The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides
Abstract
:1. Introduction
2. Results
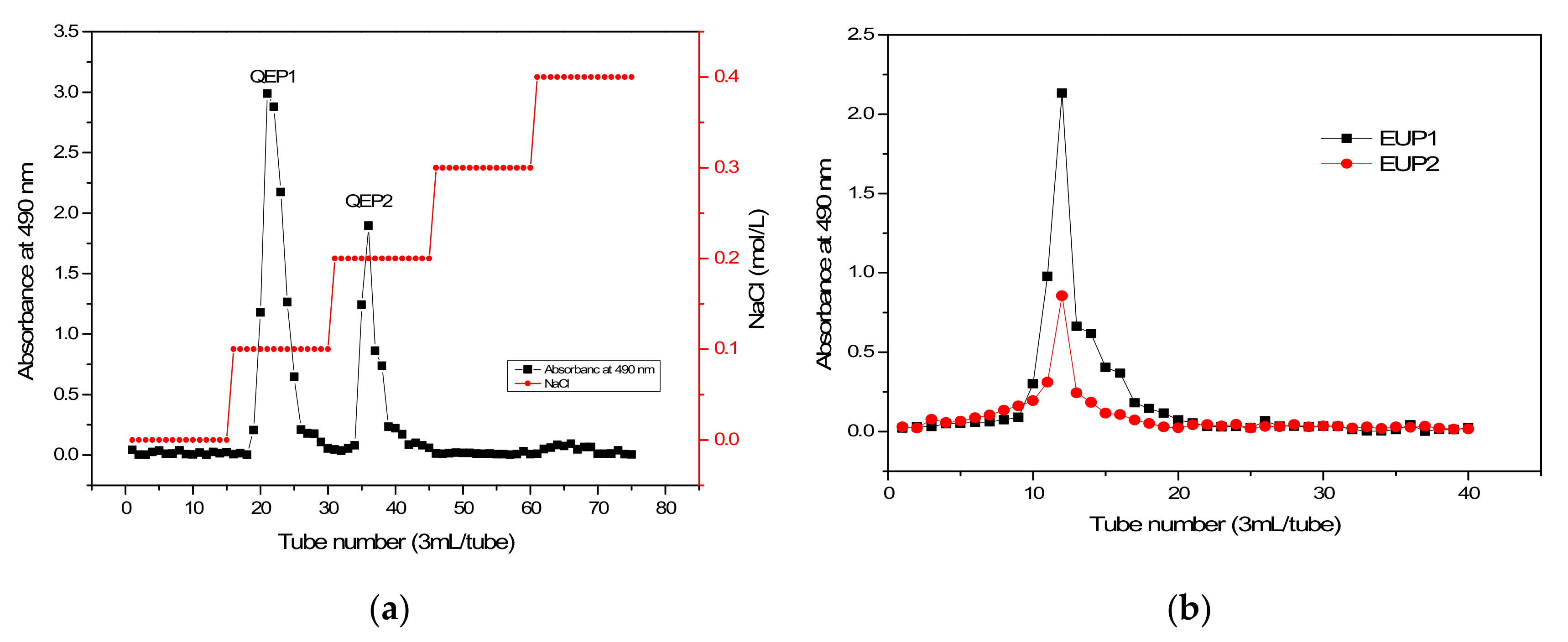
2.1. The Separation and Purification of CEP
2.1.1. Purification of CEP
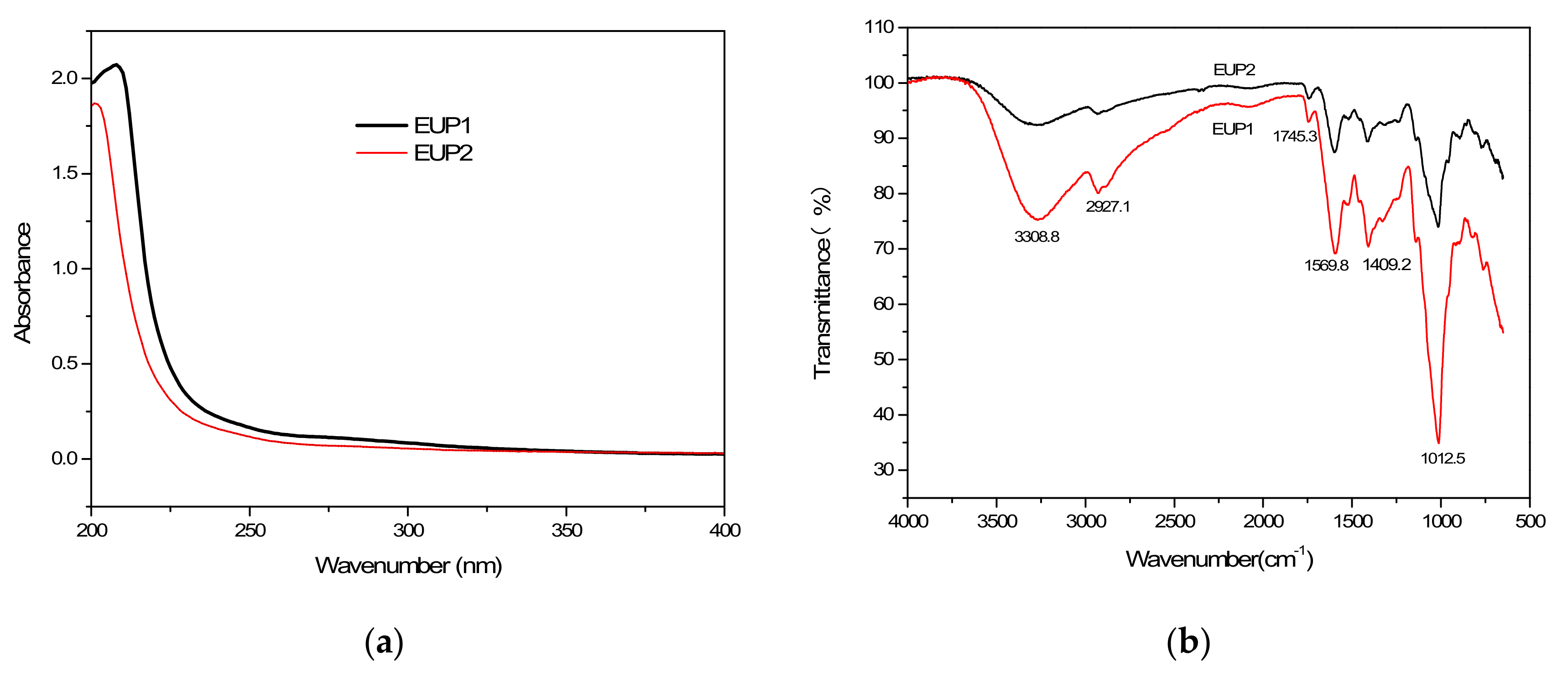
2.1.2. Analysis of the UV and FT-IR Spectra
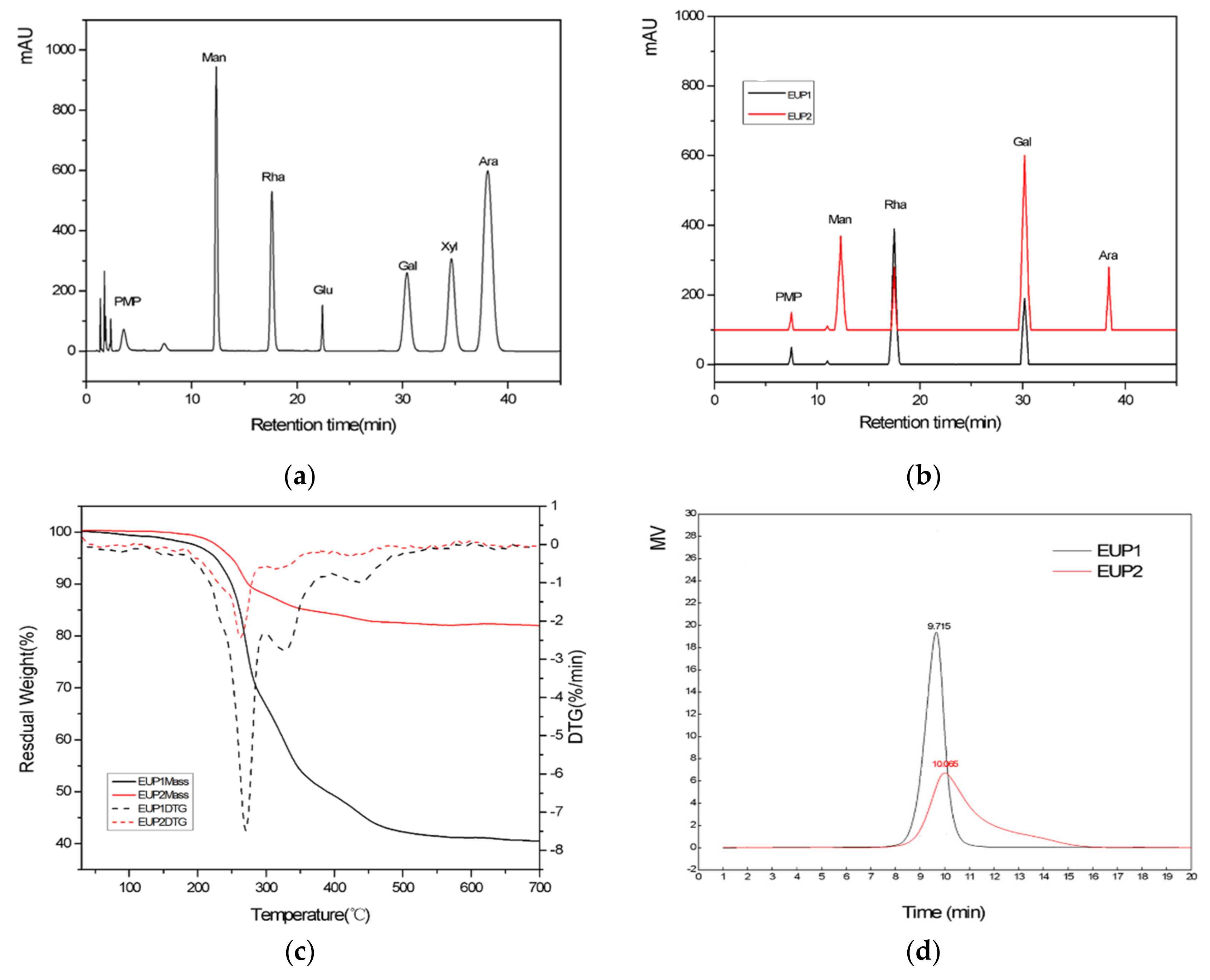
2.1.3. Analysis of the Molecular Weights of the Purified Polysaccharides
2.1.4. Monosaccharide Component Analysis
2.1.5. Scanning Electron Microscopy (SEM)
2.1.6. Thermal Gravimetric Analysis (TGA)
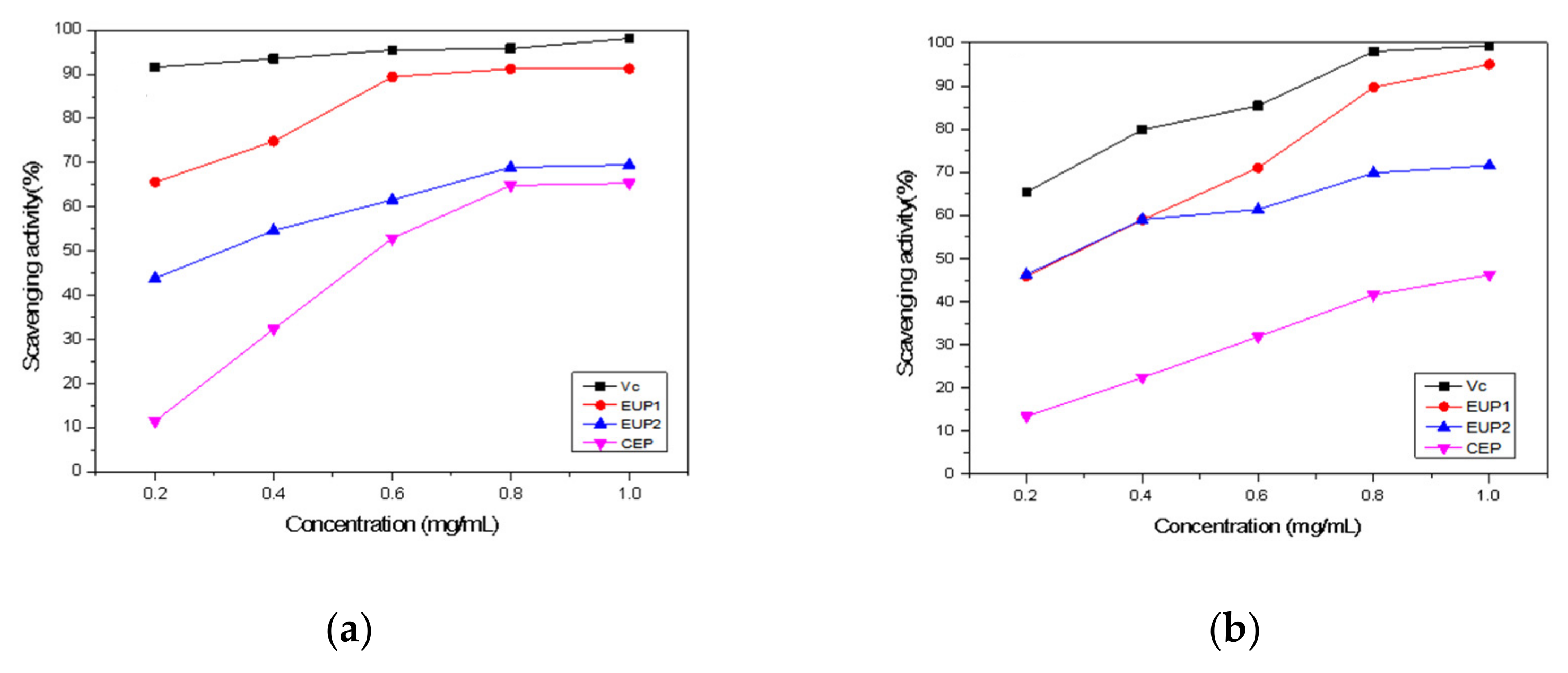
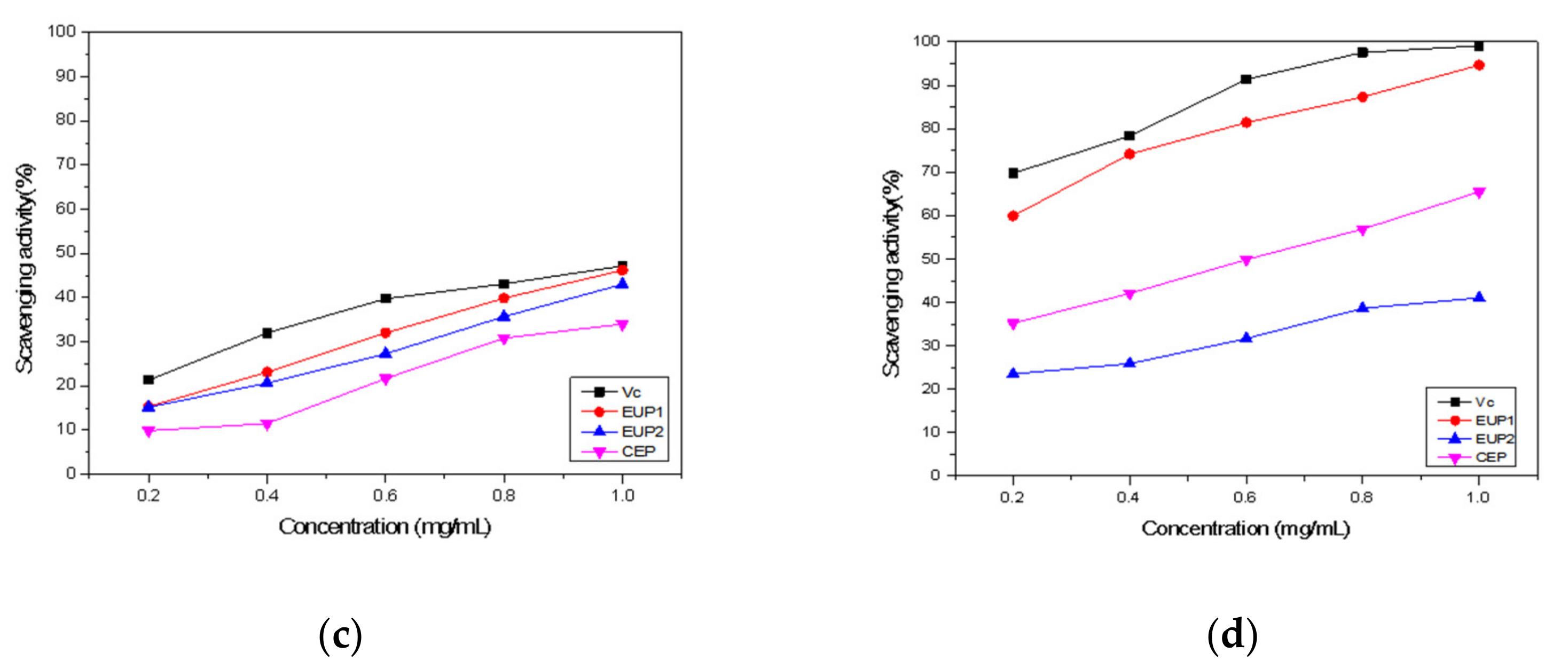
2.2. Antioxidant Activities of EUP1, EUP2, and CEP
2.2.1. DPPH Free Radical Scavenging Ability
2.2.2. Hydroxyl Radical Scavenging Ability
2.2.3. ABTS•+ Radical Scavenging Activity
2.2.4. Superoxide Anion Scavenging Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Instruments and Equipment
4.3. Extraction Procedure
4.4. Purification of the Extracted Polysaccharide
4.4.1. DEAE-Cellulose 52
4.4.2. Sephadex G-100 Gel Chromatography
4.5. Characterization of Polysaccharides
4.5.1. UV and FT-IR Spectroscopy
4.5.2. Determination of Molecular Weight
4.5.3. Analysis of Monosaccharide Composition
4.5.4. Scanning Electron Microscopy (SEM)
4.5.5. Thermal Gravimetric Analysis (TGA)
4.6. Analysis of Antioxidant Activities
4.6.1. DPPH• Radical Scavenging Activity
4.6.2. •OH Scavenging Ability
4.6.3. ABTS•+ Radical Cation Scavenging Activity
4.6.4. Superoxide Anion Scavenging Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hussain, I. Physiochemical and sensory Characteristics of Elaeagnus umbellata (Thunb) fruit from Rawalakot (Azad Kashmir) Pakistan. Afr. J. Food Sci. Technol. 2011, 2, 151–156. [Google Scholar]
- Liao, C.R.; Ho, Y.L.; Huang, G.J.; Yang, C.; Chao, C.Y.; Chang, Y.S.; Kuo, Y.H. One lignanoid compound and four triterpenoid compounds with anti-inflammatory activity from the leaves of Elaeagnus oldhamii maxim. Molecules 2013, 18, 13218–13227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.D.; Sabir, S.M.; Zubair, A. Ecotypes diversity in autumn olive (Elaeagnus umbellata Thunb): A single plant with multiple micronutrient genes. Chem. Ecol. 2006, 22, 509–521. [Google Scholar] [CrossRef]
- Dirr, M.A. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation and Uses; Stipes Publishing Co.: Champaign, IL, USA, 1998. [Google Scholar]
- Nuzzo, V.A. Element Stewardship Abstract for Alliaria Petiolata; The Nature Conservancy: Arlington, VA, USA, 1987. [Google Scholar]
- Zhang, R.; Zhang, X.X.; Tang, Y.X.; Mao, J.L. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Altgarde, N.; Eriksson, C.; Peerboom, N.; Phan-Xuan, T.; Moeller, S.; Schnabelrauch, M.; Svedhem, S.; Trybala, E.; Bergstrom, T.; Bally, M. Mucin-like Region of Herpes Simplex Virus Type 1 Attachment Protein Glycoprotein C (gC) Modulates the Virus-Glycosaminoglycan Interaction. J. Biol. Chem. 2015, 290, 21473–21485. [Google Scholar] [CrossRef]
- Zglińska, K.; Rygało-Galewska, A.; Bryś, J.; Koczon, P.; Borek, K.; Roguski, M.; Niemiec, T. Elaeagnus umbellata fruit—Chemical composition, bioactive compounds, and kinetic of DPPH inhibition compared to standard anti, oxidants. Emir. J. Food Agric. 2021, 33, 639–646. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: A review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Dankar, I.; Haddarah, A.; Omar, E.F.; Pujola, M.; Sepulcre, F. Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chem. 2018, 260, 7–12. [Google Scholar] [CrossRef]
- Malagoli, G.B.; Cardozo, T.F.; Gomes, H.J.; Ferraz, V.P.; Simoes, C.M.O.; Braga, F.C. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int. J. Biol. Macromol. 2014, 66, 332–337. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shu, X.; Chen, Y.Y.; Yan, J.F.; Zhang, S.S.; Wu, B.Y.; Jia, J.X. Enrichment, purification and in vitro antioxidant activities of polysaccharides from Umbilicaria esculenta macrolichen. Biochem. Eng. J. 2018, 130, 10–20. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Zhang, Q.; Liu, X.M.; Ma, L.; Lai, F. Extraction of polysaccharides and its antitumor activity on Magnolia kwangsiensis Figlar & Noot. Carbohydr. Polym. 2016, 142, 98–104. [Google Scholar]
- Xu, J.; Liu, W.; Yao, W.B.; Pang, X.B.; Yin, D.K.; Gao, X.D. Carboxymethylation of a polysaccharide extracted from Ganoderma lucidum enhances its antioxidant activities in vitro. Carbohydr. Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.W.; Lin, S.L.; Cheung, P. Cell wall structure of mushroom sclerotium (Pleurotus tuber regium): Part 1. Fractionation and characterization of soluble cell wall polysaccharides. Food Hydrocoll. 2014, 36, 189–195. [Google Scholar] [CrossRef]
- Hu, T.; Huang, Q.L.; Wong, K.H.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94 Pt A, 423–430. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.M.; Qin, G.Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.R.; Xu, H.L.; Xie, L.L.; Sun, J.; Sun, T.T.; Wu, X.Y.; Fu, Q.B. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Bai, A.J.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.J.; Wen, P.W.; Shen, M.Y.; Li, H.R.; Ren, Y.M.; Xiao, Y.H.; Song, Q.Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, X.H.; Zhou, S.Y.; Lin, Y.Y.; Zhang, W.; Fu, C.J.; Luo, L.C.; Wang, K.; Xie, X.J.; Fan, H.J. Biphasic extraction of different polysaccharides from Radix Sophorae Tonkinensis by microwave-assisted aqueous two-phase extraction: Process optimization, structural characterization and mechanism exploration. Sep. Purif. Technol. 2018, 207, 187–198. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Cai, L.L.; Zou, S.S.; Liang, D.P.; Luan, L.B. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Varma, K.A.C.; Kumar, J.K. Characterization and evaluation of smart releasing polysaccharide from yellow poinciana seed of Jharkhand. Int. J. Biol. Macromol. 2018, 118 Pt B, 2156–2162. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, B.K.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Huang, L.X.; Shen, M.Y.; Zhang, X.W.; Jiang, L.; Song, Q.Q.; Xie, J.H. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
- Shah, A.; Ahmad, M.; Ashwar, B.A.; Gani, A.; Masoodi, F.A.; Wani, I.A.; Wani, S.M.; Gani, A. Effect of γ-irradiation on structure and nutraceutical potential of β-d-glucan from barley (Hordeum vulgare). Int. J. Biol. Macromol. 2015, 72, 1168–1175. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Qi, H.M.; Zhang, Q.B.; Zhao, T.T.; Chen, R.; Zhang, H.; Niu, X.Z.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Duan, M.Y.; Li, R.; Wu, H.X.; Lou, Y.J. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef]
- Yuan, J.F.; Zhang, Z.Q.; Fan, Z.C.; Yang, J.X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr. Polym. 2008, 74, 822–827. [Google Scholar] [CrossRef]
- Zeng, P.J.; Li, J.; Chen, Y.L.; Zhang, L.J. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [PubMed]
- Niyomploy, P.; Thunyakitpisal, P.; Karnchanatat, A.; Sangvanich, P. Cell prolif⁃erative effect of polyxyloses extracted from the rhizomes of wild turmeric, Curcuma aromatica. Pharm. Biol. 2010, 48, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, H.B.; Zhao, H.; Wang, Y.L.; Wang, C.X. Study on the separation and antioxidant activity of polysaccharides from Acanthopanax senticosus fruit by ultrafiltration. Heilongjiang Med. Sci. 2020, 43, 8–11. [Google Scholar]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.M.; Lu, J.K. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Wang, Y.F.; Jia, J.X.; Ren, X.J.; Li, B.H.; Zhang, Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yu, J.B.; Zhang, L.F.; Hu, M.Q.; Xu, Y.; Su, W.K. Extraction, characterization and biological activity of polysaccharides from Sophora flavescens ait. Int. J. Biol. Macromol. 2016, 93, 459–467. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Zhu, X.Y.; Xie, J. Optimization of flash extraction process of water gardenia polysaccharide by response surface methodology. For. Chem. Ind. 2013, 33, 59–62. [Google Scholar]
- Li, J.; Rong, Y.H.; Rong, L. Flash extraction of dendrobium polysaccharide. Chin. Mater. Medica 2013, 36, 1524–1527. [Google Scholar]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Tang, X.Y.; Fan, H.J.; Xie, X.J.; Wan, Q.; Wu, X.H.; Tang, J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohydr. Polym. 2016, 144, 263–270. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Amjad, M.S.; Mehmood, A.; Mustafa, G.; Binish, Z.; Khan, S.; Arshad, H.; Prockow, J.; de la Lastra, J.M.P. Antibacterial, Antioxidant, and Phytotoxic Potential of Phytosynthesized Silver Nanoparticles Using Elaeagnus umbellata Fruit Extract. Molecules 2022, 27, 5847. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Wang, E.L.; Chen, X.; Wang, K.Y.; Wang, J.; Chen, D.F.; Geng, Y.; Lai, W.M.; Wei, X.C. Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol. 2016, 59, 196–202. [Google Scholar] [CrossRef]
- Zglińska, K.; Niemiec, T.; Ozicki, A.; Matusiewicz, M.; Koczoń, P. Effect of Elaeagnus umbellata (Thunb.) fruit extract on H2O2-induced oxidative and inflammatory responses in normal fibroblast cells. PeerJ. 2021, 9, e10760. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z.M.; Bao, L.L.; Wen, F.; Yang, H.H. The preparation and antioxidant activities of four 2-aminoacyl-chitooligosaccharides. Carbohydr. Res. 2022, 521, 108667. [Google Scholar] [CrossRef]
- Lin, L.H.; Xie, J.H.; Liu, S.C.; Shen, M.Y.; Tang, W.; Xie, M.Y. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 665–673. [Google Scholar] [CrossRef]
- Shi, H.M.; Yang, H.S.; Zhang, X.W.; Yu, L.L. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of Chinese propolis. J. Agric. Food Chem. 2012, 60, 12403–12410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xu, X.; Liu, X.; Chen, M.; Bai, B.; Yang, Y.; Bo, T.; Fan, S. The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides. Molecules 2023, 28, 6468. https://doi.org/10.3390/molecules28186468
Zhang J, Xu X, Liu X, Chen M, Bai B, Yang Y, Bo T, Fan S. The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides. Molecules. 2023; 28(18):6468. https://doi.org/10.3390/molecules28186468
Chicago/Turabian StyleZhang, Jinhua, Xin Xu, Xinyi Liu, Min Chen, Baoqing Bai, Yukun Yang, Tao Bo, and Sanhong Fan. 2023. "The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides" Molecules 28, no. 18: 6468. https://doi.org/10.3390/molecules28186468
APA StyleZhang, J., Xu, X., Liu, X., Chen, M., Bai, B., Yang, Y., Bo, T., & Fan, S. (2023). The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides. Molecules, 28(18), 6468. https://doi.org/10.3390/molecules28186468






