Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions
Abstract
:1. Introduction
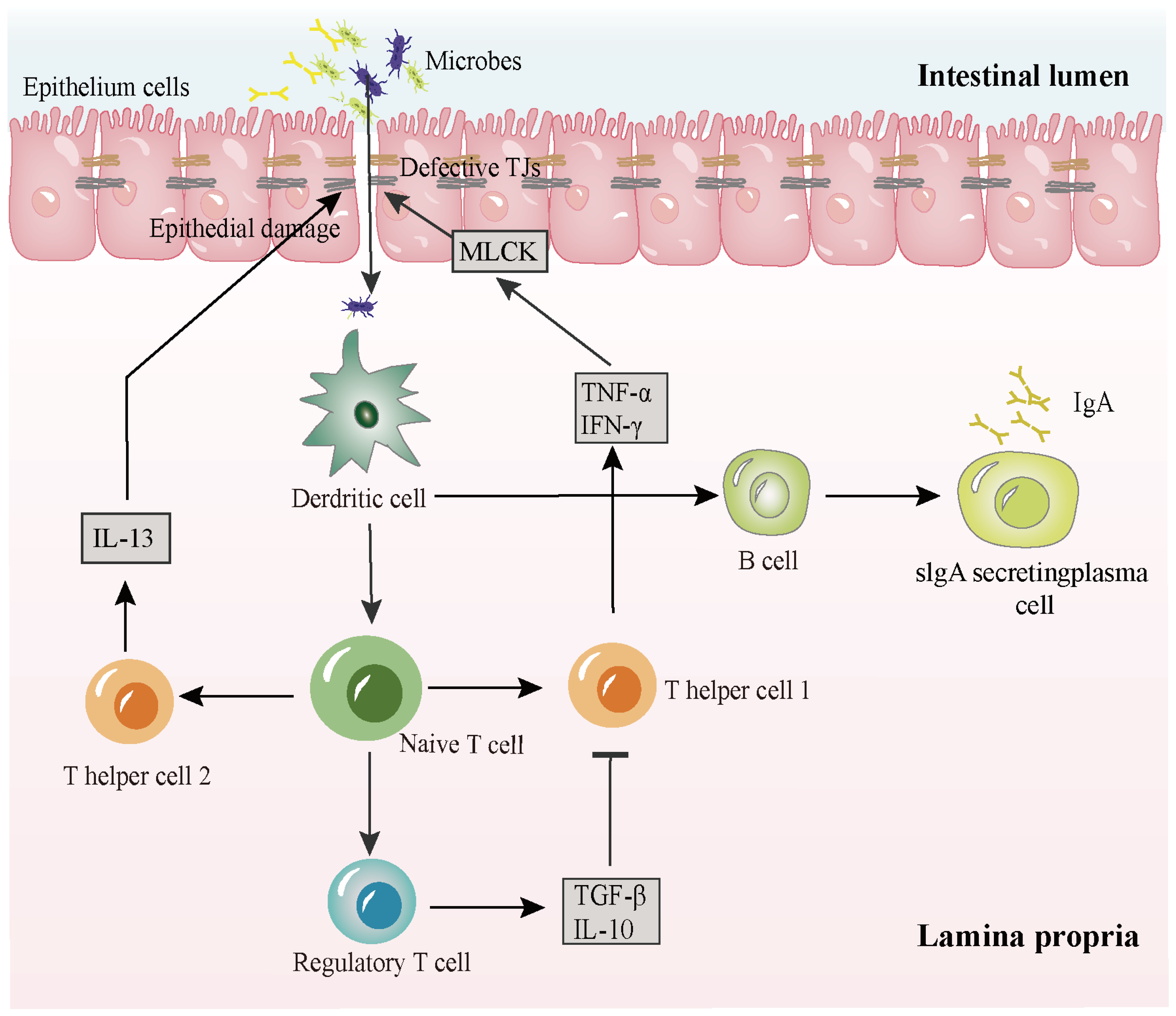
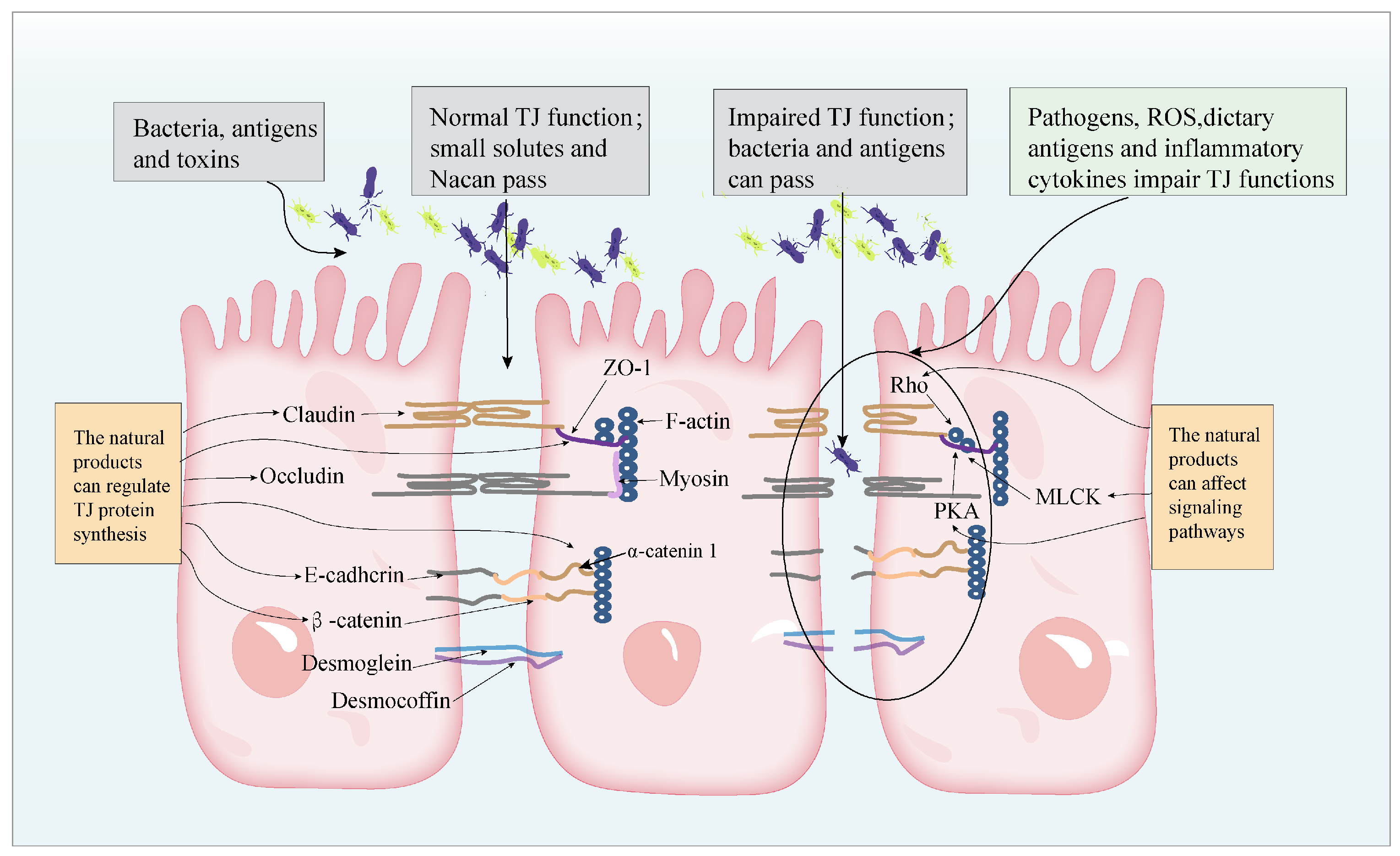
2. The Pathogenesis of IBD
3. Natural Products for the Treatment of IBD
| Classes of Single Bioactive Components | Monomers | Major Plants Present |
|---|---|---|
| Flavonoids | Kaempferol | Fruits, vegetables, and herbs [23] |
| Quercetin | Flowers, leaves, and fruits of plant [24] | |
| Puerarin | Pueraria lobata [25] | |
| Naringin | Grapes and citrus fruits [26] | |
| Icariin | Epimedium [27] | |
| Alpinetin | Alpinia katsumadai Hayata [28] | |
| Baicalin | Scutellaria baicalensis Georgi [29] | |
| Rhein | Rheum rhabarbarum [30] | |
| Terpenoids | Ginsenosides | Ginseng [31] |
| Astragaloside IV | Astragalus membranaceus [32] | |
| Geniposide | Gardenia jasminoides Ellis [33] | |
| Patchouli alcohol | P. cablin [34] | |
| Atractylodes A | Atractylodes macrocephala [35] | |
| Clematichinenoside | Clematis chinensis Osbeck [36] | |
| Oridonin | Rabdosia rubescens [37] | |
| Alkaloids | Berberine | ranunculaceae, rutaceae and berberidaceae [38] |
| Koumine | Gelsemium [39] | |
| Non-flavonoid polyphenols | Curcumin | Turmeric (Zingiberaceae) [40] |
| Resveratrol | Red wine and grape skin [41] | |
| Other classes | Emodin | Rheum palmatum [42] |
| Arctigenin | Fructus Arctii [43] | |
| Sodium houttuyfonate | Houttuynia cordata Thunb [44] | |
| Artemisinin | Artemisia annua L. [45] |
4. Flavonoids for the Treatment of IBD (Table 2)
4.1. Kaempferol
4.2. Quercetin
4.3. Puerarin
4.4. Naringin
4.5. Icariin
4.6. Alpinetin
4.7. Baicalin
4.8. Rhein
| Monomers | Objects (Model Induces) | Effects | Signaling Pathway |
|---|---|---|---|
| Kaempferol | 1. A model of colitis mice induced by dextran sulfate sodium 2. Epithelial-endothelial cells co-culture model 3. Caco-2 cell caused by Deoxynivalenol | 1. ZO-1, occludin, and claudin-1 ↑, IL-1β, IL-6, and TNF-a ↓, IL-10 ↑ 2. TEER ↑, FITC ↓, ZO-1, occludin, and claudin-2 ↑, NF-κB and I-κB ↓ 3. Claudin-3, claudin-4, and occludin ↑, PKA ↑ | 1. Inhibiting the LPS-TLR4-NF-κB pathway [49] 2. Inhibiting NF-κB signaling pathway activation [50] 3. Activating the PKA pathway and deactivation of the MAPK/ERK pathway [51] |
| Quercetin | 1. IEC-6 cell injured by indomethacin 2. A rat intestinal epithelial (IEC-6) cells 3. In a rat model of acute necrotizing pancreatitis (ANP) 4. IPEC-1 incubated with vehicle or diquat | 1. ZO-1, occludin, and claudin-1 ↑ 2. ZO-1, occludin, and claudin-1 ↑ 3. ZO-1, claudin-1, occludin ↑, IL-1β, TNF-α, and IL-17A ↓ 4. ROS ↓, GSH ↑, ZO-1, ZO-2, ZO-3, occludin, and claudin-4 ↑ | 1. Attenuating calcium-mediated JNK/Src activation [52] 2. Inhibiting the RhoA/ROCK signal pathway [53] 3. Inhibiting TLR4/MyD88/p38MAPK and ERS [54] 4. Activating Nrf2 [55] |
| Puerarin | 1. Ethanol-induced Caco-2 monolayer 2. DSS-induced colitis mice | 1. ZO-1, occludin, claudin-1 ↑, NF-κB ↓, MLCK, ERK1 and ERK2 ↑ 2. TNF-α, IL-1β, IL-6 ↓, Nrf2, HO-1, and NQO1 ↑, MDA ↓, CAT, GSH, and SOD ↑, ZO-1, occludin, and claudin-1 ↑ | 1. Activation of the MAPK (ERK1 and ERK2) signal pathway and inhibition of the NF-κB signal pathway [35] 2. Inhibition of NF-κB and activation of the Nrf2 signaling pathway [25] |
| Naringin | 1. CLP mice and lipopolysaccharide (LPS)-stimulated MODE-K cells | 1. TNF-α and IL-6 ↓, IL-10 ↑, ZO-1, and claudin-1 ↑, p65 and IκB-α ↓, P-MLC and MLCK ↓, GTP-RhoA ↓ | 1. Inhibiting the RhoA/ROCK/NF-kappaB/MLCK/MLC signaling pathway [47] |
| Icariin | 1. Bisphenol A(BPA)-exposed mice and MODE-K cells 2. Piglets and IPEC-J2 cell with ETEC K88 | 1. ZO-1, occludin, and claudin-1 ↑, ROS, RNS, MDA, andH2O2 ↓, SOD, GPx, CAT, and T-AOC) ↑ 2. ZO-1 and occludin ↑, IL-1β, IL-6, IL-8, and TNF-α ↓, ROS, MDA, and H2O2 ↓, p38 MAPK ↓ | 1. Inhibiting p38 MAPK [42] 2. Regulating the expression of p38 MAPK [60] |
| Alpinetin | 1. A mouse model of (DSS)-induced ulcerative colitis 2. A mouse model of DSS-induced UC and in TNF-α-stimulated Caco-2 and NCM460 cells | 1. DAI and SOD ↑, MDA ↓, occludin and ZO-1 ↑, claudin-2 ↓ 2. TEER ↑, claudin-7 and occludin ↑ | 1. Activation of the Nrf2/HO-1 signal pathway [62] 2. Regulating the AhR/SUV39H1/TSC2/mTORC1/ autophagy pathway [63] |
| Baicalin | 1. A mouse model of pediatric RV-DH diarrhea | 1. Occludin, claudin-1, and ZO-1↑, IL-1β, IL-2, IL-6, and IL-8 ↓, SIgA ↓ | 1. Inhibiting STAT1 and activating the STAT3 signaling pathways [66] |
| Rhein | 1. An IEC-6 cell model with LPS stimulation 2. A rat model induced by intraperitoneal injection of lipopolysaccharide (LPS) | 1. ZO-1 ↑, p-MLC, MLCK, NF-κB ↓, IL-1β, and IL-6 ↓, TLR4, NLRP3, and cleaved caspase1 ↓, NF-κB ↓ 2. DAO, ZO-1, and occludin ↑, TNF-α, IL-1β, IL-6, and NO ↓, CAT, GSH-Px, and HO-1 ↑, MDA ↓ | 1. Inhibition of the NF-κB/MLCK/p-MLC pathway, TLR4/NF-κB pathway, andNLRP3 inflammasome [68] 2. Inhibiting the MAPKs (p38MAPK and JNK) signaling pathways, activating Nrf2 pathway [69] |
5. Terpenoids for the Treatment of IBD (Table 3)
5.1. Ginsenosides
5.2. Astragaloside IV
5.3. Geniposide
5.4. Patchouli Alcohol
5.5. Atractylodes A
5.6. Atractylodes Clematichinenoside
5.7. Oridonin
| Monomers | Objects (Model Induces) | Effects | Signaling Pathway |
|---|---|---|---|
| Ginsenoside Rg1 | A mouse model of colitis induced by sodium glucan sulfate (DSS) | IL-1β and TNF-α ↓ | Interfering with TLR4-NLRP12-NF-κB [72] |
| Ginsenoside Rk3 | A mouse model of colitis induced by DSS | TNF-α, IL-1β, IL-6, NLRP3, ASC, and Caspase-1 ↓, ZO-1, occludin, and claudin-1 ↑ | Blockading of the NLRP3 inflammasome pathway [73] |
| Astragaloside IV | Septic mice modeled by cecal ligation and puncture (CLP) operation and LPS-challenged Caco-2 monolayer barrier model | Occludin and ZO-1 ↑, Caspase-1, IL-1β, and IL-18 ↓ | Suppressing RhoA/NLRP3 inflammasome signaling [76] |
| Geniposide | Rats with TNBS-induced colitis and Caco-2 cells-induced LPS | TNF-α, IL-1β, and IL-6 ↓, NF-κB, COX-2, iNOS, and MLCK ↓, occludin and ZO-1 ↑, p-AMPK ↑ | Activating the AMPK signaling pathway, inhibiting the MLCK pathway [78] |
| Patchouli alcohol | Rat intestinal mucositis model established by intraperitoneal injection of 5-fluorouracil (5-FU) | TLR2 and MyD88 ↓, NF-κB p-IκBα and p65 ↓, TNF-α, IL-1β, IL-6, and MPO ↓, IL-10 ↑, MLC, ZO-1, occludin, claudin-1, and mucin-2 ↑ | Inhibiting the TLR2/MyD88/NF-κB pathway [79] |
| Atractylodes A | A rat model of spleen deficiency syndrome (SDS) | ZO-1 and occludin ↑, p-p38MAPK and p-MLC ↓ | Inhibition of the p38 MAPK pathway [81] |
| Clematichinenoside AR | In a spontaneous colitis mice model by in interleukin-10 gene knockout (IL-10−/−) | Occludin and ZO-1 ↑, IL-17A+CD4+T cells Bcl-2, caspase-3, and Bax ↓ | Inhibiting the PI3K/Akt signal pathway [84] |
| Oridonin | In a PI-IBS rat model and Caco-2 cell lines | Claudin-1, occludin, and ZO-1 ↑, p-NF-κB, and p65 ↓, iNOS, COX-2, IL-1β, and IL-6 ↓ | Inhibiting PxR/NF-κB signaling [86] |
| Saikosaponin-d | Dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) mice | TNF-α, IL-6, and IL-1β ↓, IL-10 ↑, Muc1 and Muc2 ↑, ZO-1 and Claudin-1 ↑ | Inhibiting NF-κB activation [87] |
| Morroniside and loganin | DSS-induced murine model of colitis and an LPS-induced colorectal cancer (CRC) cell inflammation model | ZO-1, occludin, claudin-3, Ecadherin, and Muc2 ↑, IL-1β, IL-6, TNF-α, and IFN-γ ↓, p-STAT3 and p-p65 ↓ | Blocking of the STAT3/NF-κB pathway [88] |
6. Alkaloids for the Treatment of IBD (Table 4)
6.1. Berberine
6.2. Koumine
| Monomers | Objects (Model Induces) | Effects | Signaling Pathway |
|---|---|---|---|
| Berberine | 1. A model of colitis mice induced with DSS 2. In a mice model of IBS-D established by using 4% acetic acid 3. A rat model of acute endotoxemia induced by injection of lipopolysaccharide (LPS) 4. DSS-induced colitis mice | 1. ZO-1, occludin, and epithelial cadherin ↑, IL-1β, IL-6, and TNF-α ↓, P-STAT3 ↓, MPO ↓, and SOD, CAT ↑ 2. Occludin, claudin-1, ZO-1, and F-actin ↑, TNF-α, NF-kB p65, MLCK, MLC, TRAF6, and RIP1 ↓ 3. Ileal insulin-like growth factor I (IGF-I) and binding protein 3 (IGFBP-3) ↑, occludin and claudin-1 ↑ 4. ZO-1, ZO-2, JAM-A, claudin-1, and occludin ↑, TLR4 and MyD88 ↓, P-IκBα and NF-κB p65 ↓ | 1. Inhibiting the STAT3 signaling pathway [90] 2. Inhibiting the activation of the NF-κB-MLCK pathway [91] 3. Modulation of the Wnt/beta-catenin signaling pathway [93] 4. Blocking the TLR4-MyD88-NF-κB signaling pathway [94] |
| Koumine | 1. IPEC-J2 cells induced by lipopolysaccharide | 1. TNF-α, IL-6, IL-1β, NO, iNOS, and COX-2 ↓, p-IκBα and NF-κB p-p65 ↓, Nrf2 and HO-1 by KEAP-1 ↑, SOD and CAT ↑ | 1. Inhibition of the NF-κB pathway, activating the Nrf2 pathway [96] |
7. Non-Flavonoid Polyphenols for the Treatment of IBD (Table 5)
7.1. Curcumin
7.2. Resveratrol
| Monomers | Objects (Model Induces) | Effects | Signaling Pathway |
|---|---|---|---|
| Curcumin | 1. The human IEC lines Caco-2 and HT-29 induced with LPS 2. H2O2 induced oxidative stress in IPEC-J2 cell and in a piglet’s intestinal oxidative stress model by challenging with diquat 3. BALB/c mice were fed with 3% DSS | 1. MLCK ↓, IL-10 ↑, and IL-1β ↓, p38MAPK ↓, ZO-1, claudin-1, claudin-7, and actin filaments ↑ 2. SOD, CAT, Cu/Zn-SOD, Mn-SOD, GPX-1, and GPX-4 ↑, MDA and ROS ↓, FD4 flux ↓, TER ↑, occludin, ZO-1, and claudin-1, PINK-1 and Parkin ↑ 3. CD4+ Foxp3+regulatory T cells and CD103+CD8α ↑, TNF-α, IL-1β, IL-6, CXCL1, and CXCL2 ↓ | 1. Inhibition p38MAPK [98] 2. Activation of the AMPK-TFEB signal pathway [99] 3. Suppressing NF-κB [100] |
| Resveratrol | 1. IPEC-J2 cell induced by Deoxynivalenol 2. Oxidative stress induced by H2O2 in IPEC-J2 cells | 1. TEER ↑, promoting the assembly of claudin-4, IL-6, and IL-8 ↓, reduced DON-induced phosphorylation of p38, ERK, and JNK 2. claudin-1, occludin, and ZO-1 ↑, superoxide dismutase-1 SOD-1, CAT, and GSH-Px ↑, ROS and apoptosis ↓, p-Akt, p-Nrf2, and HO-1, SOD-1, and CAT ↑ | 1. Suppressing MAPK signaling [101] 2. Inhibiting the PI3K/Akt-mediated Nrf2 signaling pathway [102] |
8. Other Classes of Single Bioactive Components from Herbs for the Treatment of IBD (Table 6)
8.1. Emodin
8.2. Arctigenin
8.3. Sodium Houttuyfonate
8.4. Artemisinin
| Monomers | Objects (Model Induces) | Effects | Signaling Pathway |
|---|---|---|---|
| Emodin | 1. Caco-2 cells induced by LPS/hypoxia-reoxygenation 2. Rat intestinal epithelial cell-6, pancreatitis model rats induced by Taurocholate | 1. ZO-1 ↑, HIF-1α, IκB-α, NF-κB, and COX-2 ↓ 2. Fas, FasL, Bax, caspase-9, and caspase-3 ↓, occludin, ZO-1, and E-cadherin ↑ | 1. Inhibiting the HIF-1α and NF-κB signaling pathways [104] 2. Regulate the activity of the RhoA/ROCK and NOTCH signaling pathways [105] |
| Arctigenin | Colitis mice induced by DSS, TNBS, (Caco-2 and HT-29 cell lines), TNF-α, and IL-1β | Occludin, ZO-1, and F-actin ↑, TEER ↑ | Inhibiting the ERβ-MLCK/MLC pathway [107] |
| Sodium houttuyfonate (SH) | A mouse model of diarrhea induced by Salmonella typhimurium (ST) | TNF-α, IL-1β, and IL-6 ↓, iNOS, COX-2 ↓, p-NF-κBp65, and IκB ↓, the localization and distribution of tight junction proteins ↑ | Inhibiting the NF-κB signaling pathway [108] |
| Artemisinin | A mouse model of ulcerative colitis induced by DSS | Muc2 and claudin-1 ↑, Bcl-2/Bax ↓, cleaved-caspase-3 ↓, p-IκBα and NF-κBp65 ↓, IL-1β, I L-6, and TNF-α ↓, IL-10 ↑ | Inhibiting the NF-κB signaling pathway [111] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2017, 10, CD012839. [Google Scholar]
- Chen, X.; Nie, Y.; Xiao, H.; Bian, Z.; Scarzello, A.J.; Song, N.-Y.; Trivett, A.L.; Yang, D.; Oppenheim, J.J. TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci. Rep. 2016, 6, 34680. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Beloqui, A.; Memvanga, P.B.; Coco, R.; Reimondez-Troitiño, S.; Alhouayek, M.; Muccioli, G.G.; Alonso, M.J.; Csaba, N.; de la Fuente, M.; Préat, V. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids Surf. B Biointerfaces 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-C.; Hanauer, S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst. Rev. 2010, 12, Cd008870. [Google Scholar] [CrossRef]
- Louis, E. Stopping Biologics in IBD-What Is the Evidence? Inflamm. Bowel Dis. 2018, 244, 725–731. [Google Scholar] [CrossRef]
- Corrigendum to A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD Inflamm. Bowel Dis. 2019, 2512, e168. [CrossRef]
- Martin-Subero, M.; Anderson, G.; Kanchanatawan, B.; Berk, M.; Maes, M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2015, 21, 184–198. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan Attenuates Colon Inflammation, Oxidative Perturbations and Apoptosis in a Rat Model of Experimental Inflammatory Bowel Disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 13971, 66–79. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 704, 631–659. [Google Scholar] [CrossRef]
- Stewart, T.; Koval, W.T.; Molina, S.A.; Bock, S.M.; Lillard, J.W.; Ross, R.F.; Desai, T.A.; Koval, M. Calibrated flux measurements reveal a nanostructure-stimulated transcytotic pathway. Exp. Cell Res. 2017, 3552, 153–161. [Google Scholar] [CrossRef] [PubMed]
- John, L.J.; Fromm, M.; Schulzke, J.D. Epithelial Barriers in Intestinal Inflammation. Antioxid. Redox Signal. 2011, 155, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Fries, W.; Belvedere, A.; Vetrano, S. Sealing the Broken Barrier in IBD: Intestinal Permeability, Epithelial Cells and Junctions. Curr. Drug Targets 2013, 1412, 1460–1470. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Wang, X.; Wang, C.; Bao, K.; Ji, L.; Jiang, G.; Hong, M. Calycosin Suppresses Epithelial Derived Initiative Key Factors and Maintains Epithelial Barrier in Allergic Inflammation via TLR4 Mediated NF-kappa B Pathway. Cell. Physiol. Biochem. 2017, 443, 1106–1119. [Google Scholar] [CrossRef]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 1075, 749–762. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 41, 33–46. [Google Scholar] [CrossRef]
- Holmberg, F.E.; Pedersen, J.; Jørgensen, P.; Soendergaard, C.; Jensen, K.B.; Nielsen, O.H. Intestinal barrier integrity and inflammatory bowel disease: Stem cell-based approaches to regenerate the barrier. J. Tissue Eng. Regen. Med. 2018, 124, 923–935. [Google Scholar] [CrossRef] [PubMed]
- López-Posadas, R.; Stürzl, M.; Atreya, I.; Neurath, M.F.; Britzen-Laurent, N. Interplay of GTPases and Cytoskeleton in Cellular Barrier Defects during Gut Inflammation. Front. Immunol. 2017, 8, 9. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-D.; Lee, J.-H.; Lee, Y.-M.; Kim, D.-K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Tsui, V.W.K.; Wong, R.W.K.; Rabie, A.B.M. The inhibitory effects of naringrin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008, 223, 401–406. [Google Scholar] [CrossRef]
- Brown, E.S.; Bice, C.; Putnam, W.C.; Leff, R.; Kulikova, A.; Nakamura, A.; Ivleva, E.I.; Van Enkevort, E.; Holmes, T.; Miingi, N. Human Safety and Pharmacokinetics Study of Orally Administered Icariin: Randomized, Double-Blind, Placebo-Controlled Trial. Nat. Prod. Commun. 2019, 146, 6. [Google Scholar] [CrossRef]
- DU, J.; Tang, B.; Wang, J.; Sui, H.; Jin, X.; Wang, L.; Wang, Z.; Du, J.; Tang, B.; Wang, J.; et al. Antiproliferative effect of alpinetin in BxPC-3 pancreatic cancer cells. Int. J. Mol. Med. 2012, 294, 607–612. [Google Scholar] [CrossRef]
- Zhen-xing, R.E.N.; Meng-liang, W.; Dian-sheng, L.I.U. Study on the Growth and Baicalin Accumulation in Scutellaria Baicalensis. Georg. J. Shanxi Univ. 2009, 323, 468–471. [Google Scholar]
- Zhuang, S.; Yu, R.; Zhong, J.; Liu, P.; Liu, Z. Rhein from Rheum rhabarbarum Inhibits Hydrogen-Peroxide-Induced Oxidative Stress in Intestinal Epithelial Cells Partly through PI3K/Akt-Mediated Nrf2/HO-1 Pathways. J. Agric. Food Chem. 2019, 679, 2519–2529. [Google Scholar] [CrossRef]
- Xiao, S.; Luo, G. Chemical reactions of ginsenosides in red ginseng processing by HPLC/MS/MS Chinese Traditional and Herbal. Drugs 2005, 361, 40–43. [Google Scholar]
- Yan, J.; Ruobing, C. Comparison of contents of astragaloside IV and total saponins in Astragalus membranaceus West China. J. Pharm. Sci. 2007, 223, 322–324. [Google Scholar]
- Zhao, C.; Liu, H.; Yang, J.; Zhang, H.; Yang, M. Determination of Geniposide in Gardenia jasminoides Ellis by TLC-UC Spectrophotometry. Lishizhen Med. Mater. Med. Res. 2012, 2312, 2982–2983. [Google Scholar]
- Cai, J.; Guo, N.; Huang, J.; Li, L.; Ji, S. Quantitative model for patchouli alcohol in Pogostemon cablin by near-infrared spectroscopy. China J. Chin. Mater. Med. 2012, 3714, 2113–2116. [Google Scholar]
- Zhong, N.; Chen, H.; Wang, L. Analysis of volatile compoents from raw product and three processed products of Atractylodes macrocephala by HS-SPME-GC-MS. China J. Tradit. Chin. Med. Pharm. 2022, 379, 5405–5408. [Google Scholar]
- Liu, L.-F.; Ma, X.-L.; Wang, Y.-X.; Li, F.-W.; Li, Y.-M.; Wan, Z.-Q.; Tang, Q.-L. Triterpenoid saponins from the roots of Clematis chinensis Osbeck. J. Asian Nat. Prod. Res. 2009, 115, 389–396. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.; Liu, X.; Wu, Y.; Qu, L. Determination of oridonin in Rabdosia rubescens and its tablets by HPLC West China. J. Pharm. Sci. 2010, 251, 76–77. [Google Scholar]
- Ding, Y.; Ye, X.; Zhou, J.; Luo, S.; Lian, M.; Li, X. Progress in Synthesis and Physiological Activity of Berberine Derivatives. Chin. J. Org. Chem. 2012, 324, 677–685. [Google Scholar] [CrossRef]
- Liu, H.; Shen, J.; Liu, M.; Xu, Y.; Yu, C. Separation and Purification of Koumine from Gelsemium elegans by High-speed Counter-current Chromatography Traditional. Chin. Drug Res. Clin. Plarmacol. 2013, 242, 197–200. [Google Scholar]
- Aditi, P. Curcuminoid content in Curcuma spp.: An overview. Int. Res. J. Pharm. Appl. Sci. 2013, 36, 75–79. [Google Scholar]
- Geana, E.I.; Dinca, O.R.; Ionete, R.E.; Artem, V.; Niculescu, V.C. Monitoring trans-Resveratrol in Grape Berry Skins during Ripening and in Corresponding Wines by HPLC. Food Technol. Biotechnol. 2015, 531, 73–80. [Google Scholar] [CrossRef]
- Fan, Y.; Niu, Z.; Xu, C.; Yang, L.; Yang, T. Protic Ionic Liquids as Efficient Solvents in Microwave-Assisted Extraction of Rhein and Emodin from Rheum palmatum L. Molecules 2019, 24, 2770. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Bao, W.; Zhao, Y.; Guo, H.; Wang, S.; Conh, X. Rapid determination method of the contents of arctiin and arctigenin in Fructus Arctii by mid-infrared spectrometry. Chin. J. New Drugs 2013, 2220, 2431. [Google Scholar]
- Cheng, T.; Xu, C.; Wu, D.; Yan, G.; Wang, C.; Wang, T.; Shao, J. Sodium houttuyfonate derived from Houttuynia cordata Thunb improves intestinal malfunction via maintaining gut microflora stability in Candida albicans overgrowth aggravated ulcerative colitis. Food Funct. 2023, 142, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yue, H.; Kang, C.; Wang, S.; Lv, Z.; Guo, L. Effects of different concentrations of cadmium on biomass, artemisinin content and genes expression in Artemisia annua L. China J. Tradit. Chin. Med. Pharm. 2016, 315, 1887–1892. [Google Scholar]
- Khare, T.; Palakurthi, S.S.; Shah, B.M.; Palakurthi, S.; Khare, S. Natural Product-Based Nanomedicine in Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 3956. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Yang, B.; Zhang, H.; Wang, K.; Liu, Z.; Xiao, X.; Yang, M. Naringin attenuates MLC phosphorylation and NF-kappa B activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed. Pharmacother. 2018, 103, 50–58. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-kappa B Axis. Front. Immunol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2019, 68, 160–167. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, L.; Zhang, G.Y. A proteomic study on the protective effect of kaempferol pretreatment against deoxynivalenol-induced intestinal barrier dysfunction in a Caco-2 cell model. Food Funct. 2020, 118, 7266–7279. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.-R.; Zhang, Q.; Zhao, X.-H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, T.J.; Zhao, X.H. Barrier-promoting efficiency of two bioactive flavonols quercetin and myricetin on rat intestinal epithelial (IEC-6) cells via suppressing Rho activation. RSC Adv. 2020, 1046, 27249–27258. [Google Scholar] [CrossRef] [PubMed]
- Junyuan, Z.; Hui, X.; Chunlan, H.; Junjie, F.; Qixiang, M.; Yingying, L.; Lihong, L.; Xingpeng, W.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 187, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef]
- Venkatashivam, S.; Anuchandra, R.; Mohammad, A.; Amit, D.; Pinaki, G.; Subhash, L. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J. Biomed. Res. 2014, 282, 132–145. [Google Scholar] [CrossRef]
- Liu, P.; Bian, Y.; Fan, Y.; Zhong, J.; Liu, Z. Protective Effect of Naringin on In Vitro Gut-Vascular Barrier Disruption of Intestinal Microvascular Endothelial Cells Induced by TNF-alpha. J. Agric. Food Chem. 2020, 681, 168–175. [Google Scholar] [CrossRef]
- Li, H.-F.; Guan, X.-Y.; Yang, W.-Z.; Liu, K.-D.; Ye, M.; Sun, C.; Lu, S.; Guo, D.-A. Antioxidant flavonoids from Epimedium wushanense. Fitoterapia 2012, 831, 44–48. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. The protective effect of icariin and phosphorylated icariin against LPS-induced intestinal epithelial cells injury. Biomed. Pharmacother. 2019, 118, 109246. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, J.; Li, X.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. Icariin and its phosphorylated derivatives alleviate intestinal epithelial barrier disruption caused by enterotoxigenic Escherichia coli through modulate p38 MAPK in vivo and in vitro. FASEB J. 2020, 341, 1783–1801. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Fu, Y.; Yang, Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS- induced acute colitis. Sci. Rep. 2016, 6, 28370. [Google Scholar] [CrossRef]
- Tan, Y.; Zheng, C.Q. Effects of Alpinetin on Intestinal Barrier Function, Inflammation and Oxidative Stress in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice. Am. J. Med. Sci. 2018, 3554, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lv, Q.; Qiao, S.; Yang, L.; Tao, Y.; Yan, W.; Wang, P.; Cao, N.; Dai, Y.; Wei, Z. Alpinetin improves intestinal barrier homeostasis via regulating AhR/suv39h1/TSC2/mTORC1/autophagy pathway. Toxicol. Appl. Pharmacol. 2019, 384, 114772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 6118, 1391–1398. [Google Scholar] [CrossRef]
- Wu, D.; Ding, L.; Tang, X.; Wang, W.; Chen, Y.; Zhang, T. Baicalin Protects against Hypertension-Associated Intestinal Barrier Impairment in Part Through Enhanced Microbial Production of Short-Chain Fatty Acids. Front. Pharmacol. 2019, 10, 1271. [Google Scholar] [CrossRef]
- Shen, J.; Chen, J.-J.; Zhang, B.-M.; Zhao, J.; Chen, L.; Ye, Q.-Y.; Ling, Q.-H.; Chen, Y.-Y.; Zhong, Z.-Y.; Huang, Q.-W. Baicalin Is Curative against Rotavirus Damp Heat Diarrhea by Tuning Colonic Mucosal Barrier and Lung Immune Function. Dig. Dis. Sci. 2020, 658, 2234–2245. [Google Scholar] [CrossRef]
- Ge, H.; Tang, H.; Liang, Y.; Wu, J.; Yang, Q.; Zeng, L.; Ma, Z. Rhein attenuates inflammation through inhibition of NF-.B and NALP3 inflammasome in vivo and in vitro. Drug Des. Dev. Ther. 2017, 11, 1663–1671. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhong, J.; Zhou, Q.; Zhong, Y.; Liu, P.; Liu, Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int. Immunopharmacol. 2019, 71, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Zhong, J.; Bian, Y.; Fan, Y.; Chen, Q.; Liu, P.; Liu, Z. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019, 216, 168–175. [Google Scholar] [CrossRef]
- Kim, D.H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 361, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Rausch, W.-D. Ginsenosides Rb-1 and Rg(1) effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004, 10211, 41–53. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Wang, T.; Shi, F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int. Immunopharmacol. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, P.; Chen, X.; Yan, J.; Yao, J.; Yu, Z.; Chen, X. Ginsenoside Rb1 protects the intestinal mucosal barrier following peritoneal air exposure. Exp. Ther. Med. 2016, 124, 2563–2567. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 333, 413–416. [Google Scholar] [CrossRef]
- Xie, S.; Yang, T.; Wang, Z.; Li, M.; Ding, L.; Hu, X.; Geng, L. Astragaloside IV attenuates sepsis-induced intestinal barrier dysfunction via suppressing RhoA/NLRP3 inflammasome signaling. Int. Immunopharmacol. 2020, 78, 12. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE(2) and inflammatory cytokine by downregulating NF-kappa B, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 202, 298–306. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.-L.; Xu, M.; Yu, C.-C.; Lian, M.-Q.; Tang, Z.-Y.; Li, C.-X.; Lin, Y. Geniposide ameliorates TNBS-induced experimental colitis in rats via reducing inflammatory cytokine release and restoring impaired intestinal barrier function. Acta Pharmacol. Sin. 2017, 385, 688–698. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Qu, L.; Lin, X.; Xie, Y.; Tu, J.; Liu, X.; Zhou, Z.; Cao, G.; Li, S.; Liu, Y. Deep-Fried Atractylodis Rhizoma Protects against Spleen Deficiency-Induced Diarrhea through Regulating Intestinal Inflammatory Response and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 124. [Google Scholar] [CrossRef]
- Tu, J.; Xie, Y.; Xu, K.; Qu, L.; Lin, X.; Ke, C.; Yang, D.; Cao, G.; Zhou, Z.; Liu, Y. Treatment of Spleen-Deficiency Syndrome with Atractyloside A from Bran-Processed Atractylodes lancea by Protection of the Intestinal Mucosal Barrier. Front. Pharmacol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Peng, C.; Perera, P.K.; Li, Y.-M.; Fang, W.-R.; Liu, L.-F.; Li, F.-W. Anti-inflammatory effects of Clematis chinensis Osbeck extract(AR-6) may be associated with NF-kappa B, TNF-alpha, and COX-2 in collagen-induced arthritis in rat. Rheumatol. Int. 2012, 3210, 3119–3125. [Google Scholar] [CrossRef]
- Han, D.; Fang, W.; Zhang, R.; Wei, J.; Kodithuwakku, N.D.; Sha, L.; Ma, W.; Liu, L.; Li, F.; Li, Y. Clematichinenoside protects blood brain barrier against ischemic stroke superimposed on systemic inflammatory challenges through up-regulating A20. Brain Behav. Immun. 2016, 51, 56–69. [Google Scholar] [CrossRef]
- Song, X.; Li, J.; Wang, Y.; Zhou, C.; Zhang, Z.; Shen, M.; Xiang, P.; Zhang, X.; Zhao, H.; Yu, L.; et al. Clematichinenoside AR ameliorated spontaneous colitis in Il-10(−/−) mice associated with improving the intestinal barrier function and abnormal immune responses. Life Sci. 2019, 239, 117021. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.; Zhang, C.-Y.; Schluesener, H.; Zhang, Z.-Y. Natural Diterpenoid Oridonin Ameliorates Experimental Autoimmune Neuritis by Promoting Anti-inflammatory Macrophages through Blocking Notch Pathway. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Y.; Guo, Y.; Feng, X.-J.; Liu, J.-J.; Chang, Z.-P.; Deng, G.-F.; Xu, D.; Gao, J.-P.; Hou, R.-G. Oridonin Attenuates TNBS-induced Post-inflammatory Irritable Bowel Syndrome via PXR/NF-kappa B Signaling. Inflammation 2021, 442, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Xiong, W.; Li, J.; An, Y.; Ren, J.; Xie, Y.; Xue, H.; Yan, D.; Li, M.; et al. Saikosaponin-d ameliorates dextran sulfate sodium-induced colitis by suppressing NF-kappa B activation and modulating the gut microbiota in mice. Int. Immunopharmacol. 2020, 81, 106288. [Google Scholar] [CrossRef]
- Yuan, J.; Cheng, W.; Zhang, G.; Ma, Q.; Li, X.; Zhang, B.; Hu, T.; Song, G. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-kappa B pathway. Int. Immunopharmacol. 2020, 81, 106240. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a promising novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Atheroscler. Suppl. 2006, 73, 464. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Wang, Y.; Tong, L.-C.; Sun, S.; Liu, W.-Y.; Zhang, S.; Wang, R.-M.; Wang, Z.-B.; Li, L. Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Exp. Ther. Med. 2017, 136, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhu, S.; Zhang, C.; Huang, Y.; Guo, Y.; Li, P.; Chen, X.; Wen, Y.; Han, Q.; Liu, F. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed. Pharmacother. 2019, 118, 109206. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, X.; Zhou, G.; Feng, A. Activation of IGF-1/IGFBP-3 signaling by berberine improves intestinal mucosal barrier of rats with acute endotoxemia. Fitoterapia 2018, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, X.; Zuo, H.; Sun, Y.; Feng, A. Berberine Exerts a Protective Effect on Gut-Vascular Barrier via the Modulation of the Wnt/Beta-Catenin Signaling Pathway during Sepsis. Cell. Physiol. Biochem. 2018, 494, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappa B pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Yuan, Z.; Liang, Z.; Yi, J.; Chen, X.; Li, R.; Wu, Y.; Wu, J.; Sun, Z. Protective Effect of Koumine, an Alkaloid from Gelsemium Sempervirens, on Injury Induced by H2O2 in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 754. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.-L.; Sha, Y.-K.; Wu, Y.; Liu, Z.-Y.; Yuan, Z.-H.; Sun, Z.-L. Koumine Alleviates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction in IPEC-J2 Cells by Regulating Nrf2/NF-κB Pathway. Am. J. Chin. Med. 2020, 48, 127–142. [Google Scholar] [CrossRef]
- Dibaei, M.; Rouini, M.-R.; Sheikholeslami, B.; Gholami, M.; Dinarvand, R. The effect of surface treatment on the brain delivery of curcumin nanosuspension: In vitro and in vivo studies. Int. J. Nanomed. 2019, 14, 5477–5490. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol.-Cell Physiol. 2017, 3124, C438–C445. [Google Scholar] [CrossRef]
- Cao, S.T.; Wang, C.C.; Yan, J.T.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 1210, e0185999. [Google Scholar] [CrossRef]
- Ling, K.-H.; Wan, M.L.Y.; El-Nezami, H.; Wang, M. Protective Capacity of Resveratrol, a Natural Polyphenolic Compound, against Deoxynivalenol-Induced Intestinal Barrier Dysfunction and Bacterial Translocation. Chem. Res. Toxicol. 2016, 295, 823–833. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Chen, D.; Xiong, Y.; Wang, L.; Lv, B.; Lin, Y. Characteristics of emodin on modulating the contractility of jejunal smooth muscle. Can. J. Physiol. Pharmacol. 2012, 904, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Qiang, F.; Chao, D.; DI, W.; Guoqian, Z.; Bo, Y.; Lina, Y. Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-kappa B and HIF-1 alpha signaling pathways. Int. J. Mol. Med. 2014, 346, 1629–1639. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Wu, H.-Y.; Xia, J.; Zhang, H.-B.; Liu, M.-W.; Qian, C.-Y. Effects of emodin on intestinal mucosal barrier by the upregulation of miR-218a-5p expression in rats with acute necrotizing pancreatitis. Int. J. Immunopathol. Pharmacol. 2020, 34, 18. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Jung, S.Y.; Back, S.Y.; Do, J.-R.; Shon, D.-H. Arctigenin from Fructus Arctii (Seed of Burdock) Reinforces Intestinal Barrier Function in Caco-2 Cell Monolayers. Evid.-Based Complement. Altern. Med. 2015, 2015, 368105. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yue, M.; Lv, C.; Yun, X.; Qiao, S.; Fang, Y.; Wei, Z.; Xia, Y.; Dai, Y. Pharmacological activation of ER beta by arctigenin maintains the integrity of intestinal epithelial barrier in inflammatory bowel diseases. FASEB J. 2020, 342, 3069–3090. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, H.; Li, Y.; Dong, N.; Bi, C.; Shan, A.; Wu, Z.; Shi, B. Sodium houttuyfonate enhances the intestinal barrier and attenuates inflammation induced by Salmonella typhimurium through the NF-kappa B pathway in mice. Int. Immunopharmacol. 2020, 89, 107058. [Google Scholar] [CrossRef]
- Tu, Y.Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 1710, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, J.; Yang, C.; Gao, Y.; Li, X.; Chen, X.; Peng, Y.; Fang, J.; Xiao, S. Immunomodulatory and Anti-inflammatory Properties of Artesunate in Experimental Colitis. Curr. Med. Chem. 2012, 1926, 4541–4551. [Google Scholar] [CrossRef]
- Yin, S.; Yang, H.; Tao, Y.; Wei, S.; Li, L.; Liu, M.; Li, J. Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response. Inflammation 2020, 432, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Tea polyphenol (-)-epigallocatechin-3-gailate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines Cancer. Epidemiol. Biomark. Prev. 2003, 1211, 1366S. [Google Scholar]
- Karatzas, P.S.; Gazouli, M.; Safioleas, M.; Mantzaris, G.J. DNA methylation changes in inflammatory bowel disease. Ann. Gastroenterol. 2014, 272, 125–132. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Li, H.; Olaolu, O.A.; Ibrahim, S.; Ibrahim, S.; Wang, S. Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions. Molecules 2023, 28, 6293. https://doi.org/10.3390/molecules28176293
Peng J, Li H, Olaolu OA, Ibrahim S, Ibrahim S, Wang S. Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions. Molecules. 2023; 28(17):6293. https://doi.org/10.3390/molecules28176293
Chicago/Turabian StylePeng, Jing, Hao Li, Oladejo Ayodele Olaolu, Saber Ibrahim, Sally Ibrahim, and Shengyi Wang. 2023. "Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions" Molecules 28, no. 17: 6293. https://doi.org/10.3390/molecules28176293
APA StylePeng, J., Li, H., Olaolu, O. A., Ibrahim, S., Ibrahim, S., & Wang, S. (2023). Natural Products: A Dependable Source of Therapeutic Alternatives for Inflammatory Bowel Disease through Regulation of Tight Junctions. Molecules, 28(17), 6293. https://doi.org/10.3390/molecules28176293








