Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental and Thermal Analysis
2.1.1. Elemental Analysis
2.1.2. Thermal Analysis
2.2. Spectroscopic Study
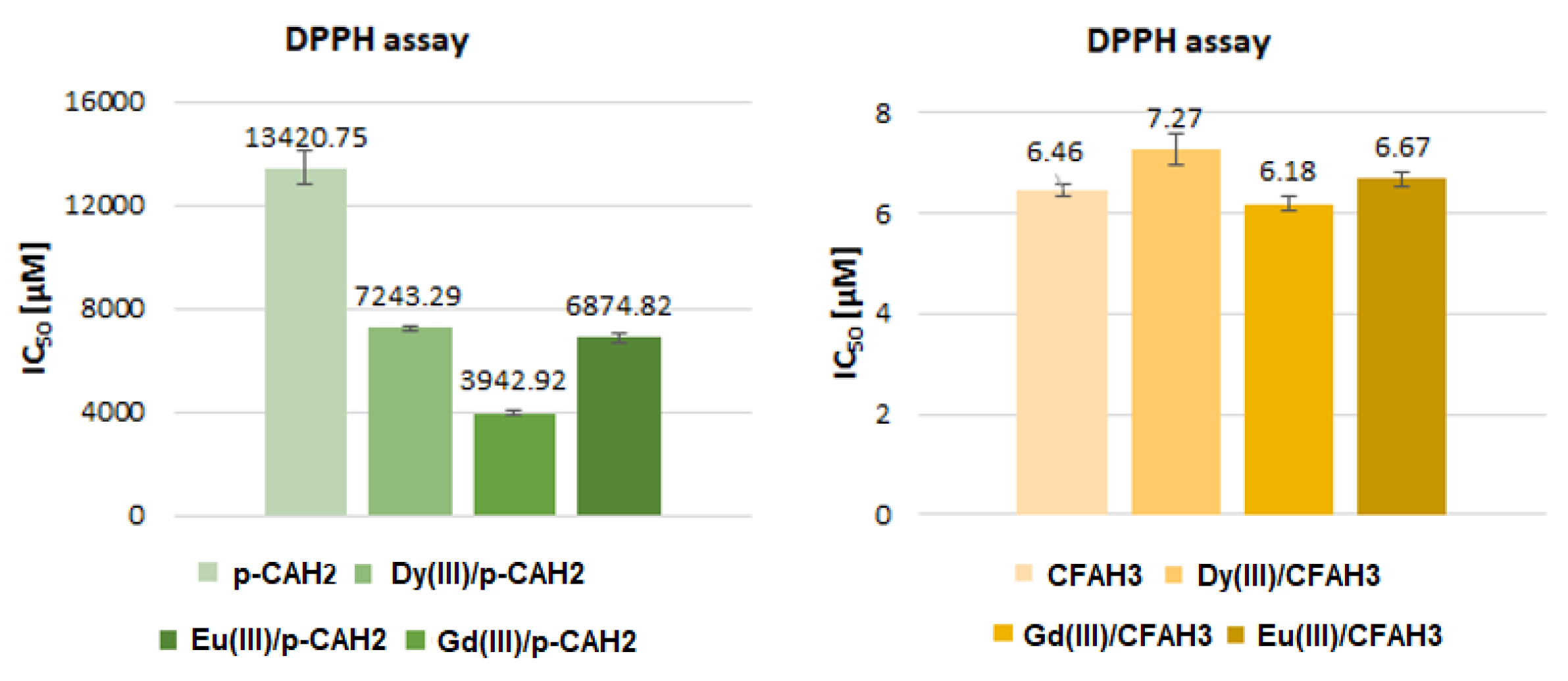
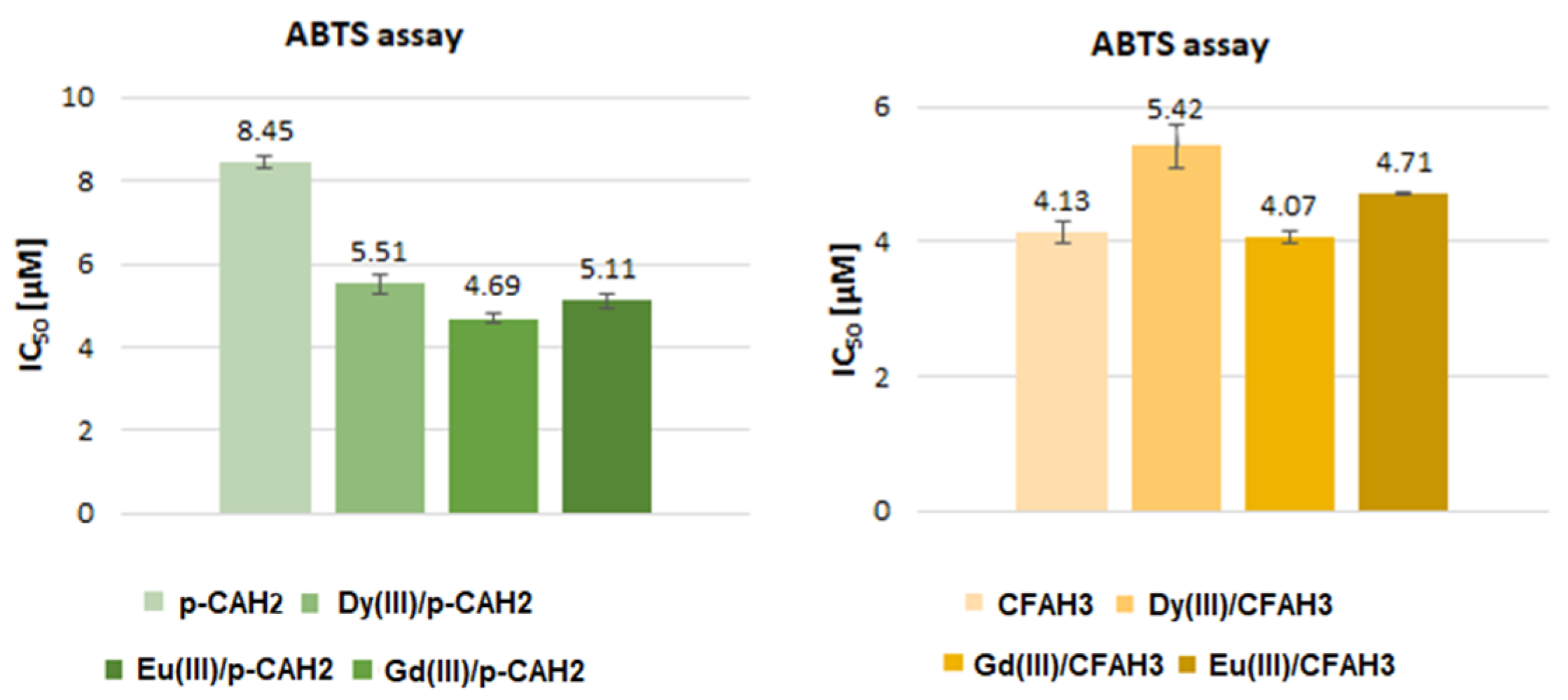
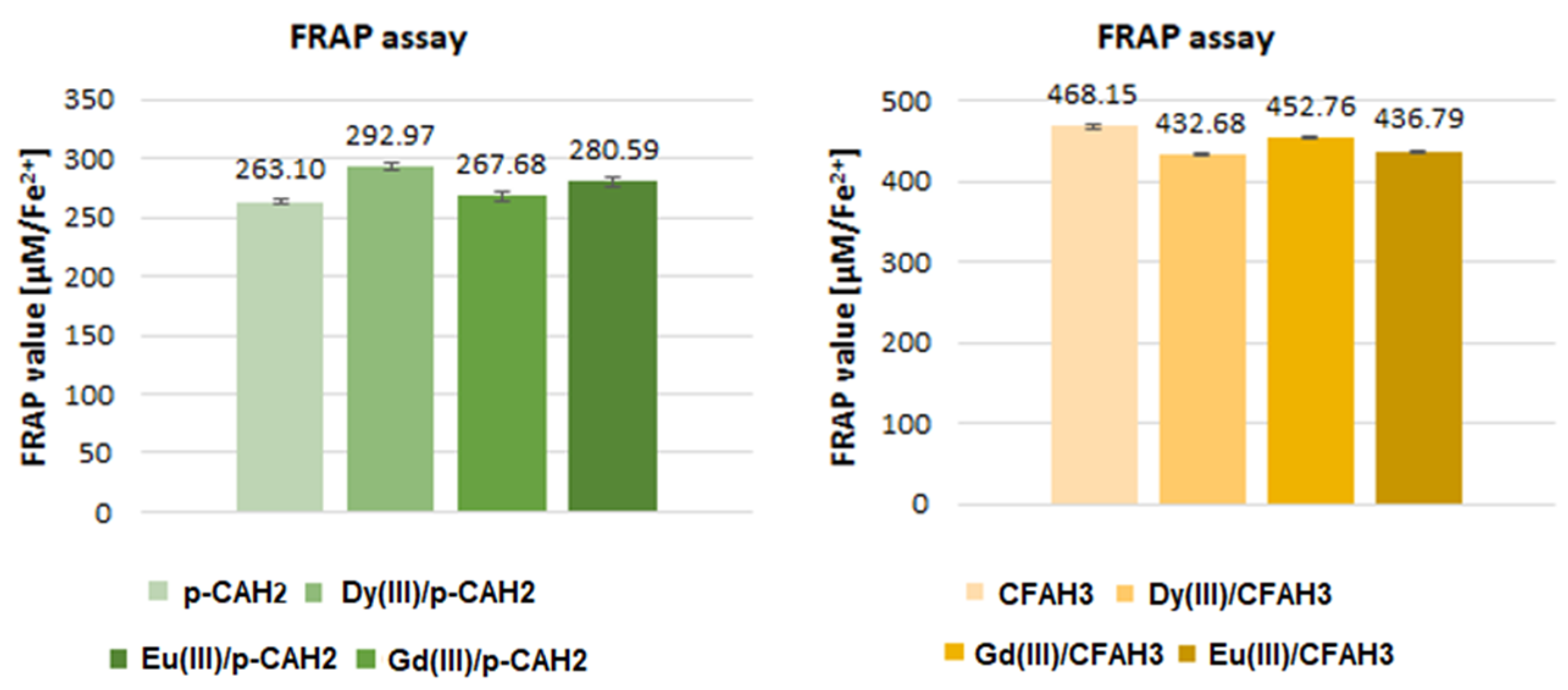
2.3. Antioxidant Activity
2.4. Antimicrobial Activity
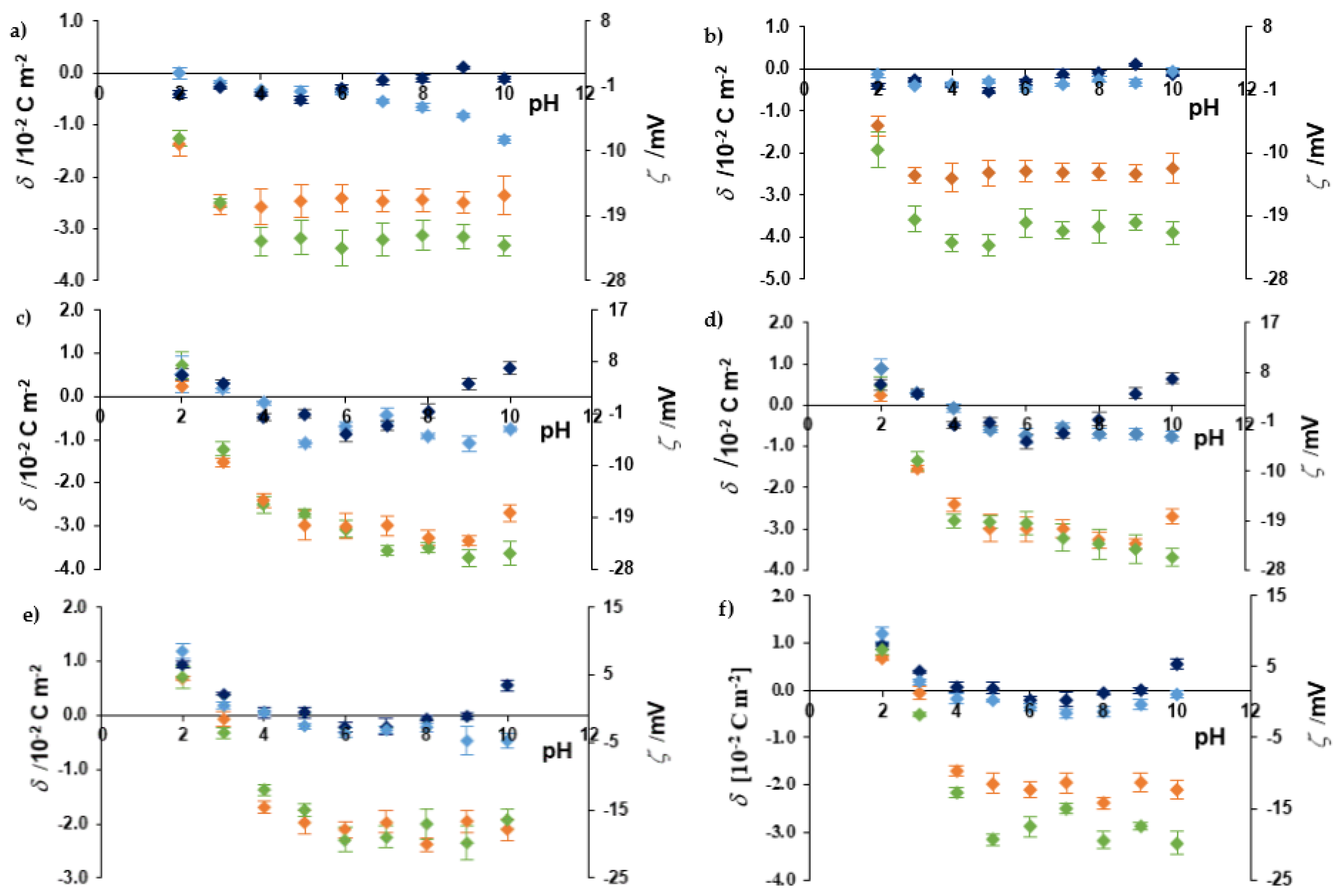
2.5. Microelectrophoretic Mobility Measurements
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Elemental and Thermal Analysis
3.4. Raman and FTIR Spectroscopy
3.5. Antioxidant properties
3.6. Antimicrobial Study
3.7. Microelectrophoretic Mobility Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Caparica, R.; Júlio, A.; Rolim, B.A.; Santos de Almeida, T.; Guilherme, C.J. In vitro cytotoxicity assessment of ferulic, caffeic and p-coumaric acids on human renal cancer cells. Biomed. Biopharm. Res. 2020, 17, 63–74. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Araújo, A.M.; Costa, G.M. Chemical properties of caffeic and ferulic acids in biological system: Implications in cancer therapy. A review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The anti-tumor effects of p-coumaric acid on melanoma A375 and B16 cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tsuchimura, S.; Azuma, N.; Endo, S.; Ichihara, K.; Ikari, A. Caffeic acid phenethyl ester potentiates gastric cancer cell sensitivity to doxorubicin and cisplatin by decreasing proteasome function. Anticancer Drug. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation—A chemical approach. PLoS ONE 2015, 10, e0142402. [Google Scholar]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 21, 541. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic acid and diseases—Mechanisms of action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 2017, 10, 3804–3815. [Google Scholar] [CrossRef]
- Tosovic, J. Spectroscopic features of caffeic acid: Theoretical study. Kragujev. J. Sci. 2017, 29, 99–108. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, Q.H.; Xiong, X.G.; Wang, Y.; Zhang, Z.H.; Sun, M.J.; Lu, X.; Wu, D. Anti-inflammatory effects of p-coumaric acid, a natural compound of Oldenlandia diffusa, on arthritis model rats. Evid. Based Complement. Alternat. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu (III) complexes: Solution equilibrium studies, structure characterization and biological activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef]
- Świderski, G.; Jabłońska-Trypuć, A.; Kalinowska, M.; Świsłocka, R.; Karpowicz, D.; Magnuszewska, M.; Lewandowski, W. Spectroscopic, theoretical and antioxidant study of 3d-transition metals (Co (II), Ni (II), Cu (II), Zn (II)) complexes with cichoric acid. Materials 2020, 13, 3102. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) Complex of Plant Phenolic Chlorogenic Acid: Antioxidant, Antimicrobial and Structural Studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Kalinowska, M.; Mazur, L.; Piekut, J.; Rzączyńska, Z.; Laderiere, B.; Lewandowski, W. Synthesis, crystal structure, spectroscopic properties, and antimicrobial studies of a zinc(II) complex of p-coumaric acid. J. Coord. Chem. 2013, 66, 334–344. [Google Scholar] [CrossRef]
- Lewandowski, W.; Kalinowska, M.; Lewandowska, H. The influence of metals on the electronic system of biologically important ligands. Spectroscopic study of benzoates, salicylates, nicotinates and isoorotates. Review. J. Inorg. Biochem. 2005, 99, 1407–1423. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes-molecular structure, antioxidant activity and biological effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Fricker, S.P. The therapeutic application of lanthanides. Chem. Soc. Rev. 2006, 35, 524–533. [Google Scholar] [CrossRef]
- Chundawat, N.S.; Jadoun, S.; Zarrintaj, P.; Chauhan, N.P.S. Lanthanide complexes as anticancer agents: A review. Polyhedron 2021, 207, 115387. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with Schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Madanhire, T.; Davids, H.; Pereira, M.C.; Hosten, E.C.; Abrahams, A.R. Synthesis, characterisation and anticancer activity screening of lanthanide (III) acetate complexes with benzohydrazone and nicotinohydrazone ligands. Polyhedron 2020, 184, 114560. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, M.; Zhang, J. A series of novel lanthanide complexes with 2-bromine-5-methoxybenzoic acid and 2,20-bipyridine: Syntheses, crystal structures, and luminescent properties. J. Mol. Struct. 2017, 1149, 171–182. [Google Scholar] [CrossRef]
- Zheng, J.; Ren, S.; Ren, N.; Zhang, J.; Zhang, D.; Wang, S. Synthesis, thermodynamic properties and antibacterial activities oflanthanide complexes with 3,5-dimethoxybenzoic acid and 1,10-phenanthroline. Thermochim. Acta 2013, 572, 101–106. [Google Scholar] [CrossRef]
- Świsłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. J. Spectroscop. 2012, 27, 35–48. [Google Scholar] [CrossRef]
- Świsłocka, R. Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++ G** level) studies on alkali metal salts of caffeic acid. Spectrochim. Acta Part A 2013, 100, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Varsányi, G. Assignments for Vibrational Spectra of 700 Benzene Derivates; Akademiai Kiado: Budapest, Hungary, 1973. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Or-ganometallic, and Bioinorganic Chemistry; Deacon, G.B., Philips, R.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 2. Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Nalewajko-Sieliwoniuk, E.; Gama, S.; Arciszewska, Ż.; Bogdan, P.; Naumowicz, M.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Lewandowski, W.; Lando, G.; et al. Chemical speciation of caffeic and p-coumaric acids with selected lanthanides. J. Mol. Liq. 2023, 382, 121915. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal structure, spectroscopic characterization, antioxidant and cytotoxic activity of new Mg(II) and Mn(II)/Na(I) complexes of isoferulic acid. Materials 2021, 14, 3236. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent Overview of Potent Antioxidant Activity of Coordination Compounds. Antioxidants 2023, 12, 213. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, 445–451. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans antifungal resistance and tolerance in bloodstream infections: The triad yeast-host-antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Y.; Dai, L.; Xu, G. Bacillus subtilis and Bifidobacteria bifidum Fermentation Effects on Various Active Ingredient Contents in Cornus officinalis Fruit. Molecules 2023, 28, 1032. [Google Scholar] [CrossRef] [PubMed]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef]
- Denning, E.J.; Beckstein, O. Influence of lipids on protein mediated transmembrane transport. Chem. Phys. Lipids 2013, 169, 57–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oursel, D.; Loutelier-Bourhis, C.; Orange, N.; Chevalier, S.; Norris, V.; Lange, C.M. Lipid composition of membranes of Escherichia coli by liquid chromatography/tandem mass spectrometry using negative electrospray ionization. Rapid Commun. Mass Spectrom. 2007, 21, 1721–1728. [Google Scholar] [CrossRef]
- Santos, A.X.; Riezman, H. Yeast as a model system for studying lipid homeostasis and function. FEBS Lett. 2012, 586, 2858–2867. [Google Scholar] [CrossRef]
- Martin, C.E.; Oh, C.S.; Jiang, Y. Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim. Biophys. Acta 2007, 1771, 271–285. [Google Scholar] [CrossRef]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef]
- Löffler, J.; Einsele, H.; Hebart, H.; Schumacher, U.; Hrastnik, C.; Daum, G. Phospholipid and sterol analysis of plasma membranes of azole-resistant Candida albicans strains. FEMS Microbiol. Lett. 2000, 185, 59–63. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Huang, C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry 1969, 8, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kotyńska, J.; Naumowicz, M. Theoretical considerations and the microelectrophoresis experiment on the influence of selected chaotropic anions on phosphatidylcholine membrane surface charge density. Molecules 2020, 25, 132. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.E.; Johnson, P. Colloid Science. J. Pol. Sci. 1949, 4, 747–748. [Google Scholar]
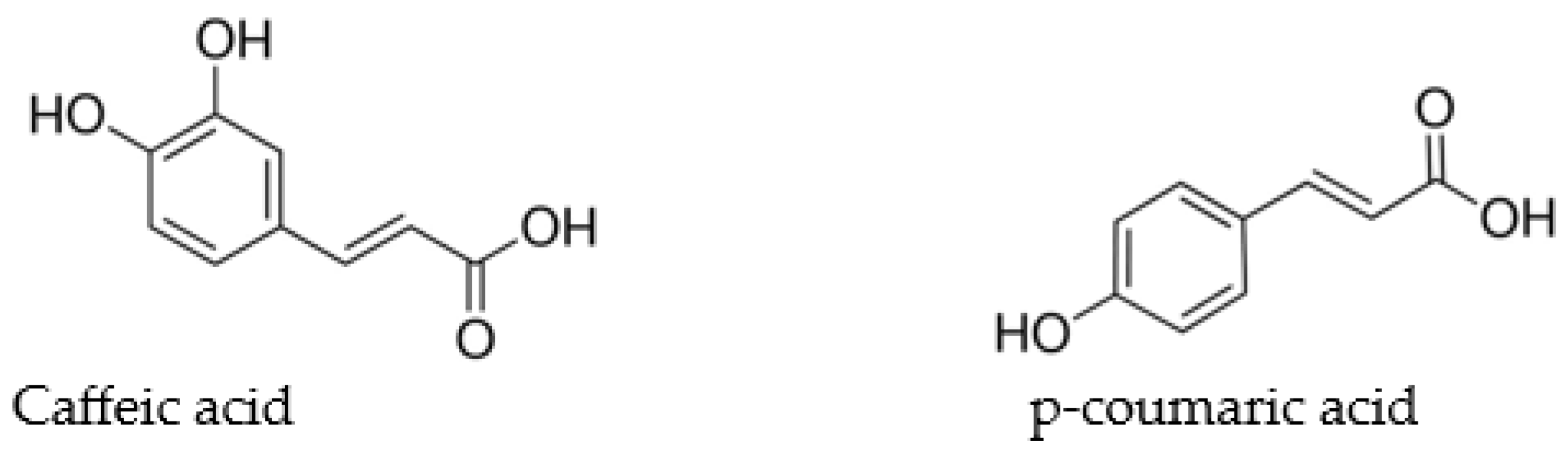
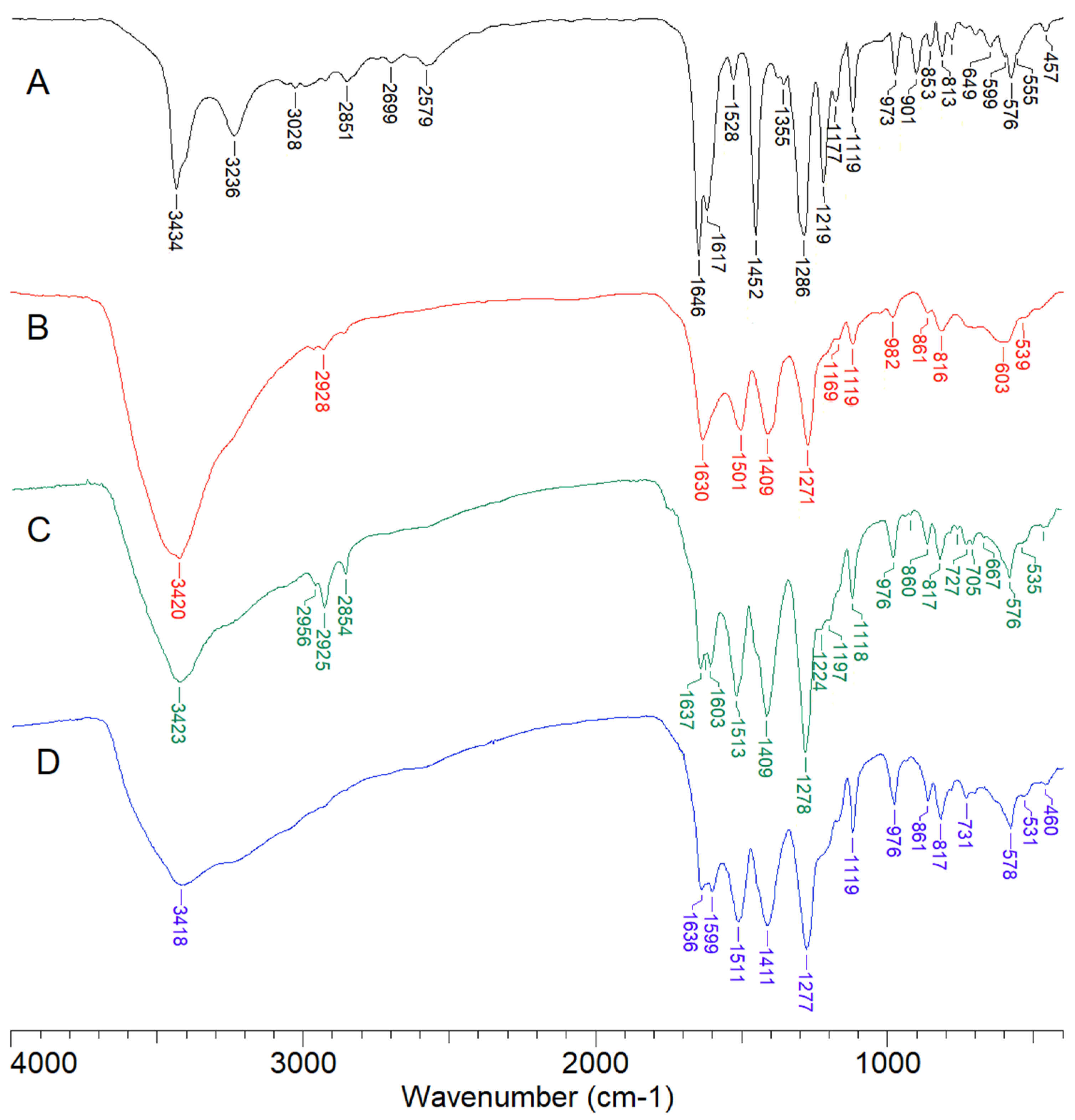


| Compound Name/Formula | Content C (%) | Content H (%) | Synthesis Yeld * | ||
|---|---|---|---|---|---|
| Calculated | Experimental | Calculated | Experimental | ||
| Dy(p-CAH)3 Dy(C9H7O3)3∙5H2O | 43.70 | 43.85 | 4.21 | 4.14 | 65–70% |
| Dy(CFAH2)3 Dy(C9H7O4)3∙6H2O | 40.13 | 40.53 | 4.12 | 3.98 | 65–75% |
| Eu(p-CAH)3 Eu(C9H7O3)3∙5H2O | 44.33 | 45.21 | 4.27 | 4.17 | 65–70% |
| Eu(CFAH2)3 Eu(C9H7O4)3∙7H2O | 39.76 | 39.15 | 4.33 | 4.25 | 70–80% |
| Gd(p-CAH)3 Gd(C9H7O3)3∙5H2O | 44.01 | 44.87 | 4.24 | 4.58 | 70–75% |
| Gd(CFAH2)3 Gd(C9H7O4)3∙8H2O | 38.66 | 37.70 | 4.44 | 4.18 | 75–80% |
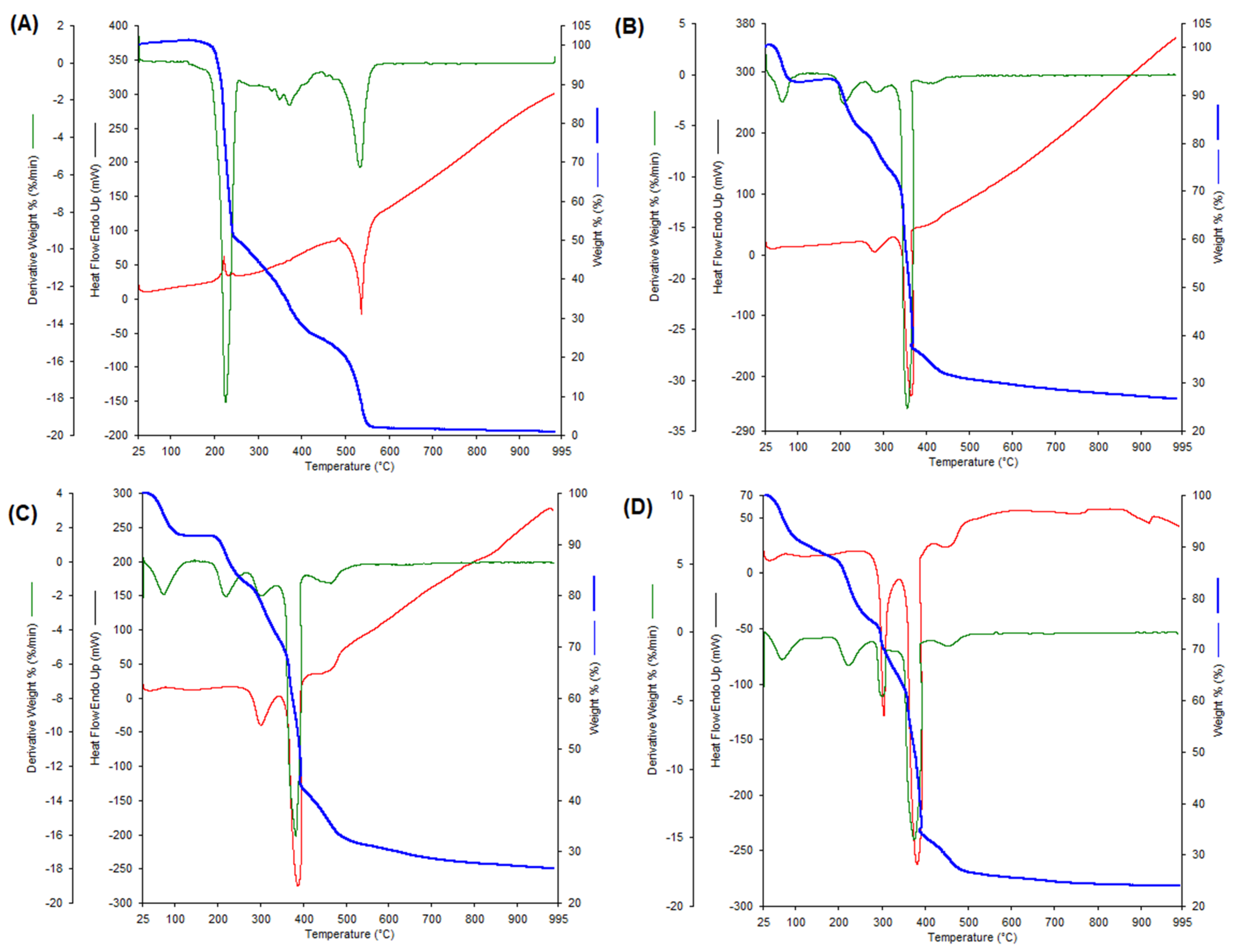
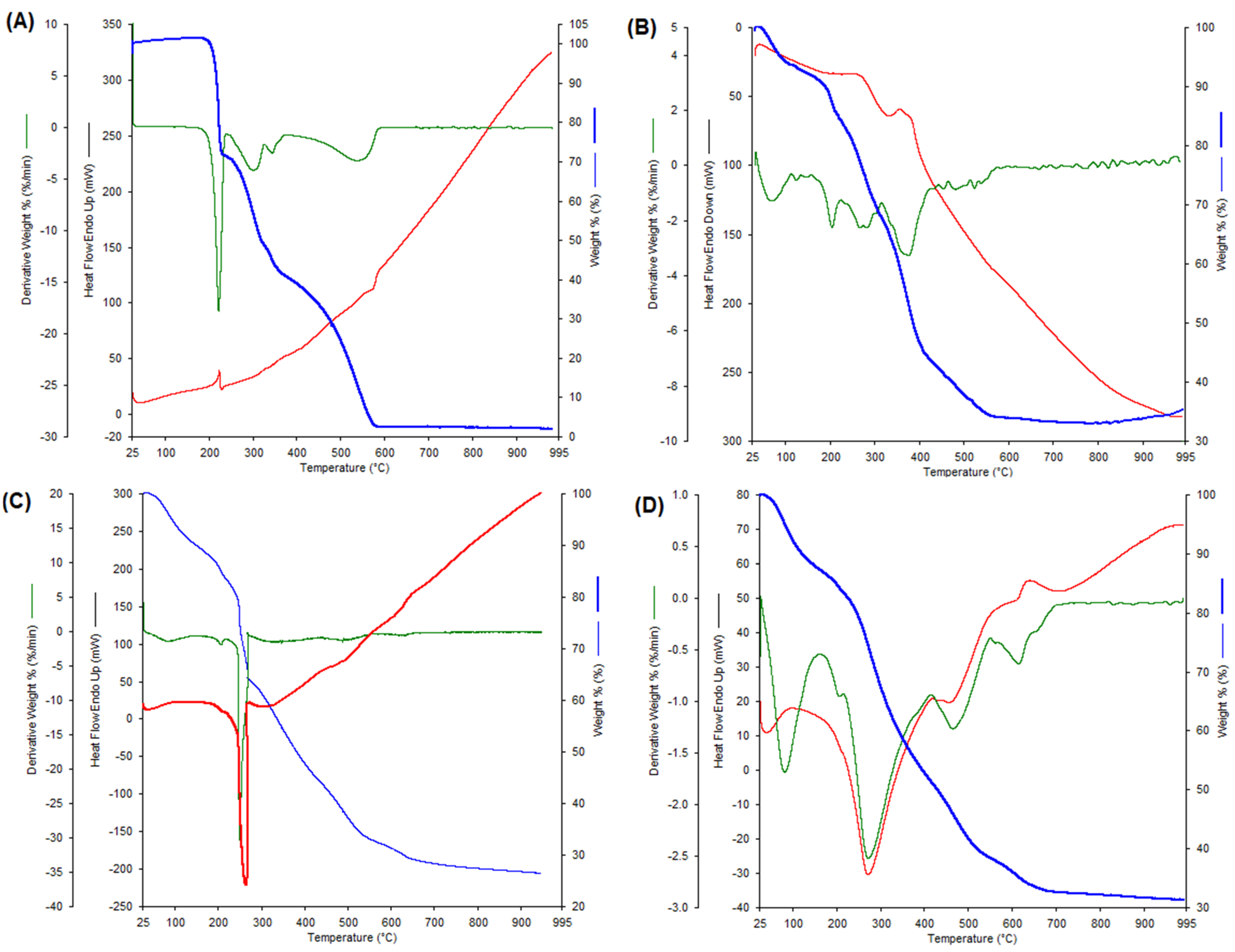
| Compound | Stage | TG Trange [°C] | DTG (DSC) Tmax. Peaks [°C] | Proces Nature | Mass Loss [%] | Final Residue | |
|---|---|---|---|---|---|---|---|
| Calc. | Found | ||||||
| p-CAH2 | Decomposition | 180–550 | 225.5 (221.8) | egzo | 100 | 98.8 | - |
| [Dy(p-CAH)3∙2H2O]∙3H2O | Dehydration Dehydration Decomposition | 40–100 120–220 220–900 | 70.10 (68.80) 304.5 (300.10) 382.2 (375.3) | endo endo egzo egzo | 5.97 9.95 74.24 | 6.03 9.90 75.5 | [Dy(p-CAH)3∙2H2O] Dy(p-CAH)3 Dy2O3 |
| [Eu(p-CAH)3∙3H2O]∙2H2O | Dehydration Dehydration Decomposition Decomposition | 40–120 140–240 240–900 | 64.48 207.34 276.90 (278.78) 355.89 (364.99) | endo endo egzo egzo | 7.77 12.31 75.94 | 7.04 12.24 73.27 | Eu(p-CAH)3∙3H2O] Eu(p-CAH)3 Eu2O3 |
| [Gd(p-CAH)3∙3H2O]∙2H2O | Dehydration Dehydration Decomposition Decomposition | 40–140 140–240 240–900 | 74.50 218.0 300.71 (300.71) 383.14 (388.19) | endo endo egzo egzo | 7.71 14.31 75.40 | 8.33 15.70 74.29 | [Gd(p-CAH)3∙3H2O] Gd(p-CAH)3 Gd2O3 |
| CFAH3 | Decomposition | 200–570 | 221.8 (223.6) | endo | 100 | 98.2 | - |
| [Dy(CFAH2)3∙3H2O]∙3H2O | Dehydration Dehydration Decomposition Decomposition Decomposition | 40–100 100–200 200–900 | 82.50 (38.70) 271.6 (272.2) 465.4 (458.3) 616.70 (613.9) | endo endo egzo egzo egzo | 7.16 13.36 79.06 | 6.15 12.6 68.00 | [Dy(CFAH2)3∙3H2O] Dy(CFAH2)3 Dy2O3 + Corg |
| [Eu(CFAH2)3∙4H2O]∙3H2O | Dehydration Dehydration Decomposition | 40–120 140–240 240–900 | 204.86 368.93 (368.58) | endo egzo egzo | 7.23 15.45 79.90 | 6.32 14.80 66.81 | [Eu(CFAH2)3∙4H2O] Eu(CFAH2)3 Eu2O3 + Corg |
| [Gd(CFAH2)3∙4H2O]∙4H2O | Dehydration Dehydration Decomposition | 40–120 120–250 25–900 | 79.34 206.13 269.37 (264.38) | endo endo egzo | 9.46 17.27 79.70 | 10.39 18.13 74.55 | [Gd(CFAH2)3∙4H2O] Gd(CFAH2)3 Gd2O3 + Corg |
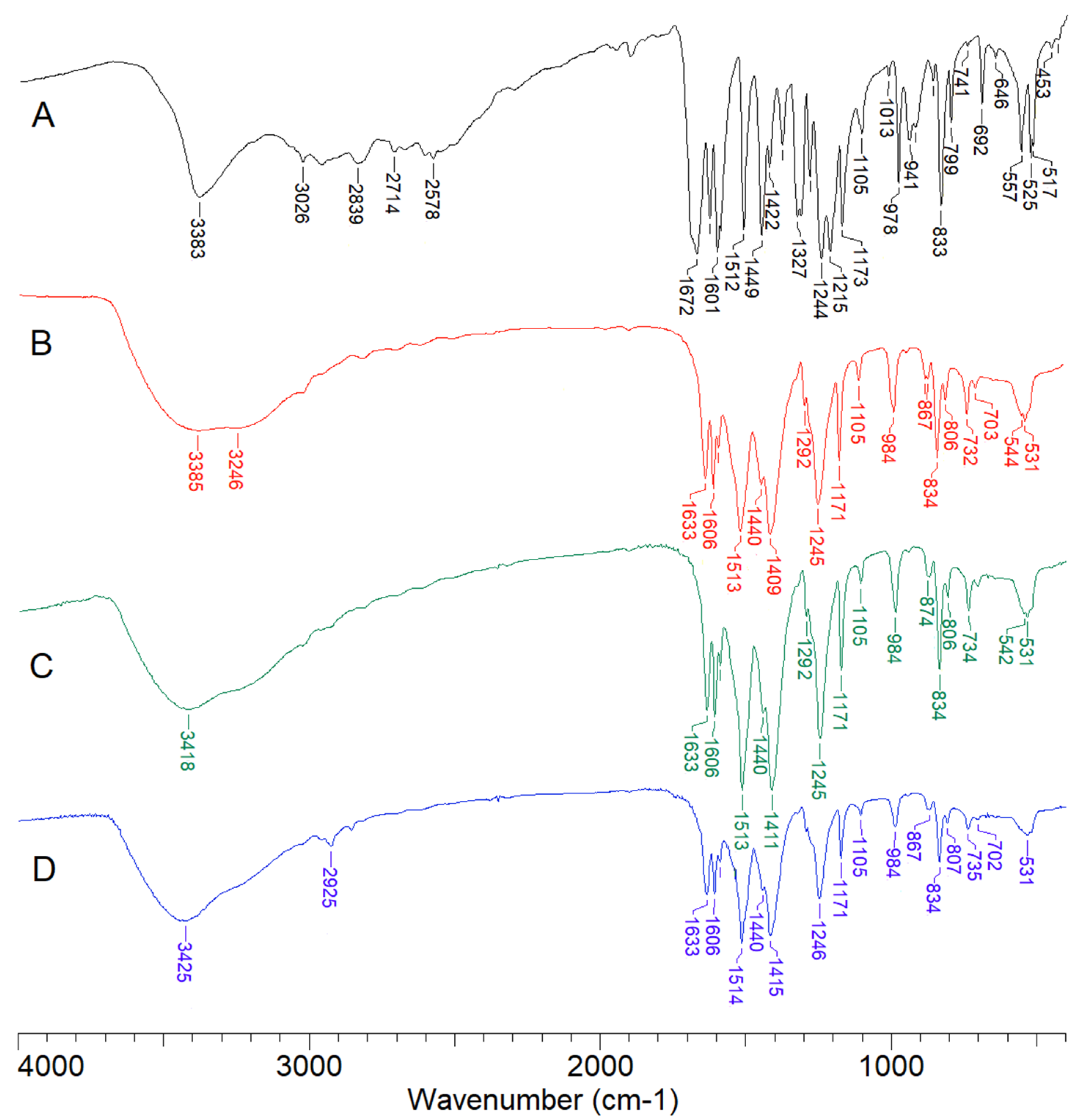
| p-CAH2 [34] | Eu(p-CAH)3 | Gd(p-CAH)3 | Dy(p-CAH)3 | Assignment | No. [35] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTIRKBr | FTIRATR | Raman | FTIRKBr | FTIRATR | Raman | FTIRKBr | FTIRATR | FTIRKBr | FTIRATR | Raman | ||
| 3383 s a | 3385 s | 3417 s | 3384 vs | ν (OH)ar b | ||||||||
| 3026 w | 3025 vw | 3023 m | 3017 w | 3025 w | 3027 w | 3030 w | ν(CH), ν(CH)C=C | 20b | ||||
| 2839–2513 m | ν(OH) | |||||||||||
| 1672 vs | 1667 vs | ν(C=O) | ||||||||||
| 1628 s | 1626 s | 1636 m | 1633 s | 1635 m | 1639 vs | 1633 s | 1629 m | 1633 s | 1629 m | 1634 s | ν(CH)C=C | |
| 1601 vs | 1600 s | 1606 vs | 1606 s | 1605 s | 1609 vs | 1606 s | 1603 m | 1606 s | 1603 s | 1606 vs | ν(CC) | 8a |
| 1591 s | 1589 s | 1593 m | 1589 m | 1581 m | 1589 m | 1585 m | 1589 m | 1586 m | ν(CC) | 8b | ||
| 1513 vs | 1513 vs | 1519 w | 1513 vs | 1507 s | 1514 vs | 1508 vs | 1524 w | νas(COO) | ||||
| 1512 s | 1510 m | 1519 w | 1501 vs | 1504 w | ν(CC) | 19a | ||||||
| 1449 vs | 1446 s | 1448 w | 1440 m | 1445 s | 1437 w | 1440 s | 1438 m | 1440 m | 1439 m | 1436 w | ν(CC) | 19b |
| 1409 vs | 1405 vs | 1411 vs | 1386 vs | 1415 vs | 1389 vs | 1401 w | νs(COO) | |||||
| 1379 m | 1376 m | 1388 w | ν(CC), β(OH)ar | 14 | ||||||||
| 1283 m | 1309 s | 1282 w | 1292 m | 1292 w | 1292 m | 1292 m | 1292 m | 1292 m | ν(C-OH) | |||
| 1244 vs | 1241 s | 1260 m | 1245 s | 1239 vs | 1250 m | 1245 s | 1230 s | 1246 s | 1233 s | 1252 s | β(OH) | |
| 1215 s | 1211 s | β(CH) | 13 | |||||||||
| 1173 s | 1171 s | 1172 s | 1171 s | 1173 w | 1171 m | 1169 s | 1171 m | 1169 s | 1170 s | β(CH) | 9a | |
| 1105 m | 1103 m | 1105 w | 1105 m | 1105 w | 1104 m | 1105 w | 1104 m | 1101 w | β(CH) | 18b | ||
| 1013 w | 1012 w | 1018 w | β(CH) | 18a | ||||||||
| 984 m | 987 s | 987 w | 984 m | 982 s | 984 m | 983 s | 979 m | βs(COO) | ||||
| 978 s | 977 s | 977 w | ν(CCO) | |||||||||
| 941 m | 937 m | 952 vw | 942 w | 942 vw | 944 w | 943 w | 944 w | 945 w | γ(CH) | 17a | ||
| 920 m | 912 m | γ(C=O) | ||||||||||
| 860 w | 864 w | 867 w | 866 w | 867 w | 865 m | 867 w | 865 m | 866 m | γ(CH) | 10a | ||
| 833 s | 827 s | 837 w | 834 s | 834 s | 837 w | 834 s | 832 vs | 834 s | 833 vs | γ(CH) | 17b | |
| 799 m | 797 m | 800 w | 806 w | 806 m | 806 w | 806 w | 804 m | 807 w | 805 m | 808 m | α(CCC) | 1 |
| 732 w | 731 s | 726 w | 734 w | 732 s | 735 w | 733 m | 733 w | γs(COO) | ||||
| 703 w | 704 s | 707 w | 703 w | 699 m | βas(COO) | |||||||
| 692 m | 690 m | β(C=O) | ||||||||||
| 646 w | 645 w | 645 vw | 642 vw | 633 s | 642 w | 641 w | 641 vw | 640 w | φ(CC) | 4 | ||
| 557 m | 557 w | 552 vw | 544 m | 542 m | 554 sh | γ(OH) | ||||||
| 525 m | 524 m | 534 vw | 531 m | 531 m | 528 sh | 531 m | 531 m | α(CCC) | ||||
| 517 m | 513 s | 516 vw | 518 sh | 514 s | 517 w | 515 s | φ(CC) | 16b | ||||
| 453 w | 451 w | 459 vw | 454 w | 434 w | φ(CC) | 16a | ||||||
| Compound | Wavenumber [cm−1] | Coordination Type [36] | |||
|---|---|---|---|---|---|
| νas(COO−) | νs(COO−) | Δν | |||
| Sodium salt [33] | FTIRKBr | 1549 | 1414 | 135 | - |
| FT-Raman | 1538 | 1424 | 114 | ||
| Dy(p-CAH)3 | FTIRKBr | 1513 | 1414 | 99 | bidentate chelation |
| FT-Raman | 1523 | 1401 | 122 | ||
| Eu(p-CAH)3 | FTIRKBr | 1513 | 1409 | 104 | bidentate chelation |
| FT-Raman | 1519 | - | - | ||
| Gd(p-CAH)3 | FTIRKBr | 1513 | 1411 | 102 | bidentate chelation |
| CFAH3 [34] | Eu(CFAH2)3 | Gd(CFAH2)3 | Dy(CFAH2)3 | Assignment | No. [35] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FTIRKBr | FTIRATR | Raman | FTIRKBr | FTIRATR | FTIRKBr | FTIRATR | FTIRKBr | FTIRATR | ||
| 3431 s a | 3399 m | 3421 vs | 3396 m | 3418 s | 3423 vs | ν(OH)ar b | ||||
| 3235 m | 3218 m | ν(OH)ar | ||||||||
| 3000 w | 3056 m | 3032 vw | 2963 w | ν(CH), ν(CH)C=C | 20b | |||||
| 2839–2569 w | ν(OH) | |||||||||
| 1647 vs | 1634 s | 1641 m | ν(C=O) | |||||||
| 1630 s | 1629 sh | 1637 s | 1631 m | 1636 s | 1630 m | ν(CH)C=C | ||||
| 1611 vs | 1602 vs | 1613 vs | 1603 s | 1595 m | 1599 s | 1594 m | ν(CC) | 8a | ||
| 1526 w | 1526 m | 1532 vw | ν(CC) | 8b | ||||||
| 1501 m | 1499 vs | 1513 s | 1508 s | 1511s | 1509 s | νas(COO) | ||||
| 1500 s | 1499 s | νas(COO) | ||||||||
| 1450 vs | 1442 vs | 1453 vw | 1505 vs | 1458 w | 1459 w | ν(CC) | 19b | |||
| 1409 s | 1409 s | 1409 s | 1405 s | 1411 s | 1401 s | νs(COO) | ||||
| 1364 w | 1362 w | 1353 w | ν(CC), β(OH) | 14 | ||||||
| 1299 s | β(OH) | |||||||||
| 1283 vs | 1271 s | 1271 s | 1271 vs | 1278 vs | 1270 vs | 1278 vs | 1266 vs | ν(C-OH) | ||
| 1215 s | 1209 s | 1204 sh | 1206 m | β(OH), β(CH)C=C | ||||||
| 1174 m | 1186 w | 1169 w | 1170 m | 1163 m | 1174 sh | 1162 m | β(CH) | 18a | ||
| 1115 m | 1110 s | 1108 vw | 1119 m | 1120 m | 1118 m | 1116 m | 1119 m | 1115 m | β(CH) | 18b |
| 982 w | 980 w | 976 m | 977 m | 976 m | 975 m | βs(COO) | ||||
| 972 w | 965 m | 974 vw | 961 w | 977 w | γ(CH)C=C, γ(CH) | 17b | ||||
| 936 w | 939 w | 932 w | 935 w | 936 w | 936 w | 934 w | γ(CH) | 17a | ||
| 901 w | 892 s | ν(CCO) | ||||||||
| 856 w | 853 m | 852 vw | 861 w | 853 vw | 860 w | 858 m | 861 m | 858 m | γ(CH) | 5 |
| 812 w | β(C=O) | |||||||||
| 816 w | 823 w | 817 m | 814 m | 817 w | 813 m | α(CCC) | 1 | |||
| 801 w | 806 s | 803 vw | 793 m | γ(CH) | 10a | |||||
| 787 w | 788 m | 779 vw | 756 sh | 782 w | 782 w | α(CCC) | 12 | |||
| 701 w | 695 m | 727 w | 728 m | 731 w | 728 m | γs(COO) | ||||
| 699 w | 695 w | 686 vw | γ(C=O) | |||||||
| 645 w | 642 m | 664 vw | 667 vw | 663 vw | φ(CC) | 16a | ||||
| 603 w | 603 vw | 603 w | 595 s | α(CCC) | 6a | |||||
| 575 m | 534 vw | 535 w | 576 m | 577 s | 578 m | 577 s | γ(OH) | |||
| 459 w | 459 vw | 547 vw | 547 s | 460 vw | 447 w | 460 vw | 445 w | α(CCC) | 6b | |
| 409 w | 416 vw | 407 vw | 409 vw | φ(CC) | 16b | |||||
| Compound | Wavenumber [cm−1] | Coordination Type [36] | ||
|---|---|---|---|---|
| νas(COO−) | νs(COO−) | Δν | ||
| Sodium salt [37] | 1522 | 1375 | 147 | - |
| Dy(CFAH2)3 | 1509, 1499 | 1404 | 105, 95 | bidentate chelation |
| Eu(CFAH2)3 | 1501 | 1409 | 92 | bidentate chelation |
| Gd(CFAH2)3 | 1513 | 1409 | 104 | bidentate chelation |
| Species of Microorganism | Compound | |||
|---|---|---|---|---|
| CFAH3 | Dy(CFAH2)3 | Eu(CFAH2)3 | Gd(CFAH2)3 | |
| MIC (mg/mL) | ||||
| Escherichia coli | 4 | 2 | 1 | 1 |
| Bacillus subtilis | 5 | 2 | 1 | 1 |
| Candida albicans | 7 | 4 | 2 | 2 |
| Species of Microorganism | Compound | |||
|---|---|---|---|---|
| p-CAH2 | Dy(p-CAH)3 | Eu(p-CAH)3 | Gd(p-CAH)3 | |
| MIC (mg/mL) | ||||
| Escherichia coli | 7 | 3 | 2 | 2 |
| Bacillus subtilis | 7 | 3 | 3 | 2 |
| Candida albicans | 8 | 5 | 4 | 3 |
| Escherichia coli | Bacillus subtilis | Candida albicans |
|---|---|---|
| MIC (mg/L) | ||
| 0.5 gentamicin | 5 gentamicin | 0.25 fluconazole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderski, G.; Kalinowska, M.; Gołębiewska, E.; Świsłocka, R.; Lewandowski, W.; Kowalczyk, N.; Naumowicz, M.; Cudowski, A.; Pietryczuk, A.; Nalewajko-Sieliwoniuk, E.; et al. Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates. Molecules 2023, 28, 6506. https://doi.org/10.3390/molecules28186506
Świderski G, Kalinowska M, Gołębiewska E, Świsłocka R, Lewandowski W, Kowalczyk N, Naumowicz M, Cudowski A, Pietryczuk A, Nalewajko-Sieliwoniuk E, et al. Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates. Molecules. 2023; 28(18):6506. https://doi.org/10.3390/molecules28186506
Chicago/Turabian StyleŚwiderski, Grzegorz, Monika Kalinowska, Ewelina Gołębiewska, Renata Świsłocka, Włodzimierz Lewandowski, Natalia Kowalczyk, Monika Naumowicz, Adam Cudowski, Anna Pietryczuk, Edyta Nalewajko-Sieliwoniuk, and et al. 2023. "Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates" Molecules 28, no. 18: 6506. https://doi.org/10.3390/molecules28186506
APA StyleŚwiderski, G., Kalinowska, M., Gołębiewska, E., Świsłocka, R., Lewandowski, W., Kowalczyk, N., Naumowicz, M., Cudowski, A., Pietryczuk, A., Nalewajko-Sieliwoniuk, E., Wysocka, I., Arciszewska, Ż., & Godlewska-Żyłkiewicz, B. (2023). Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates. Molecules, 28(18), 6506. https://doi.org/10.3390/molecules28186506








