Fluorescent and Catalytic Properties of a 2D Lamellar Zn Metal–Organic Framework with sql Network Structure
Abstract
:1. Introduction
2. Results and Discussion
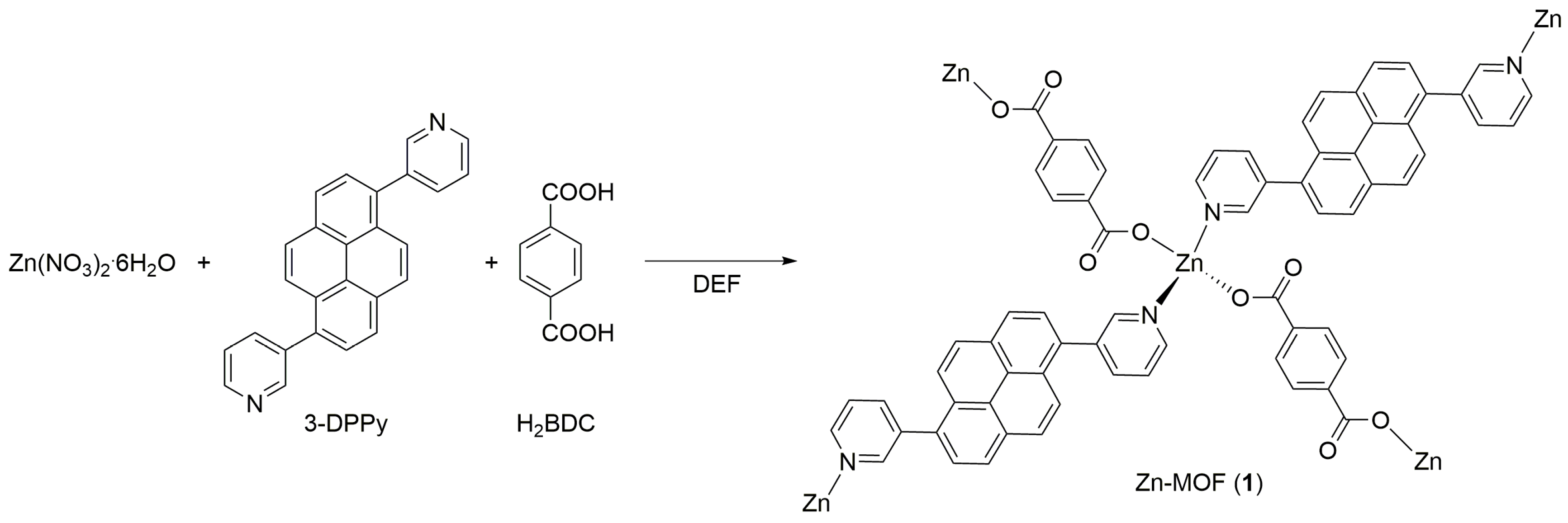
2.1. Preparation of the Bridging Linker and Zn-MOF
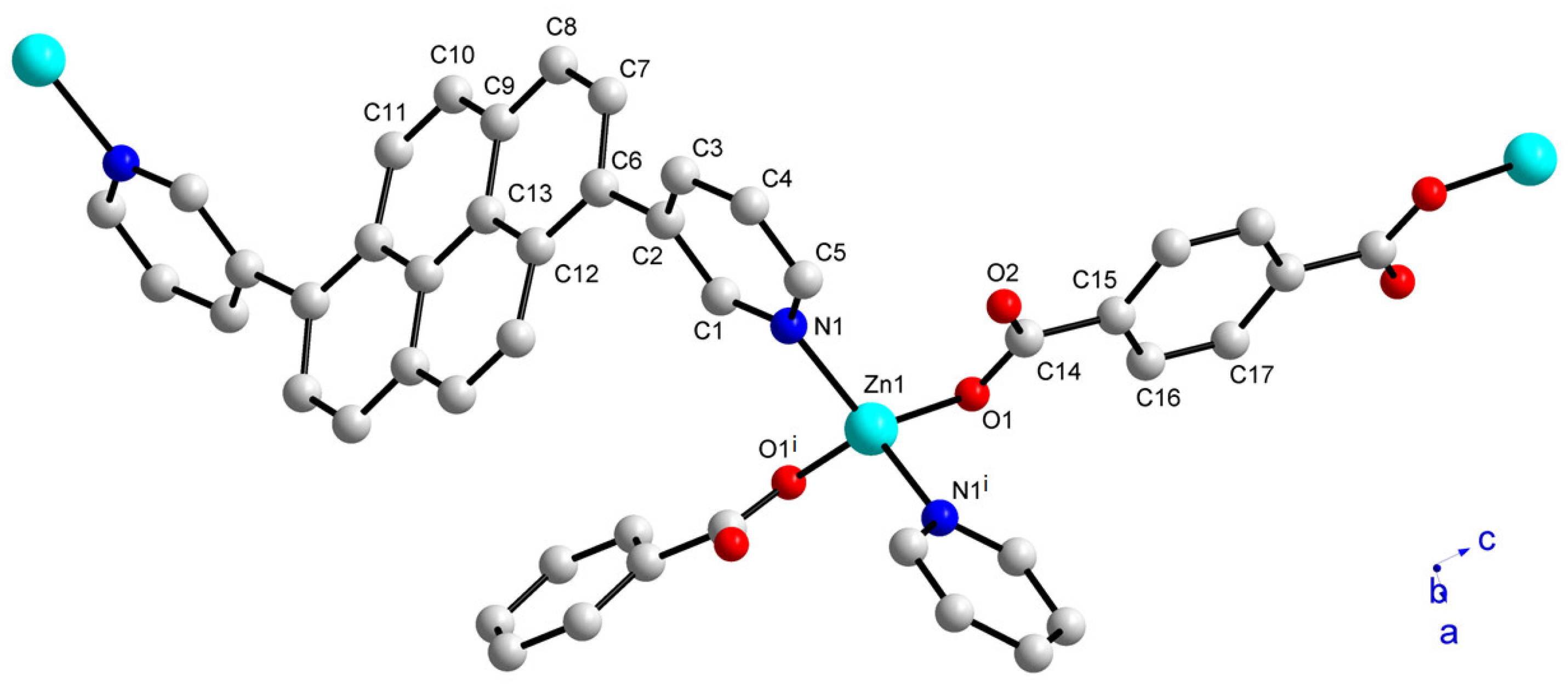
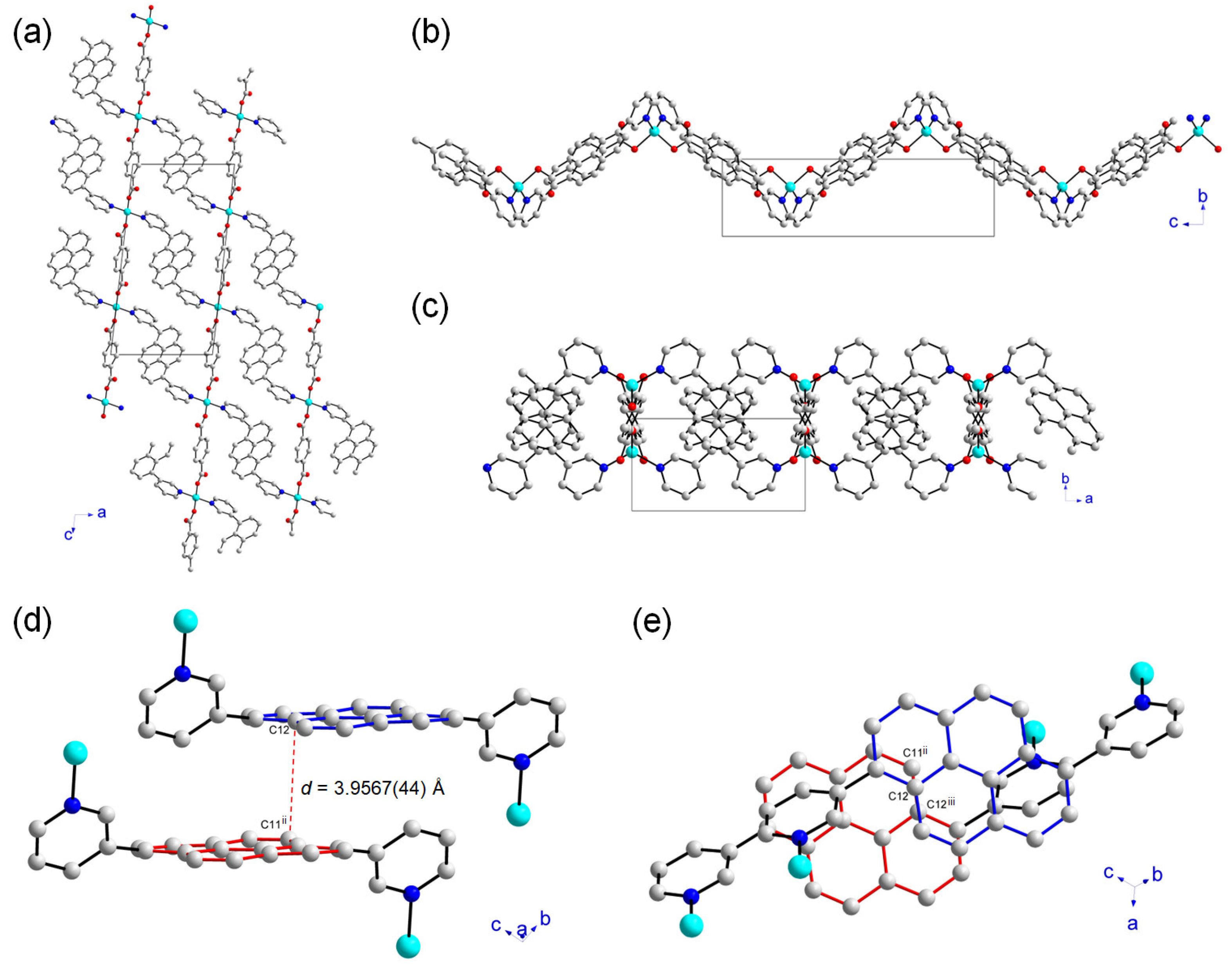
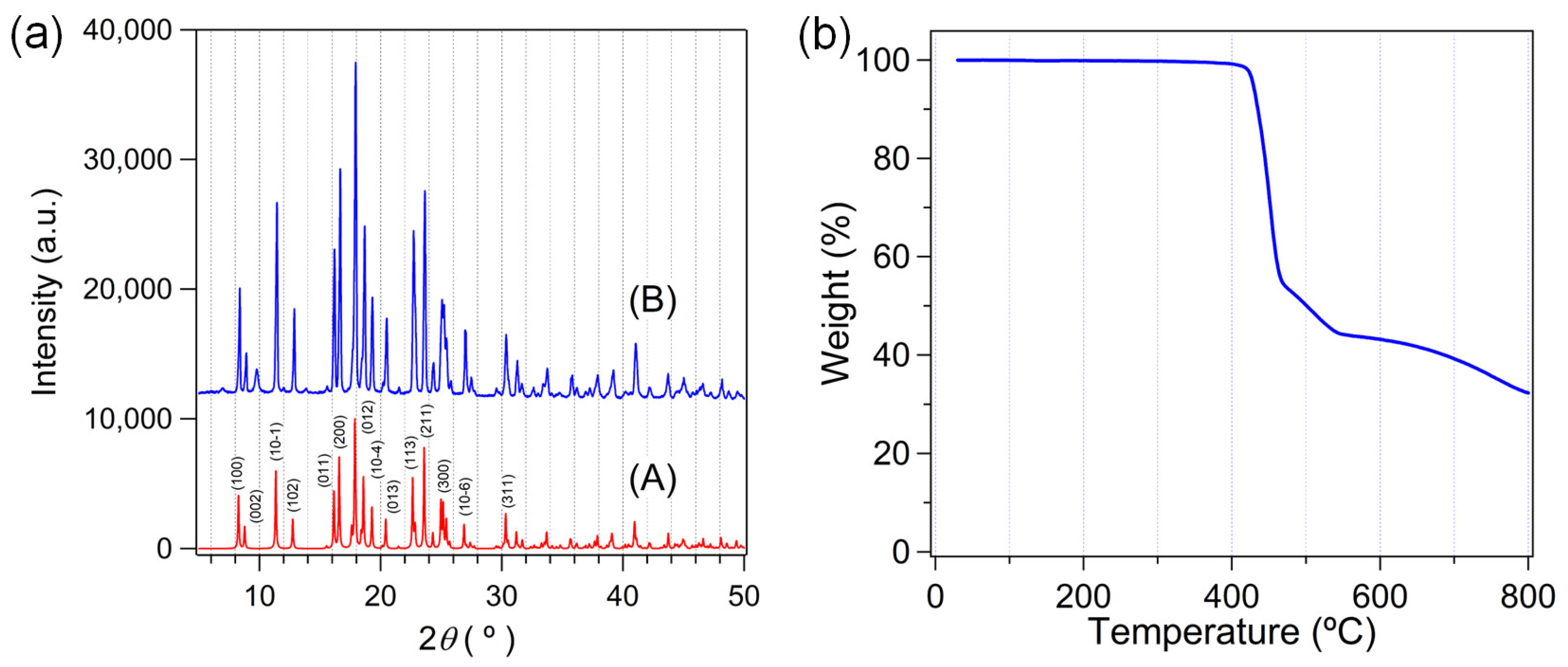
2.2. Crystal Structure and Topology Analysis of Zn-MOF
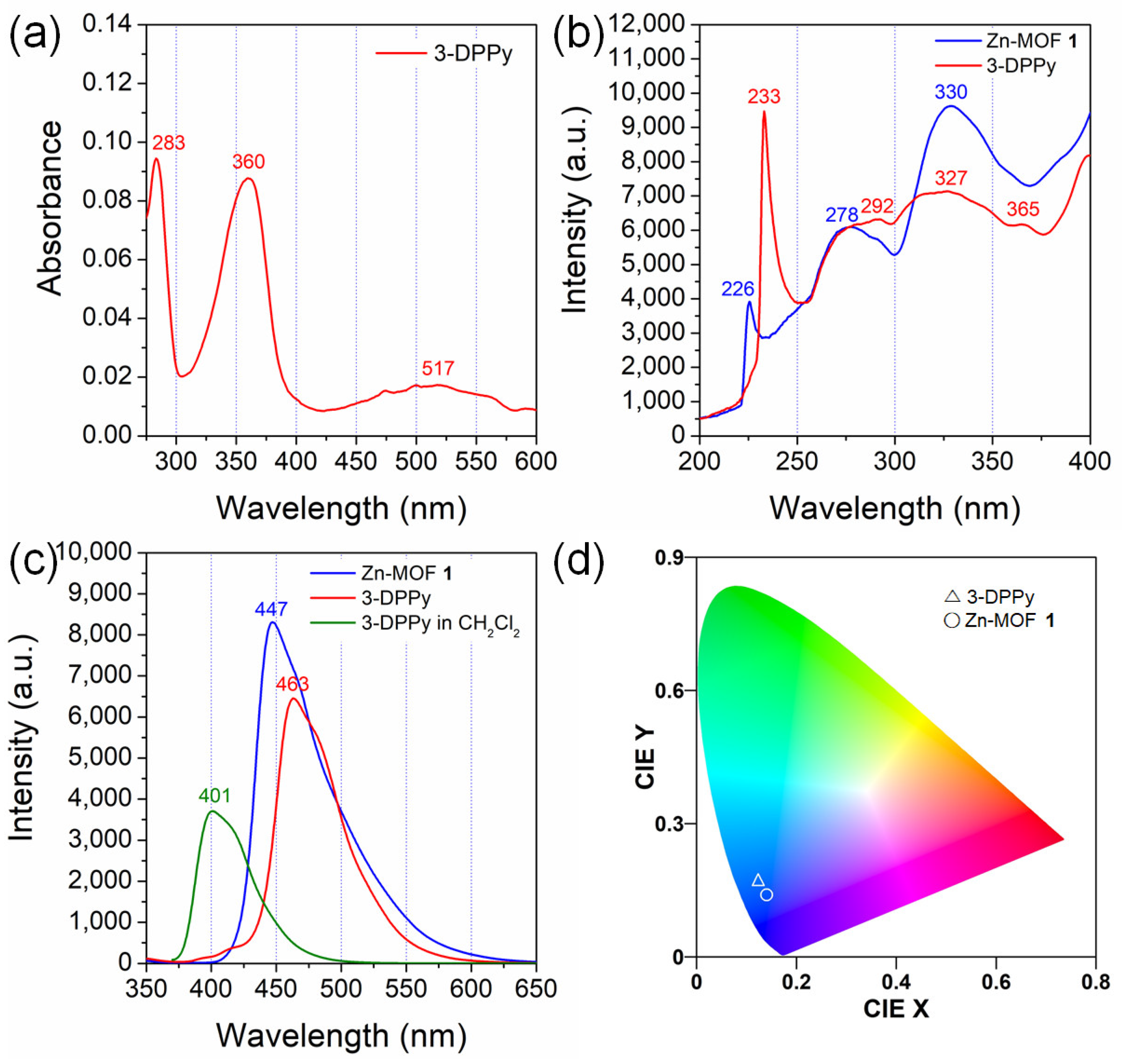
2.3. Fluorescent Properties of Zn-MOF and 3-DPPy in the Solid State
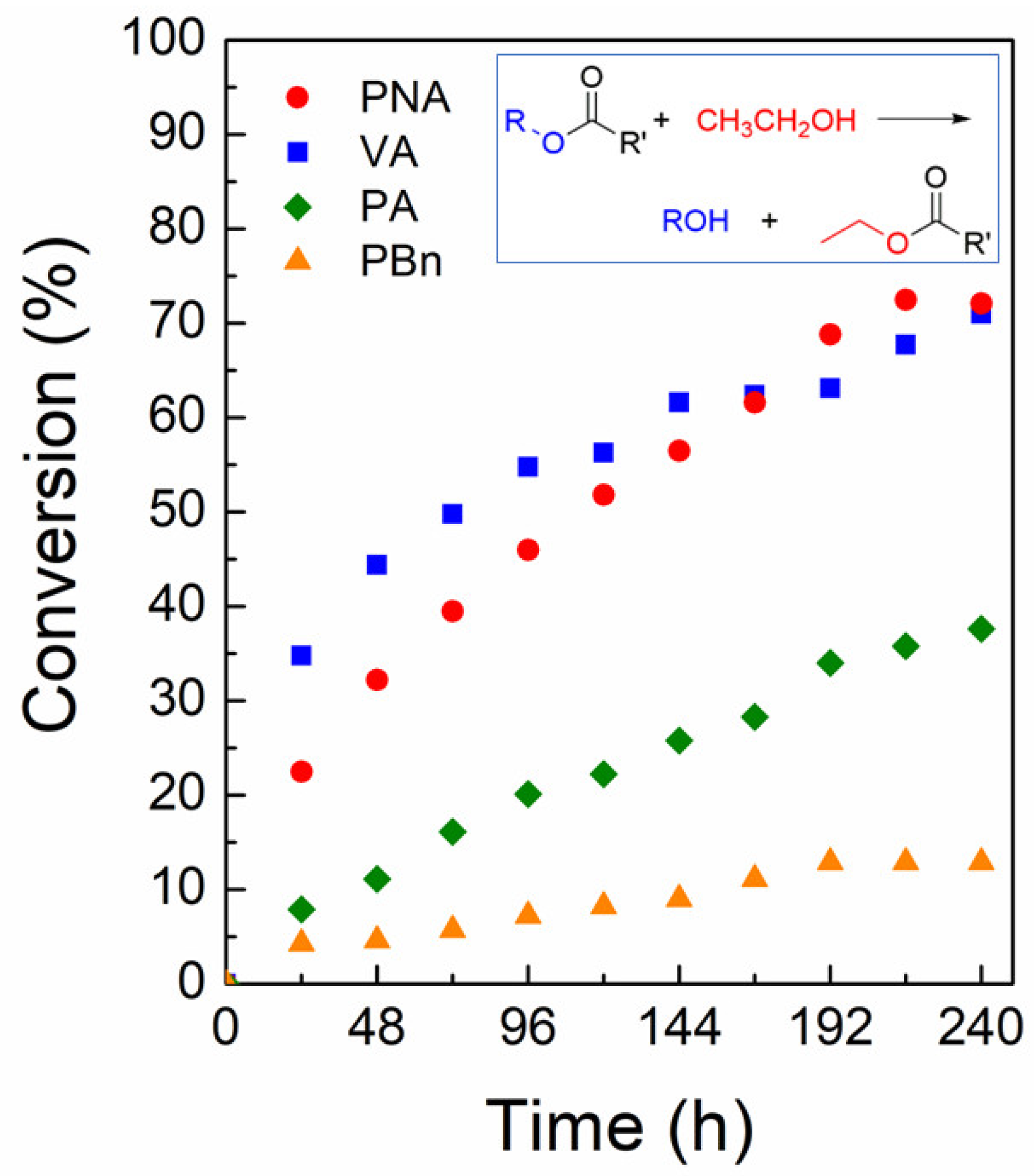
2.4. Catalytic Transesterification Activity of Zn-MOF
3. Materials and Methods
3.1. Preparation of 3-DPPy
3.2. Preparation of Zn-MOF 1
3.3. Instrumentation
3.4. Photoluminescence Lifetime Measurements
3.5. Crystal Structure Determination of Zn-MOF 1
3.6. Transesterification of Esters by Zn-MOF 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, J.-X.; Liang, C.-C.; Gu, X.-W.; Wen, H.-M.; Jiang, C.; Li, B.; Qian, G.; Chen, B. Recent advances in microporous metal–organic frameworks as promising adsorbents for gas separation. J. Mater. Chem. A 2022, 10, 17878–17916. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef]
- Leith, G.A.; Martin, C.R.; Mayers, J.M.; Kittikhunnatham, P.; Larsen, R.W.; Shustova, N.B. Confinement-guided photophysics in MOFs, COFs, and cages. Chem. Soc. Rev. 2021, 50, 4382–4410. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Zhang, Y.; Tan, J.-C. Confinement of Luminescent Guests in Metal−Organic Frameworks: Understanding Pathways from Synthesis and Multimodal Characterization to Potential Applications of LG@MOF Systems. Chem. Rev. 2022, 122, 10438–10483. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Tuci, G.; Luconi, L.; Giambastiani, G. Metal−Organic Frameworks as Heterogeneous Catalysts in Hydrogen Production from Lightweight Inorganic Hydrides. ACS Catal. 2017, 7, 5035–5045. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal−Organic Framework (MOF)- Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Zhang, C.; Huang, X.; Peng, B.; Wang, G. Recent advances in metal-organicframework-based catalysts for thermocatalytic selective oxidation of organic substances. Chem. Catal. 2022, 2, 1009–1045. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; Lee, J.; Kim, S.; Kim, J.; Kim, M. Strategies in Metal–Organic Framework-based Catalysts for the Aerobic Oxidation of Alcohols and Recent Progress. Bull. Korean Chem. Soc. 2021, 42, 359–368. [Google Scholar] [CrossRef]
- Seo, S.; Lee, E.; Ju, H.; Kim, S.; Lee, S.S. Mononickel(II), dimercury(II), and 1-D polymeric silver(I) complexes with a ditopic 18-membered N2O2S2-macrocycle. Inorg. Chem. Commun. 2017, 83, 92–96. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Guy Orpen, A.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Huh, S.; Kwon, T.-H.; Park, N.; Kim, S.-J.; Kim, Y. Nanoporous In-MOF with multiple one-dimensional pores. Chem. Commun. 2009, 33, 4953–4955. [Google Scholar] [CrossRef]
- Gu, J.-M.; Kim, S.-J.; Kim, Y.; Huh, S. Structural isomerism of an anionic nanoporous In-MOF with interpenetrated diamond-like topology. CrystEngComm 2012, 14, 1819–1824. [Google Scholar] [CrossRef]
- Cho, E.-Y.; Gu, J.-M.; Choi, I.-H.; Kim, W.-S.; Hwang, Y.-K.; Huh, S.; Kim, S.-J.; Kim, Y. Encapsulation of Various Guests by an Anionic In-Metal−Organic Framework Containing Tritopic BTB Ligand: Crystal Structure of Reichardt’s Dye Captured in an In-Metal−Organic Framework. Cryst. Growth Des. 2014, 14, 5026–5033. [Google Scholar] [CrossRef]
- Weston, M.H.; Delaquil, A.A.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Tuning the Hydrophobicity of Zinc Dipyridyl Paddlewheel Metal−Organic Frameworks for Selective Sorption. Cryst. Growth Des. 2013, 13, 2938–2942. [Google Scholar] [CrossRef]
- Hwang, I.H.; Bae, J.M.; Kim, W.-S.; Jo, Y.D.; Kim, C.; Kim, Y.; Kim, S.-J.; Huh, S. Bifunctional 3D Cu-MOFs containing glutarates and bipyridyl ligands: Selective CO2 sorption and heterogeneous catalysis. Dalton Trans. 2012, 41, 12759–12765. [Google Scholar] [CrossRef]
- Hwang, I.H.; Bae, J.M.; Hwang, Y.-K.; Kim, H.-Y.; Kim, C.; Huh, S.; Kim, S.-J.; Kim, Y. CO2 selective dynamic two-dimensional ZnII coordination polymer. Dalton Trans. 2013, 42, 15645–15649. [Google Scholar] [CrossRef]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray Crystal Structures, and Gas Sorption Properties of Pillared Square Grid Nets Based on Paddle-Wheel Motifs: Implications for Hydrogen Storage in Porous Materials. Chem. Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Karagiaridi, O.; Bury, W.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Solvent-Assisted Linker Exchange: An Alternative to the De Novo Synthesis of Unattainable Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2014, 53, 4530–4540. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Kole, G.K.; Friedrich, A.; Müller-Buschbaum, K.; Liu, Z.; Yu, X.; Marder, T.B. Comparison Study of the Site-Effect on Regioisomeric Pyridyl−Pyrene Conjugates: Synthesis, Structures, and Photophysical Properties. J. Org. Chem. 2020, 85, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Song, J.; Wang, F.; Kan, L.; Song, J.; Wang, W.; Ma, W.; Zhang, W.; He, G. Pyrenoviologen-based fluorescent sensor for detection of picric acid in aqueous solution. Chin. Chem. Lett. 2019, 30, 1984–1988. [Google Scholar] [CrossRef]
- Zych, D. Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules 2019, 24, 2551. [Google Scholar] [CrossRef]
- Vanga, M.; Lalancette, R.A.; Jäkle, F. Controlling the Optoelectronic Properties of Pyrene by Regioselective Lewis Base-Directed Electrophilic Aromatic Borylation. Chem. Eur. J. 2019, 25, 10133–10140. [Google Scholar] [CrossRef]
- Gu, J.-M.; Kwon, T.-H.; Park, J.-H.; Huh, S. DABCO-functionalized metal–organic framework bearing a C2h-symmetric terphenyl dicarboxylate linker. Dalton Trans. 2010, 39, 5608–5610. [Google Scholar] [CrossRef]
- Gu, J.-M.; Kim, W.-S.; Huh, S. Size-dependent catalysis by DABCO-functionalized Zn-MOF with one-dimensional channels. Dalton Trans. 2011, 40, 10826–10829. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal–Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar] [CrossRef]
- Kozlova, S.G.; Gabuda, S.P. Thermal properties of Zn2(C8H4O4)2·C6H12N2 metal-organic framework compound and mirror symmetry violation of dabco molecules. Sci. Rep. 2017, 7, 11505. [Google Scholar] [CrossRef]
- Uemura, K.; Yamasaki, Y.; Onishi, F.; Kita, H.; Ebihara, M. Two-Step Adsorption on Jungle-Gym-Type Porous Coordination Polymers: Dependence on Hydrogen-Bonding Capability of Adsorbates, Ligand-Substituent Effect, and Temperature. Inorg. Chem. 2010, 49, 10133–10143. [Google Scholar] [CrossRef]
- Han, W.; Ma, X.; Wang, J.; Leng, F.; Xie, C.; Jiang, H.-L. Endowing Porphyrinic Metal−Organic Frameworks with High Stability by a Linker Desymmetrization Strategy. J. Am. Chem. Soc. 2023, 145, 9665–9671. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, H.; Wang, Z.U.; Liu, Y.; Zhou, H.-C.; Li, J.-R. Unusual preservation of polyhedral molecular building units in a metal–organic framework with evident desymmetrization in ligand design. Chem. Commun. 2014, 50, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-Y.; Lee, C.; Lee, S. Efficient blue organic light-emitting diodes using newly-developed pyrene-based electron transport materials. Org. Electron. 2009, 10, 163–169. [Google Scholar] [CrossRef]
- Hagiwara, S.; Ishida, Y.; Masui, D.; Shimada, T.; Takagi, S. Photochemical properties of cationic pyrene derivative and energy transfer reaction between pyrene and porphyrin on the clay surface. Clay Sci. 2013, 17, 7–10. [Google Scholar]
- Kim, H.-C.; Huh, S.; Kim, S.-J.; Kim, Y. Selective carbon dioxide sorption and heterogeneous catalysis by a new 3D Zn-MOF with nitrogen-rich 1D channels. Sci. Rep. 2017, 7, 17185. [Google Scholar] [CrossRef]
- Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrating metal-organic and inorganic 3D networks: A computer-aided systematic investigation. Part I. Analysis of the Cambridge structural database. CrystEngComm 2004, 6, 377–395. [Google Scholar] [CrossRef]
- Camerman, A.; Trotter, J. The Crystal and Molecular Structure of Pyrene. Acta Cryst. 1965, 18, 636–643. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Yoshizawa, M.; Akita, M.; Fujita, M. Engineering Double to Quintuple Stacks of a Polarized Aromatic in Confined Cavities. J. Am. Chem. Soc. 2010, 132, 960–966. [Google Scholar] [CrossRef]
- Katz, A.; Davis, M.E. Molecular imprinting of bulk, microporous silica. Nature 2000, 403, 286–289. [Google Scholar] [CrossRef]
- Banerjee, M.; Vyas, V.S.; Lindeman, S.V.; Rathore, R. Isolation and X-ray structural characterization of tetraisopropylpyrene cation radical. Chem. Commun. 2008, 1889–1891. [Google Scholar] [CrossRef]
- Crawford, A.G.; Dwyer, A.D.; Liu, Z.; Steffen, A.; Beeby, A.; Pålsson, L.-O.; Tozer, D.J.; Marder, T.B. Experimental and Theoretical Studies of the Photophysical Properties of 2- and 2,7-Functionalized Pyrene Derivatives. J. Am. Chem. Soc. 2011, 133, 13349–13362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lin, J.; Wang, J.; Li, F.; Tang, F.; Zhao, X. A designed amphiphilic peptide containing the silk fibroin motif as a potential carrier of hydrophobic drugs. Prog. Nat. Sci. 2009, 19, 1529–1536. [Google Scholar] [CrossRef]
- Winnik, F.M. Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Kübler, J.A.; Pfund, B.; Wenger, O.S. Zinc(II) Complexes with Triplet Charge-Transfer Excited States Enabling Energy-Transfer Catalysis, Photoinduced Electron Transfer, and Upconversion. JACS Au 2022, 2, 2367–2380. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B. The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules 2020, 25, 4984. [Google Scholar] [CrossRef]
- Thomas, K.R.J. GoCIE V2; Department of Chemistry, Indian Institute of Technology: Roorkee, India, 2009. [Google Scholar]
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- Tang, M.-C.; Chan, M.-Y.; Yam, V.W.-W. Molecular Design of Luminescent Gold(III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials. Chem. Rev. 2021, 121, 7249–7279. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Karlsson, M.; Bettinelli, M. Inorganic Phosphor Materials for Lighting. Top. Curr. Chem. 2016, 374, 21. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, T.; Yu, J.; Yang, Y.; Wang, Z.; Qian, G. Dye Encapsulated Metal-Organic Framework for Warm-White LED with High Color-Rendering Index. Adv. Funct. Mater. 2015, 25, 4796–4802. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Yong, X.; Yang, X.; Yu, J.; Lu, S.; Bai, F.; Wang, S.; Wang, K.; Liu, Z.; et al. Pressure Engineering Toward Harvesting the Bright Deep-Blue-Light Emission in Y-based Metal-Organic Frameworks. Adv. Funct. Mater. 2023, 33, 2300109. [Google Scholar] [CrossRef]
- Kosugi, K.; Akatsuka, C.; Iwami, H.; Kondo, M.; Masaoka, S. Iron-Complex-Based Supramolecular Framework Catalyst for Visible-Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 10451–10457. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T. Development of Tetranuclear Zinc Cluster-Catalyzed Environmentally Friendly Reactions and Mechanistic Studies. Chem. Pharm. Bull. 2016, 64, 523–539. [Google Scholar] [CrossRef]
- Nisar, S.; Hanif, M.A.; Rashid, U.; Hanif, A.; Akhtar, M.N.; Ngamcharussrivichai, C. Trends in Widely Used Catalysts for Fatty Acid Methyl Esters (FAME) Production: A Review. Catalysts 2021, 11, 1085. [Google Scholar] [CrossRef]
- Hou, X.; Qi, Y.; Qiao, X.; Wang, G.; Qin, Z.; Wang, J. Lewis acid-catalyzed transesterification and esterification of high free fatty acid oil in subcritical methanol. Korean J. Chem. Eng. 2007, 24, 311–313. [Google Scholar] [CrossRef]
- Kim, W.-S.; Lee, K.Y.; Ryu, E.-H.; Gu, J.-M.; Kim, Y.; Lee, S.J.; Huh, S. Catalytic Transesterifications by a Zn–BisSalen MOF Containing Open Pyridyl Groups Inside 1D Channels. Eur. J. Inorg. Chem. 2013, 4228–4233. [Google Scholar] [CrossRef]
- Meneghetti, M.R.; Meneghetti, S.M.P. Sn(IV)-based organometallics as catalysts for the production of fatty acid alkyl esters. Catal. Sci. Technol. 2015, 5, 765–771. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, C.; Kim, J.; Huh, S. Fluorescent and Catalytic Properties of a 2D Lamellar Zn Metal–Organic Framework with sql Network Structure. Molecules 2023, 28, 6357. https://doi.org/10.3390/molecules28176357
Shin C, Kim J, Huh S. Fluorescent and Catalytic Properties of a 2D Lamellar Zn Metal–Organic Framework with sql Network Structure. Molecules. 2023; 28(17):6357. https://doi.org/10.3390/molecules28176357
Chicago/Turabian StyleShin, Chaewon, Jongseo Kim, and Seong Huh. 2023. "Fluorescent and Catalytic Properties of a 2D Lamellar Zn Metal–Organic Framework with sql Network Structure" Molecules 28, no. 17: 6357. https://doi.org/10.3390/molecules28176357
APA StyleShin, C., Kim, J., & Huh, S. (2023). Fluorescent and Catalytic Properties of a 2D Lamellar Zn Metal–Organic Framework with sql Network Structure. Molecules, 28(17), 6357. https://doi.org/10.3390/molecules28176357






