Nanomaterials for Advanced Photocatalytic Plastic Conversion
Abstract
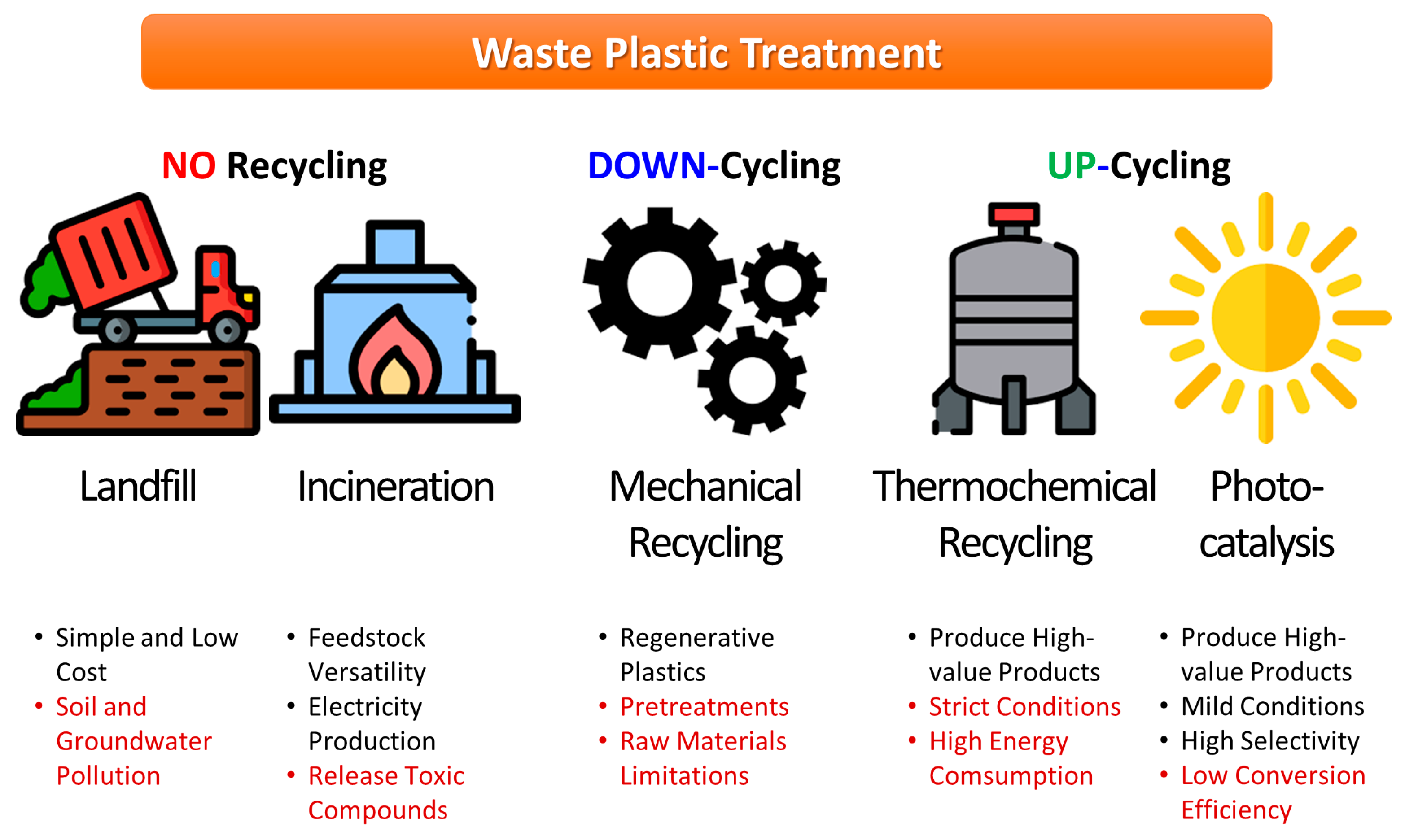
:1. Introduction
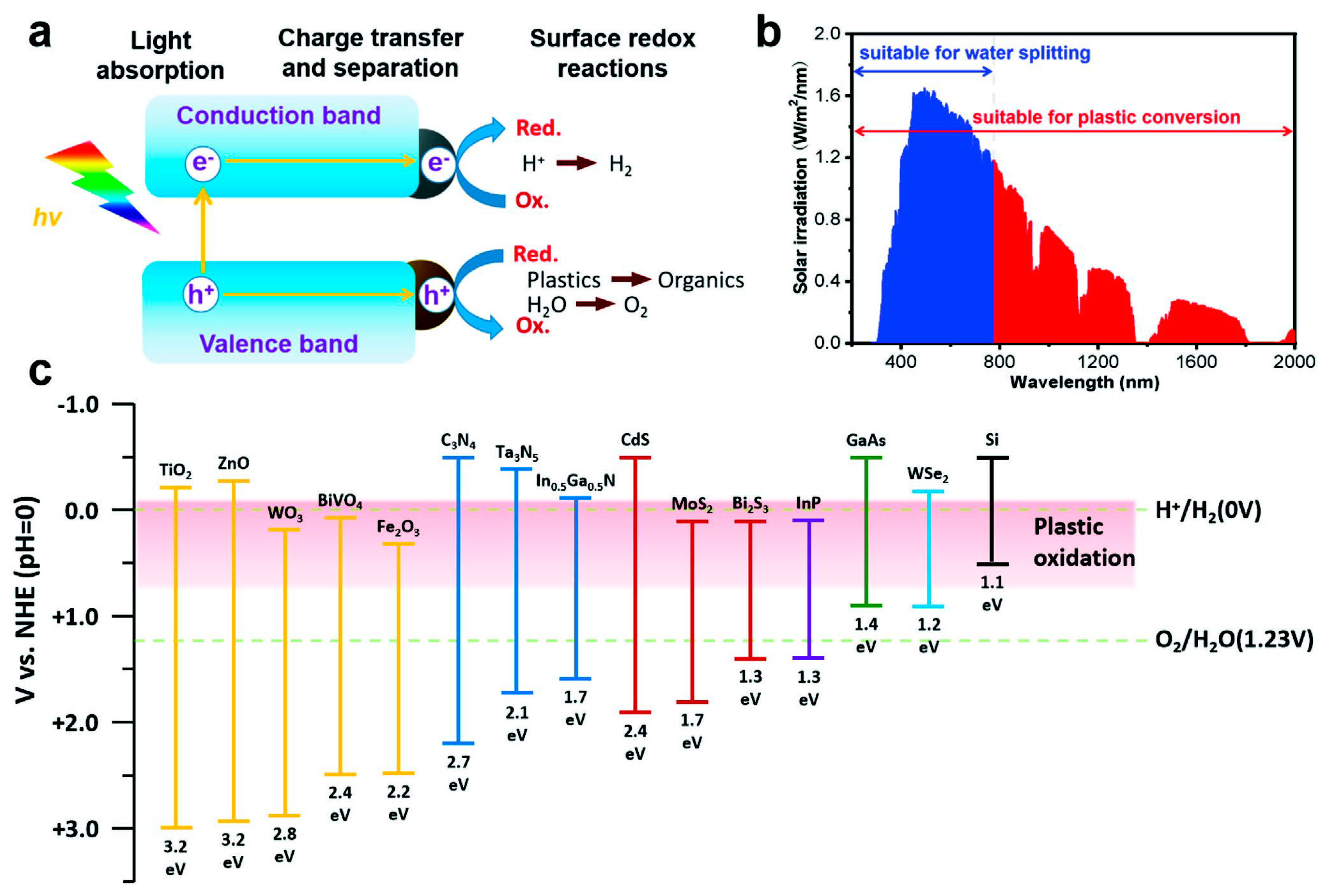
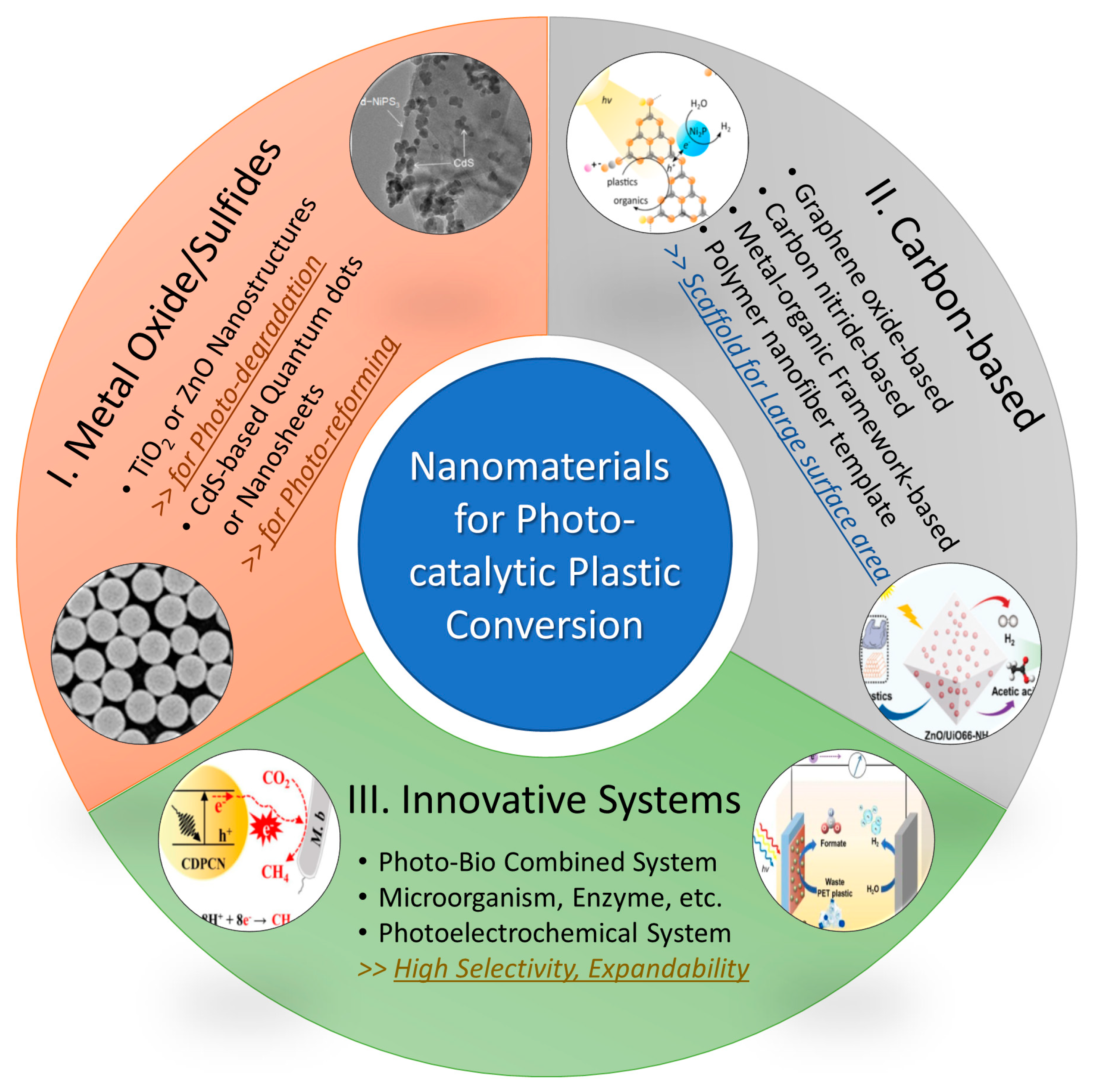
2. Nanomaterials for Photocatalytic Plastic Conversion
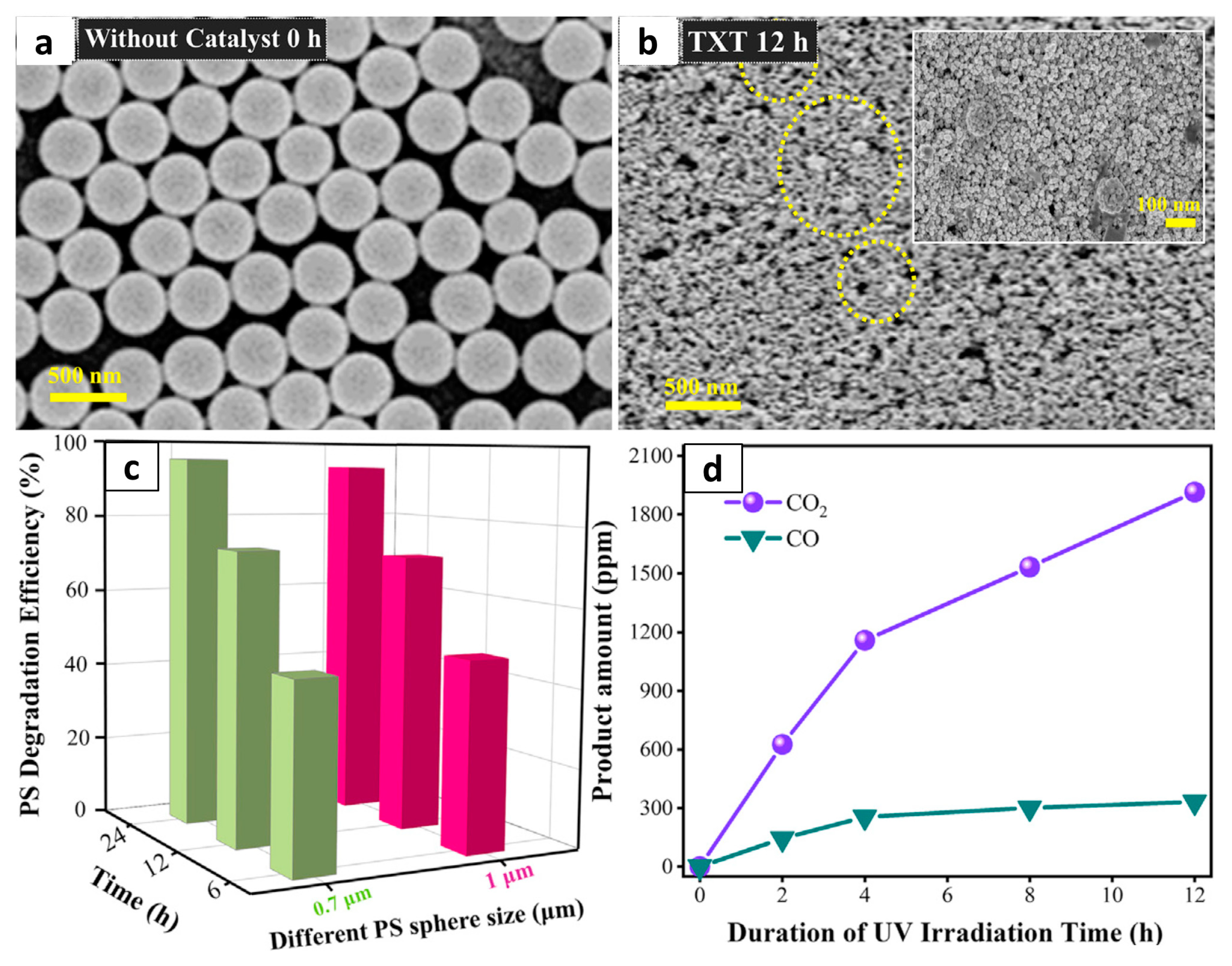
2.1. Metal Oxide/Sulfide Nanostructures
2.2. Carbon-Based Nanomaterials
2.3. Innovative Systems Based on Nanostructures
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, W. The plastic eaters. Science 2021, 373, 36–39. [Google Scholar] [CrossRef]
- Law, K.L.; Thomson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thomson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Santos, R.G.; Machovsky-Capuska, G.E.; Andrades, R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 2021, 373, 56–60. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Cozar, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Kasmuri, N.; Tarmizi, N.A.A.; Mojiri, A. Occurrence, impact, toxicity, and degradation methods of microplastics in environment—A review. Environ. Sci. Pollut. Res. 2022, 29, 30820–30836. [Google Scholar] [CrossRef]
- Editorials Team. Making plastics sustainable isn’t the whole solution. Nature 2021, 590, 363–364. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Cui, X.; Liu, W.; Wang, X.; Li, X.; Li, S. Life cycle assessment of end-of-life treatments of waste plastics in China. Resour. Conserv. Recycl. 2019, 146, 348–357. [Google Scholar] [CrossRef]
- Ragaert, T.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Ashworth, D.C.; Elliott, P.; Toledano, M.B. Waste incineration and adverse birth and neonatal outcomes: A systematic review. Environ. Int. 2014, 69, 120–132. [Google Scholar] [CrossRef]
- Su, K.; Liu, H.; Zhang, C.; Wang, F. Photocatalytic conversion of waste plastics to low carbon number organic products. Chin. J. Catal. 2022, 43, 589–594. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Liu, Q.; Zhang, H. Low chlorine oil production through fast pyrolysis of mixed plastics combined with hydrothermal dechlorination pretreatment. Process. Saf. Environ. Prot. 2021, 149, 105–114. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, K.; Peng, B.; Wang, G.; Muhler, M.; Wang, F. Ceria-based materials for thermocatalytic and photocatalytic organic synthesis. ACS Catal. 2021, 11, 9618–9678. [Google Scholar] [CrossRef]
- Chen, A.; Yang, M.-Q.; Wang, S.; Qian, Q. Recent advancements in photocatalytic valorization of plastic waste to chemicals and fuels. Front. Nanotechnol. 2021, 3, 723120. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2020, 19, 123–148. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, J.; Xiao, R.; Jin, B.; Dong, C.; Xiao, G. Catalytic co-pyrolysis of biomass and different plastics (polyethylene, polypropylene, and polystyrene) to improve hydrocarbon yield in a fluidized-bed reactor. Energy Fuels 2014, 28, 1940–1947. [Google Scholar] [CrossRef]
- Sayre, H.J.; Tian, L.; Son, M.; Hart, S.M.; Liu, X.; Arias-Rotondo, D.M.; Rand, B.P.; Schlau-Cohen, G.S.; Scholes, G.D. Solar fuels and feedstocks: The quest for renewable black gold. Energy Environ. Sci. 2021, 14, 1402–1419. [Google Scholar] [CrossRef]
- Zhu, S.S.; Wang, D.W. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatlytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Zhang, B.; Zhao, X.; Soo, H.S.; Wang, F.; Xiao, R.; Zhang, H. Photocatalytic Conversion of Plastic Waste: From Photodegradation to Photosynthesis. Adv. Energy Mater. 2022, 12, 2200435. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Liu, Y.; Yu, L. Probing the effect of nitrate anion in CAN: An additional opportunity to reduce the catalyst loading for aerobic oxidations. Chin. Chem. Lett. 2023, 34, 108489. [Google Scholar] [CrossRef]
- Chen, X.; Mao, J.; Liu, C.; Chen, C.; Cao, H.; Yu, L. An unexpected generation of magnetically separable Se/Fe3O4 for catalytic degradation of polyene contaminants with molecular oxygen. Chin. Chem. Lett. 2020, 31, 3205–3208. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, H.; Feng, J.; Wang, W.; Guan, H.; Li, Z.; Zou, Z. Material design and surface/interface engineering of photoelectrodes for solar water splitting. Sol. RRL 2021, 5, 2100100. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Liu, Y.; Guan, X.; Burda, C.; Guo, L. Metal oxide-based tandem cells for self-biased photoelectrochemical water splitting. ACS Energy Lett. 2020, 5, 844–866. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in hegerogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Li, C. Chapter one—Artificial photosynthesis systems for catalytic water oxidation. Adv. Inorg. Chem. 2019, 74, 3–59. [Google Scholar]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Mi, Z. Roadmap on solar water splitting: Current status and future prospects. Nano Futures 2017, 1, 022001. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic water splitting: Quantitative approaches toward photocatalyst by design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Reddy, N.L.; Rao, V.N.; Vijayakumar, M.; Santhosh, R.; Anandan, S.; Karthik, M.; Shankar, M.V.; Reddy, K.R.; Shetti, N.P.; Nadagouda, M.N.; et al. A review on Frontiers in plasmonic nano-photocatalysts for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10453–10472. [Google Scholar] [CrossRef]
- Cui, Y.; Li, M.; Zhu, N.; Cheng, Y.; Lam, S.S.; Chen, J.; Gao, U.; Zhao, J. Bi-based visible light-driven nano-photocatalyst: The design, synthesis, and its application in pollutant governance and energy development. Nanotoday 2022, 43, 101432. [Google Scholar] [CrossRef]
- Zhang, G.; Sewell, C.D.; Zhang, P.; Mi, H.; Lin, Z. Nanostructured photocatalysts for nitrogen fixation. Nano Energy 2020, 71, 104645. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M. Titanium dioxide nanostructures as efficient photocatalyst: Progress, challenges and perspective. Int. J. Energy Res. 2020, 45, 3569–3589. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Qiao, W.; Xiang, H.; Besenbacher, F.; Li, Y.; Su, R. Nanostructured heterogeneous photocatlyst materials for green synthesis of valuable chemicals. Chem. Synth. 2022, 2, 9. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Du, X.; Dong, F.; Zhang, Y. Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A 2019, 7, 11584–11612. [Google Scholar] [CrossRef]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Mohamed, A.R. Rational design of carbon-based 2D nanostructures for enhanced photocatalytic CO2 reduction: A dimensionality perspective. Chem. Eur. J. 2020, 26, 9710–9748. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.-M.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Bacha, A.U.R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Ali, S.S.; Qazi, I.A.; Arshad, M.; Khan, Z.; Voice, T.C.; Mehmood, C.T. Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes. Environ. Nanotechnol. Monit. Manag. 2016, 5, 44–53. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef]
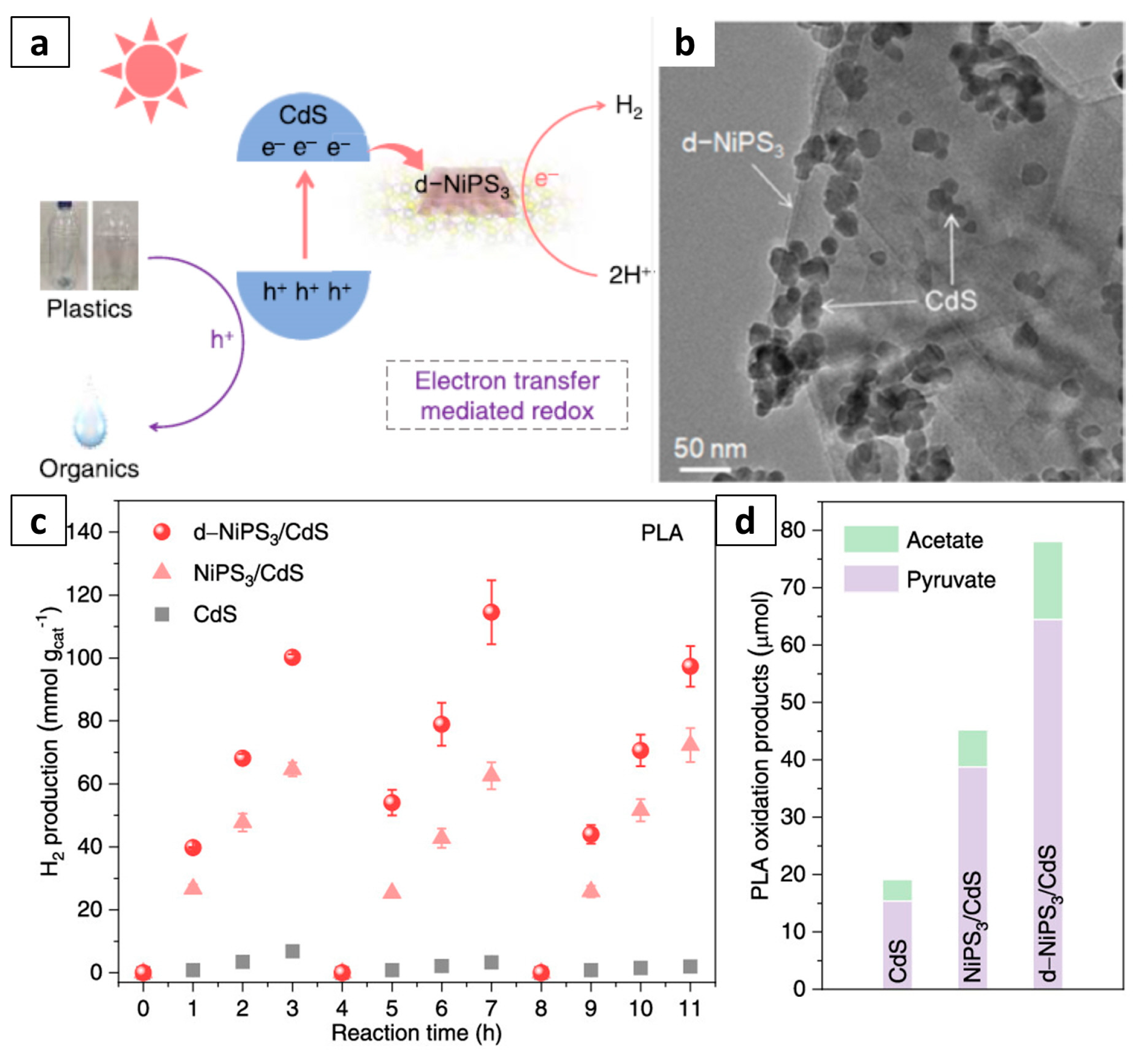
- Zhang, S.; Li, H.; Wang, L.; Liu, J.; Liang, G.; Davey, K.; Ran, J.; Qiao, S.-Z. Boosted photoreforming of plastic waste via defect-rich NiPS3 nanosheets. J. Am. Chem. Soc. 2023, 145, 6410–6419. [Google Scholar] [CrossRef]
- Fadli, M.H.; Ibadurrohman, M.; Slamet, S. Microplastic pollutant degradation in water using modified TiO2 photocatalyst under UV-irradiation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012055. [Google Scholar] [CrossRef]
- Uogintė, I.; Pleskytė, S.; Skapas, M.; Stanionytė, S.; Lujanienė, G. Degradation and optimization of microplastic in aqueous solutions with graphene oxide-based nanomaterials. Int. J. Environ. Sci. Technol. 2022, 20, 9693–9706. [Google Scholar] [CrossRef]
- Meng, X.; Peng, X.; Xue, J.; Wei, Y.; Sun, Y.; Dai, Y. A biomass-derived, all-day-round solar evaporation platform for harvesting clean water from microplastic pollution. J. Mater. Chem. A 2021, 9, 11013–11024. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Garcia-Lopez, E.I.; Marcì, G.; Palmisano, L.; Parrino, F. Heterogeneous photocatalytic materials for sustainable formation of high value chemicals in green solvents. Mater. Today Sustain. 2021, 13, 100071. [Google Scholar] [CrossRef]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of nonrecyclable plastic waste over a carbon nitiride/nickel phosphide catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Dou, Y.; Zhou, J.; Candelario, V.M.; Anderson, H.R.; Hélix-Nielsen, C.; Zhang, W. Photocatalytic valorization of plastic waste over zinc oxide encapsulated in a metal-organic framework. Adv. Funct. Mater. 2023, 33, 2214839. [Google Scholar] [CrossRef]
- Wu, F.; Dou, Y.; Zhou, J.; Qin, J.; Jiang, T.; Yao, Y.; Hélix-Nielsen, C.; Zhang, W. High-entropy (FeCoNiCuZn)WO4 photocatalysts-based fibrous membrane for efficient capturing and upcycling of plastic. Chem. Eng. J. 2023, 470, 144134. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Gao, C.; Wang, C.; Hu, A.; Dong, G.; Chen, Z.; Zhou, S.; Xiong, Y. Sustainable conversion of microplastics to methane with ultrahigh selectivity by a biotic-abiotic hybrid photocatalytic system. Angew. Chem. Int. Ed. 2022, 61, e202213244. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.; Hilberath, T.; Hollmann, F.; Park, C.B. Photoelectrocatalytic biosynthesis fuelled by microplastics. Nat. Synth. 2022, 1, 776–786. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Pan, Y.; Shao, J.; Wang, X.; Jiang, Y.; Xu, X.; Chu, S. Photoelectrochemical conversion of plastic waste into high-value chemicals coupling hydrogen production. Chem. Eng. J. 2023, 462, 142247. [Google Scholar] [CrossRef]
- Chowdhury, P.; Moreira, J.; Gomaa, H.; Ray, A.K. Visible-solar-light-driven photocatalytic degradation of phenol with dye-sensitized TiO2: Parametric and kinetic study. Ind. Eng. Chem. Res. 2012, 51, 4523–4532. [Google Scholar] [CrossRef]
- Vinu, R.; Polisetti, S.; Madras, G. Dye sensitized visible light degradation of phenolic compounds. Chem. Eng. J. 2010, 165, 746–797. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E.; Duman, F. High optoelectronic and anti-microbial performances of green synthesized ZnO nanoparticles using Aesculus hippocastanum. Environ. Chem. Lett. 2017, 15, 547–552. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from water by the decomposition of poly-vinylchloride, protein, algae, dead insects, and excrement. Chem. Lett. 1981, 10, 81–84. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, B.; Zhu, Z.; Wang, G.; Mei, X.; Fang, Y.; Lu, W. Photocatalytic-driven self-degradation of polyester microplastics under solar light. J. Polym. Environ. 2023, 31, 2415–2423. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, Z.; Dou, Y.; Lim, S.Y.; Zou, J.; Stamate, E.; Jensen, J.O.; Zhang, W. Random occupation of multimetal sites in transition metal-organic frameworks for boosting the oxygen evolution reaction. Chem.—Eur. J. 2022, 28, e202104288. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, W.; Kaiser, A. Electrospinning of metal-organic frameworks for energy and environmental applications. Adv. Sci. 2020, 7, 1902590. [Google Scholar] [CrossRef]
- Cheng, X.M.; Dao, X.Y.; Wang, S.Q.; Zhao, J.; Sun, W.Y. Enhanced photocatalytic CO2 reduction activity over NH2-MIL-125(Ti) by facet regulation. ACS Catal. 2021, 11, 650–658. [Google Scholar] [CrossRef]
- Shi, H.; Li, C.; Wang, L.; Wang, W.; Meng, X. Selective reduction of nitrate into N2 by novel z-scheme NH2-MIL-101(Fe)/BiVO4 heterojunction with enhanced photocatalytic activity. J. Hazard. Mater. 2022, 424, 127711. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Younis, S.A.; Kwon, E.E.; Qasim, M.; Kim, K.H.; Kim, T.; Kukkar, D.; Dou, X.; Ali, I. Metal-organic framework as a photocatalyst: Progress in modulation strategies and environmental/energy applications. Prog. Energy Combust. Sci. 2020, 81, 100870. [Google Scholar] [CrossRef]
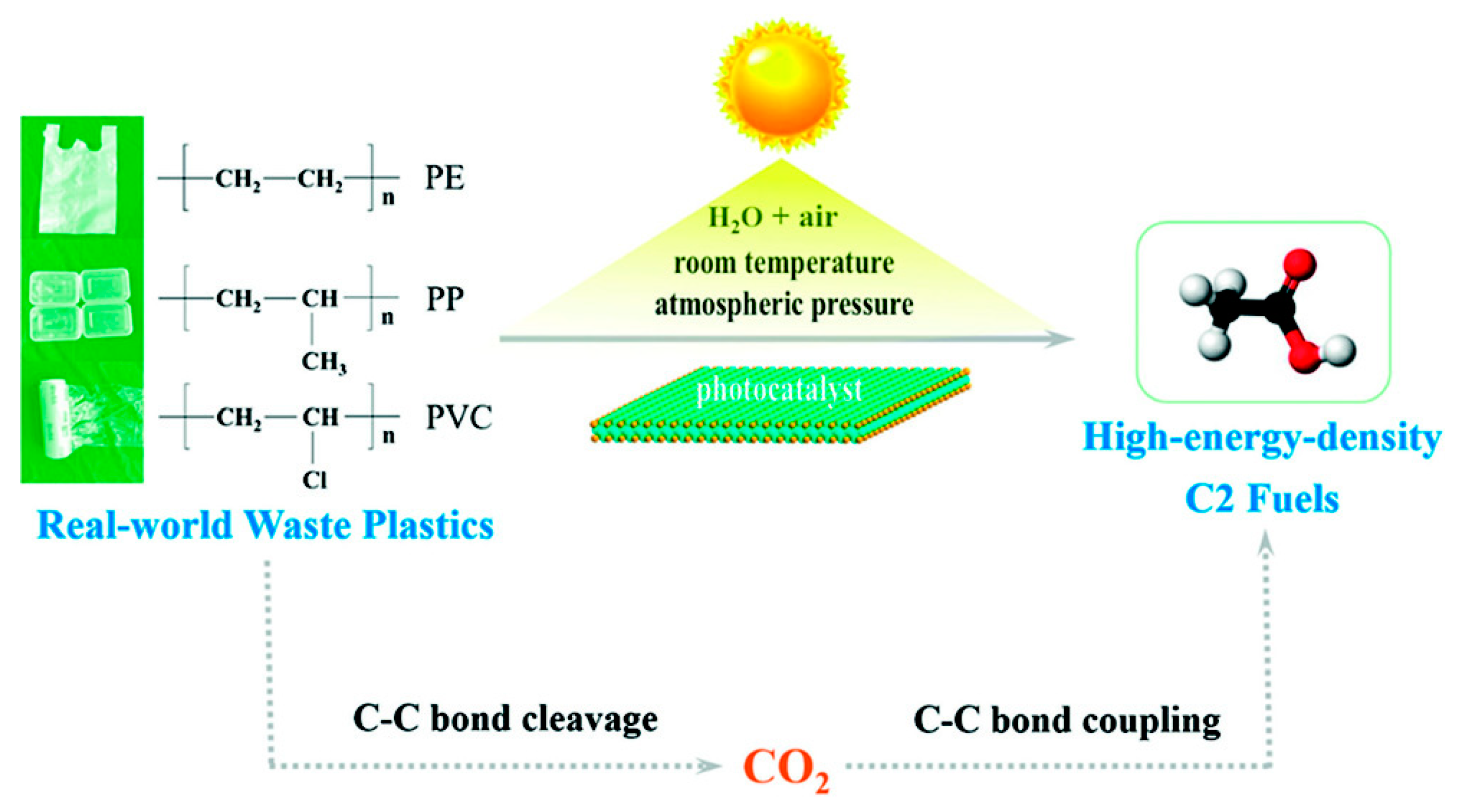
- Jiao, X.; Zheng, K.; Chen, Q.; Li, X.; Li, Y.; Shao, W.; Xu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Photocatalytic conversion of waste plastics into C2 fuels under simulated natural environment conditions. Angew. Chem. Int. Ed. 2020, 59, 15497–15501. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- DelRe, C.; Jiang, Y.; Kang, P.; Kwon, J.; Hall, A.; Jayapurna, I.; Ruan, Z.; Ma, L.; Zolkin, K.; Li, T. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 2021, 592, 558–563. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current technologies for plastic waste treatment: A review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Wang, B.; Biesold, G.M.; Zhang, M.; Lin, Z. Amorphous inorganic semiconductors for the development of solar cell, photoelectrocatalytic and photocatalytic applications. Chem. Soc. Rev. 2021, 50, 6914–6949. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Wang, J.; Bassi, A. Current research trends on microplastic pollution from wastewater systems: A critical review. Rev. Environ. Sci. Biotechnol. 2019, 18, 207–230. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bul. 2018, 129, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample waterwater-based microplastics. Water. Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water. Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [PubMed]
| Photocatalyst/Nanostructure | Plastic | Method | Efficiency/Yield | Time | Ref. |
|---|---|---|---|---|---|
| TiO2 nanoparticle film | PS | Photo-degradation | 98.4% | 12 h | [53] |
| TiO2 nanorods | PE | Photo-degradation | 6% | 20 h | [54] |
| TiO2 nanotubes | PE | Photo-degradation | 67% | 15 days | [55] |
| ZnO nanorods | PE | Photo-degradation | 30% | 175 h | [56] |
| CdS/CdOx quantum dots | PLA | Photo-reforming | 64.3 mmolH2 gcat–1 h–1 | – | [57] |
| d-NiPS3/CdS nanosheets | PLA | Photo-reforming | 40 mmolH2 gcat–1 h–1 | – | [58] |
| d-NiPS3/CdS nanosheets | PET | Photo-reforming | 32 mmolH2 gcat–1 h–1 | – | [58] |
| RGO-Ag/TiO2 | PE | Photo-degradation | 76% | 4 h | [59] |
| GO-Cu2O | PE | Photo-degradation | 48.06% | 8 h | [60] |
| GO-MnO2 | PE | Photo-degradation | 39.54% | 8 h | [60] |
| MoS2/RGO/cotton | PE | Photo-degradation | 32% | 1 h | [61] |
| g-C3N4/TiO2 | PE | Photo-degradation | 99% | 400 h | [62] |
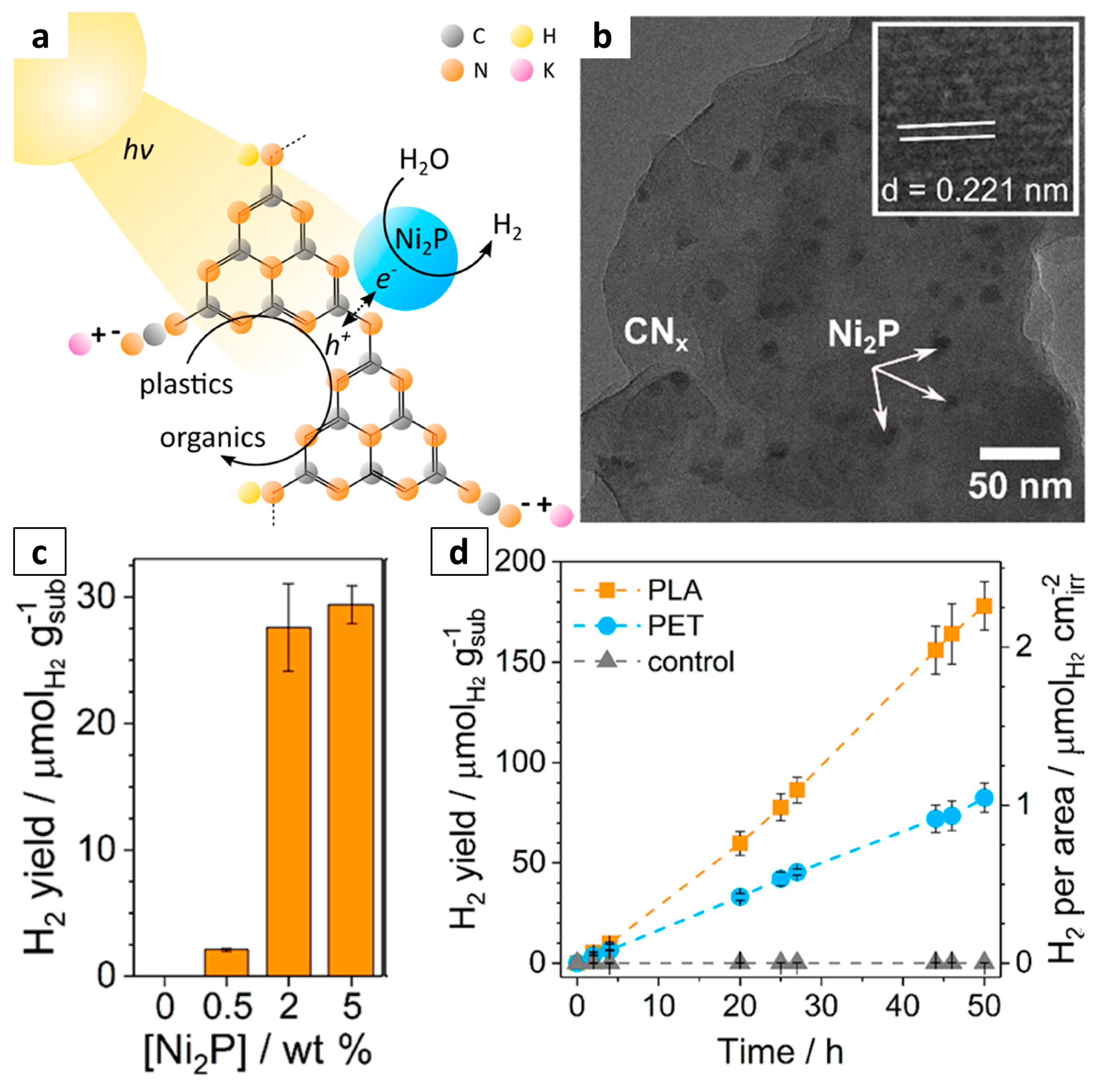
| CNx-Ni2P | PE | Photo-reforming | 111 μmolH2 gcat–1 | – | [63] |
| CNx-Ni2P | PLA | Photo-reforming | 211 μmolH2 gcat–1 | – | [63] |
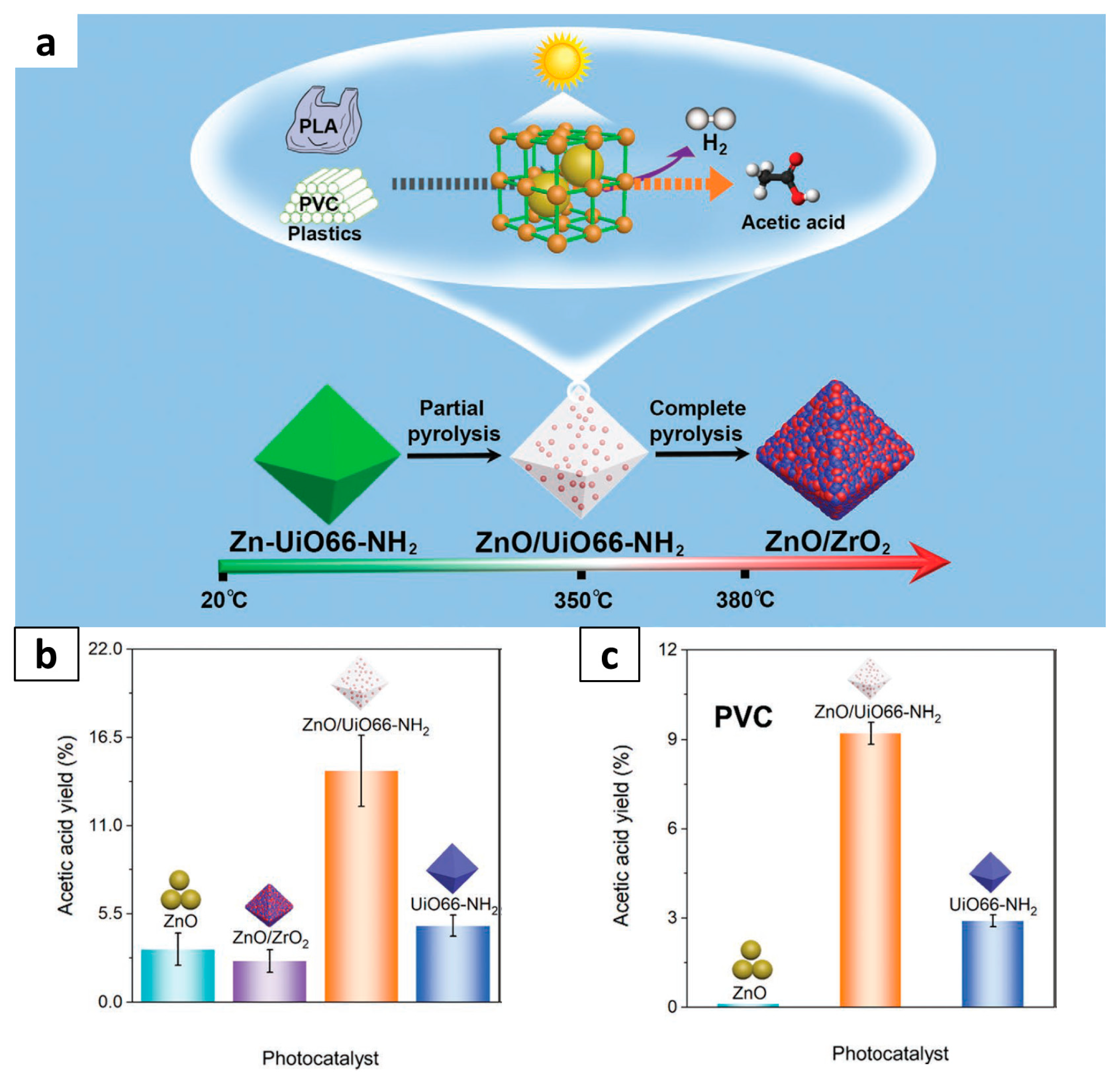
| ZnO/UiO66-NH2 (MOF) | PLA | Photo-conversion | 14.4%, SCH3OOH: 91.6% | – | [64] |
| ZnO/UiO66-NH2 (MOF) | PVC | Photo-conversion | 9% | – | [64] |
| XWO4/PAN nanofiber 1 | PLA | Photo-conversion | 38.51 mg gcat−1 h−1 | – | [65] |
| M. b-CDPCN | PLA | Photo-conversion | 90.2%, SCH4: 99.5% | 1 h | [66] |
| Zr:Fe2O3‖carbon|enzyme | PET | Photo-conversion | TON: 362 k | – | [67] |
| Ni-Pi/α-Fe2O3 | PET | Photo-conversion | 60 μmolformate cm−2 | 5 h | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Youn, D.H. Nanomaterials for Advanced Photocatalytic Plastic Conversion. Molecules 2023, 28, 6502. https://doi.org/10.3390/molecules28186502
Kim JY, Youn DH. Nanomaterials for Advanced Photocatalytic Plastic Conversion. Molecules. 2023; 28(18):6502. https://doi.org/10.3390/molecules28186502
Chicago/Turabian StyleKim, Jae Young, and Duck Hyun Youn. 2023. "Nanomaterials for Advanced Photocatalytic Plastic Conversion" Molecules 28, no. 18: 6502. https://doi.org/10.3390/molecules28186502
APA StyleKim, J. Y., & Youn, D. H. (2023). Nanomaterials for Advanced Photocatalytic Plastic Conversion. Molecules, 28(18), 6502. https://doi.org/10.3390/molecules28186502







