Therapeutic Effects of Baicalin on Diseases Related to Gut–Brain Axis Dysfunctions
Abstract
:1. Introduction
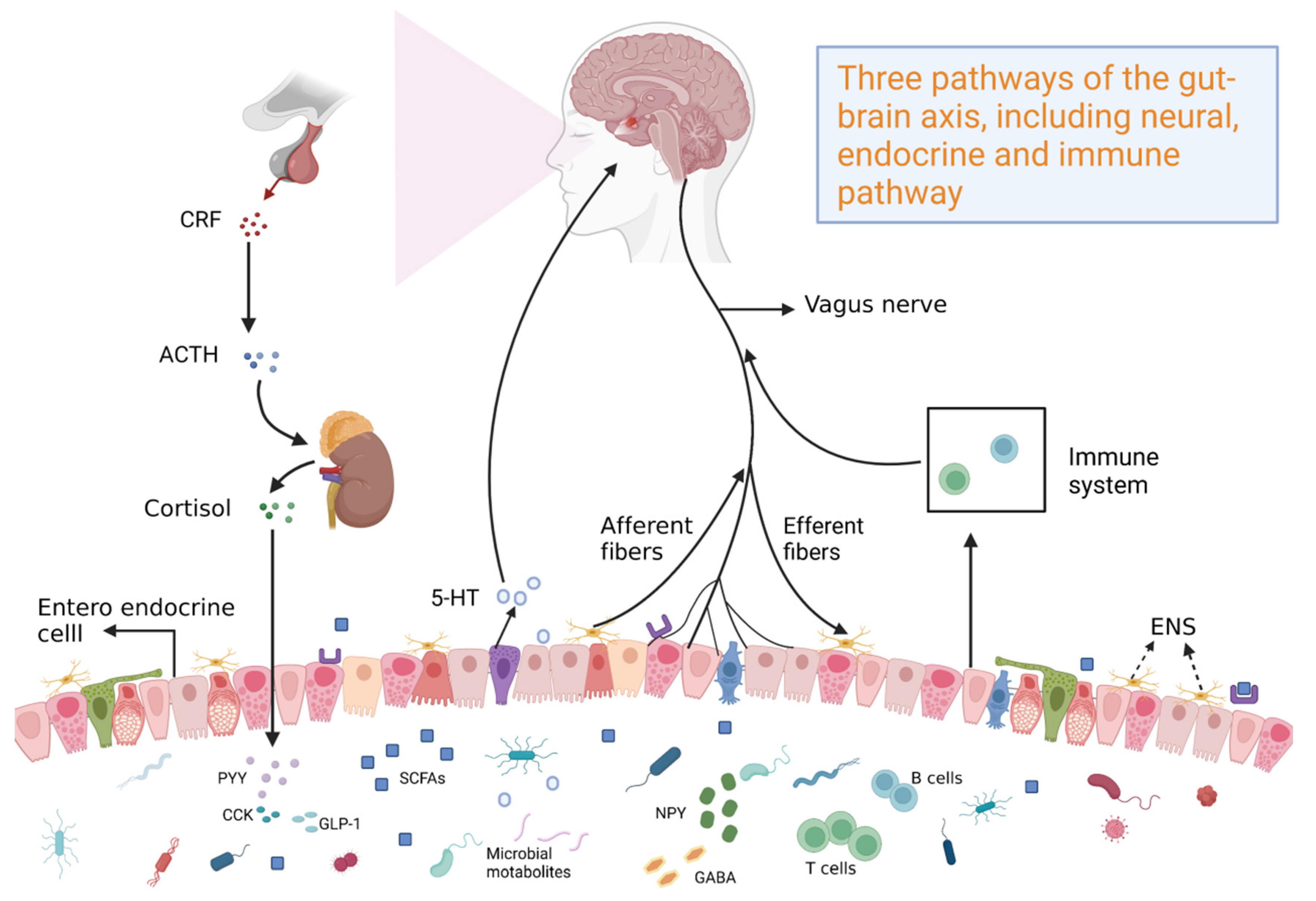
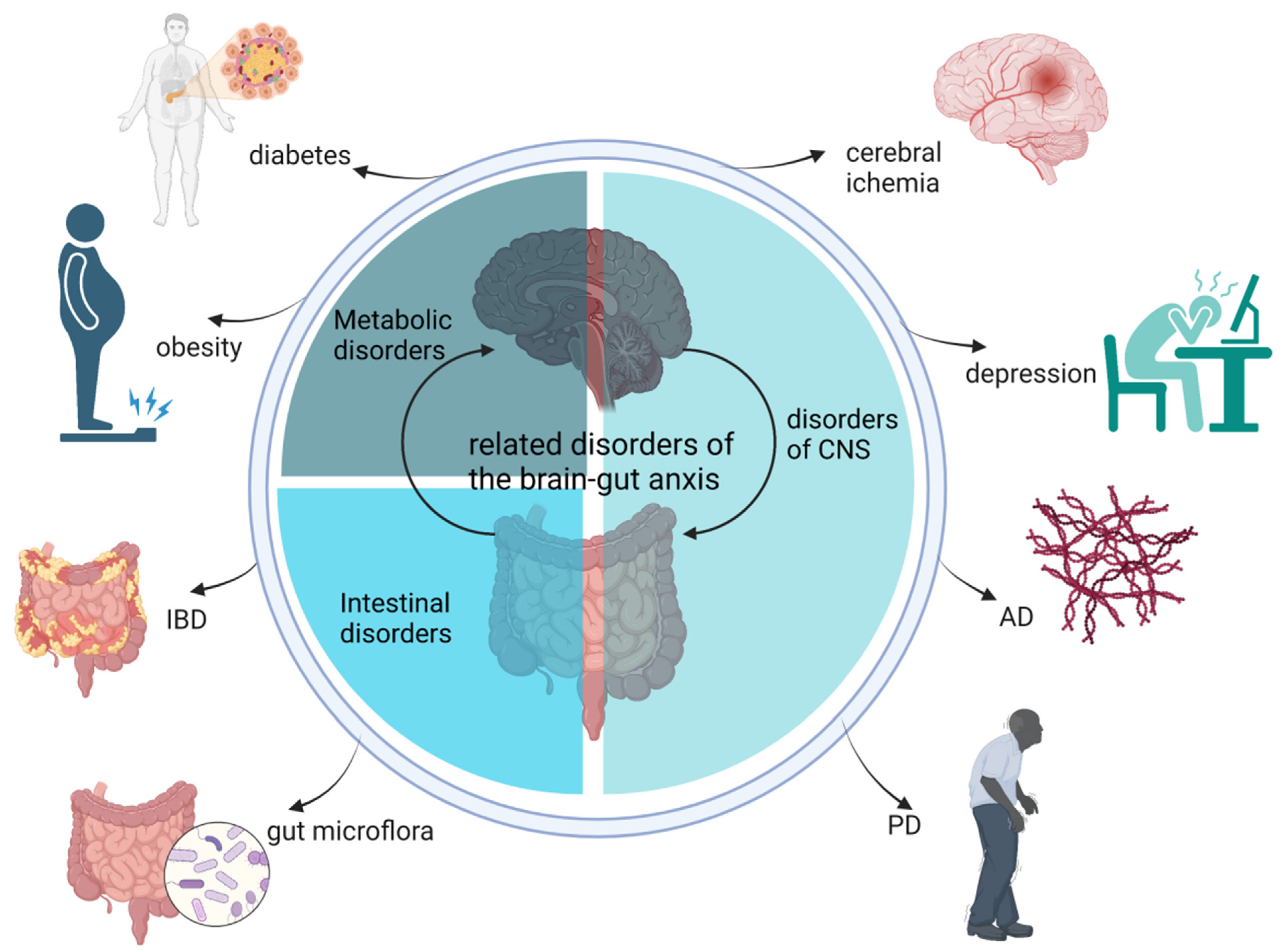
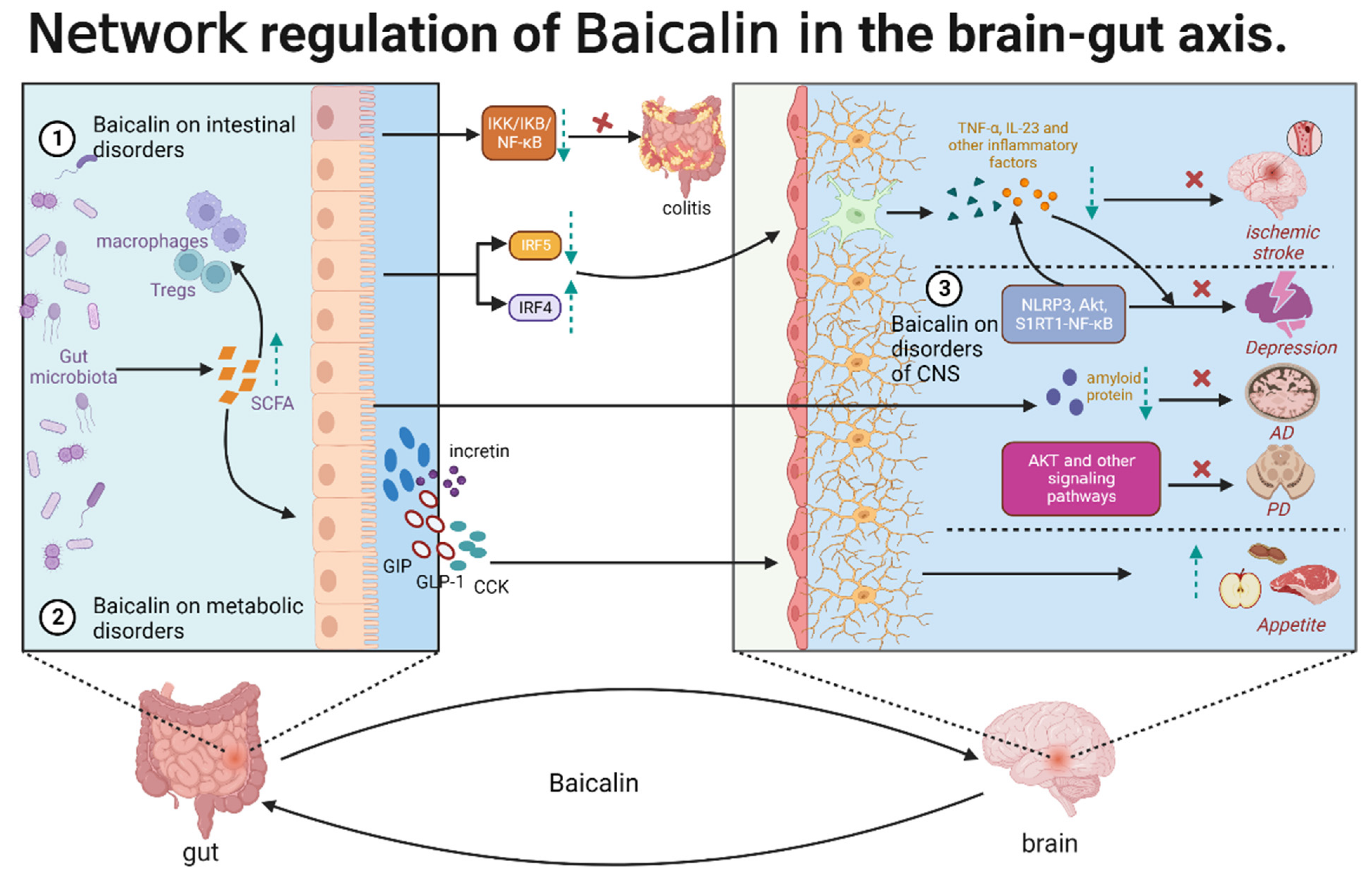
2. Effects of Baicalin Functions on the Gut–Brain Axis
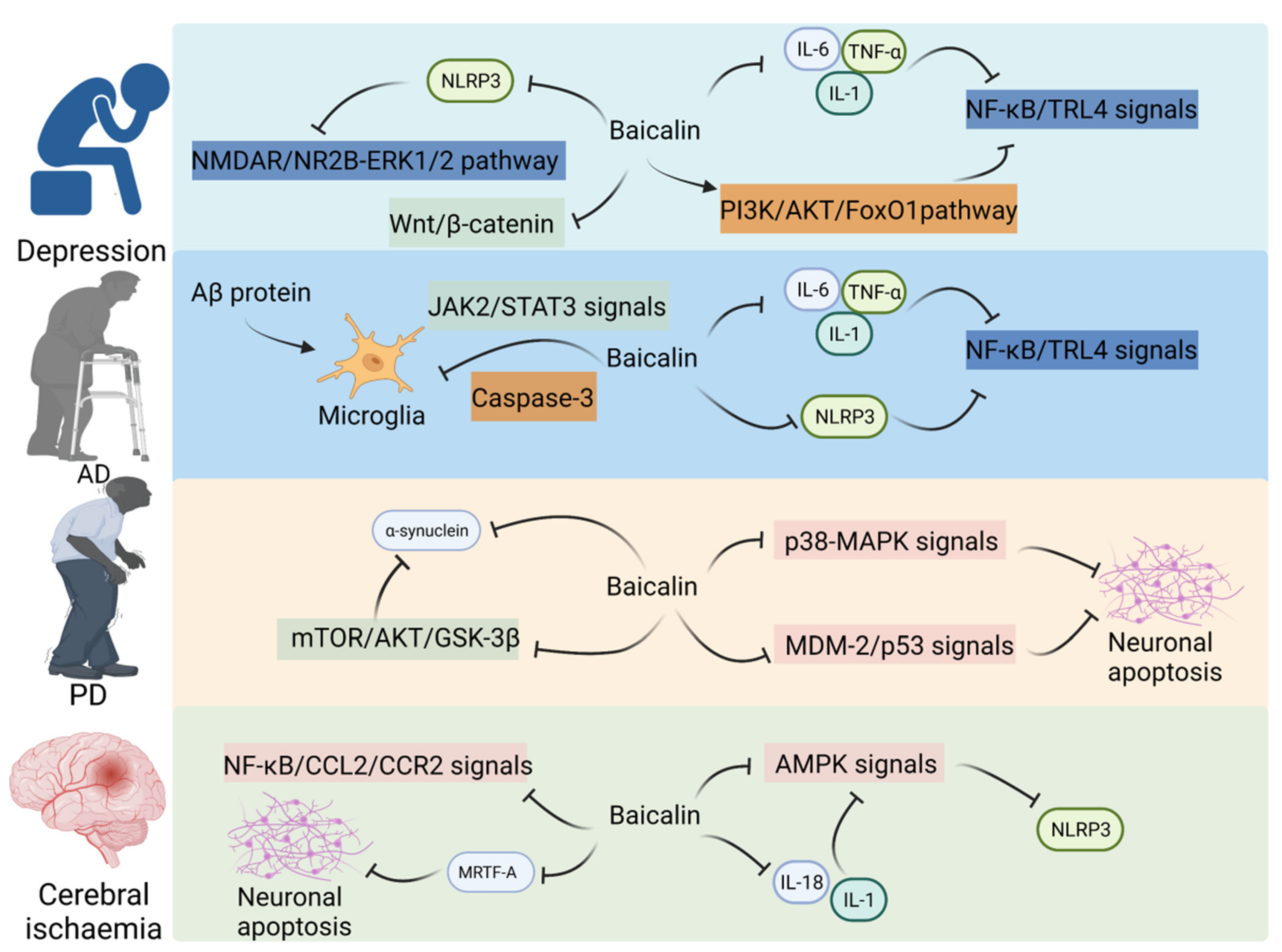
2.1. Baicalin Alleviates CNS Disorders through the Gut–Brain Axis
2.1.1. Baicalin Mitigates Depression through the Gut–Brain Axis
2.1.2. Baicalin Alleviates Cerebral Ischemia through the Gut–Brain Axis
2.1.3. Baicalin Alleviates AD through the Gut–Brain Axis
2.1.4. Baicalin Alleviates PD through the Gut–Brain Axis
2.2. Therapeutic Effects of Baicalin on Metabolic Disorders
2.3. Baicalin Alleviates Intestinal Disorders
2.3.1. Baicalin Alleviates IBD through the Gut–Brain Axis
2.3.2. Baicalin Modulates Gut Microbes
3. Discussion and Outlook
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Malagelada, J.R. The Brain-Gut Team. Dig. Dis. 2020, 38, 293–298. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Del Bas, J.M. The role of the gut microbiota in the pathophysiology of mental and neurological disorders. Psychiatr. Genet. 2020, 30, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocr. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Zhai, Z.; Su, P.-W.; Ma, L.-Y.; Yang, H.; Wang, T.; Fei, Z.-G.; Zhang, Y.-N.; Wang, Y.; Ma, K.; Han, B.-B.; et al. Progress on traditional Chinese medicine in treatment of ischemic stroke via the gut-brain axis. BioMedicine 2023, 157, 114056. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, W.; Da, X.; Hou, Y.; Ma, Q.; Wang, T.; Zhou, X.; Song, M.; Bian, Q.; Chen, J. A combination of depression and liver Qi stagnation and spleen deficiency syndrome using a rat model. Anat. Rec. 2020, 303, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Yang, J.; Cao, B.; Xue, Y.; Gao, P.; Liang, H.; Li, G. Growth years and post-harvest processing methods have critical roles on the contents of medicinal active ingredients of Scutellaria baicalensis. Ind. Crops Prod. 2020, 158, 112985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic. Med. Sci. 2022, 25, 14–26. [Google Scholar] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2018, 44, 159–168. [Google Scholar] [CrossRef]
- Chung, H.; Choi, H.S.; Seo, E.-K.; Kang, D.-H.; Oh, E.-S. Baicalin and baicalein inhibit transforming growth factor-β1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem. Biophys. Res. Commun. 2015, 458, 707–713. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, X.Y.; Zhang, Q.Y.; Kong, L.D. Corrigendum to “Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats” [Brain Behav. Immun. 41 (2014) 90-100]. Brain Behav. Immun. 2021, 97, 455. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, J.; Li, X.; Liu, Y.; Wang, T.; Yan, Z.; Chen, J. Effects of Xiaoyaosan on Depressive-Like Behaviors in Rats with Chronic Unpredictable Mild Stress Through HPA Axis Induced Astrocytic Activities. Front. Psychiatry 2020, 11, 545823. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, G.; Yang, F.; Guan, Z.; Zhang, Z.; Zhao, J.; Liu, Y.; Chu, L.; Pei, L. Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed. Pharmacother. 2019, 116, 109054. [Google Scholar] [CrossRef]
- Hammen, C. Risk Factors for Depression: An Autobiographical Review. Annu. Rev. Clin. Psychol. 2018, 14, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, J.C.; Schmidt, S.L. DSM-5 Criteria and Depression Severity: Implications for Clinical Practice. Front. Psychiatry 2018, 9, 450. [Google Scholar] [CrossRef]
- Pan, Z.; Park, C.; Brietzke, E.; Zuckerman, H.; Rong, C.; Mansur, R.B.; Fus, D.; Subramaniapillai, M.; Lee, Y.; McIntyre, R.S. Cognitive impairment in major depressive disorder. CNS Spectr. 2019, 24, 22–29. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Jia, X.; Gao, Z.; Hu, H. Microglia in depression: Current perspectives. Sci. China Life Sci. 2021, 64, 911–925. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota–brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Guo, L.-T.; Wang, S.-Q.; Su, J.; Xu, L.-X.; Ji, Z.-Y.; Zhang, R.-Y.; Zhao, Q.-W.; Ma, Z.-Q.; Deng, X.-Y.; Ma, S.-P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 2019, 16, 95. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zeng, M.J.; Zhou, L.P.; Li, Y.Q.; Zhao, F.; Shang, Z.Y.; Deng, X.Y.; Ma, Z.Q.; Fu, Q.; Ma, S.P.; et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018, 64, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, D.; Yu, H.; Yuan, H.; Shen, H.; Lan, X.; Liu, H.; Chen, X.; Meng, F.; Wu, X.; et al. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol. Psychiatry 2023, 28, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C. Baicalin ameliorates chronic unpredictable mild stress-induced depressive behavior: Involving the inhibition of NLRP3 inflammasome activation in rat prefrontal cortex. Int. Immunopharmacol. 2017, 48, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, Z.; Liu, K.; Li, Y.; Liu, D.; Xu, L.; Deng, X.; Qu, R.; Ma, Z.; Ma, S. Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 2019, 221, 241–248. [Google Scholar] [CrossRef]
- Fang, A.; Li, Y.; Wu, X.; Wu, B.; Zhang, Y. Baicalin attenuates inflammatory pain associated depressive symptoms via Akt-mediated adult hippocampal neurogenesis. Metab. Brain Dis. 2020, 35, 1085–1093. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Duan, W.; Zhao, Z.; Yang, L.; Wei, W.; Li, J.; Li, Y.; Yu, Y.; Dai, B.; et al. Shugan granule contributes to the improvement of depression-like behaviors in chronic restraint stress-stimulated rats by altering gut microbiota. CNS Neurosci. Ther. 2022, 28, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic Effect of Berberine-Based Chinese Medicine Assembled Nanostructures on Diarrhea-Predominant Irritable Bowel Syndrome In Vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, F.; Guan, X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother. Res. 2019, 33, 1480–1489. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Y.; Si, C.; Wang, X.; Wang, R.-T.; Lv, Z. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging 2020, 12, 3791–3806. [Google Scholar] [CrossRef]
- Li, H.; Wu, L.; Wang, D.; Guo, Y.; Liu, T.; Pan, Y. A protective effect of baicalin on cerebral ischemic rats is related to the improvement of serum progesterone level in serum. NeuroReport 2019, 30, 1121–1128. [Google Scholar] [CrossRef]
- Li, S.; Sun, X.; Xu, L.; Sun, R.; Ma, Z.; Deng, X.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Baicalin attenuates in vivo and in vitro hyperglycemia-exacerbated ischemia/reperfusion injury by regulating mitochondrial function in a manner dependent on AMPK. Eur. J. Pharmacol. 2017, 815, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Gao, P.; Tu, Y.; Zhao, M.; Li, J.; Zhang, S.; Liang, H. Baicalin attenuates Alzheimer-like pathological changes and memory deficits induced by amyloid β1–42 protein. Metab. Brain Dis. 2015, 30, 537–544. [Google Scholar] [CrossRef]
- Ding, H.; Wang, H.; Zhao, Y.; Sun, D.; Zhai, X. Protective Effects of Baicalin on Aβ1–42-Induced Learning and Memory Deficit, Oxidative Stress, and Apoptosis in Rat. Cell Mol. Neurobiol. 2015, 35, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, C.; Chen, H.; Hu, Y.; Tian, L.; Pan, J.; Geng, M. Aβ-induced microglial cell activation is inhibited by baicalin through the JAK2/STAT3 signaling pathway. Int. J. Neurosci. 2014, 124, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Shen, Y.; He, Y.; Zhang, L.; Zhang, J.; Tong, W.; Xu, Y.; Jin, L. Baicalin Represses C/EBPβ via Its Antioxidative Effect in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 8951907. [Google Scholar] [CrossRef]
- Zhai, H.; Kang, Z.; Zhang, H.; Ma, J.; Chen, G. Baicalin attenuated substantia nigra neuronal apoptosis in Parkinson’s disease rats via the mTOR/AKT/GSK-3β pathway. J. Integr. Neurosci. 2019, 18, 423–429. [Google Scholar] [PubMed]
- Lin, Y.; Wang, Z.-Y.; Wang, M.-J.; Jiang, Z.-M.; Qin, Y.-Q.; Huang, T.-Q.; Song, Y.; Liang, H.-T.; Liu, E.-H. Baicalin attenuate diet-induced metabolic syndrome by improving abnormal metabolism and gut microbiota. Eur. J. Pharmacol. 2022, 925, 174996. [Google Scholar] [CrossRef]
- Zhu, W.; Jin, Z.; Yu, J.; Liang, J.; Yang, Q.; Li, F.; Shi, X.; Zhu, X.; Zhang, X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, L.-Z.; Zhao, S.; Shen, Z.-F.; Shen, H.; Zhan, L.-B. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 2020, 104, 5449–5460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, H.; Gu, P.; Liu, Y.; Zhang, L.; Cheng, J. Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation. Exp. Ther. Med. 2020, 20, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Zhang, S.; He, W.X.; Lu, J.L.; Xu, Y.J.; Yang, J.Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.G.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharm. Res. 2015, 38, 1873–1887. [Google Scholar] [CrossRef]
- Cui, L.; Feng, L.; Zhang, Z.H.; Jia, X.B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef]
- Dai, S.-X.; Zou, Y.; Feng, Y.-L.; Liu, H.-B.; Zheng, X.-B. Baicalin Down-regulates the Expression of Macrophage Migration Inhibitory Factor (MIF) Effectively for Rats with Ulcerative Colitis. Phytother. Res. 2012, 26, 498–504. [Google Scholar] [CrossRef]
- Ju, M.; Liu, Y.; Li, M.; Cheng, M.; Zhang, Y.; Deng, G.; Kang, X.; Liu, H. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur. J. Pharmacol. 2019, 857, 172457. [Google Scholar] [CrossRef]
- Wu, D.; Ding, L.; Tang, X.; Wang, W.; Chen, Y.; Zhang, T. Baicalin Protects Against Hypertension-Associated Intestinal Barrier Impairment in Part Through Enhanced Microbial Production of Short-Chain Fatty Acids. Front. Pharmacol. 2019, 10, 1271. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.; Kou, L.; Tang, C.; Huang, R.; Pei, Z.; Li, Z. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci. Lett. 2017, 658, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Song, Y.; Du, X.; Bai, L.; Hua, X.; Xiao, Y.; Yu, X. Intestinal barrier dysfunction following traumatic brain injury. Neurol. Sci. 2019, 40, 1105–1110. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, J.; Ouyang, F.; Chen, X.; Lu, T.; Jiang, Z.; Li, J.; Li, Y.; Zeng, J. Persistence of Gut Microbiota Dysbiosis and Chronic Systemic Inflammation After Cerebral Infarction in Cynomolgus Monkeys. Front. Neurol. 2019, 10, 661. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef]
- Wei, X.; Huang, G.; Liu, J.; Ge, J.; Zhang, W.; Mei, Z. An update on the role of Hippo signaling pathway in ischemia-associated central nervous system diseases. Biomed. Pharmacother. 2023, 162, 114619. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Rathipriya, A.G.; Mahalakshmi, A.M.; Sharma, S.; Hediyal, T.A.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.S.; Qoronfleh, M.W.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Inouye, K.; Riddell, M.C.; Vranic, M.; Matthews, S.G. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 2003, 28, 87–102. [Google Scholar]
- Li, M.; Wang, S.; Zhang, C.; Chi, C.; Liu, R.; Wang, T.; Fu, F. Escin alleviates stress-induced intestinal dysfunction to protect brain injury by regulating the gut-brain axis in ischemic stroke rats. Int. Immunopharmacol. 2023, 115, 109659. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Zheng, Q.; Wang, Q.; Zeng, Q.P. Biotic/Abiotic Stress-Driven Alzheimer’s Disease. Front. Cell Neurosci. 2016, 10, 269. [Google Scholar] [CrossRef]
- Eratne, D.; Loi, S.M.; Farrand, S.; Kelso, W.; Velakoulis, D.; Looi, J.C. Alzheimer’s disease: Clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry 2018, 26, 347–357. [Google Scholar] [CrossRef]
- International, World Alzheimer Report 2022. Life after Diagnosis: Navigating Treatment, Care and Support. 2022. Available online: https://www.alzint.org/u/ (accessed on 6 June 2023).
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.B.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota–Gut–Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 782082. [Google Scholar] [CrossRef]
- Visñuk, D.P.; de Giori, G.S.; LeBlanc, J.G.; de Moreno de LeBlanc, A. Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition 2020, 79–80, 110995. [Google Scholar] [CrossRef]
- Shannon, K.M. Gut-Derived Sterile Inflammation and Parkinson’s Disease. Front. Neurol. 2022, 13, 831090. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Shi, H.; Xu, Y.; Ji, L. The gut microbiota metabolite propionate ameliorates intestinal epithelial barrier dysfunction-mediated Parkinson’s disease via the AKT signaling pathway. Neuroreport 2021, 32, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Wu, Z.-Y.; Yang, X.-L.; Zhang, Q.; Gu, R.; Wang, Q.; Tian, T.; Yao, H.; Qu, X.; Tian, J.-Y. Neuroprotective effect and mechanism of baicalin on Parkinson’s disease model induced by 6-OHDA. Neuropsychiatr. Dis. Treat. 2019, 15, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Plaisancié, P.; Dumoulin, V.; Chayvialle, J.-A.; Cuber, J.-C. Luminal glucagon-like peptide-1(7–36) amide-releasing factors in the isolated vascularly perfused rat colon. J. Endocrinol. 1995, 145, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Lovillo, A.; Romero-Luna, H.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-S.; Hu, Y.-Y. Intestinal Microecology: An Important Target for Chinese Medicine Treatment of Non-alcoholic Fatty Liver Disease. Chin. J. Integr. Med. 2020, 26, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- EWilliams, K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e23. [Google Scholar] [CrossRef]
- Khan, R.; Tomas, A.; Rutter, G.A. Effects on pancreatic Beta and other Islet cells of the glucose-dependent insulinotropic polypeptide. Peptides 2020, 125, 170201. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- LMayoral, P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar]
- Hu, Q.; Chen, Y.; Deng, X.; Li, Y.; Ma, X.; Zeng, J.; Zhao, Y. Diabetic Nephropathy: Focusing on Pathological Signals, Clinical Treatment, and Dietary Regulation. Biomed. Pharmacother. 2023, 159, 114252. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- D’antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Ippolito, C.; Segnani, C.; Benvenuti, L.; D’amati, A.; Errede, M.; Virgintino, D.; Fornai, M.; et al. Enteric Glia and Brain Astroglia: Complex Communication in Health and Disease along the Gut-Brain Axis. Neuroscientist 2023, 10738584231163460. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Rothhammer, V.; Karow, M.; Neurath, M.F.; Winner, B. The Gut-Brain Axis in Inflammatory Bowel Disease—Current and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 8870. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, M.; Aznar, S.; Pakkenberg, B.; Jess, T.; Brudek, T. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut 2019, 68, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, D.; Ottani, A.; Altavilla, D.; Bazzani, C.; Squadrito, F.; Guarini, S. Melanocortins and the Cholinergic Anti-Inflammatory Pathway. Adv. Exp. Med. Biol. 2010, 681, 71–87. [Google Scholar] [CrossRef]
- Meroni, E.; Stakenborg, N.; Viola, M.F.; Boeckxstaens, G.E. Intestinal macrophages and their interaction with the enteric nervous system in health and inflammatory bowel disease. Acta Physiol. 2019, 225, e13163. [Google Scholar] [CrossRef]
- O’Brien, R.; Buckley, M.M.; O’Malley, D. Divergent effects of exendin-4 and interleukin-6 on rat colonic secretory and contractile activity are associated with changes in regional vagal afferent signaling. Neurogastroenterol. Motil. 2021, 33, e14160. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Guzzo, G.L.; Andrews, J.M.; Weyrich, L.S. The Neglected Gut Microbiome: Fungi, Protozoa, and Bacteriophages in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1112–1122. [Google Scholar] [CrossRef]
- Rashed, R.; Valcheva, R.; Dieleman, L.A. Manipulation of Gut Microbiota as a Key Target for Crohn’s Disease. Front. Med. 2022, 9, 887044. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Putra, R.B.D.S.; Aui, S.-P.; Chang, K.-T. M2C Polarization by Baicalin Enhances Efferocytosis via Upregulation of MERTK Receptor. Am. J. Chin. Med. 2018, 46, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Chauhan, A.; Qi, S.; Ngwa, C.; Xu, Y.; Sharmeen, R.; Hazen, A.L.; Li, J.; Aronowski, J.A.; McCullough, L.D.; et al. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc. Natl. Acad. Sci. USA 2020, 117, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Magdy, S.; Gamal, M.; Samir, N.F.; Rashed, L.; Aboulhoda, B.E.; Mohammed, H.S.; Sharawy, N. IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine. J. Cell Physiol. 2011, 236, 7516–7532. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, Y.; He, F.; Zhu, C.; Ren, W. Intestinal mycobiota in health and diseases: From a disrupted equilibrium to clinical opportunities. Microbiome 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, P.; Capparelli, R.; Alifano, M.; Iannelli, A.; Iannelli, D. Gut Microbiota Host-Gene Interaction. Int. J. Mol. Sci. 2022, 23, 13717. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.; Reimann, F.; Gribble, F. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain, Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Guan, K.; Wang, Y.; Gu, M.; Chen, Y.; Cai, L.; Ye, R.; Huang, Z.; Guo, A.; Su, Z.; et al. Baicalein exerts anxiolytic and antinociceptive effects in a mouse model of posttraumatic stress disorder: Involvement of the serotonergic system and spinal delta-opioid receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 122, 110689. [Google Scholar] [CrossRef] [PubMed]
- Baslam, A.; Aitbaba, A.; Hanchi, A.L.; Tazart, Z.; Aboufatima, R.; Soraa, N.; Ait-El-Mokhtar, M.; Boussaa, S.; Baslam, M.; Chait, A. Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. Int. J. Mol. Sci. 2023, 24, 9086. [Google Scholar] [CrossRef] [PubMed]
| Category | Related Disorders | Animal/Cell Model | Dose | Target Pathway/Mechanisms | Reference |
|---|---|---|---|---|---|
| Disorders in CNS | Depression | chronic unpredictable mild stress (CUMS) mice/BV2 microglia cell lines | 60 mg/kg (in vivo); 100 μM (in vitro) | ↓ TLR4; ↓ PI3K/AKT/FoxO1 signaling pathway | [30] |
| CUMS mice | 20 and 40 mg/kg (in vivo) | ↓ GSK3β/NF-κB/NLRP3 signaling pathway | [31] | ||
| CUMS mice | 20 and 40 mg/kg (in vivo) | ↓ NLRP3 signaling pathway | [33] | ||
| CUMS mice | 60 mg/kg (in vivo) | ↓ Akt/FOXG1 signaling pathway | [34] | ||
| CUMS mice | 50 mg/kg (in vivo) | ↓ Akt signaling pathway | [35] | ||
| CUMS mice | 20 and 40 mg/kg (in vivo) | ↓ SIRT1-NF-κB signaling pathway | [38] | ||
| Cerebral ischaemia | MCAO (middle cerebral arteryocclusion) rats | 100 mg/kg (in vivo) | / | [39] | |
| MCAO rats | 50–100 mg/kg (in vivo) | remodeling the gut microbiota | [40] | ||
| MCAO rats | 15 mg/kg (in vivo) | ↑ ACTH; ↑ NeuN; ↑ GFAP | [41] | ||
| MCAO rats | 100 mg/kg (in vivo) | ↓ AMPK signaling pathway | [42] | ||
| Alzheimer’s disease | APP (amyloid beta precursor protein)/PS1 (presenilin-1)mice | 100 mg/kg (in vivo) | ↓ NLRP3 signaling pathway; ↓ TLR4/NF-κB signaling pathway | [43] | |
| amyloid β (Aβ)1–42 protein-induced AD mouse | 30, 50 and 100 mg/kg (in vivo) | ↓ Aβ1–42 protein | [44] | ||
| amyloid β (Aβ)1–43 protein-induced AD mouse | 50, 100 and 200 mg/kg (in vivo) | ↓ Aβ1–43 protein; ↓ Caspase-3 signaling pathway | [45] | ||
| amyloid β (Aβ)1–44 protein-induced BV2 microglial cells | 50 and 100 μM (in vitro) | ↓ JAK2/STAT3 signaling pathway | [46] | ||
| Parkinson’s disease | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD mouse | 20 and 40 mg/kg (in vivo) | ↓ C/EBPβ | [47] | |
| 6-hydroxydopamine (6-OHDA)-induced PD rats | 25 mg/kg (in vivo) | ↓ mTOR/AKT/GSK-3β signaling pathway | [48] | ||
| Metabolic disorders | Obesity | HFD-induced obesity mice | 400 mg/kg/day (in vivo) | / | [49] |
| Disorders in gut | IBD | DDS-induced UC | 25, 50 and 100 mg/kg (in vivo); 6.25, 12.5, 25, and 50 μM (in vitro) | ↓ IRF5; ↑ IRF4; ↓ TNF-α/IL-23/IRF5; ↑ IL-10/Arg1/IRF4 | [50] |
| TNBS-induced UC | 30–90 mg/kg (in vivo) | ↓ cleaved-caspase3/cleaved-caspase9/Bcl-2/Bax/cyt-c/NF-kB p-65/p-IKKβ/IKKβ and p-IKBα/IKBα ↓ p-IKBα/IKBα ↓ MDA/PEG2/MPO/IL-1β/TNF-α | [51] | ||
| TNBS-induced UC | 25, 50 and 100 mg/kg (in vivo) | ↓ Th17/Treg signaling pathway | [52] | ||
| TNBS-induced UC | 100 mg/kg/d (in vivo); 316 µg/mL (in vitro) | ↓ PI3K/AKT signaling pathway | [53] | ||
| DDS-induced UC | 50, 100 and 150 mg/kg (in vivo) | ↓ MPO,NO,Ly6/G,IL-6,IL-1β,TNF-α ↓ IL-33/NF-κB p65/p-NF-κB p65/p-IκB-α; ↑ IκB-α | [54] | ||
| TNBS-induced UC | 10 mL/kg (in vivo) | ↓ MPO/TNF-α/IL-1β/IFN-γ/IL-12; ↓ Th17/Treg | [55] | ||
| TNBS-induced UC | 25, 50 and100 mg/kg (in vivo); 5 × 10−4, 5 × 10−5, 5 × 10−6 μM (in vitro) | ↓ TLR4/NF-κB signaling pathway; | [56] | ||
| TNBS-induced UC | 10 mL/kg (in vivo) | ↓ MIF/MCP-1/CCL2/MIP-3α/CCL20 | [57] | ||
| Microbiota regulation | high-fat diet-induced disorder | 200 mg/kg (in vivo) | SCFAs | [58] | |
| TNBS-induced disorder | 20–100 mg/kg (in vivo) | SCFAs | [52] | ||
| intestinal barrier damage-induced disorder | 100 mg/kg (in vivo) | SCFAs | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Hou, S.; Xiong, B.; Wen, Y.; Wang, J.; Zeng, J.; Ma, X.; Wang, F. Therapeutic Effects of Baicalin on Diseases Related to Gut–Brain Axis Dysfunctions. Molecules 2023, 28, 6501. https://doi.org/10.3390/molecules28186501
Hu Q, Hou S, Xiong B, Wen Y, Wang J, Zeng J, Ma X, Wang F. Therapeutic Effects of Baicalin on Diseases Related to Gut–Brain Axis Dysfunctions. Molecules. 2023; 28(18):6501. https://doi.org/10.3390/molecules28186501
Chicago/Turabian StyleHu, Qichao, Shuyu Hou, Baoyi Xiong, Yueqiang Wen, Jundong Wang, Jinhao Zeng, Xiao Ma, and Fang Wang. 2023. "Therapeutic Effects of Baicalin on Diseases Related to Gut–Brain Axis Dysfunctions" Molecules 28, no. 18: 6501. https://doi.org/10.3390/molecules28186501
APA StyleHu, Q., Hou, S., Xiong, B., Wen, Y., Wang, J., Zeng, J., Ma, X., & Wang, F. (2023). Therapeutic Effects of Baicalin on Diseases Related to Gut–Brain Axis Dysfunctions. Molecules, 28(18), 6501. https://doi.org/10.3390/molecules28186501






