The Nighttime Fragrance of Guettarda scabra (Rubiaceae): Flower Scent and Its Implications for Moth Pollination
Abstract
1. Introduction
2. Results
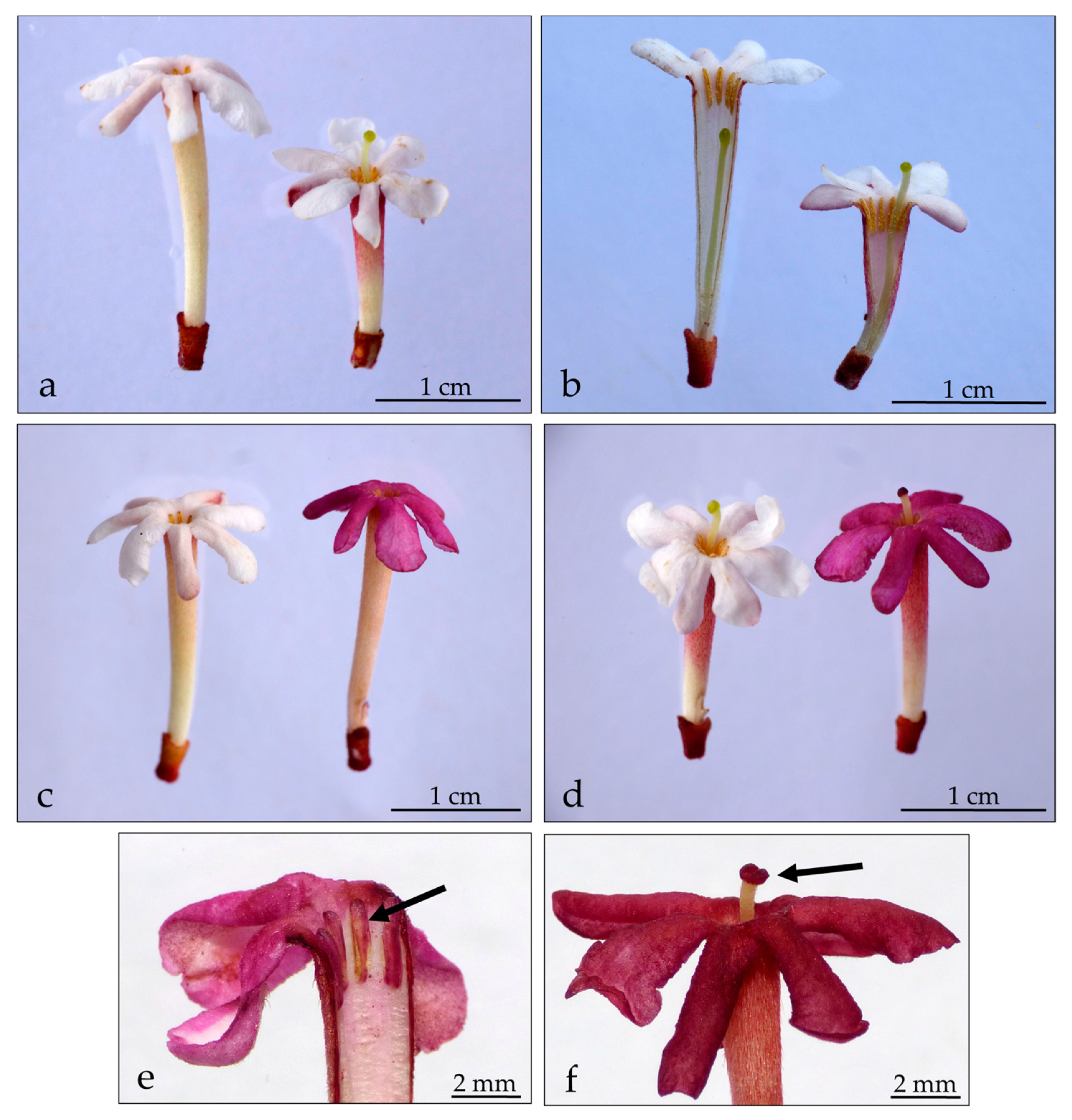
2.1. Scent-Emitting Regions within the Flower
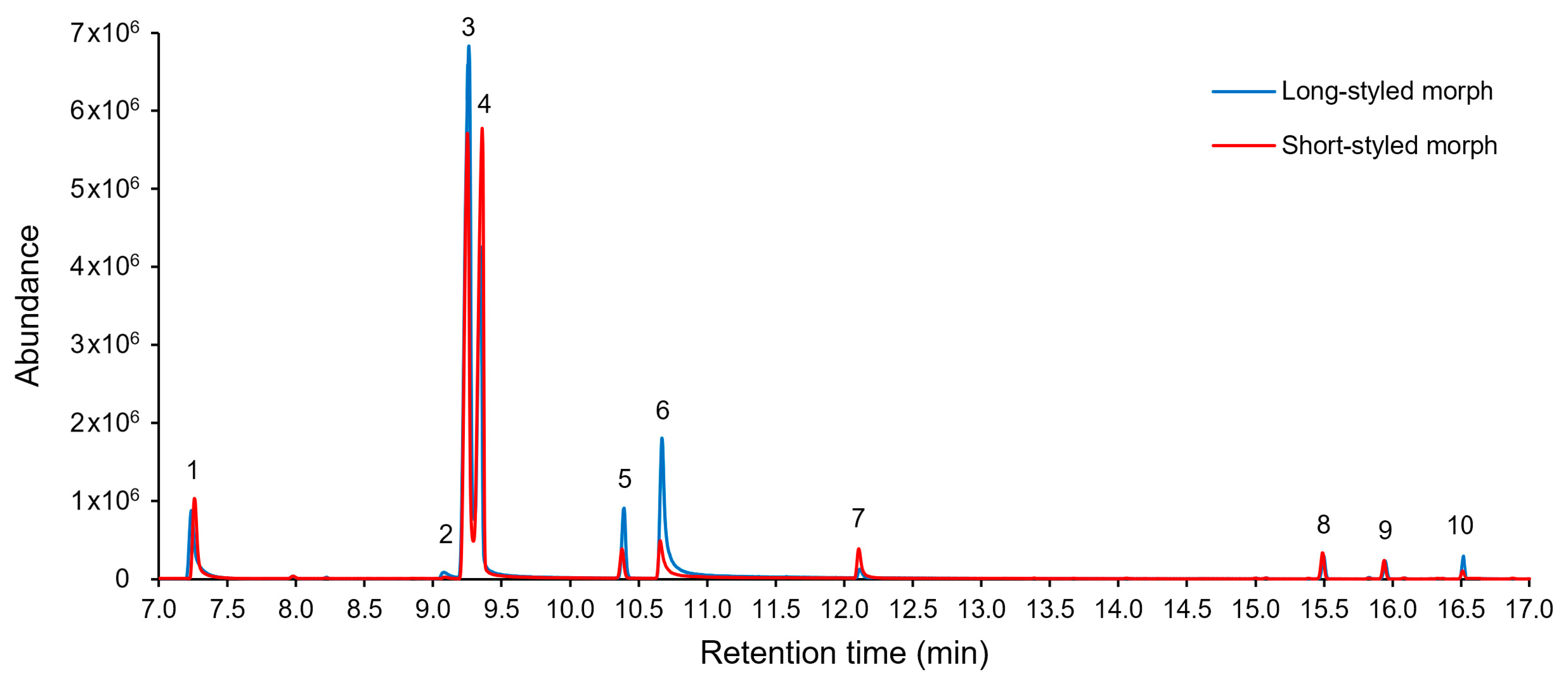
2.2. Composition of Floral Scent
3. Discussion
3.1. Scent Emission within the Flower
3.2. Floral Scent Composition
4. Materials and Methods
4.1. Study Species
4.2. Plant Material
4.3. Localization of Scent-Emitting Regions within the Flower
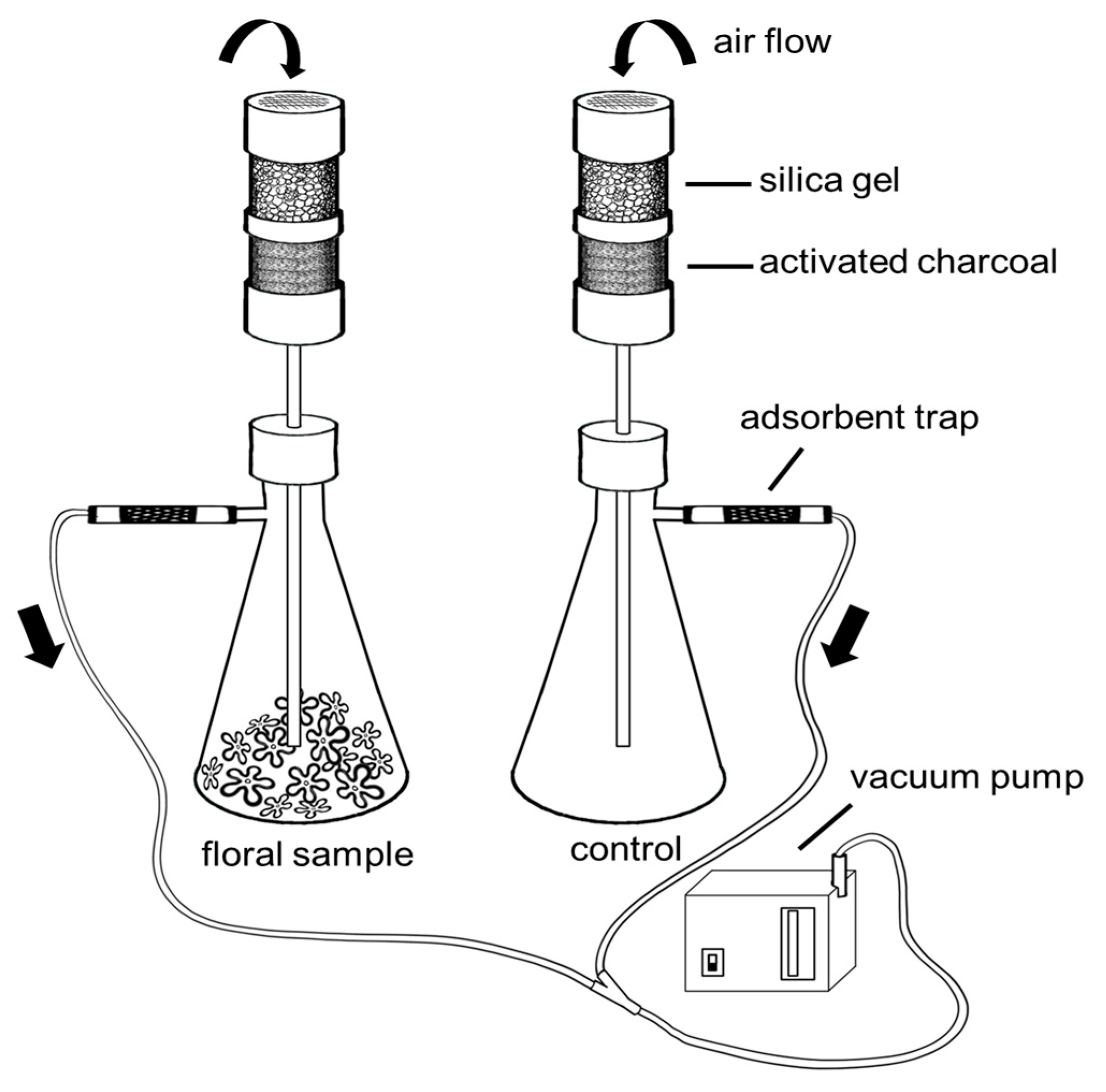
4.4. Collection and Chemical Analysis of Floral Scents
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Borges, R.M.; Somanathan, H.; Kelber, A. Patterns and processes in nocturnal and crepuscular pollination services. Q. Rev. Biol. 2016, 91, 389–418. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.E.M. Floral volatiles in insect biology. In Insect–Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1994; Volume 5, pp. 47–81. [Google Scholar]
- Dudareva, N.; Piechulla, B.; Pichersky, E. Biogenesis of floral scent. Hortic. Rev. 1999, 24, 31–54. [Google Scholar]
- Dudareva, N.; Pichersky, E. Floral scent metabolic pathways: Their regulation and evolution. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 55–78. [Google Scholar]
- Effmert, U.; Große, J.; Röse, U.S.; Ehrig, F.; Kägi, R.; Piechulla, B. Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae). Am. J. Bot. 2005, 92, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kawakita, A.; Kato, M. Floral adaptations to nocturnal moth pollination in Diplomorpha (Thymelaeaceae). Plant Species Biol. 2008, 23, 192–201. [Google Scholar] [CrossRef]
- Powers, J.M.; Seco, R.; Faiola, C.L.; Sakai, A.K.; Weller, S.G.; Campbell, D.R.; Guenther, A. Floral scent composition and fine-scale timing in two moth-pollinated Hawaiian Schiedea (Caryophyllaceae). Front. Plant Sci. 2020, 11, 1116. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Raguso, R.A.; Knudsen, J.T.; Ayasse, M. Scent as an attractant. In Practical Pollination Biology; Dafni, A., Kevan, P.G., Husband, B.C., Eds.; Enviroquest Ltd.: Cambridge, ON, Canada, 2005; pp. 197–230. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents: A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Gershenzon, J. The chemical diversity of floral scent. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 27–52. [Google Scholar]
- Johnson, B.O.; Golonka, A.M.; Blackwell, A.; Azquez, I.V.; Wolfram, N. Floral scent variation in the heterostylous species Gelsemium sempervirens. Molecules 2019, 24, 2818. [Google Scholar] [CrossRef]
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 147–198. [Google Scholar]
- Knudsen, J.T.; Tollsten, L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth-pollinated taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Miyake, T.; Yamaoka, R.; Yahara, T. Floral scents of hawkmoth-pollinated flowers in Japan. J. Plant Res. 1998, 111, 199–205. [Google Scholar] [CrossRef]
- Cunningham, J.P.; Moore, C.J.; Zalucki, M.P.; West, S.A. Learning, odour preference and flower foraging in moths. J. Exp. Biol. 2004, 207, 87–94. [Google Scholar] [CrossRef]
- Riffell, J.A. The neuroecology of a pollinator’s buffet: Olfactory preferences and learning in insect pollinators. Integr. Comp. Biol. 2011, 51, 781–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelber, A. Pattern discrimination in a hawkmoth: Innate preferences, learning performance and ecology. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 2573–2577. [Google Scholar] [CrossRef]
- Riffell, J.A.; Alarcón, R.; Abrell, L.; Davidowitz, G.; Bronstein, J.L.; Hildebrand, J.G. Behavioral consequences of innate preferences and olfactory learning in hawkmoth–flower interactions. Proc. Natl. Acad. Sci. USA 2008, 105, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Agrawal, A.A. Learning in insect pollinators and herbivores. Annu. Rev. Entomol. 2017, 62, 53–71. [Google Scholar] [CrossRef]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Biology of Trees Native to Tropical Florida; Harvard University Printing Office: Allston, MA, USA, 1980. [Google Scholar]
- Snyder, J.R.; Herndon, A.; Robertson, W.B., Jr. South Florida Rockland. In Ecosystems of Florida; Myers, R.L., Ewel, J.J., Eds.; University of Central Florida Press: Orlando, FL, USA, 1990; pp. 230–274. [Google Scholar]
- Koptur, S. The conservation of specialized and generalized pollination systems in subtropical ecosystems: A case study. In Plant–Pollinator Interactions: From Specialization to Generalization; Waser, N., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 341–361. [Google Scholar]
- Possley, J.; Woodmansee, S.W.; Maschinski, J. Patterns of plant composition in fragments of globally imperiled pine rockland forest: Effects of soil type, recent fire frequency, and fragment size. Nat. Areas J. 2008, 28, 379–394. [Google Scholar] [CrossRef]
- Florida Natural Areas Inventory (FNAI). Pine Rockland. In Guide to the Natural Communities of Florida; Florida Natural Areas Inventory: Tallahassee, FL, USA, 2010; pp. 69–72. [Google Scholar]
- Jones, I.M.; Koptur, S. Dead land walking: The value of continued conservation efforts in South Florida’s imperiled pine rocklands. Biodivers. Conserv. 2017, 26, 3241–3253. [Google Scholar] [CrossRef]
- Faegri, K.; van der Pijl, L. The Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Bawa, K.S.; Bullock, S.H.; Perry, D.R.; Coville, R.E.; Grayum, M.H. Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. Am. J. Bot. 1985, 72, 346–356. [Google Scholar] [CrossRef]
- Haber, W.A.; Frankie, G.W. A tropical hawkmoth community: Costa Rican dry forest Sphingidae. Biotropica 1989, 21, 155–172. [Google Scholar] [CrossRef]
- Pimienta, M.C. Floral Ecology of a Night-Blooming Plant in Its Disappearing Habitat: A Comprehensive Study of Flower Visitors, Pollination, and Floral Scent in Guettarda scabra (Rubiaceae). PhD Dissertation, Florida International University, Miami, FL, USA, 2023. [Google Scholar]
- Pimienta, M.C.; Koptur, S. More than moths: Flower visitors of a night-blooming plant in south Florida pine rocklands, USA. Plants 2022, 11, 2799. [Google Scholar] [CrossRef]
- Watanabe, K.; Kato, H.; Kuraya, E.; Sugawara, T. Pollination and reproduction of Psychotria homalosperma, an endangered distylous tree endemic to the oceanic Bonin (Ogasawara) Islands, Japan. Plant Species Biol. 2018, 33, 16–27. [Google Scholar] [CrossRef]
- Maruyama, P.K.; Amorim, F.W.; Oliveira, P.E. Night and day service: Distyly and mixed pollination system in Faramea cyanea (Rubiaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 818–824. [Google Scholar] [CrossRef]
- Wolff, D.; Braun, M.; Liede, S. Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): A sphingophilous plant visited by hummingbirds. Plant Biol. 2003, 5, 71–78. [Google Scholar] [CrossRef]
- Lorence, D.H.; Wagner, W.L.; Laidlaw, W.G. Kadua haupuensis (Rubiaceae: Spermacoceae), a new endemic species from Kaua’i, Hawaiian Islands. Brittonia 2010, 62, 137–144. [Google Scholar] [CrossRef]
- de Avila, R.S.; Freitas, L. Frequency of visits and efficiency of pollination by diurnal and nocturnal lepidopterans for the dioecious tree Randia itatiaiae (Rubiaceae). Aust. J. Bot. 2011, 59, 176–184. [Google Scholar] [CrossRef]
- Effmert, U.; Buss, D.; Rohrbeck, D.; Piechulla, B. Localization of the synthesis and emission of scent compounds within the flower. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 105–124. [Google Scholar]
- Dobson, H.E.M.; Bergström, G.; Groth, I. Differences in fragrance chemistry between flower parts of Rosa rugosa Thunb. (Rosaceae). Isr. J. Plant Sci. 1990, 39, 143–156. [Google Scholar]
- Bergström, G.; Dobson, H.E.M.; Groth, I. Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae). Plant Syst. Evol. 1995, 195, 221–242. [Google Scholar] [CrossRef]
- Terra-Araujo, M.H.; Webber, A.C.; Vicentini, A. Pollination of Pagamea duckei Standl. (Rubiaceae): A functionally dioecious species. Biota Neotrop. 2012, 12, 98–104. [Google Scholar] [CrossRef]
- Rossi, A.A.B.; Oliveira, L.O.D.; Vieira, M.F. Distyly and variation in floral traits in natural populations of Psychotria ipecacuanha (Brot.) Stokes (Rubiaceae). Rev. Bras. Bot. 2005, 28, 285–294. [Google Scholar] [CrossRef][Green Version]
- Castro, C.C.; Oliveira, P.E.A.M.; Pimentel, R.M. Reproductive biology of the herkogamous vine Chiococca alba (L.) Hitchc. (Rubiaceae) in the Atlantic Rain Forest, SE Brazil. Rev. Bras. Bot. 2008, 31, 317–321. [Google Scholar] [CrossRef][Green Version]
- Novo, R.R.; Consolaro, H.; Almeida, N.M.; Castro, C.C. Floral biology of the velvetseed Guettarda platypoda DC. (Rubiaceae): Atypical distyly or style dimorphism? Flora Morphol. Distrib. Funct. Ecol. Plants 2018, 239, 62–70. [Google Scholar] [CrossRef]
- Vogel, S.; Hadacek, F. Contributions to the functional anatomy and biology of Nelumbo nucifera (Nelumbonaceae) III. An ecological reappraisal of floral organs. Plant Sys. Evol. 2004, 249, 173–189. [Google Scholar] [CrossRef]
- Haverkamp, A.; Yon, F.; Keesey, I.W.; Mißbach, C.; Koenig, C.; Hansson, B.S.; Baldwin, I.T.; Knaden, M.; Kessler, D. Hawkmoths evaluate scenting flowers with the tip of their proboscis. eLife 2016, 5, e15039. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedove, R.; Schatz, B.; Dufay, M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 1–20. [Google Scholar] [CrossRef]
- Liu, C.L.; Jia, Y.; Li, Y.F.; Xiang, Y.F.; Pan, Y.Z.; Liu, Q.L.; Ma, K.H.; Yin, X.C. The rapid appearance of homostyly in a cultivated distylous population of Primula forbesii. Ecol. Evol. 2022, 12, e9515. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yi, Y. Features of floral odor and nectar in the distylous Luculia pinceana (Rubiaceae) promote compatible pollination by hawkmoths. Ecol. Evol. 2023, 13, e9920. [Google Scholar] [CrossRef]
- Richards, J.H.; Koptur, S. Floral variation and distyly in Guettarda scabra (Rubiaceae). Am. J. Bot. 1993, 80, 31–40. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef]
- Raguso, R.A.; Pichersky, E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): Recent evolution of floral scent and moth pollination. Plant Sys. Evol. 1995, 194, 55–67. [Google Scholar] [CrossRef]
- Kite, G.C.; Leon, C. Volatile compounds emitted from flowers and leaves of Brugmansia × candida (Solanaceae). Phytochemistry 1995, 40, 1093–1095. [Google Scholar] [CrossRef]
- Levin, R.A.; Raguso, R.A.; McDade, L.A. Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry 2001, 58, 429–440. [Google Scholar] [CrossRef]
- Steen, R.; Norli, H.R.; Thöming, G. Volatiles composition and timing of emissions in a moth-pollinated orchid in relation to hawkmoth (Lepidoptera: Sphingidae) activity. Arthropod Plant Interact. 2019, 13, 581–592. [Google Scholar] [CrossRef]
- Kantsa, A.; Garcia, J.E.; Raguso, R.A.; Dyer, A.G.; Steen, R.; Tscheulin, T.; Petanidou, T. Intrafloral patterns of color and scent in Capparis spinosa L. and the ghosts of its selection past. Am. J. Bot. 2023, 110, e16098. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, A.; Witt, T.; Gottsberger, G. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem. Syst. Ecol. 2003, 31, 345–357. [Google Scholar] [CrossRef]
- Setzer, W.N.; Noletto, J.A.; Haber, W.A. Chemical composition of the floral essential oil of Psychotria eurycarpa from Monteverde, Costa Rica. J. Essent. Oil-Bear. Plants 2006, 9, 28–31. [Google Scholar] [CrossRef]
- Setzer, W.N.; Noletto, J.A.; Lawton, R.O. Chemical composition of the floral essential oil of Randia matudae from Monteverde, Costa Rica. Flavour Fragr. J. 2006, 21, 244–246. [Google Scholar] [CrossRef]
- Lawton, R.O.; Alexander, L.D.; Setzer, W.N.; Byler, K.G. Floral essential oil of Guettarda poasana inhibits yeast growth. Biotropica 1993, 25, 483–486. [Google Scholar] [CrossRef]
- Dobson, H.E.M. Analysis of flower and pollen volatiles. In Essential Oils and Waxes: Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 231–251. [Google Scholar] [CrossRef]
- Allison, J.; Borden, J.; Seybold, S. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Toshova, T.B.; Subchev, M.; Abaev, V.; Vuts, J.; Imrei, Z.; Koczor, S.; Galli, Z.; van de Ven, R.; Toth, M. Responses of Pseudovadonia livida adults to olfactory and visual cues. Bull. Insectology 2016, 69, 161–172. [Google Scholar]
- Andersson, S.; Dobson, H.E.M. Behavioral foraging responses by the butterfly Heliconius melpomene to Lantana camara floral scent. J. Chem. Ecol. 2003, 29, 2303–2318. [Google Scholar] [CrossRef]
- Giurfa, M.; Vorobyev, M.; Kevan, P.; Menzel, R. Detection of coloured stimuli by honeybees: Minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 1996, 178, 699–709. [Google Scholar] [CrossRef]
- Dafni, A.; Lehrer, M.; Kevan, P.G. Spatial flower parameters and insect spatial vision. Biol. Rev. 1997, 72, 239–282. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee–flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Pemberton, R.W.; Wheeler, G.S. Orchid bees don’t need orchids: Evidence from the naturalization of an orchid bee in Florida. Ecology 2006, 87, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; Heidinger, I.M.; Blüthgen, N. Floral scent terpenoids deter the facultative florivore Metrioptera bicolor (Ensifera, Tettigoniidae, Decticinae). J. Orthoptera Res. 2010, 19, 69–74. [Google Scholar] [CrossRef]
- Galen, C.; Kaczorowski, R.; Todd, S.L.; Geib, J.; Raguso, R.A. Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the alpine skypilot Polemonium viscosum. Am. Nat. 2011, 177, 258–272. [Google Scholar] [CrossRef]
- Kessler, D.; Diezel, C.; Clark, D.G.; Colquhoun, T.A.; Baldwin, I.T. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecol. Lett. 2013, 16, 299–306. [Google Scholar] [CrossRef]
- Acevedo-Rodríguez, P.; Strong, M.T. Catalogue of Seed Plants of the West Indies; Smithsonian Institution: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Roberts, A.; Guettarda scabra. The IUCN Red List of Threatened Species. 2014. Available online: https://www.iucnredlist.org/species/56503696/56503850 (accessed on 1 April 2023).
- WCSP: World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. 2023. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30058289-2 (accessed on 1 April 2023).
- Vogel, S. The Role of Scent Glands in Pollination, 1st ed.; Smithsonian Institution: Washington, DC, USA, 1990. [Google Scholar]
- Stern, W.L.; Curry, K.J.; Pridgeon, A.M. Osmophores of Stanhopea (Orchidaceae). Am. J. Bot. 1987, 74, 1323–1331. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 February 2023).

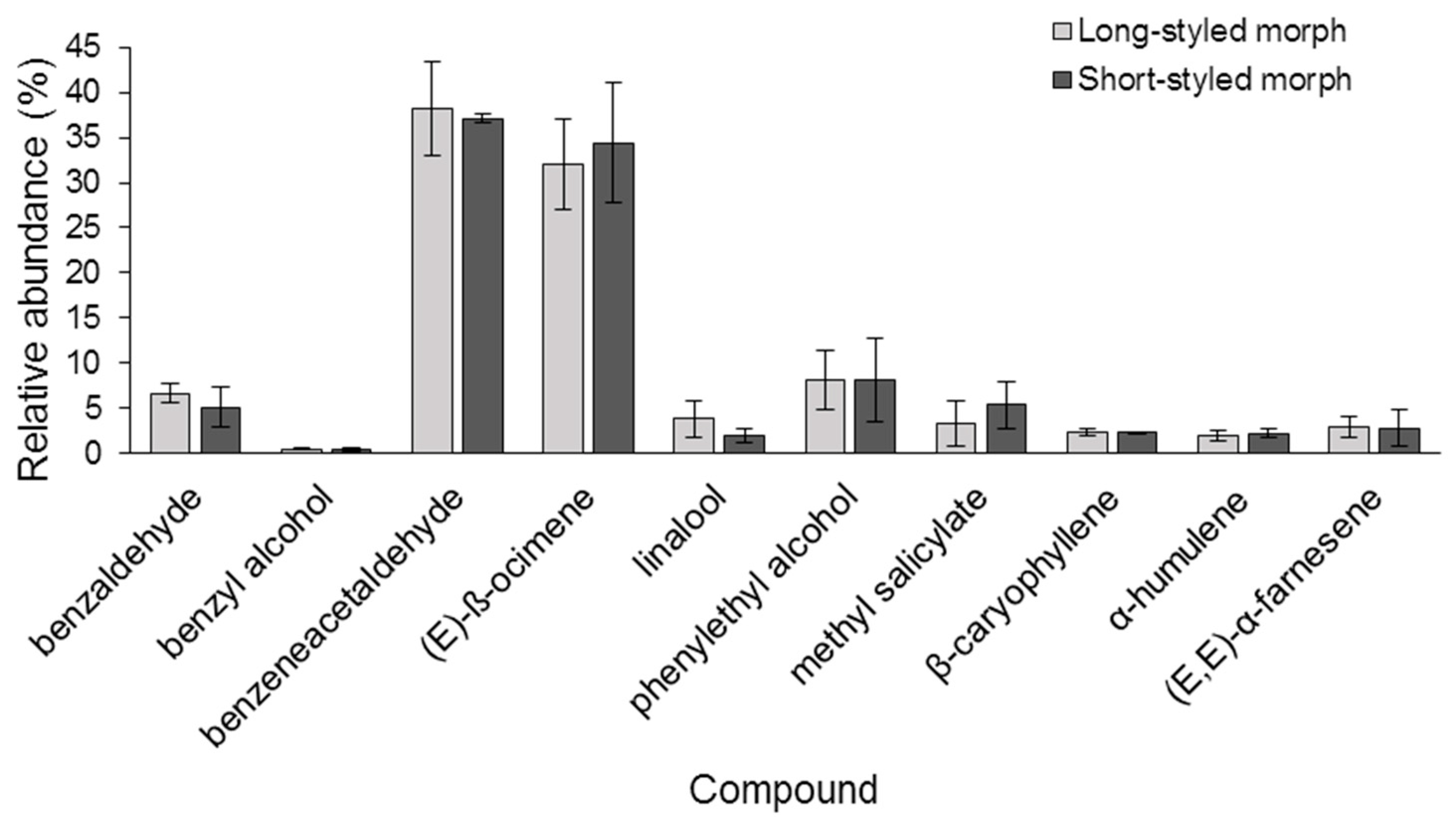
| Chemical Class | Compound * | RT (min) | Long-styled Morph | Short-styled Morph | |||
|---|---|---|---|---|---|---|---|
| ENP (A) | LPT (C) | ENP (B) | LPT (D) | ||||
| Benzenoids | Aldehydes | benzaldehyde | 7.26 | 5.59 | 7.76 | 7.35 | 2.93 |
| benzeneacetaldehyde | 9.25 | 43.46 | 32.92 | 37.64 | 36.56 | ||
| Alcohols | benzyl alcohol | 9.09 | 0.56 | 0.45 | 0.18 | 0.72 | |
| phenylethyl alcohol | 10.67 | 11.48 | 4.79 | 3.47 | 12.70 | ||
| Esters | methyl salicylate | 12.12 | 0.82 | 5.81 | 2.72 | 8.04 | |
| Terpenes | Monoterpenes | (E)-β-ocimene | 9.34 | 27.05 | 37.04 | 41.04 | 27.82 |
| linalool | 10.39 | 5.78 | 1.86 | 2.75 | 1.26 | ||
| Sesquiterpenes | β-caryophyllene | 15.50 | 1.89 | 2.78 | 2.40 | 2.26 | |
| α-humulene | 15.94 | 1.49 | 2.49 | 1.71 | 2.81 | ||
| (E,E)-α-farnesene | 16.52 | 1.88 | 4.09 | 0.74 | 4.90 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimienta, M.C.; Salazar, D.; Koptur, S. The Nighttime Fragrance of Guettarda scabra (Rubiaceae): Flower Scent and Its Implications for Moth Pollination. Molecules 2023, 28, 6312. https://doi.org/10.3390/molecules28176312
Pimienta MC, Salazar D, Koptur S. The Nighttime Fragrance of Guettarda scabra (Rubiaceae): Flower Scent and Its Implications for Moth Pollination. Molecules. 2023; 28(17):6312. https://doi.org/10.3390/molecules28176312
Chicago/Turabian StylePimienta, María Cleopatra, Diego Salazar, and Suzanne Koptur. 2023. "The Nighttime Fragrance of Guettarda scabra (Rubiaceae): Flower Scent and Its Implications for Moth Pollination" Molecules 28, no. 17: 6312. https://doi.org/10.3390/molecules28176312
APA StylePimienta, M. C., Salazar, D., & Koptur, S. (2023). The Nighttime Fragrance of Guettarda scabra (Rubiaceae): Flower Scent and Its Implications for Moth Pollination. Molecules, 28(17), 6312. https://doi.org/10.3390/molecules28176312






