Abstract
Switchable nonlinear optical (NLO) materials have widespread applications in electronics and optoelectronics. Thermo-switches generate many times higher NLO responses as compared to photo-switches. Herein, we have investigated the geometric, electronic, and nonlinear optical properties of spiropyranes thermochromes via DFT methods. The stabilities of close and open isomers of selected spiropyranes are investigated through relative energies. Electronic properties are studied through frontier molecular orbitals (FMOs) analysis. The lower HOMO-LUMO energy gap and lower excitation energy are observed for open isomers of spiropyranes, which imparts the large first hyperpolarizability value. The delocalization of π-electrons, asymmetric distribution and elongated conjugation system are dominant factors for high hyperpolarizability values of open isomers. For deep understanding, we also analyzed the frequency-dependent hyperpolarizability and refractive index of considered thermochromes. The NLO response increased significantly with increasing frequency. Among all those compounds, the highest refractive index value is observed for the open isomer of the spiropyran 1 (1.99 × 10−17 cm2/W). Molecular absorption analysis confirmed the electronic excitation in the open isomers compared to closed isomers. The results show that reversible thermochromic compounds act as excellent NLO molecular switches and can be used to design advanced electronics.
1. Introduction
Since the 1970s, the designing and synthesis of thermochromic materials have been an important interdisciplinary research field because of their excellent efficiency in changing their characteristics concerning temperature [,]. Several applications of thermochromic materials have been reported, such as in cosmetics [], as food quality indicators, forehead thermometers [,], thermal mapping, refrigeration thermometers, thermochromic sensors [,], memory devices and cholesteric liquid crystals (which are used in biomedical therapy) [] etc.
Thermochromes are classified as reversible or irreversible []. Irreversible thermochromic materials cannot return to their original state []. Although there are several applications of irreversible thermochromic materials in different fields but in most cases, thermochromism reversibility is considered a vital requirement. Crystal violet lactone is the most essential class of reversible thermochromism []. Towns et al. reported reversible spiropyranes. Those convert from colorful to colorless complexes with respect to changes in temperature []. Spiropyranes are extensively studied due to their structural variability and clear color change under temperature variations [], upon heating, the closed isomer of spiropyran changes to an unstable open isomer having a specific color. The color change depicts the electronic excitation in these thermochromes due to extended conjugation and unequal electronic distribution.
The literature revealed the generation of optical and NLO properties of compounds if electronic excitations occur [,,,,,]. For example, the NLO response of photochemical/thermochemical interconversion of substituted dihydroazulene (DHA) to vinylheptafuluene (VHF) was analyzed by Pozzo and coworkers. Electron withdrawing group (NO2) has a pronounced effect on the first hyperpolarizability compared to the electron donating group (NH2) []. Previtali et al. synthesized different polymorphs of cyclic triimidazle pyrene bio probe. They observed that they have tunable linear and nonlinear optical properties where one of the polymorphs TTPyr(HT), obtained at high temperature, has ten times higher second harmonic generation efficiency than urea []. Sliwa et al. synthesized N-(4-hydroxy)-salicylidene-amino-4-(methylbenzoate) and N-(3,5-di-tert-butylsalicylidene)-4-aminopyridine. Upon irradiation with UV light using TD-DFT analysis, they change color and possess better NLO response than standard urea []. Photochromic interconversion between spiropyran and merocyanine results in a large light switchable change in the second and third-order nonlinearity [,].
Le-Bozec and co-workers thoroughly studied the photochromic transformation of 4,4′-bis(ethenyl)-2,2′-bipyridine ligands functionalized by a dimethylaminophenyl–dithienylethene group and the corresponding zinc (II) complex. The compound efficiently undergoes reversible interconversion into open and closed forms, where the closed form shows outstanding NLO response due to π-conjugation (which is absent in open form) []. They also designed several switching photochromes with excellent ON/OFF photoswitchable NLO response []. Jing et al. computationally explored the NLO response of photochromic dihydroazulene/vinylheptafulvene with Ru-based metal complexes. They observed the largest second-order nonlinear response for an open form of these complexes which was further increased with frequency []. This detailed survey illustrates several synthetic and computational reports on reversible NLO photoswitches in literature []. But computational work on reversible NLO thermoswitches is not explored yet. Keeping in view the importance of thermochromic materials, in this study, we have performed a DFT analysis of the NLO response of open and closed isomers of Spiropyranes-based reversible thermochromes (See Figure 1).
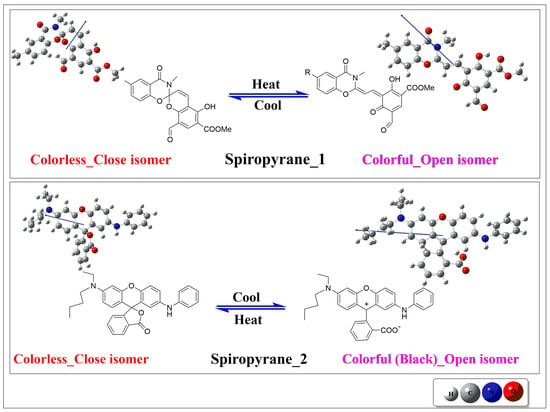
Figure 1.
The close and open isomers of spiropyranes 1 [] (top where R = Methyl group) & 2 (bottom) [].
2. Computational Details
All calculations were performed by using Gaussian-09 software []. The selected structures of compounds were optimized at ωB97XD/6-311+G(d, p) method. The selection of ωB97XD density functional captures long-range and short-range interactions in a system and intersystem charge transfer property. The Pople’s 6-311+G (d, p) basis set gives an idea about the localization of electronic density in orbitals [,,]. After optimization, frequency calculations are performed at the same level of theory to verify true optimization, which is confirmed by the absence of any imaginary frequency. Thus, after obtaining the optimized geometries of thermochromic compounds, we investigated the relative stability of open and closed forms by considering the energy difference (closed/open isomer) at the same level of theory. Moreover, for all other properties, ωB97XD/6-311+G (d, p) method is used. GaussView 5.0 is used for the visualization of structures [].
NBO charge analysis is also performed to know the charge transfer in molecules []. Frontier molecular orbital (FMO) analysis was performed to calculate molecular species’ electronic stability and chemical reactivity. The dipole moment of open and closed isomers was calculated at above mention level of theory. TD-DFT calculations were carried out at ωB97XD/6-311+G(d, p) method to determine the spectrum region where these thermochromic compounds show absorbance and transparency. Moreover, these thermochromic compounds’ nonlinear optical (NLO) properties of open and closed isomers were calculated at the ωB97XD/6-311+G(d, p) method. ωB97XD is a long-range corrected functional method and is best for calculating NLO properties [,,,]. Linear optical property polarizability (αo) and nonlinear optical properties (static first hyperpolarizability (βo), frequency-dependent first, second hyperpolarizability and reflective index were calculated at the above-mentioned level of theory.
Mathematical expressions for polarizability (αo) and static first hyperpolarizability (βo) are given below:
where,
αo = 1/3(αxx + αyy + αzz)
βo = [βx2 + βy2 + βz2]½
βx = βxxx + βxxy + βxxz, βy = βyyy + βyzz + βyxx and βz = βzzz + βzxx + βzyy
The frequency-dependent nonlinear optical analysis was investigated at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au). Frequency-dependent hyperpolarizability contains the approximation of electro-optic Pockel’s effect β(−ω;ω,0) and second harmonic generation β(−2ω; ω, ω). For estimation of frequency-dependent second hyperpolarizability (γ), dc-Kerr effect (ω) = γ(−ω; ω,0,0) and electric field induced second harmonic generation (ω) = γ(−2ω; ω, ω,0) and degenerate four-wave mixing are calculated. These values were also used to estimate the refractive index of the respective compounds.
3. Results and Discussion
3.1. Geometric and Structural Properties of Close and Open Isomers of Spiropyranes
The geometric stability of close and open isomers of spiropyranes is estimated from their geometric properties. The optimized geometries are given in Figure 2, and the geometric parameters of spiropyranes are given in Table 1. Optimized geometries of spiropyran 1 show a clear change in bond distance between the ring closing atoms (C-O). In the close isomer of spiropyran 1, the ring-closing carbon and oxygen atoms (C-O) bond length is 1.43 Å. This bond distance increases as the close structure is converted to an open form. The C-O bond length of ring closing atoms is increased to 4.11 Å, which confirms the breakage of the C-O bond. Similar behavior is observed for spiropyran 2, where the bond length between ring closing C and O atoms is increased from closed isomer to open isomer. The optimized structure of the close isomer of spiropyran 2 shows terminal C-O atoms bond length of 1.36 Å. In contrast, this terminal C-O atoms bond length in an open isomer increases to 4.30 Å. These results are comparable to the X-rays data of the C-O bond length of spyropyran reported by Ozhogin et al. [].
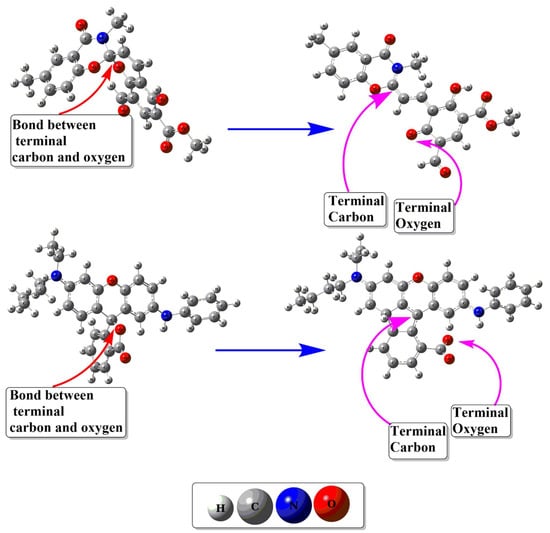
Figure 2.
Optimized structures of close and open isomers of spiropyranes (1 & 2).

Table 1.
Bond distance of ring-closing carbon and oxygen atoms (bC-O in Å), activation energy (Eact), relative energy of close and open isomer (Erel in kcal mol−1) and charge (|e|) on ring-closing carbon and oxygen atoms of Spiropyran (1 & 2).
The relative energies are calculated to check the thermodynamic stability for close and open isomers of spiropyranes (1 & 2) and are given in Table 1. Relative energy measures the energy difference between the most stable isomer and another. In spiropyran 1, the close isomer is thermodynamically more stable than the open isomer of the spiropyran. The open isomer is 5.78 kcal mol−1 less stable than the closed isomer. Menzonatto and Lopes studied the mechanistic pathway of spiropyrane close-form conversion to open-form, and they observed more stability for close compared to open-form []. In spiropyran 2, the open isomer is thermodynamically more stable (by 0.29 kcal mol−1) than the close isomer of spiropyran 2 (see Table 1). We also calculated the activation barrier for the thermal opening of both spiropyranes 1 & 2. The activation barriers for spiropyranes 1 & 2 are 12.74 kcal mol−1and 14.83 kcal mol−1, respectively. The structures of transition states are given in Supplementary Materials Figures S1 and S2. The reported activation barrier for Cspiro-O bond cleavage is 7.6 kcal mol−1. The high activation barrier of spiropyranes is due to their rotation from close to open isomer during bond cleavage. The rotation is necessary to elongate the Cspiro-O bond and a larger torsional angle after cleavage of this bond [].
3.2. NBO Charge Analysis of Close and Open Isomers of Spiropyranes (1 & 2)
The NBO charges of close and open isomers of spiropyranes (1 & 2) are carried out to estimate the acceptor and donor sites of the closing terminal carbon and oxygen (C-O) atoms, and the results are given in Table 1. In the open isomer of the spiropyran 1, the NBO charge value obtained for the carbon atom is 0.64 |e| and for the oxygen atom is −0.63 |e|. In spiropyran 2, the charge on terminal carbon is −0.05 |e| and −0.65 |e| is observed on the oxygen atom in open form. The opposite charges on terminal carbon and oxygen atoms in open isomers of spiropyranes 1 & 2 depict the possibility of electrostatic interactions. In a close isomer of 1, the charge on the ring closing oxygen atom is −0.59 |e| and on the carbon atom is 0.75 |e|, respectively. In the close isomer of spiropyran 2, ring-closing oxygen and carbon atoms have −0.57 |e| and 0.37 |e| charges, respectively. Variations in charges of terminal (ring closing) oxygen and carbon atoms indicate the electrostatic interaction that results in bond formation between terminal carbon and oxygen atoms in individual spiropyranes (1 & 2). These opposite charges on terminal carbon and oxygen atoms in close isomers represent the polarity of the bonds. Loopes and coworkers also theoretically designed spiropyranes derivatives, and their NBO data is similar to our results [].
3.3. Dipole Moment of Close and Open and Isomers of Spiropyranes (1 & 2)
The separation of charges in a system is defined as the dipole moment. More separation of charges results in a higher dipole moment. The NBO results indicate a similar trend of increasing dipole moment for both spiropyran isomers (1 & 2). The open forms of both spiropyranes (1 & 2) have a higher dipole moment than the close form. In spiropyran 1, the close isomer has a lower dipole moment of 6.49 D than the open isomer (8.78 D). In the case of spiropyran 2, the open isomer has a higher dipole moment of 10.23 D than 6.26 D of close form. The variation in dipole moments is due to the larger internuclear distance between oppositely charged carbon and oxygen, along with large quantities of charges on these terminal atoms (C and O), as discussed vide supra. The difference in charges of spiro-carbon and oxygen is more for open isomers than closed isomers, and distances are also more for open isomers. As a result (open isomers of spiropyranes 1 & 2) dipole moment is also higher. Dipole moment values for spiropyranes are reported in Table 2. The higher dipole moment of the open isomers of spiropyranes (1 & 2) illustrates that these could be good candidates for generating high NLO response.

Table 2.
The HOMOs energies (EH in eV) and LUMO energies (EL in eV), HOMO-LUMO energy gap (EH-L in eV), ground state dipole moments (µ, in Debye), polarizability (αo, in au) and the first hyper polarizability (βo, in au) for close and open isomers of spiropyranes 1 & 2.
3.4. Frontier Molecular Orbitals (FMOs) Analysis of Close and Open Isomers of Spiropyranes (1 & 2)
After calculating the geometric parameters, FMOs analysis is executed to observe the electronic stability and reactivity of open and closed isomers of spiropyranes (1 & 2). The energies of HOMOs (EH), the energies of LUMOs (EL) and the HOMO-LUMO gap (EH-L) of spiropyranes (1 & 2) are given in Table 2. EH-L of a close isomer of spiropyran 1 is 8.36 eV which is noticeably higher than the open isomer (6.34 eV). Similarly, the close isomer of spiropyran 2 has an EH-L of 7.51 eV, more than the open isomer of spiropyran 2 (4.26 eV). The reduction of EH-L in open isomers spiropyras (1 & 2) illustrates the shifting of electronic density in these conjugated systems, which is related to the conductivity. The lower EH-L of a system has more charge transfer, generating conductive properties. The close isomers of both spiropyranes 1 & 2 indicate insulator properties due to large EH-L converted to semi-conductors in open isomer. The isodensities of HOMOs, LUMOs and their energy gaps (EH-L) of close and open isomers of spiropyranes are shown in Figure 3. A similar kind of variation in EH-L values was observed experimentally by Kudernac et al. during their work on nano-electronic switches [].
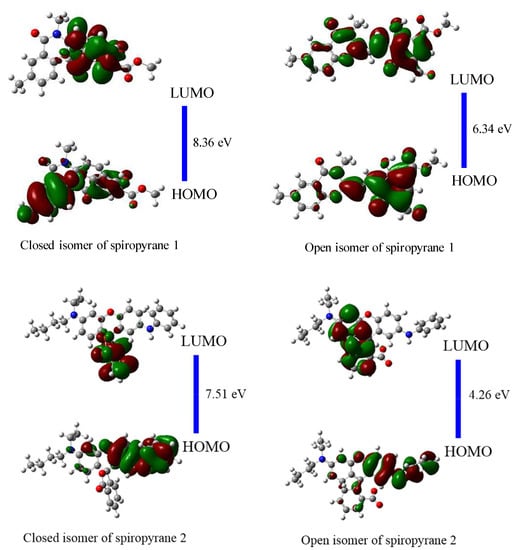
Figure 3.
HOMOs-LUMOs densities and their gaps (in eV) of close and open isomers of spiropyranes (1 & 2).
The HOMOs densities are distributed on the whole skeleton except the methoxy group of close isomers of spiropyran 1. The LUMO densities are localized on the central benzopyran ring of a close isomer of spiropyran 1. The HOMOs densities are distributed on the skeleton of an open isomer of spiropyran 1 but are more concentrated on the phenolic ring. The LUMO densities are delocalized on the entire skeleton in this isomer. For close isomers of spiropyran 2, the HOMOs densities are distributed on the aromatic amine. The LUMOs densities are localized on benzofuran. The HOMOs densities of an open isomer of spiropyran 2 are distributed on the whole skeleton, but more densities are present on the aromatic amine. The LUMOs densities of this open isomer are localized on acetic acid-containing rings in this isomer. Comparatively, between open and closed isomers, the HOMOs densities are more delocalized in the case of open isomers of both spiropyranes 1 & 2.
3.5. Linear and Nonlinear Optical (NLO) Properties of Close and Open Isomers of Spiropyranes (1 & 2)
3.5.1. Polarizability (αo) and First Hyperpolarizability (βo) Analyses
Linear and nonlinear optical parameters are calculated after geometric and electronic analyses. Polarizability (αo) and the first hyperpolarizability (βo) of close and open isomers of spiropyranes 1 & 2 are given in Table 2, and a graphical representation is given in Figure 4.
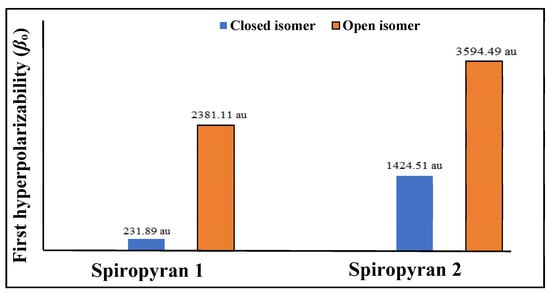
Figure 4.
Graphical representation of hyperpolarizability βo (in au) for close and open isomers of piropyranes (1 & 2).
The polarizability value of close and open isomers of spiropyran 1 is 276 au and 350 au, respectively. Similarly, for close and open isomers of spiropyran 2, αo is 413 au and 460 au, respectively. Open isomers of the spiropyranes show comparatively high polarizability than close isomers. The first hyperpolarizability (βo) results of both isomers show a prominent increase in hyperpolarizability value from close to open isomers of both 1 and 2. The βo of close and open isomers of the spiropyrane 1 is 231.89 au and 2381.11 au, respectively. Bond length alternation and asymmetric electronic density distribution affect βo of an open isomer of spiropyran 1 absent in a closed isomer. The βo value for the close isomer of spiropyran 2 is 1424.51 au and the open isomer has βo value is 3594.49 au. The hyperpolarizability response of the open isomer of spiropyran 2 is larger due to extended conjugation (delocalization of electrons), which is absent in the close isomer.
The NLO response of close and open isomers of spiropyranes 1 & 2 are also observed in acetonitrile solvent, and their results are given in Table 2 To see the difference in the gas phase and aqueous phase effect on open and closed isomers of spiropyranes 1 & 2, we performed NLO calculations in this solvent. As the previous reports illustrate that reversible spiropyranes show high thermal stability in acetonitrile we select this solvent in our study []. The βo of close and open isomers of the spiropyran 1 are 1242.20 au and 4876.61 au, respectively. The βo values increase in the solvent phase.
The βo of close and open isomers of the spiropyran 2 are 2844.07 au and 3578.72 au, respectively. The βo values increase in the solvent phase for the close isomer, but for the open isomer, it is a little bit decreased. Similar to spiropyrane 1, the βo values of open isomers are higher in comparison to close isomers. The increasing trend of βo values from close to open form of both spiropyranes 1 & 2 is similar to gas phase calculations. Following is an increasing trend of βo values; open isomer-spiropyran 1 (4876.61 au) > open isomer-spiropyran 2 (3578.72 au) > close isomer-spiropyran 2 (2844.07 au) > close isomer-spiropyran 1 (1242.20 au). The increase in hyperpolarizability is increased in the dipole moment and polarizability of these isomers, as given in Table S1.
3.5.2. Two-Level Model Analysis of Close and Open Isomers of Spiropyranes 1 & 2
Two level model is implemented to investigate the internal parameters responsible for the increase in the first hyperpolarizability value of closed and open isomers of spiropyranes 1 & 2. These parameters include excitation energy (ΔE), oscillating strength (ƒo) and excited dipole moment (Δµ), and their results are summarized in Table 3. The excitation energy of close isomers of spiropyranes 1 & 2 is 6.40 eV and 4.62 eV, respectively. The ΔE value is lower for open isomers of both spiropyranes 1 (2.97 eV) & 2 (4.55 eV). The trend of decreasing ΔE values from close to open isomers is inconsistent with an increase in βo values. The βo values of open isomers 1 & 2 are higher, and their excitation energies are also lower. Moreover, the ƒo and Δµ values also show a similar increasing trend compared to βo values moving from close to open isomers of spiropyran 1 & 2. The ƒo (Δµ) values of open isomers of spiropyranes 1 and 2 are 0.68 (8.78 Debye) and 0.53 (10.22 Debye), respectively. The ƒo (Δµ) values of close isomers of spiropyranes 1 and 2 are 0.54 (6.49 Debye) and 0.41 (6.26 Debye), respectively. In addition, it is confirmed that the dipole moment values do not show any change between the ground and the excited states in the gas phase. The variational trend of βTLM values is comparable to the trend of increasing βo values. So, ΔE, ƒo and Δµ are the decisive factors for the enhancement of NLO response in these isomers.

Table 3.
Transition energy (ΔE in eV) oscillator strength (ƒo) maximum absorbance (λmax in nm) and excited dipole moment (Δµ) for close and open isomers of spiropyranes 1 & 2.
The internal parameters are also observed in the presence of acetonitrile solvent for closed and open isomers of spiropyranes 1 & 2, and their results are described in the Section 3.6 (Table 6, vide infra). The ΔE and Δµ of spiropyranes 1 & 2 in the solvent phase are similar to gas phase results. The ΔE value (5.03 eV) of the open isomer is lower, and Δµ value (11.74) is higher. The βo value (2381.11 au) of this isomer is also higher. The ΔE value (5.26 eV) of the close isomer is lower, and Δµ value (11.74 Debye) is higher and βo value of this isomer is also low (231.89 au). The trend of increasing ƒo is opposite to the βo value for closed and open isomers. Similar results are obtained for spiropyran 2 as obtained for spiropyran 1. The ΔE value (4.60 eV) of the open isomer is lower, and ƒo (0.89) & Δµ value (13.91 Debye) are higher, which is according to the two-level model. Alternatively, the ΔE value (4.57 eV) of the open isomer is lower, and ƒo (0.62) & Δµ value (8.36 Debye) are higher. Overall, excitation energy and variational dipole moment are decisive factors in describing the NLO response of these systems.
3.5.3. Frequency-Dependent First Hyperpolarizability of Close and Open Isomers of Spiropyranes 1 & 2
Frequency-dependent first hyperpolarizability is the fundamental parameter in the NLO phenomenon. Experimentalists required frequency-dependent first hyperpolarizability information for the development of optical devices. These properties give guidelines for the behavior of NLO materials when they are treated with light beams of different frequencies in practice. The second harmonic generation is one of the effects governed by hyperpolarizabilities []. Here, the frequency-dependent first hyperpolarizability is calculated for spiropyranes 1 & 2, and results are given in Table 4. All the calculations are implemented at routinely used wavelengths of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au) []. The electro pockel effect β(−ω;ω,0) values for close and open isomers of spiropyran 1 are in the range of 255.9 to 6324.6 au at a wavelength of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The close and open isomers of spiropyran 1 showed the most prominent second harmonic generation effect β(−2ω;ω,ω) values in the range of 317.9–6698.7 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The higher electro-pockel effect value (6324.6 au) and second harmonic generation effect value (6698.7 au) are obtained for the open isomer of spiropyran 1 at 5.63 × 1014 Hz (0.09 au). These results are similar to the work of Tonnele and Castet [].

Table 4.
Static first hyperpolarizability, frequency-dependent first hyperpolarizability coefficients in terms of electro-optic Pockel’s effect βo (−ω; ω, 0) and second harmonic generation βo (−2ω; ω, ω) (in au) for close and open isomers of spiropyranes 1 & 2.
The electro pockel effect β(−ω;ω,0) values for close and open isomers of spiropyran 2 range from 1565.4 au to 22,805.2 au at a wavelength of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au). Again, the close and open isomers of spiropyran 2 showed prominent second harmonic generation effect β(−2ω;ω,ω) values from 1893.9–54,124.8 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The higher electro-optical pockel effect value (22,805.2 au) and second harmonic generation effect value (54,124.8 au) are obtained for the open isomer of spiropyran 2 at 5.63 × 1014 Hz (0.09 au). The electro-optical Pockel effect and second harmonic generation are highly affected at the wavelength of 5.63 × 1014 Hz (0.09 au) for both open and closed isomers of spiropyran 2. Among spiropyranes 1 & 2, the highest electro-optical pockel effect and second harmonic generation effect values are seen for the open isomer of spiropyran 2. These results are comparable to frequency-dependent work on other photoswitches [].
3.5.4. Frequency-Dependent Second Hyperpolarizability and Quadratic Nonlinear Refractive Index Analysis of Spiropyranes 1 & 2
The second-order electric susceptibility per unit volume is known as the second hyperpolarizability. Experimentalists required frequency-dependent second hyperpolarizability for practical applications where the hyperpolarizability is calculated at different wavelengths []. The frequency-dependent second hyperpolarizability results for close and open isomers of spiropyranes 1 & 2 are given in Table 5. All the calculations are implemented at routinely used wavelengths of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au) []. It is observed that the gamma (γ) values for close and open isomers of spiropyran 1 at static frequency are 3564.7 au and 6907.9 au, respectively. The large second hyperpolarizability (6907.9 au) value is obtained for the open isomer of the spiropyran 1. The dc-Kerr effect values for close isomer of the spiropyran 1 are 5095.1 au and 3909.5 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The EFSHG values for close isomers are 3037.8 au and 4636.6 au at frequencies of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The dc-Kerr effect values for an open isomer of the spiropyran 1 are 52,842.8 au and 9030.3 au at a wavelength of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The open isomer of spiropyran 1 also shows the prominent electric field-induced second harmonic generation effect values of 2398.2 au and 23,299.4 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The high values of the dc-Kerr effect coefficient are observed for open isomers of the spiropyran 1 (52,842.8 au) & 2 (126,583.9 au) at 5.63 × 1014 Hz (0.09 au). The higher values of EFSHG coefficient are seen for open isomers of the spiropyran 1 (23,299.4 au) & 2 (33,722.1au) at 2.82 × 1014 Hz. The highest frequency-dependent dc-Kerr effect coefficient value (126,583.9 au) is obtained for open isomers of the spiropyran 1 at 5.63 × 1014 Hz (0.09 au). These results are similar to the reported data for acid-controlled second-order nonlinear optical switches []. We observed that the delocalization of π electrons, elongated conjugation system and asymmetric distribution of electrons are dominant factors in increasing static and frequency-dependent first and second hyperpolarizability response of open isomers of spiropyranes 1 & 2.

Table 5.
Static second-order hyperpolarizability (γo in au), frequency-dependent second-order hyperpolarizability coefficients in terms of dc-Kerr γ(−ω; ω,0,0) effect in au, EFSHG (in au) and refractive index (n2, in cm2/W) for close and open isomers of spiropyranes 1 & 2.
Static second hyperpolarizability (γ(0; 0, 0, 0)) values for close and open isomers of spiropyran 2 are 11,533.7 au and 27,098.8 au, respectively. The large γo value is obtained for the open isomer of the spiropyran 2. The dc-Kerr effect values for close isomer of spiropyran 2 are 20,011.3 au and 13,033.1 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The EFSHG values for close isomers are 1027.6 au and 1713.9 au. At 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), the dc-Kerr effect values for an open isomer of spiropyran 2 are 126,583.9 au and 120,760.7 au, respectively. The open isomer of spiropyran 2 also shows the noticeable electric field-induced second harmonic generation effect values, which are 112,874.0 au and 33,722.1 au at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The higher dc-Kerr effect value (126,583.9 au) is detected for both isomers of spiropyran 2 at 5.63 × 1014 Hz (0.09 au). The high EFSHG effect value (33,722.1 au) is detected for both isomers of spiropyran 2 at 2.82 × 1014 Hz.
The refractive index (n2) values obtained for a close isomer of the spiropyran 1 are 3.23 × 10−18 cm2/W and 1.01 × 10−18 cm2/W at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The open isomer of the spiropyran 1 has refractive index values of 1.99 × 10−17 cm2/W and 3.25 × 10−18 cm2/W at a wavelength of 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. Among both isomers (close and open) of spiropyran 1, the open isomer has the large n2 (1.99 × 10−17 cm2/W) value at the wavelength of 5.63 × 1014 Hz (0.09 au). The refractive index values found for close isomer of spiropyran 2 are 1.11 × 10−17 cm2/W and 3.45 × 10−18 cm2/W. At 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), the open isomer of spiropyran 2 has refractive index values of 2.21 × 10−17 cm2/W and 1.50 × 10−17 cm2/W at 5.63 × 1014 Hz (0.09 au) and 2.82 × 1014 Hz (0.04 au), respectively. The highest refractive index value (2.21 × 10−17 cm2/W) is observed at the wavelength of 5.63 × 1014 Hz (0.09 au) for open isomer of spiropyran 2 at 5.63 × 1014 Hz (0.09 au).
3.6. Absorption Spectra for Close and Open Isomers of Spiropyranes 1 & 2
The absorption spectra of both isomers of spiropyranes 1 & 2 have significant absorption in the entire UV region and somewhat in the visible region of the electromagnetic spectrum. The λmax values are summarized in Table 3 and graphically represented in Figure 5. It is found that the close isomer of the spiropyran 1 showed absorbance in the UV region at 194 nm, while the open isomer of the spiropyran 1 showed maximum absorbance in the visible region at 418 nm. For spiropyran 2, both closed, and open isomers of the compound show maximum absorbance in the UV region at 269 nm and 272 nm, respectively. Both isomers (close and open) of spiropyran 1 & 2 show transparency in the deep UV region (below 200 nm), except for the close isomer of spiropyran 1. The increase in λmax values for open isomers supports the NLO response. As the electronic excitation increases in a system, the NLO response also increases. Such behavior is previously seen by Bibi et al. for on-off switches based on cobalt and iron-containing coordinate complexes [], which justifies our results.
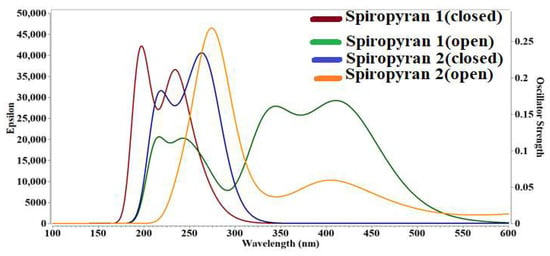
Figure 5.
UV-Visible absorption spectra of closed and open isomers of spiropyranes (1 & 2).
The UV-Vis analysis of close and open isomers of spiropyranes 1 & 2 are also performed in acetonitrile solvent, and their results of λmax are given in Table 6. The λmax for the open isomer (246 nm) is higher than the closed isomer (236 nm) of spiropyran 1. In continuation to this result, the λmax of the close isomer (271 nm) is higher than the open isomer (270 nm) of spiropyran 2. Although compared to gas phase calculations, the λmax of closed isomer is increased, and for open isomer, this value is decreased significantly. The λmax values in acetonitrile are comparable to already reported λmax values reported by spiropyranes in the same solvent [].

Table 6.
Transition energy (ΔE in eV) oscillator strength (ƒo) maximum absorbance (λmax in nm), excited dipole moment (Δµ in Debye) ground state dipole moments (µ, in Debye), polarizability (αo, in au) and the first hyper polarizability (βo, in au) for close and open isomers of spiropyranes 1 & 2 in the presence of acetonitrile solvent.
3.7. The Charge-Transfer Distance or Density Difference between Ground and Excited States for Close and Open Isomers of Spiropyranes 1 & 2
The charge transfer distance and charge density difference of both spiropyranes 1 & 2 isomers are calculated with Multiwfn software []. Figure 6 shows the charge density difference of the excited states where the green and blue densities correspond to the region where the electron density increases and decreases after electron excitation, respectively. The change in the position of electronic densities, HOMO (electron) and LUMO (hole) in Figure 6 compared to Figure 3 for the ground state density shoe the shifting of charge from one place to another. The integral of overlap of hole-electron (S) and the distance between the centroid of the hole and electron (D in Å) for close and open isomers of spiropyranes 1 & 2 to five excited states are given in Table 7. According to the rule, the excited states with a high D value and lower S value have Charge transfer mode (CT type) and those excited states with lower D values and large S values are Local excitation type (LE type). When both D and S values are small, they are Rydberg-type excitation. The close isomer of spiropyran 1, excited states 1 (D/S = 1.57/0.21) and 5 (D/S = 2.07/0.17), have high D-values and lower S values, showing CT-type excitations. The rest of the excited states 2–4 must have LE-type excitations. The open isomer of spiropyran 1, excited states 2 (D/S = 2.36/0.15) and 5 (D/S = 2.84/0.27) have high D-values and lower S values, showing CT-type excitations. The rest of the excited states 1,3, and 4 have LE-type excitations. The close isomer of spiropyran 2, excited states 1 (D/S = 1.94/0.26) and 4 (D/S = 2.51/0.20), have high D and lower S values, showing CT-type excitations. The rest of the excited states 2, 3, and 5 have LE-type excitations. The open isomer of spiropyran 2, excited states 3 (D/S = 2.43/0.25) and 4 (D/S = 2.98/0.34), have high values of D values and lower S values, so they show CT-type excitations. The rest of the excited states, 1 and 5, have LE-type excitations. Compared to other states, the excited state 2 shows Rydberg-type excitation as both D (1.73 Å) and S (0.21) values are small.
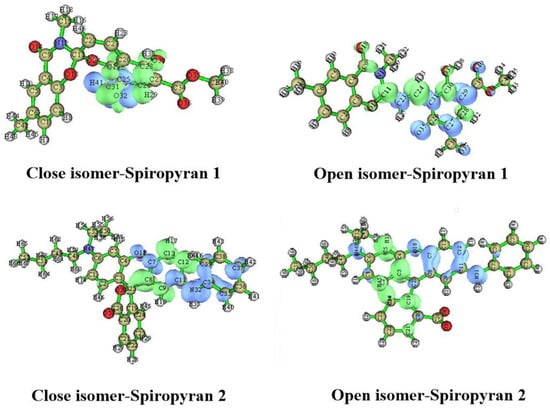
Figure 6.
Density difference surfaces of closed and open isomers of spiropyranes (1 & 2) at excited state.

Table 7.
The integral of hole-electron (S) overlap and the distance between the centroid of hole and electron (D in Å) for close and open isomers of spiropyranes 1 & 2 to five excited states.
4. Conclusions
We have investigated the geometric, electronic, optical, and nonlinear optical properties of reversible thermochromic spiropyranes at the DFT method. Electronic properties of these close and open isomers of spiropyranes 1 & 2 are studied through FMOs analysis. It is observed that the lower HOMO-LUMO gap with lower excitation energy in open isomers of both spiropyranes 1 & 2 imparts large hyperpolarizability values. The extended electronic delocalization and unsymmetrical electronic distribution are responsible for the decrease in the HOMO-LUMO gap. Comparatively, the first hyperpolarizability values of open isomers are more than the closed isomers. The delocalization of π-electrons, asymmetric distribution of electrons (in spiropyran 1) and elongated conjugation system (in spiropyran 2) are dominant factors for the increase of hyperpolarizability values of open isomers (1 & 2). In both spiropyranes, the highest βo (3594.49 au) is observed for the open isomer of spiropyran 2. To get a deep understanding of our studied reversible thermochromic spiropyranes (1 & 2), we also analyzed the frequency-dependent hyperpolarizability. The more prominent frequency-dependent NLO response is at 5.63 × 1014 Hz (0.09 au). To further observe their NLO response, we also calculated the refractive index of these thermochromic compounds. Among all those compounds, the highest refractive index value is observed for the open isomer of the spiropyran 1 (1.99 × 10−17 cm2/W). Molecular absorption analysis confirmed electronic excitation in open and closed isomers, where the effect is more pronounced for open isomers of thermochromic spiropyranes (1 & 2). Therefore, the calculated values reveal reversible thermochromic compounds as excellent NLO molecular switches for future applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176283/s1, Table S1. The ground state dipole moments (µ, in Debye), polarizability (αo, in au) for close and open isomers of spiropyranes 1 & 2 in the presence of acetonitrile solvent. Figure S1. Optimized structures of close, transition state and open isomers of spiropyran 1. Figure S2. Optimized structures of close, transition state and open isomers of spiropyran 2.
Author Contributions
Conceptualization, K.A. and T.M.; Methodology, M.A.G.; Validation, S.K. and K.A.; Formal analysis, N.K., M.H.S.A.H., M.I. and M.A.; Investigation, N.K., S.K., M.I. and M.A.A.; Resources, M.H.S.A.H.; Data curation, M.A.G., M.A., M.A.A. and N.S.S.; Writing—original draft, T.M.; Writing—review & editing, N.S.S.; Supervision, T.M.; Project administration, T.M.; Funding acquisition, M.A.A. and N.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. 3699].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available to the corresponding author upon request.
Acknowledgments
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. 3699]. The authors also thank the Universiti Brunei Darussalam for the research grant (UBD/RSCH/1.4/FICBF(b)/2022/049). M. Imran appreciates the Deanship of Scientific Research at King Khalid University Saudi Arabia through the research groups program under Grant Number R.G.P. 2/256/44.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chowdhury, M.A.; Joshi, M.; Butola, B.S. Photochromic and Thermochromic Colorants in Textile Applications. J. Eng. Fibers Fabr. 2014, 9, 107–123. [Google Scholar] [CrossRef]
- Study, A.S.A.; Francesco, B.Y. Thermal and Photochemical Interconversion of Spiropyrans and Merocyanines. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1984, 80, 1513–1527. [Google Scholar]
- Kao, H.-L.; Mohan, M.; Schmandt, C.; Paradiso, J.A.; Vega, K. ChromoSkin. In Proceedings of the 2016 CHI Conference Extended Abstracts on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; Association for Computing Machinery: New York, NY, USA; pp. 3703–3706. [Google Scholar]
- Lewit, E.M. An Evaluation of a Plastic Strip Thermometer. J. Am. Med. Assoc. 1982, 247, 321. [Google Scholar] [CrossRef]
- Dawson, T.L. Changing Colours: Now You See Them, Now You Don’t. Color. Technol. 2010, 126, 177–188. [Google Scholar] [CrossRef]
- Yoo, W.J.; Seo, J.K.; Jang, K.W.; Heo, J.Y.; Moon, J.S.; Park, J.-Y.; Park, B.G.; Lee, B. Fabrication and Comparison of Thermochromic Material-Based Fiber-Optic Sensors for Monitoring the Temperature of Water. Opt. Rev. 2011, 18, 144–148. [Google Scholar] [CrossRef]
- Dzisah, P.; Sadoh, A.; Ravindra, N.M. 3D Printing of Polymer-Based Gasochromic, Thermochromic and Piezochromic Sensors. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, San Antonio, TX, USA, 10–14 March 2019; pp. 1545–1561. [Google Scholar]
- Yang, W.-J.; Yang, P.P.T. Literature Survey on Biomedical Applications of Thermography. Biomed. Mater. Eng. 1992, 2, 7–18. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Fang, C.; Chen, J.; Wang, Z. Discoloration Mechanism, Structures and Recent Applications of Thermochromic Materials via Different Methods: A Review. J. Mater. Sci. Technol. 2018, 34, 2225–2234. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic Polymers—Function by Design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M.; Novak, U.; Klanjšek Gunde, M. The Relation between Colour and Structural Changes in Thermochromic Systems Comprising Crystal Violet Lactone, Bisphenol A, and Tetradecanol. Dye. Pigment. 2017, 136, 382–389. [Google Scholar] [CrossRef]
- Towns, A.D. Thermochromic Composite Materials Formulated from Spirolactone Colour Formers. In Proceedings of the ChemiChromics USA 99, New Orleans, LA, USA, 20 January 1999; pp. 1171–1182. [Google Scholar]
- Rosario, R.; Gust, D.; Hayes, M.; Jahnke, F.; Springer, J.; Garcia, A.A. Photon-Modulated Wettability Changes on Spiropyran-Coated Surfaces. Langmuir 2002, 18, 8062–8069. [Google Scholar] [CrossRef]
- Lewis, K.L.; Pitt, A.M.; Wyatt-Davies, T.; Milward, J.R. Thin Film Thermochromic Materials for Non-Linear Optical Devices. MRS Online Proc. Libr. 1994, 374, 105. [Google Scholar] [CrossRef]
- Huggins, K.E.; Son, S.; Stupp, S.I. Two-Dimensional Supramolecular Assemblies of a Polydiacetylene. 1. Synthesis, Structure, and Third-Order Nonlinear Optical Properties. Macromolecules 1997, 30, 5305–5312. [Google Scholar] [CrossRef]
- Nasr, M.; Gomaa, H.M.; Yahia, I.S.; Saleh, H.A. Novel Thermochromic (TC) and Electrochromic (EC) Characteristics of the V4O7 Liquid Crystal for LCDs and Versatile Optoelectronic Applications. J. Mol. Liq. 2021, 330, 115620. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Fan, H.; Lin, C.; Chen, K.; Peng, G.; Li, B.; Zhang, G.; Long, X.; Ye, N. (NH4)Bi2(IO3)2F5: An Unusual Ammonium-Containing Metal Iodate Fluoride Showing Strong Second Harmonic Generation Response and Thermochromic Behavior. Angew. Chem. Int. Ed. 2020, 59, 5268–5272. [Google Scholar] [CrossRef]
- Mączka, M.; Zaręba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. [Methylhydrazinium]2PbBr4, a Ferroelectric Hybrid Organic–Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Pandey, R.; Das, P.K.; Castet, F.; Blanchard-Desce, M. Linear and Nonlinear Optical Properties of Tricyanopropylidene-Based Merocyanine Dyes: Synergistic Experimental and Theoretical Investigations. ChemPhysChem 2018, 19, 187–197. [Google Scholar] [CrossRef]
- Previtali, A.; He, W.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Carlucci, L.; Mercandelli, P.; Ortenzi, M.A.; Terraneo, G.; et al. Tunable Linear and Nonlinear Optical Properties from Room Temperature Phosphorescent Cyclic Triimidazole-Pyrene Bio-Probe. Chem. A Eur. J. 2021, 27, 16690–16700. [Google Scholar] [CrossRef]
- Sliwa, M.; Létard, S.; Malfant, I.; Nierlich, M.; Lacroix, P.G.; Asahi, T.; Masuhara, H.; Yu, P.; Nakatani, K. Design, Synthesis, Structural and Nonlinear Optical Properties of Photochromic Crystals: Toward Reversible Molecular Switches. Chem. Mater. 2005, 17, 4727–4735. [Google Scholar] [CrossRef]
- Tamaoki, N.; Van Keuren, E.; Matsuda, H.; Hasegawa, K.; Yamaoka, T. Photoreversible Optical Nonlinearities of Polymeric Films Containing Spiropyran with Long Alkyl Chains. Appl. Phys. Lett. 1996, 69, 1188–1190. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Aubert, V.; Guerchais, V.; Ishow, E.; Hoang-Thi, K.; Ledoux, I.; Nakatani, K.; Le Bozec, H. Efficient Photoswitching of the Nonlinear Optical Properties of Dipolar Photochromic Zinc(II) Complexes. Angew. Chem. Int. Ed. 2008, 47, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent Developments in the Field of Metal Complexes Containing Photochromic Ligands: Modulation of Linear and Nonlinear Optical Properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Jing, L.-X.; Wang, L.; Ye, J.-T.; Chen, Z.-Z.; Chen, H.; Qiu, Y.-Q. Theoretical Investigation on Second-Order Nonlinear Optical Properties of Ruthenium Alkynyl–Dihydroazulene/Vinylheptafulvene Complexes. J. Mol. Graph. Model. 2017, 77, 363–371. [Google Scholar] [CrossRef]
- Okuno, K.; Shigeta, Y.; Kishi, R.; Nakano, M. Photochromic Switching of Diradical Character: Design of Efficient Nonlinear Optical Switches. J. Phys. Chem. Lett. 2013, 4, 2418–2422. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Tkachev, V.V.; Lukyanov, B.S.; Mukhanov, E.L.; Rostovtseva, I.A.; Lukyanova, M.B.; Shilov, G.V.; Strekal, N.D.; Aldoshin, S.M.; Minkin, V.I. Synthesis, Structure and Photochromic Properties of Novel Highly Functionalized Spiropyrans of 1,3-Benzoxazin-4-One Series. J. Mol. Struct. 2018, 1161, 18–25. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Dong, N.; Li, Z. Fabrication and Characterization of Reversible Thermochromic Wood Veneers. Sci. Rep. 2017, 7, 16933. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Ozhogin, I.V.; Dorogan, I.V.; Lukyanov, B.S.; Mukhanov, E.L.; Tkachev, V.V.; Chernyshev, A.V.; Lukyanova, M.B.; Aldoshin, S.M.; Minkin, V.I. New Spiropyrans Based on 1,3-Benzoxazine-2-One: Acid Catalyzed Synthesis and Theoretical Insight into the Photochromic Activity. Tetrahedron Lett. 2016, 57, 2382–2385. [Google Scholar] [CrossRef]
- Plaquet, A.; Guillaume, M.; Champagne, B.; Castet, F.; Ducasse, L.; Pozzo, J.-L.; Rodriguez, V. In Silico Optimization of Merocyanine-Spiropyran Compounds as Second-Order Nonlinear Optical Molecular Switches. Phys. Chem. Chem. Phys. 2008, 10, 6223. [Google Scholar] [CrossRef]
- Vaez, Z.; Javanbakht, V. Synthesis, Characterization and Photocatalytic Activity of ZSM-5/ZnO Nanocomposite Modified by Ag Nanoparticles for Methyl Orange Degradation. J. Photochem. Photobiol. A Chem. 2020, 388, 112064. [Google Scholar] [CrossRef]
- Dennington, R.I.; Keith, T.; Millam, J. GaussView, Version 5.0.8; Semichem, Inc.: Shawnee Mission, KS, USA, 2008. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural Bond Orbital Analysis of Near-Hartree–Fock Water Dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. A Benzothiazolinic Spiropyran for Highly Selective, Sensitive and Visible Light Controlled Detection of Copper Ions in Aqueous Solution. J. Photochem. Photobiol. A Chem. 2020, 390, 112265. [Google Scholar] [CrossRef]
- Hou, X.-F.; Chen, X.-M.; Bisoyi, H.K.; Qi, Q.; Xu, T.; Chen, D.; Li, Q. Light-Driven Aqueous Dissipative Pseudorotaxanes with Tunable Fluorescence Enabling Deformable Nano-Assemblies. ACS Appl. Mater. Interfaces 2023, 15, 11004–11015. [Google Scholar] [CrossRef]
- Kosar, N.; Wajid, S.; Ayub, K.; Gilani, M.A.; Mahmood, T. First, Second and Third Order NLO Response of Alkaline Earth Metals Doped C6O6Li6 Organometallic Complexes. Chem. Phys. 2023, 570, 111894. [Google Scholar] [CrossRef]
- Kosar, N.; Wajid, S.; Ayub, K.; Mahmood, T. Excellent Static and Dynamic Hyperpolarizabilities of TM@C6O6Li6 (TM=Sc, Ti, V, Cr and Mn) Complexes to Prove Their NLO Applications. Optik 2023, 276, 170660. [Google Scholar] [CrossRef]
- Menzonatto, T.G.; Lopes, J.F. The Role of Intramolecular Interactions on the Stability of the Conformers of a Spiropyran Derivative. Chem. Phys. 2022, 562, 111654. [Google Scholar] [CrossRef]
- Dorogan, I.V.; Minkin, V.I. Theoretical Modeling of Electrocyclic 2H-Pyran and 2H-1,4-Oxazine Ring Opening Reactions in Photo- and Thermochromic Spiropyrans and Spirooxazines. Chem. Heterocycl. Compd. 2016, 52, 730–735. [Google Scholar] [CrossRef]
- Kudernac, T.; Katsonis, N.; Browne, W.R.; Feringa, B.L. Nano-Electronic Switches: Light-Induced Switching of the Conductance of Molecular Systems. J. Mater. Chem. 2009, 19, 7168. [Google Scholar] [CrossRef]
- Mukhanov, E.L.; Dorogan, I.V.; Chernyshev, A.V.; Bezuglyi, S.O.; Ozhogin, I.V.; Lukyanova, M.B.; Lukyanov, B.S. Theoretical and Experimental Study of New Photochromic Bis-Spiropyrans with Hydroxyethyl and Carboxyethyl Substituents. Int. J. Photoenergy 2013, 2013, 752949. [Google Scholar] [CrossRef]
- van Gisbergen, S.J.A.; Snijders, J.G.; Baerends, E.J. Accurate Density Functional Calculations on Frequency-Dependent Hyperpolarizabilities of Small Molecules. J. Chem. Phys. 1998, 109, 10657–10668. [Google Scholar] [CrossRef]
- Therssen, E.; Bouvier, Y.; Schoemaecker-Moreau, C.; Mercier, X.; Desgroux, P.; Ziskind, M.; Focsa, C. Determination of the Ratio of Soot Refractive Index Function E(m) at the Two Wavelengths 532 and 1064 Nm by Laser Induced Incandescence. Appl. Phys. B 2007, 89, 417–427. [Google Scholar] [CrossRef]
- Tonnelé, C.; Castet, F. Nonlinear Optical Properties of Spirocyclohexadine Photochromes: Insights from DFT Calculations. Photochem. Photobiol. Sci. 2019, 18, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Hättig, C.; Christiansen, O.; Jørgensen, P. Frequency-Dependent Second Hyperpolarizabilities Using Coupled Cluster Cubic Response Theory. Chem. Phys. Lett. 1998, 282, 139–146. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, H.-L.; Su, Z.-M. A Novel Acid-Controlled Second-Order Nonlinear Optical Switch Based on Dimethyldihydropyrene/Cyclophanediene Photoswitch. J. Mater. Chem. C 2022, 10, 12338–12349. [Google Scholar] [CrossRef]
- Bibi, T.; Jadoon, T.; Ayub, K. Two State “ON–OFF” NLO Switch Based on Coordination Complexes of Iron and Cobalt Containing Isomeric Ligand: A DFT Study. RSC Adv. 2022, 12, 23204–23214. [Google Scholar] [CrossRef]
- Roohi, H.; Rostami, T. Mechanism of the Photo Triggered Ring-Opening Reaction of Spiropyran Derivatives (SP-X1-7; X1-7= H, NO2, CF3, CN, OH, OMe and NMe2) in the Gas Phase and Various Solvent Media: A GD3-TD-DFT Approach. J. Photochem. Photobiol. A Chem. 2020, 392, 112410. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).