K.K.; Guercio, R.; Marker, T.L. Feedstock Effects in Coal Flash Pyrolysis. Ind. Eng. Chem. Res. 1990, 29, 2154–2159. [Google Scholar] [CrossRef]
- de Moraes Pinheiro, S.M.; Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Olive-Stone Biomass Ash (OBA): An Alternative Alkaline Source for the Blast Furnace Slag Activation. Constr. Build. Mater. 2018, 178, 327–338. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the Behaviour of Ash from Agricultural Wastes during Combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Moraes, J.C.B.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. A 100% Waste-Based Alkali-Activated Material by Using Olive-Stone Biomass Ash (OBA) and Blast Furnace Slag (BFS). Mater. Lett. 2017, 203, 46–49. [Google Scholar] [CrossRef]
- Marinova, E.; van der Valk, J.M.A.; Valamoti, S.M.; Bretschneider, J. An Experimental Approach for Tracing Olive Processing Residues in the Archaeobotanical Record, with Preliminary Examples from Tell Tweini, Syria. Veg. Hist. Archaeobot. 2011, 20, 471–478. [Google Scholar] [CrossRef]
- Sanginés, P.; Domínguez, M.P.; Sánchez, F.; San Miguel, G. Slow Pyrolysis of Olive Stones in a Rotary Kiln: Chemical and Energy Characterization of Solid, Gas, and Condensable Products. J. Renew. Sustain. Energy 2015, 7, 043103. [Google Scholar] [CrossRef]
- Nunes Raupp, Í.; Valério Filho, A.; Lemos Arim, A.; Rosa Costa Muniz, A.; Silveira da Rosa, G. Materials Development and Characterization of Activated Carbon from Olive Pomace: Experimental Design, Kinetic and Equilibrium Studies in Nimesulide Adsorption. Materials 2021, 14, 6820. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Şensöz, S.; Angin, D. Pyrolysis of Safflower (Charthamus tinctorius L.) Seed Press Cake in a Fixed-Bed Reactor: Part 2. Structural Characterization of Pyrolysis Bio-Oils. Bioresour. Technol. 2008, 99, 5498–5504. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Loh, S.K. Fractionation of Pyrolysis Oil via Supercritical Carbon Dioxide Extraction: Optimization Study Using Response Surface Methodology (RSM). Biomass Bioenergy 2017, 107, 155–163. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Lagazzo, A.; Garbarino, G.; Carnasciali, M.M.; Perego, P.; Busca, G. Pyrolysis of Grape Marc before and after the Recovery of Polyphenol Fraction. Fuel Process. Technol. 2016, 153, 121–128. [Google Scholar] [CrossRef]
- Mortensen, A.; Faurskov Nielsen, O. Vibrational Spectra in the Carbonyl Stretching Region of Isotopomers of Formamide in the Gaseous and Liquid States. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 1345–1354. [Google Scholar] [CrossRef]
- Vamvuka, D.; Esser, K.; Marinakis, D. Characterization of Pyrolysis Products of Forest Residues and Refuse-Derived Fuel and Evaluation of Their Suitability as Bioenergy Sources. Appl. Sci. 2023, 13, 1482. [Google Scholar] [CrossRef]
- Ozbay, N.; Pütün, A.E.; Pütün, E. Bio-Oil Production from Rapid Pyrolysis of Cottonseed Cake: Product Yields and Compositions. Int. J. Energy Res. 2006, 30, 501–510. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Bakar, N.K.A.; Chin, C.M. Optimization of Pyrolysis of Oil Palm Empty Fruit Bunches. J. Oil Palm Res. 2009, 21, 653–658. [Google Scholar]
- Gibbins-Matham, J.; Kandiyoti, R. Coal Pyrolysis Yields from Fast and Slow Heating in a Wire-Mesh Apparatus with a Gas Sweep. Energy Fuels 1988, 2, 505–511. [Google Scholar] [CrossRef]
- Strezov, V.; Moghtaderi, B.; Lucas, J.A. Thermal Study of Decomposition of Selected Biomass Samples. J. Therm. Anal. Calorim. 2003, 72, 1041–1048. [Google Scholar] [CrossRef]
- Haykiri-Acma, H. The Role of Particle Size in the Non-Isothermal Pyrolysis of Hazelnut Shell. J. Anal. Appl. Pyrolysis 2006, 75, 211–216. [Google Scholar] [CrossRef]
- Xu, M.; Huai, X.; Cai, J. Agglomeration Behavior of Calcium Hydroxide/Calcium Oxide as Thermochemical Heat Storage Material: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 3025–3033. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged Thermal Fractionation for Segregation of Lignin and Cellulose Pyrolysis Products: An Experimental Study of Residence Time and Temperature Effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Nassar, M.M.; MacKay, G.D.M. Mechanism of Thermal Decomposition of Lignin. Wood Fiber Sci. 1984, 16, 441–453. [Google Scholar]
- Waheed, Q.M.K.; Nahil, M.A.; Williams, P.T. Pyrolysis of Waste Biomass: Investigation of Fast Pyrolysis and Slow Pyrolysis Process Conditions on Product Yield and Gas Composition. J. Energy Inst. 2017, 9671, 233–241. [Google Scholar] [CrossRef]
- Yan, W.; Islam, S.; Coronella, C.J.; Vá Squez, V.R. Pyrolysis Kinetics of Raw/Hydrothermally Carbonized Lignocellulosic Biomass. Environ. Prog. Sustain. Energy 2012, 31, 200–204. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, S.; Liao, Y.; Zhou, J.; Gu, Y.; Cen, K. Research on Biomass Fast Pyrolysis for Liquid Fuel. Biomass Bioenergy 2004, 26, 455–462. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Czernik, S.; Piskorz, J. An Overview of Fast Pyrolysis. Prog. Thermochem. Biomass Convers. 2008, 30, 977–997. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.T.; Wei, Y.H.; Chi-Wei Lan, J. Recent Advances on the Sustainable Approaches for Conversion and Reutilization of Food Wastes to Valuable Bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review on the Comparison Between Slow Pyrolysis and Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
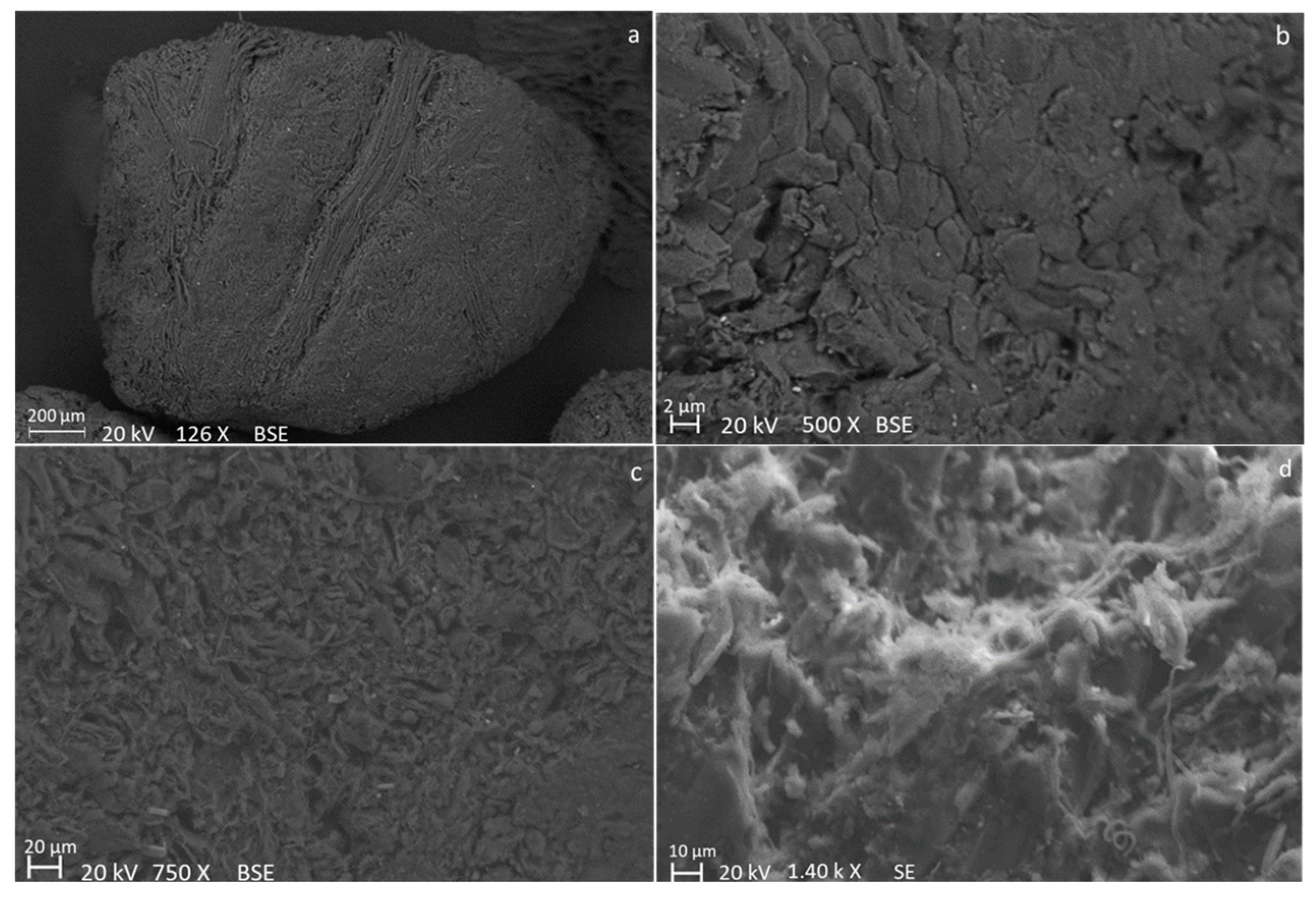

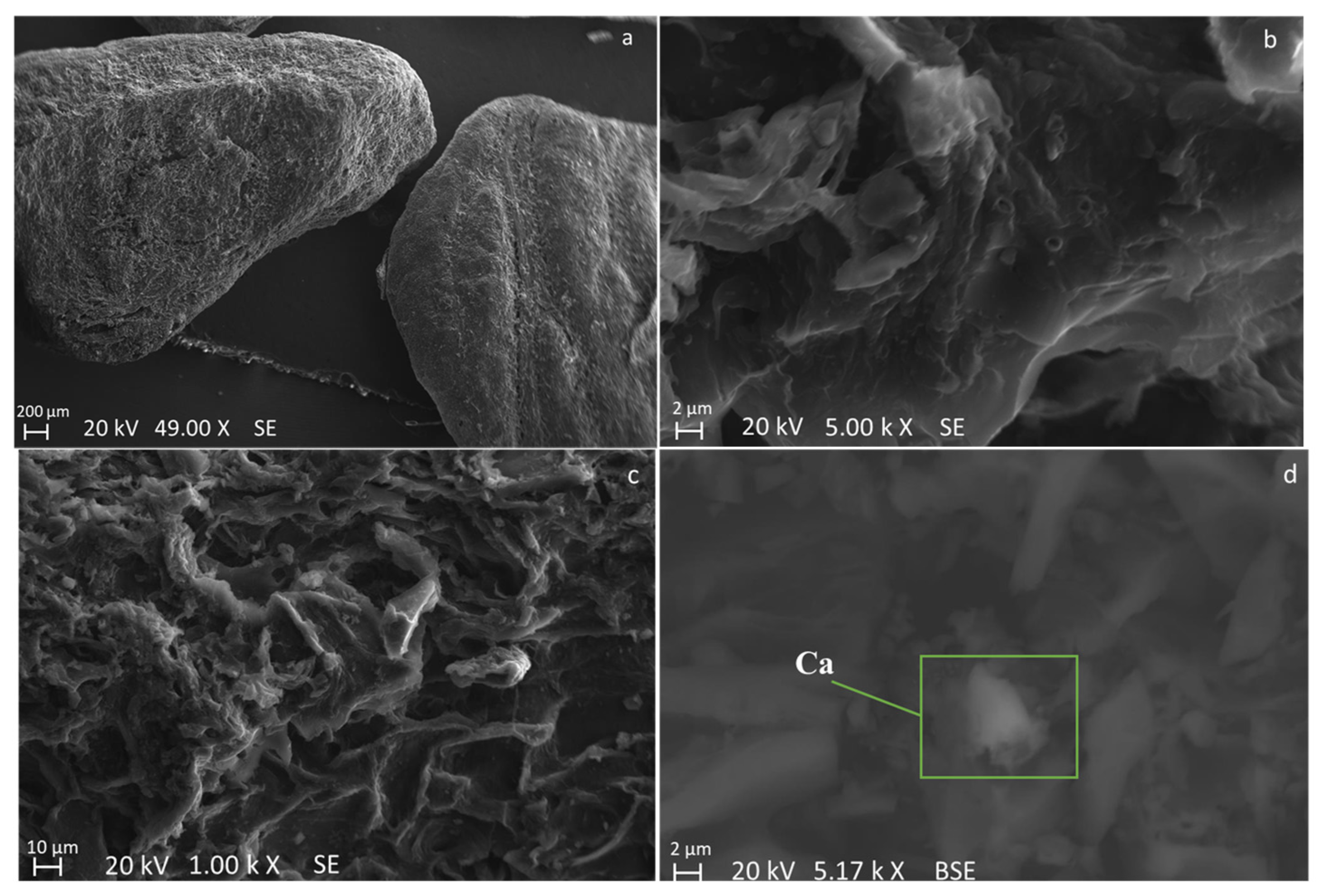
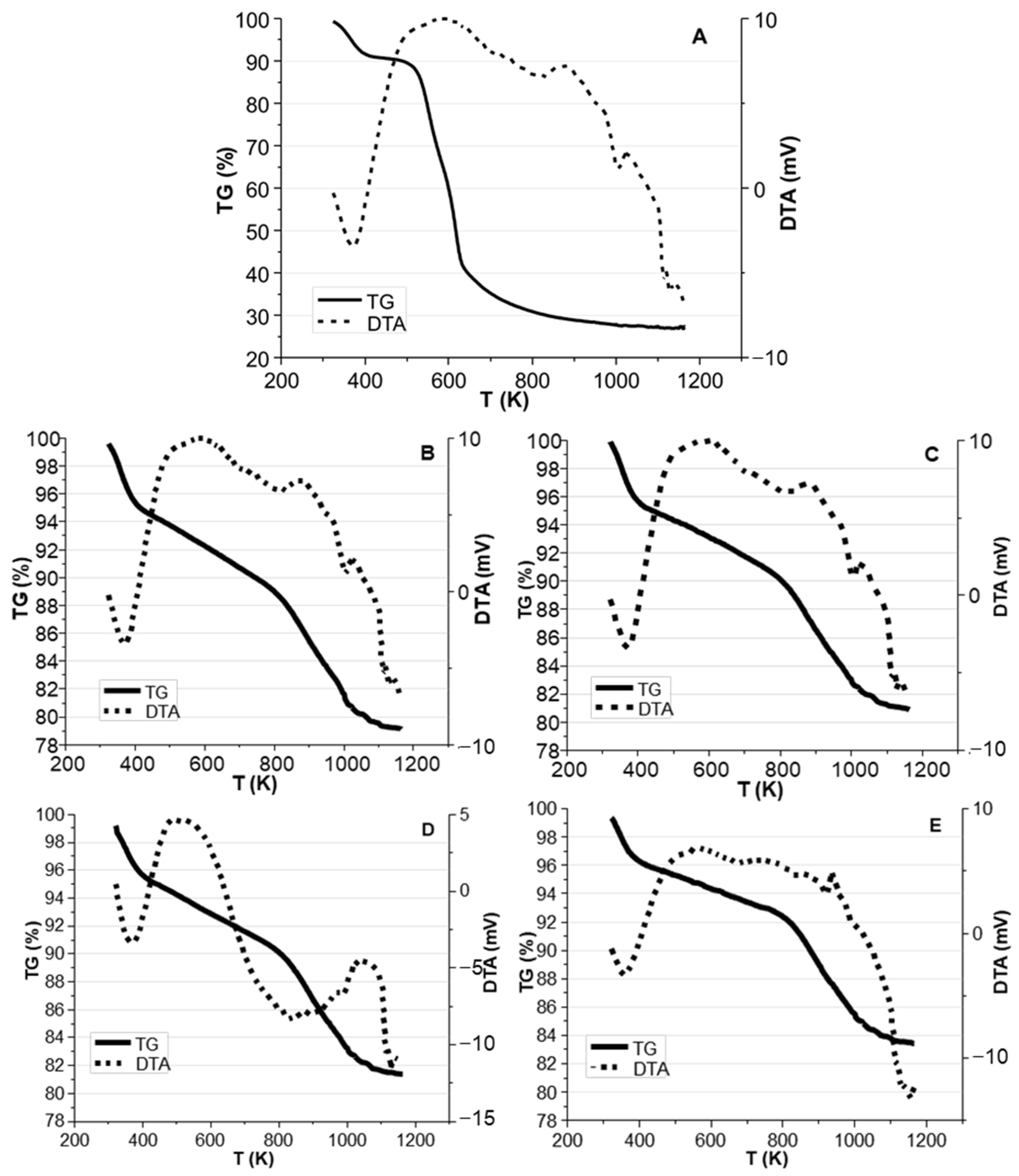
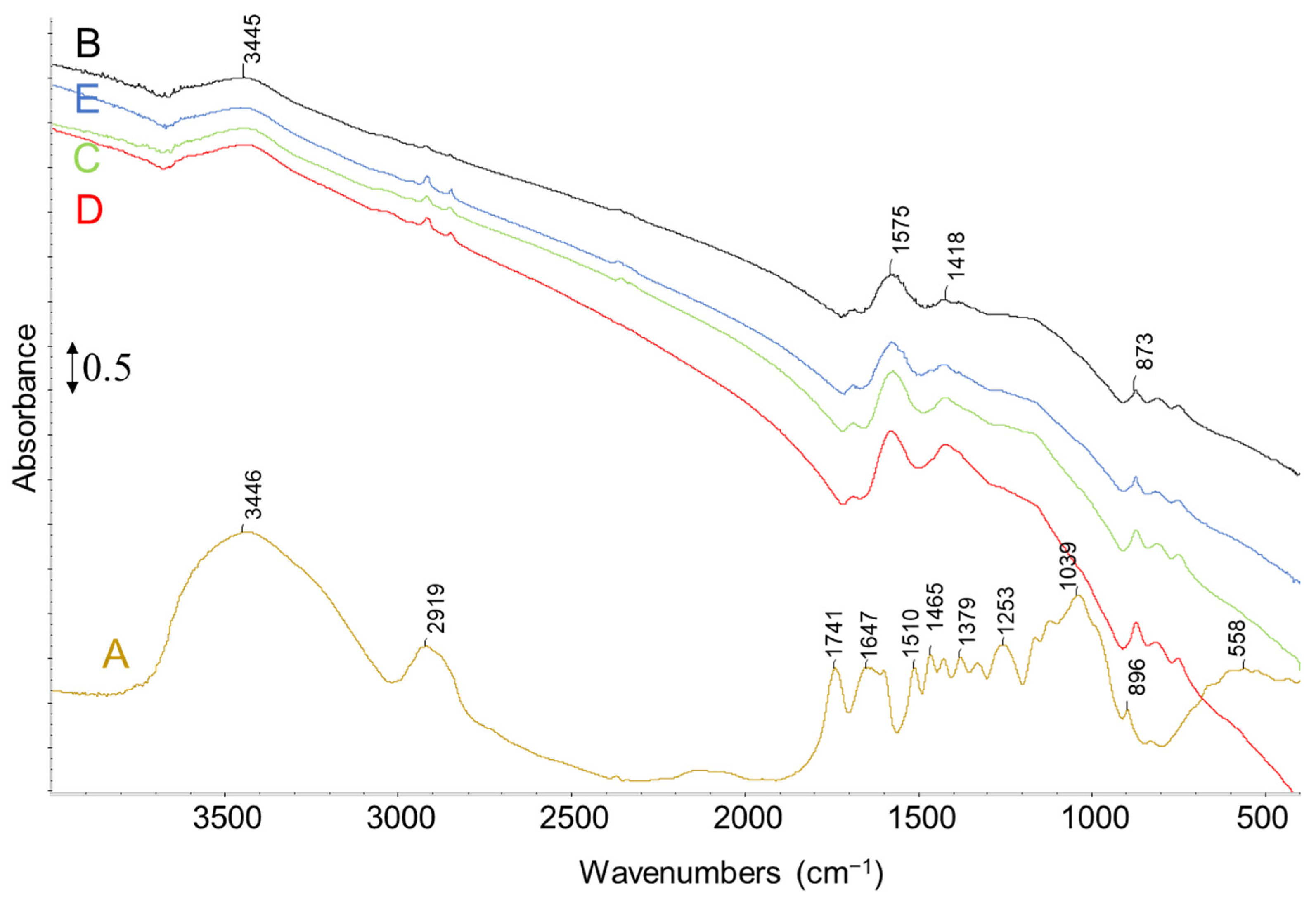


| Sample | C (wt.%) | O (wt.%) | Na (wt.%) | K (wt.%) | Ca (wt.%) | Mg (wt.%) | Si (wt.%) | P (wt.%) | S (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| Fresh Biomass | 53 | 47 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ash | 5.5 | 51.5 | 1.4 | 24.0 | 12.8 | 2.0 | 1.0 | 0.8 | 1.0 |
| Biomass Size | Heating Rate | Reaction Time (h) | N2 Condition | C (%) | O (%) | K(%) | Ca (%) |
|---|---|---|---|---|---|---|---|
| 1–5 mm | 50 K min−1 | 3 | Batch | 82.5 | 15.5 | 1.5 | 0.5 |
| 3 | Semi-batch | 81.5 | 15.5 | 1.9 | 1.1 | ||
| 1 | Batch | 84.0 | 14.5 | 1.5 | 0.0 | ||
| 1 | Semi-batch | 81.0 | 16.5 | 1.5 | 1.0 |
| Compound | Distribution (%) | |
|---|---|---|
| 3 h without N2 | 1 h without N2 | |
| N2, H2, CO2, CO, CH4, C2H4 | 77.49 ± 0.81 | 74.06 ± 0.74 |
| Acetaldehyde & 2-Butene | 6.79 ± 0.25 | 8.76 ± 0.76 |
| Formic acid, methyl ester | 2.04 ± 0.70 | 2.27 ± 0.05 |
| 1-Butane, 2-methyl- | 0.03 ± 0.02 | 0.06 ± 0.02 |
| Furan & Acetone | 4.63 ± 0.15 | 4.57 ± 0.06 |
| 1-Butan, 3-methyl- | 0.56 ± 0.05 | 0.31 ± 0.06 |
| Acetic acid, methyl ester | 1.58 ± 0.07 | 1.55 ± 0.09 |
| Cyclopropane, ethynyl- | 0.67 ± 0.00 | 0.47 ± 0.04 |
| Propanal, 2-methyl- | 0.34 ± 0.03 | 0.39 ± 0.01 |
| 2,3-Dihydrofuran | 0.58 ± 0.04 | 1.40 ± 0.04 |
| 2,3-Butanedione | 0.75 ± 0.06 | 1.41 ± 0.08 |
| 2-Butanone | 0.42 ± 0.03 | 0.42 ± 0.01 |
| Furan, 2- or 3-methyl- | 3.42 ± 0.09 | 3.54 ± 0.02 |
| Methyl propionate | 0.12 ± 0.02 | 0.14 ± 0.01 |
| 1,4-Cyclohexadiene | 0.11 ± 0.01 | 0.09 ± 0.02 |
| 2,4-Hexadiene | 0.11 ± 0.01 | 0.13 ± 0.02 |
| Benzene | 0.16 ± 0.04 | 0.17 ± 0.02 |
| Furan,2,5-dimethyl | 0.21 ± 0.01 | 0.24 ± 0.03 |
| Biomass Size (mm) | Heating Rate (K/min) | C (%) | O (%) | K (%) | Ca (%) |
|---|---|---|---|---|---|
| 1.00–1.60 | 5 | 81.4 | 16.4 | 1.4 | 0.8 |
| 10 | 82.0 | 15.6 | 1.4 | 1.0 | |
| 20 | 82.5 | 14.0 | 1.6 | 1.9 | |
| 50 | 79.5 | 17.5 | 1.5 | 1.5 | |
| 1.60–2.30 | 50 | 83.0 | 14.4 | 1.8 | 0.8 |
| 2.30–5.00 | 83.0 | 14.5 | 1.7 | 0.8 | |
| 0.30–0.35 | 77.5 | 17.5 | 2.2 | 2.8 |
| Biomass | Pyrolysis Condition | Products Yield (wt. %) | Main PG Composition (Order Based on Abundance) | Main L Composition | BC Analysis | Ref. |
|---|---|---|---|---|---|---|
| Wood | Fast, at 1123 K, with 300–500 K s−1, static N2 | PG ≅ 78 L ≅ 12 BC ≅ 10 | CO, H2, CO2, CH4 | n.a. | n.a. | [55] |
| Slow, at 1123 K, with 10 K m−1, for 30 min, static N2 | PG ≅ 23 L ≅ 61 BC ≅ 16 | CO, CO2, H2, CH4 | ||||
| Wheat straw | Slow, at 823 K, with 5 K m−1, for 60 min, atm of N2 with residence time of 100 s, pressure of 0.2 MPa | PG ≅ 33 L ≅ 39 BC ≅ 28 | CO2, CO, H2, CH4 | yorg:ywater = 0.27 Detailed analysis n.a. | H:C ≅ 0.37 O:C ≅ 0.08 SBET = 229 m2g−1 Vultra = 0.08 cm3g−1 | [13] |
| Slow, at 748 K, with 5 K m−1, for 60 min, atm of CO2/N2 = 0.3 with residence time of 150 s, pressure of 0.55 MPa | PG ≅ 36 L ≅ 35 BC ≅ 29 | CO2, CO, CH4, H2 | yorg:ywater = 0.43 Detailed analysis n.a. | H:C ≅ 0.46 O:C ≅ 0.11 SBET = 203 m2g−1 Vultra = 0.05 cm3g−1 | ||
| Cotton seed cake | At 823 K, with 300 K min−1, for 10 min, N2 of 50, 100, 200, 400 cm3min−1 | For N2 of 100 cm3min−1 PG ≅ 30 L ≅ 44 BC ≅ 26 | n.a. | For N2 of 100 C ≅ 70% O ≅ 16% N ≅ 6% H ≅ 9% | n.a. | [47] |
| At 823 K, with 5, 100, 300, 700 K min−1, for 10 min, N2 of 100 cm3min−1 | For rate of 5 K min−1 PG ≅ 21 L ≅ 47 BC ≅ 32 | For rate of 300 K min−1C ≅ 70% O ≅ 16% N ≅ 6% H ≅ 9% | ||||
| Vine shoots | At 873 K, with 5 K min−1, for 60 min, static N2, pressure of 0.1 MPa | PG ≅ 23 L ≅ 44 BC ≅ 33 | CO2, CO, CH4, H2 | n.a. | H:C ≅ 0.40 O:C ≅ 0.07 SBET = 109 m2g−1 | [25] |
| At 873 K, with 5, K min−1, for 60 min, static CO2, pressure of 0.1 MPa | PG ≅ 27 L ≅ 43 BC ≅ 30 | CO2, CO, CH4, H2 | H:C ≅ 0.38 O:C ≅ 0.05 SBET = 57 m2g−1 | |||
| Olive husk | At 950 K, with 10 K s−1, biomass size of <0.5, 1.0, 1.5, 2.0 mm | 0.5 mm BC ≅ 18 1.0 mm BC ≅ 24 1.5 mm BC ≅ 29 2.0 mm BC ≅ 36 | n.a. | n.a. | n.a. | [21] |
| Olive stone | Slow, at 773 K, with 50 K min−1, biomass size of 1–5 mm, for 60 or 180 min, static N2 or a flow of 100 mL min−1 | For 60 min and static N2 PG ≅ 27 L ≅ 47 BC ≅ 26 | CO2, CO, H2, CH4, C2H4 | Phenolics ≅ 68% Ketones ≅ 5% Ethers ≅ 10% Alcohols ≅ 8% Ester ≅ 6% | C ≅ 84.0% O ≅ 15.5% K ≅ 1.5% Ca ≅ 0.5% H = n.a. | This work |
| Slow, at 773 K, with 5, 10, 20, 50 K min−1, biomass size of 0.30–0.35, 1.0–1.6, 1.6–2.4, and 2.4–5.0 mm, for 60 min, N2 flow of 100 mL min−1 | For 5 K min−1 and 1.0–1.6 mm PG ≅ 30 L ≅ 35 BC ≅ 35 | n.a. | Phenolics ≅ 84% Ketones ≅ 6% Ethers ≅ 4% Acids ≅ 2% Ester ≅ 5% | C ≅ 81.4% O ≅ 16.4% K ≅ 1.4% Ca ≅ 0.8% H = n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atakoohi, S.E.; Spennati, E.; Casazza, A.A.; Riani, P.; Garbarino, G. Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products. Molecules 2023, 28, 6516. https://doi.org/10.3390/molecules28186516
Atakoohi SE, Spennati E, Casazza AA, Riani P, Garbarino G. Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products. Molecules. 2023; 28(18):6516. https://doi.org/10.3390/molecules28186516
Chicago/Turabian StyleAtakoohi, Sina Ebrahim, Elena Spennati, Alessandro A. Casazza, Paola Riani, and Gabriella Garbarino. 2023. "Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products" Molecules 28, no. 18: 6516. https://doi.org/10.3390/molecules28186516
APA StyleAtakoohi, S. E., Spennati, E., Casazza, A. A., Riani, P., & Garbarino, G. (2023). Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products. Molecules, 28(18), 6516. https://doi.org/10.3390/molecules28186516







