Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand
Abstract
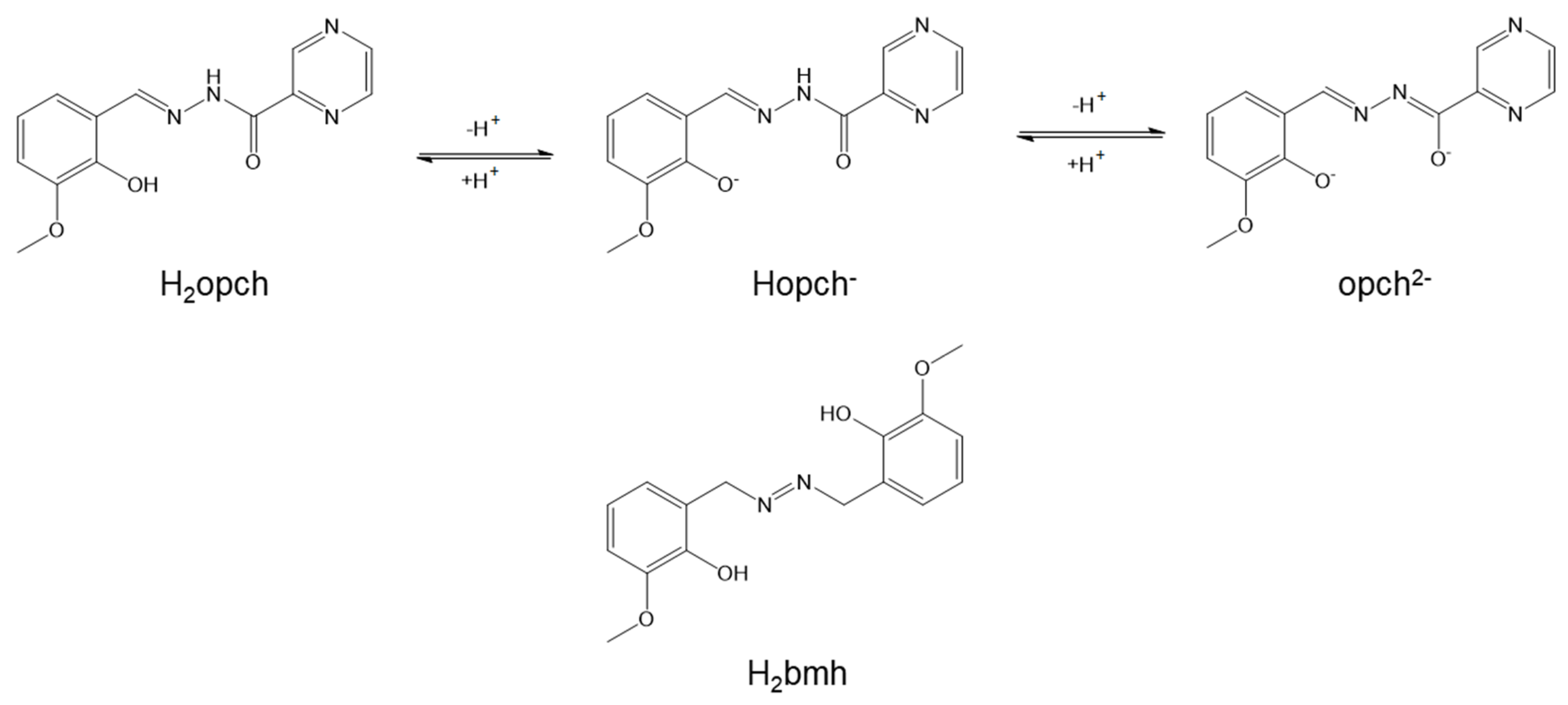
:1. Introduction
2. Results and Discussion
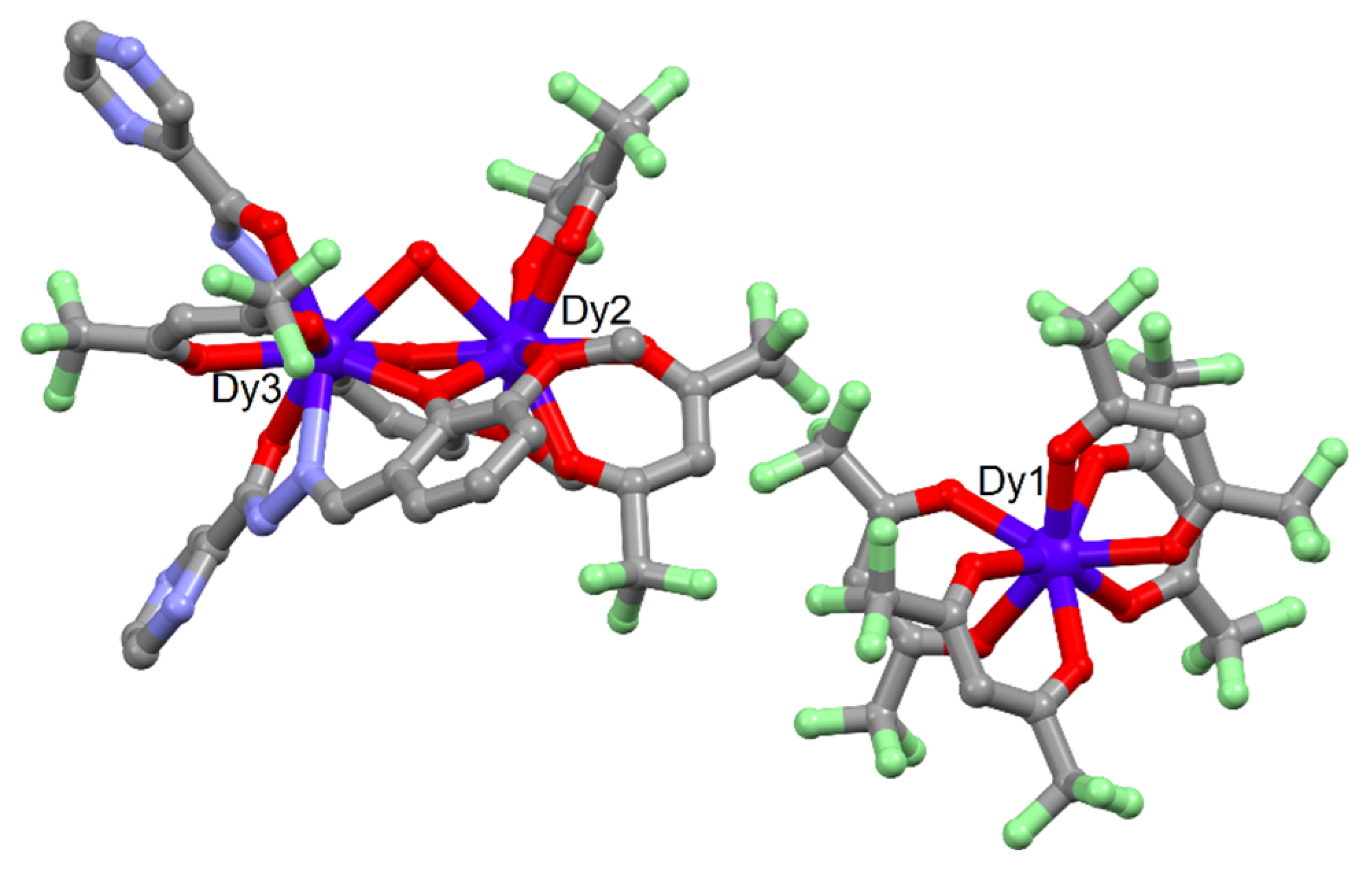
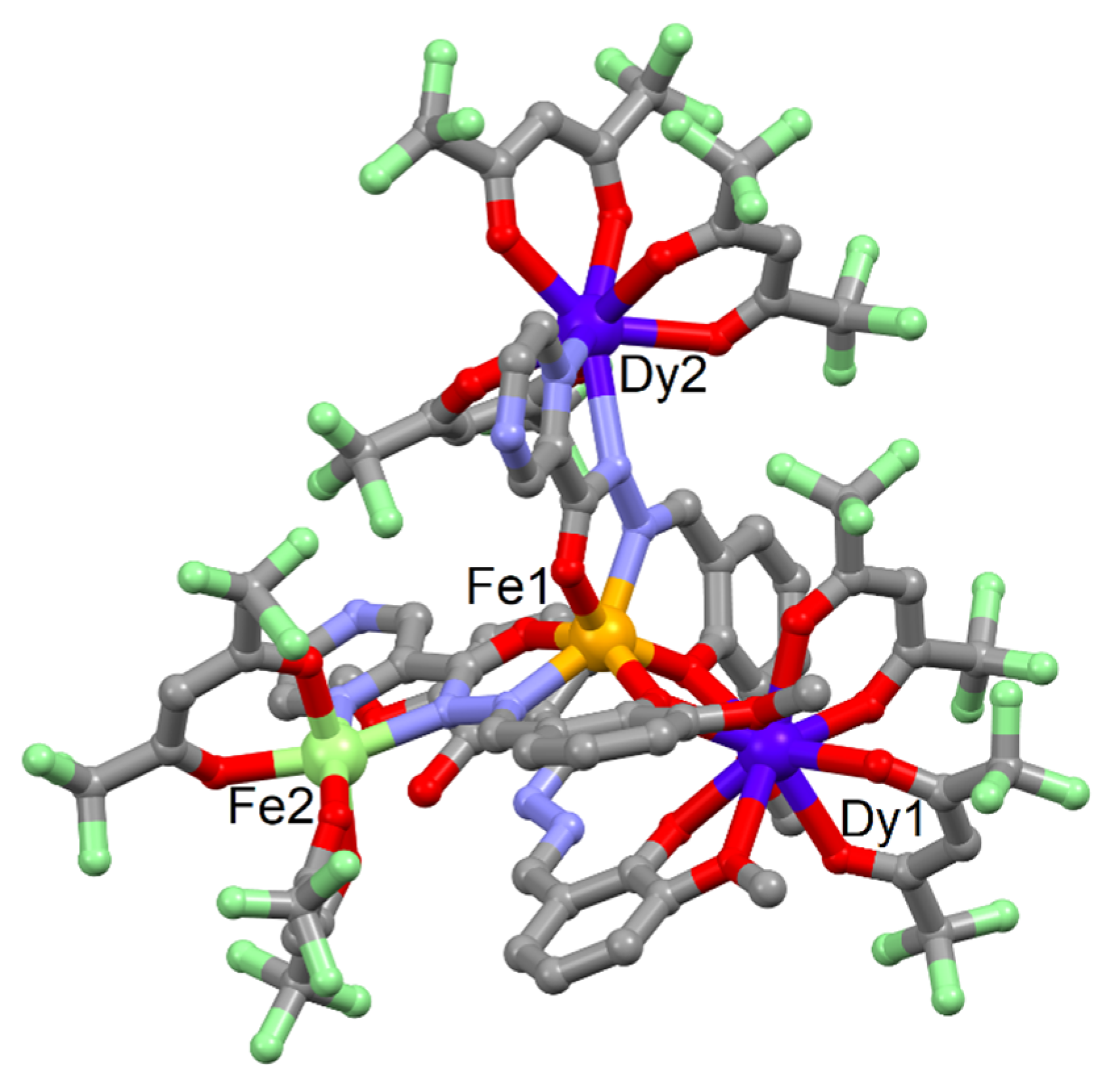
2.1. X-ray Structures
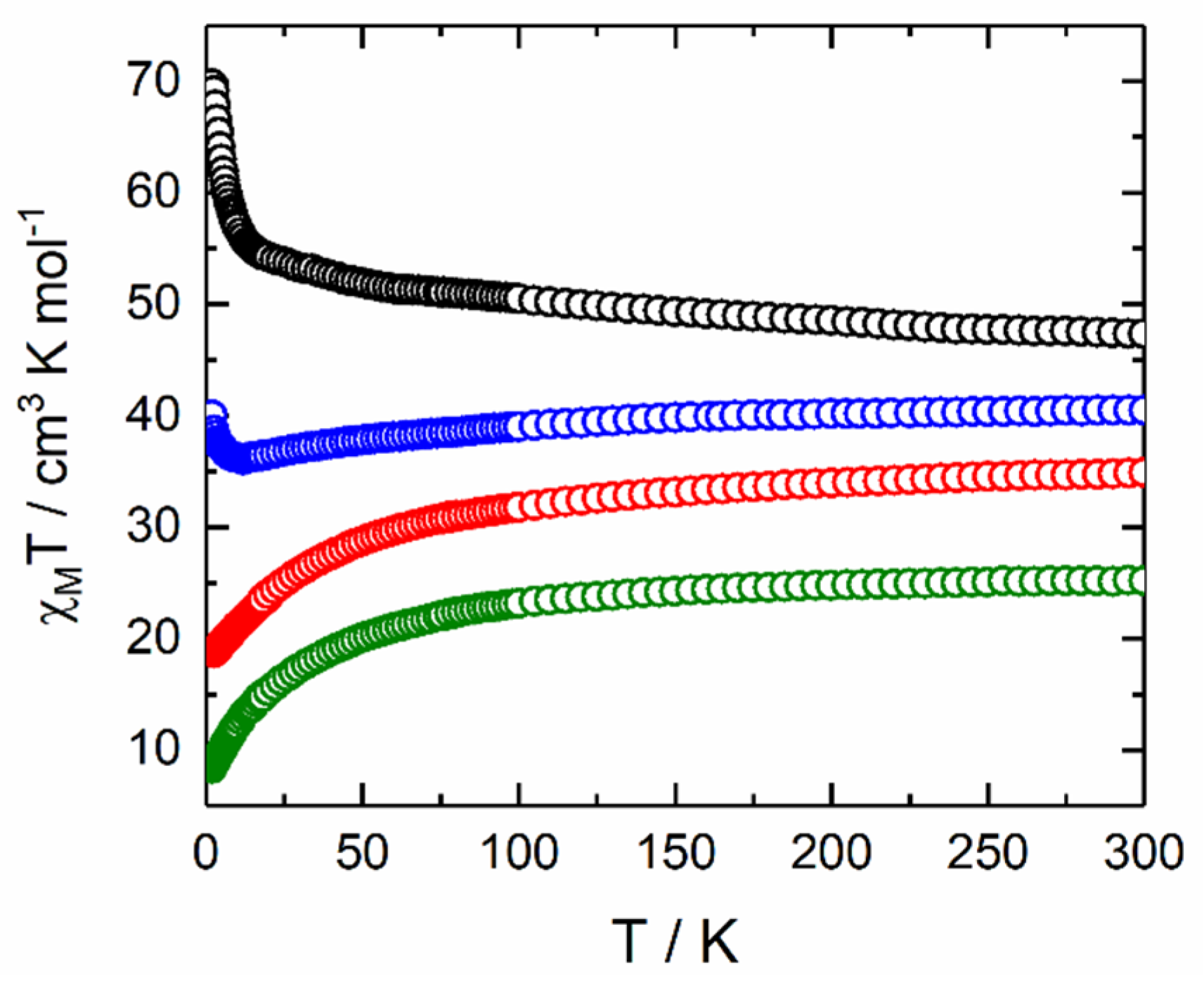
2.2. Magnetic Properties
2.2.1. Static Magnetic Measurements
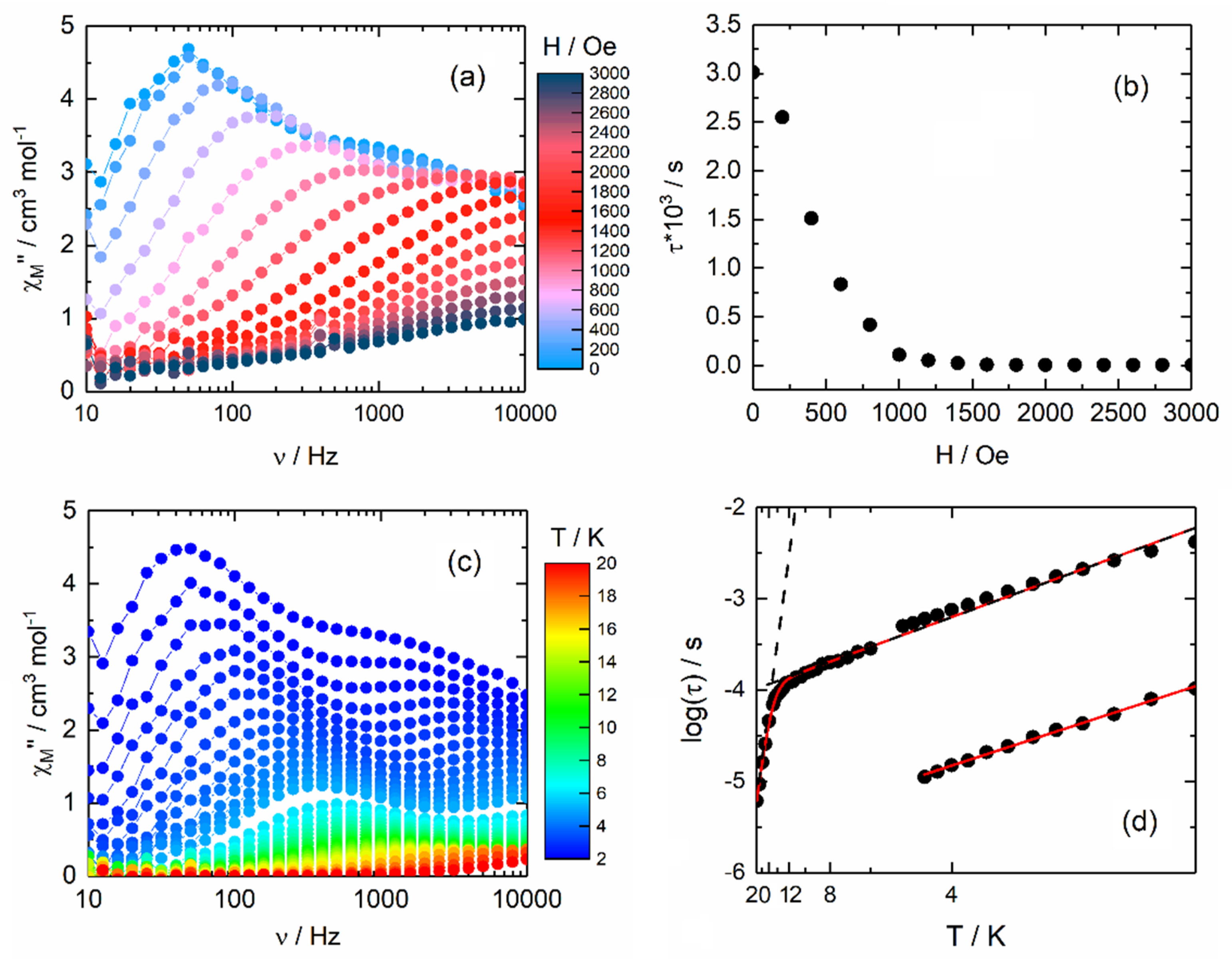
2.2.2. Dynamic Magnetic Measurements
3. Materials and Methods
3.1. Synthesis: General Procedures and Materials
3.2. Synthesis of Complex [Dy2(hfac)3(H2O)(Hopch)2]·[Dy(hfac)4] (1)
3.3. Synthesis of Complex [FeDy3(hfac)8(H2O)2(opch)2] (2)
3.4. Synthesis of Complex [Fe3Dy(hfac)6(opch)2(H2bmh)]·C6H14 (3)·C6H14
3.5. Synthesis of Complex [Fe2Dy2(hfac)7(opch)2(H2bmh)]·0.5C7H16 (4)·0.5C7H16
3.6. Crystallography
3.7. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Fang, M.; Evans, W.K.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Goodwin, C.A.P.; Mills, D.P.; Winpenny, R.E.P. The first near-linear bis(amide) f-block complex: A blueprint for a high temperature single molecule magnet. Chem. Commun. 2015, 51, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Randall McClain, K.; Gould, C.A.; Chakarawet, K.; Teat, S.J.; Groshens, T.J.; Long, J.R.; Harvey, B.G. High-temperature magnetic blocking and magneto-structural correlations in a series of dysprosium(III) metallocenium single-molecule magnets. Chem. Sci. 2018, 9, 8492–8503. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Vincent, A.H.; Whyatt, Y.L.; Chilton, N.F.; Long, J.R. Strong Axiality in a Dysprosium(III) Bis(borolide) Complex Leads to Magnetic Blocking at 65 K. J. Am. Chem. Soc. 2023, 145, 1572–1579. [Google Scholar] [CrossRef]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mondal, A.; Konar, S.; Ghosh, A. The role of 3d−4f exchange interaction in SMM behaviour and magnetic refrigeration of carbonato bridged CuII2LnIII2 (Ln = Dy, Tb and Gd) complexes of an unsymmetrical N2O4 donor ligand. Dalton Trans. 2019, 48, 15170–15183. [Google Scholar] [CrossRef] [PubMed]
- Ferbinteanu, M.; Kajiwara, T.; Choi, K.-Y.; Nojiri, H.; Nakamoto, A.; Kojima, N.; Cimpoesu, F.; Fujimura, Y.; Takaishi, S.; Yamashita, M. A Binuclear Fe(III)Dy(III) Single Molecule Magnet. Quantum Effects and Models. J. Am. Chem. Soc. 2006, 128, 9008–9009. [Google Scholar] [CrossRef]
- Chen, S.; Mereacre, V.; Anson, C.E.; Powell, A.K. A single molecule magnet to single molecule magnet transformation via a solvothermal process: Fe4Dy2 → Fe6Dy3. Dalton Trans. 2016, 45, 98–106. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wu, J.-Y.; Chen, Y.-C.; Mereacre, V.; Powell, A.K.; Ungur, L.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. A Heterometallic FeII-DyIII Single-Molecule Magnet with a record Anisotropy Barrier. Angew. Chem. Int. Ed. 2014, 53, 12966–12970. [Google Scholar] [CrossRef]
- Chen, W.-B.; Chen, Y.-C.; Huang, G.-Z.; Liu, J.-L.; Jia, J.-H.; Tong, M.-L. Cyclic OFF/Part/ON switching of single-molecule magnet behaviours via multistep single-crystal-to-crystal transformation between discrete Fe(II)-Dy(III) complexes. Chem. Commun. 2018, 54, 10886–10889. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.-C.; Liu, J.; Chen, W.-B.; Huang, G.-Z.; Wu, S.-G.; Wang, J.; Liu, J.-L.; Tong, M.-L. Cyanometallate-Bridged Didysprosium Single-Molecule Magnets Constructed with Single-Ion Magnet Building Block. Inorg. Chem. 2020, 59, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Kaemmerer, H.; Powell, A.K. From the {FeIII2Ln2} Butterfly’s Perspective: The Magnetic Benefits and Challenges of Cooperativity within 3d-4f Based Coordination Clusters. Chem. Eur. J. 2021, 27, 15044–15066. [Google Scholar] [CrossRef]
- Lin, P.-H.; Burchell, T.J.; Ungur, L.; Chibotaru, L.F.; Wernsdorfer, W.; Murugesu, M. A Polynuclear Lanthanide Single-Molecule Magnet with a record Anisotropic Barrier. Angew. Chem. Int. Ed. 2009, 48, 9489–9492. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. Quadruple-CO32− bridged octanuclear dysprosium(III) compound showing single-molecule magnet behavior. Chem. Commun. 2012, 48, 708–710. [Google Scholar] [CrossRef]
- Tian, H.; Wang, M.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. A Discrete Dysprosium Trigonal Prism Showing Single-Molecule Magnet Behaviour. Chem. Eur. J. 2012, 18, 442–445. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, L.; Lin, H.; Tang, J.; Li, G. Butterfly-Shaped Pentanuclear Dysprosium Single-Molecule Magnets. Chem. Eur. J. 2013, 19, 13235–13241. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.; Carter, A.B.; Konidaris, K.F.; Kühne, I.A.; Gonzalez, R.; Anson, C.E.; Powell, A.K. The first Use of a ReX5 Synthon to Modulate FeIII Spin Crossover via Supramolecular Halogen⋅⋅⋅Halogen Interactions. Chem. Eur. J. 2020, 26, 11835–11840. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH: Weinhem, Germany, 1993. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Orbach, R. Spin-lattice relaxation in rare-earth salts. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1961, 264, 458–484. [Google Scholar]
- Orbach, R. On the theory of spin-lattice relaxation in paramagnetic salts. Proc. Phys. Soc. 1961, 77, 821–826. [Google Scholar] [CrossRef]
- Dekker, C.; Arts, A.F.M.; de Wijn, H.W.; van Duyneveldt, A.J.; Mydosh, J.A. Activated dynamics in a two-dimensional Ising spin glass: Rb2Cu1−xCoxF4. Phys. Rev. B Condens. Matter Mater. Phys. 1989, 40, 11243–11251. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer: Berlin, Germany, 2015. [Google Scholar]
- Evans, P.; Reta, D.; Whitehead, G.F.S.; Chilton, N.F.; Mills, D.P. Bis-Monophospholyl Dysprosium Cation Showing Magnetic Hysteresis at 48 K. J. Am. Chem. Soc. 2019, 141, 19935–19940. [Google Scholar] [CrossRef]
- Reta, D.; Chilton, N.F. Uncertainty estimates for magnetic relaxation times and magnetic relaxation parameters. Phys. Chem. Chem. Phys. 2019, 21, 23567–23575. [Google Scholar] [CrossRef]
- Poneti, G.; Bernot, K.; Bogani, L.; Caneschi, A.; Sessoli, R.; Wernsdorfer, W.; Gatteschi, D. A rational approach to the modulation of the dynamics of the magnetisation in a dysprosium-nitronyl-nitroxide radical complex. Chem. Commun. 2007, 18, 1807–1809. [Google Scholar] [CrossRef]
- Yi, X.; Bernot, K.; Cador, O.; Luzon, J.; Calvez, G.; Daiguebonne, C.; Guillou, O. Influence of ferromagnetic connection of Ising-type DyIII-based single ion magnets on their magnetic slow relaxation. Dalton Trans. 2013, 42, 6728–6731. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, J.-K.; Saleh, N.; Fernandez Garcia, G.; Norel, L.; Pointillart, F.; Guizouarn, T.; Cador, O.; Totti, F.; Ouahab, L.; Crassous, J.; et al. Improved slow magnetic relaxation in optically pure helicene-based DyIII single molecule magnets. Chem. Commun. 2016, 52, 14474–14477. [Google Scholar] [CrossRef] [PubMed]
- Briganti, M.; Santanni, F.; Tesi, L.; Totti, F.; Sessoli, R. A Complete Ab Initio View of Orbach and Raman Spin-Lattice Relaxation in a Dysprosium Coordination Compound. J. Am. Chem. Soc. 2021, 143, 13633–13645. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Sheldrick, G.L. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefeuvre, B.; Guizouarn, T.; Dorcet, V.; Cordier, M.; Pointillart, F. Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand. Molecules 2023, 28, 6359. https://doi.org/10.3390/molecules28176359
Lefeuvre B, Guizouarn T, Dorcet V, Cordier M, Pointillart F. Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand. Molecules. 2023; 28(17):6359. https://doi.org/10.3390/molecules28176359
Chicago/Turabian StyleLefeuvre, Bertrand, Thierry Guizouarn, Vincent Dorcet, Marie Cordier, and Fabrice Pointillart. 2023. "Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand" Molecules 28, no. 17: 6359. https://doi.org/10.3390/molecules28176359
APA StyleLefeuvre, B., Guizouarn, T., Dorcet, V., Cordier, M., & Pointillart, F. (2023). Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand. Molecules, 28(17), 6359. https://doi.org/10.3390/molecules28176359







