Hepatotoxic Evaluation of N-(2-Hydroxyphenyl)-2-Propylpentanamide: A Novel Derivative of Valproic Acid for the Treatment of Cancer
Abstract
:1. Introduction
2. Results
2.1. Acute Toxicity
2.2. Necropsy
2.3. Effect of HO-AAVPA for 7 Days of Treatment at Repeated Doses
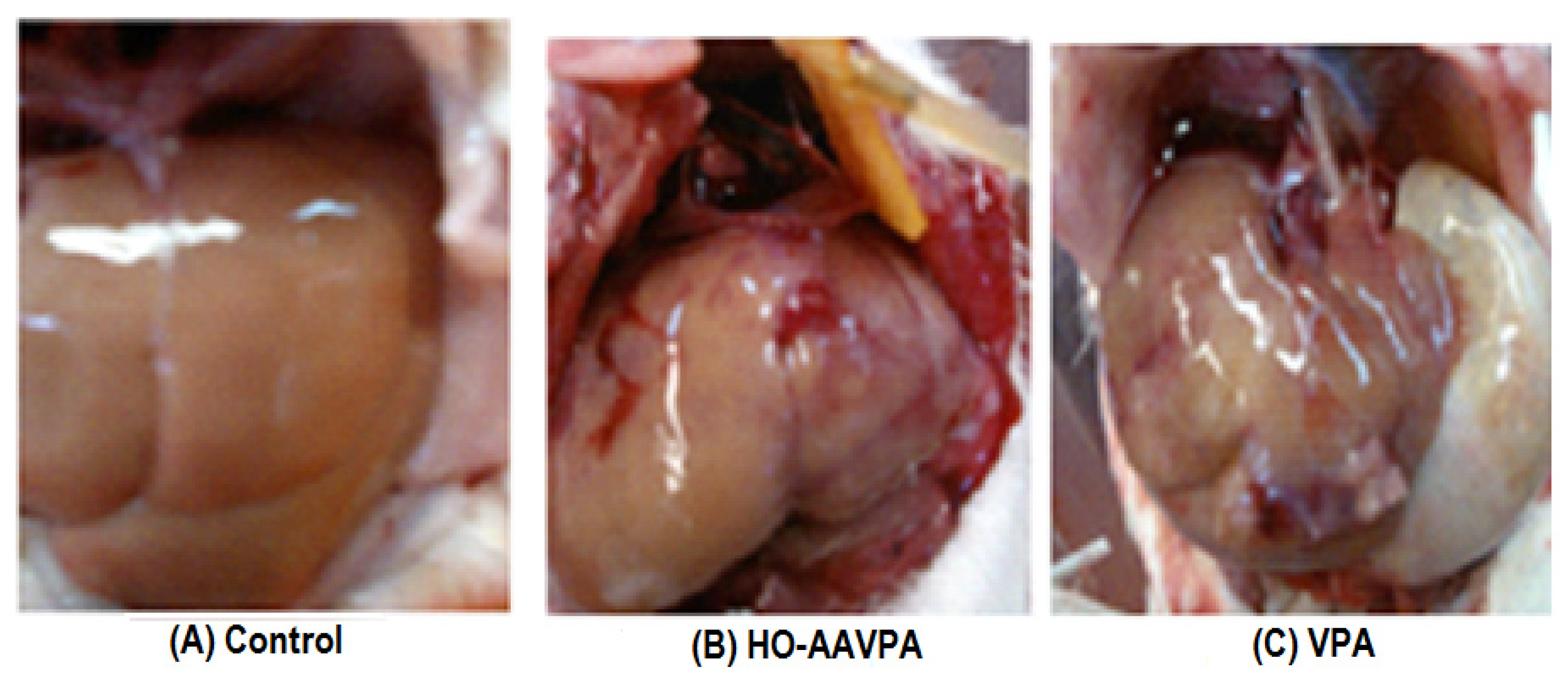
2.3.1. Macroscopic Analysis
2.3.2. Effect of HO-AAVPA on Alanine Aminotransferase, Aspartate Aminotransferase, and Alkaline Phosphatase Activity
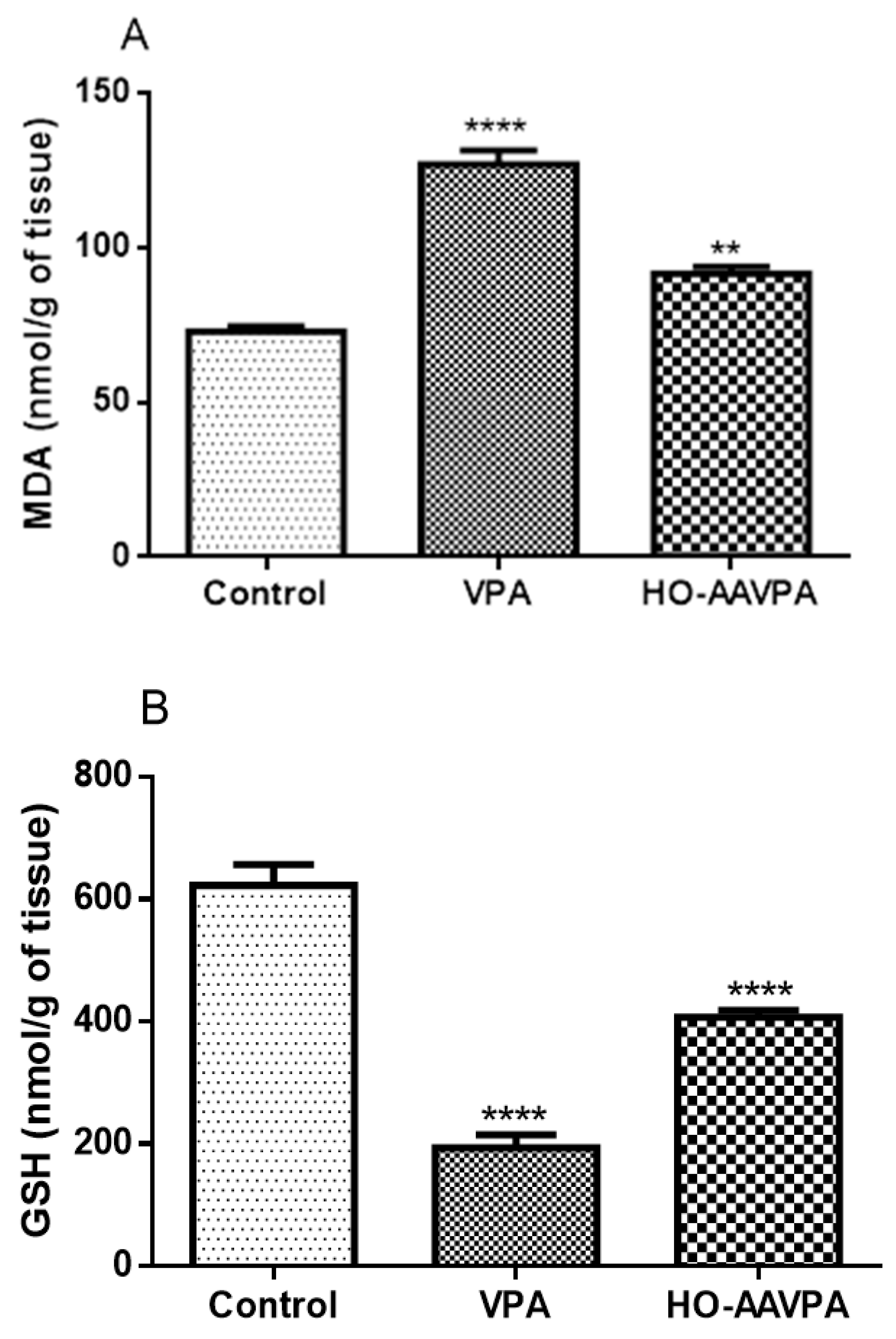
2.3.3. Effect of HO-AAVPA on Lipoperoxidation and Reduced Glutathione Levels
2.3.4. Glutathione Peroxidase (GPx) and Glutathione Reductase (GR)
2.3.5. Catalase Activity (CAT)
2.3.6. Total Proteins
2.3.7. Histopathological Examination of the Liver
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Experimental Animals
4.3. Acute Toxicity Evaluation
4.4. Evaluation of HO-AAVPA for 7 Days
4.4.1. Animals
4.4.2. Determination of Liver Damage by Macroscopic Analysis
4.4.3. Measurement of Alanine Aminotransferase and Aspartate Aminotransferase Activity
4.4.4. Measurement of Alkaline Phosphatase Activity
4.4.5. Determination of Malondialdehyde Levels
4.4.6. Measurement of Reduced Glutathione Levels
4.4.7. Determination of Glutathione Peroxidase and Glutathione Reductase Activity
4.4.8. Measurement of Catalase Activity
4.4.9. Protein Quantification
4.4.10. Histopathological Studies
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pérez-González, O.A.; Bermudez-Lugo, J.A.; Correa-Basurto, J.; Vasquez-Moctezuma, I.; Padilla-Martínez, I.I.; Trujillo-Ferrara, J.G. Derivado del Ácido Valproico con Potencial Acción Antineoplásica, Inhibidor de la Histona Desacetilasa. Título de Patente No. 363005, 12 March 2019. [Google Scholar]
- Prestegui-Martel, B.; Bermúdez-Lugo, J.A.; Chávez-Blanco, A.; Dueñas-González, A.; García-Sánchez, J.R.; Pérez-González, O.A.; Padilla-Martínez, I.I.; Fragoso-Vázquez, M.J.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; et al. N-(2-hydroxyphenyl)-2-propylpentanamide, a valproic acid aryl derivative designed in silico with improved anti-proliferative activity in HeLa, rhabdomyosarcoma and breast cancer cells. J. Enzyme Inhib. Med. Chem. 2016, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, P.; Sanchez, S.C.; Chávez-Blanco, A.D.; Mendoza-Figueroa, H.L.; Correa-Basurto, J. Apoptotic effects of N-(2-hydroxyphenyl)-2-propylpentanamide on U87-MG and U-2 OS cells and antiangiogenic properties. Anticancer. Agents Med. Chem. 2021, 21, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal-Luna, J.M.; Correa-Basurto, J.; Mendoza-Figueroa, H.L.; Chamorro-Cevallos, G. Anti-epileptic activity, toxicity and teratogenicity in CD1 mice of a novel valproic acid arylamide derivative, N-(2-hydroxyphenyl)-2-propylpentanamide. Toxicol. Appl. Pharmacol. 2020, 399, 115033. [Google Scholar]
- Ortiz-Morales, A.A.; García-Vázquez, J.B.; Fragoso-Vázquez, M.J.; Rosales-Hernández, M.C.; Fragoso-Morales, L.G.; Estrada-Pérez, A.R.; Correa-Basurto, J. PAMAM-G4 protect the N-(2-hydroxyphenyl)-2-propylpentanamide (HO-AAVPA) and maintain its antiproliferative effects on MCF-7. Sci. Rep. 2023, 13, 3383. [Google Scholar]
- Avallone, A.; Piccirillo, M.C.; Di Gennaro, E.; Romano, C.; Calabrese, F.; Roca, M.S.; Tatangelo, F.; Granata, V.; Cassata, A.; Cavalcanti, E.; et al. Randomized phase II study of valproic acid in combination with bevacizumab and oxaliplatin/fluoropyrimidine regimens in patients with RAS-mutated metastatic colorectal cancer: The REVOLUTION study protocol. Ther. Adv. Med. Oncol. 2020, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Aztopal, N.; Erkisa, M.; Erturk, E.; Ulukaya, E.; Tokullugil, A.H.; Ari, F. Valproic acid, a histone deacetylase inhibitor, induces apoptosis in breast cancer stem cells. Chem. Biol. Interact. 2018, 280, 51–58. [Google Scholar]
- Krauze, A.V.; Myrehaug, S.D.; Chang, M.G.; Holdford, D.J.; Smith, S.; Shih, J.; Tofilon, P.J.; Fine, H.A.; Camphausen, K. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 986–992. [Google Scholar] [CrossRef]
- Kiang, T.K.; Teng, X.W.; Karagiozov, S.; Surendradoss, J.; Chang, T.K.; Abbott, F.S. Role of oxidative metabolism in the effect of valproic acid on markers of cell viability, necrosis, and oxidative stress in sandwich-cultured rat hepatocytes. Toxicol. Sci. 2010, 118, 501–509. [Google Scholar] [CrossRef]
- Kiang, T.K.; Teng, X.W.; Surendradoss, J.; Karagiozov, S.; Abbott, F.S.; Chang, T.K. Glutathione depletion by valproic acid in sandwich-cultured rat hepatocytes: Role of biotransformation and temporal relationship with onset of toxicity. Toxicol. Appl. Pharmacol. 2011, 252, 318–324. [Google Scholar] [CrossRef]
- Omidipour, R.; Zarei, L.; Boroujeni, M.B.; Rajabzadeh, A. Protective effect of thyme honey against valproic acid hepatotoxicity in Wistar rats. Biomed. Res. Int. 2021, 2021, 8839898. [Google Scholar] [CrossRef]
- Vitins, A.P.; Kienhuis, A.S.; Speksnijder, E.N.; Roodbergen, M.; Luijten, M.; van der Ven, L.T. Mechanisms of amiodarone and valproic acid induced liver steatosis in mouse in vivo act as a template for other hepatotoxicity models. Arch. Toxicol. 2014, 88, 1573–1588. [Google Scholar] [CrossRef]
- Mirza, R.; Sharma, B. Benefits of Fenofibrate in prenatal valproic acid-induced autism spectrum disorder related phenotype in rats. Brain Res. Bull. 2019, 147, 36–46. [Google Scholar] [CrossRef]
- Price, K.E.; Pearce, R.E.; Garg, U.C.; Heese, B.A.; Smith, L.D.; Sullivan, J.E.; Kennedy, M.J.; Bale, J.F., Jr.; Ward, R.M.; Chang, T.K.; et al. Effects of valproic acid on organic acid metabolism in children: A metabolic profiling study. Clin. Pharmacol. Ther. 2011, 89, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Chateauvieux, S.; Morceau, F.; Dicato, M.; Diederich, M. Molecular and therapeutic potential and toxicity of valproic acid. J. Biomed. Biotechnol. 2010, 2010, 479364. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Grechkina, V.V.; Khasanova, A.K.; Bochanova, E.N.; Dontceva, E.A.; Petrova, M.M.; Asadullin, A.R.; Shipulin, G.A.; Altynbekov, K.S.; Al-Zamil, M.; et al. Therapeutic and Toxic Effects of Valproic Acid Metabolites. Metabolites 2023, 13, 134. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, T.; Li, G.; Qiu, F.; Sun, Y.; Zhao, L. Associations of CYP2C9 and CYP2A6 Polymorphisms with the Concentrations of Valproate and its Hepatotoxin Metabolites and Valproate-Induced Hepatotoxicity. Basic. Clin. Pharmacol. Toxicol. 2017, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Correa-Basurto, A.M.; Romero-Castro, A.; Correa-Basurto, J.; Hernández-Rodríguez, M.; Soriano-Ursúa, M.A.; García-Machorro, J.; Tolentino-López, L.E.; Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E. Pharmacokinetics and tissue distribution of N-(2-hydroxyphenyl)-2-propylpentanamide in Wistar Rats and its binding properties to human serum albumin. J. Pharm. Biomed. Anal. 2019, 62, 130–139. [Google Scholar] [CrossRef]
- Silva-Trujillo, A.; Correa-Basurto, J.; Romero-Castro, A.; Albores, A.; Mendieta-Wejebe, J.E. A simple validated RP-HPLC bioanalytical method for the quantitative determination of a novel valproic acid arylamide derivative in rat hepatic microsomes. Biomed. Chromatogr. 2015, 29, 523–528. [Google Scholar] [CrossRef]
- Mendieta-Wejebe, J.E.; Silva-Trujillo, A.; Bello, M.; Mendoza-Figueroa, H.L.; Galindo-Alvarez, N.L.; Albores, A.; Tamay-Cach, F.; Rosales-Hernández, M.C.; Romero-Castro, A.; Correa-Basurto, J. Exploring the biotransformation of N-(2-hydroxyphenyl)-2-propylpentanamide (an aryl valproic acid derivative) by CYP2C11, using in silico predictions and in vitro studies. J. Pharm. Pharmacol. 2020, 72, 938–955. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, R.; Mir, R.H.; Sawhney, G.; Dar, M.A.; Bhat, Z.A. Possible pathways of hepatotoxicity caused by chemical agents. Curr. Drug. Metab. 2019, 20, 867–879. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Test no. 425: Acute oral toxicity: Up-and-down procedure. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2008. [Google Scholar]
- Walker, R.M.; Smith, G.S.; Barsoum, N.J.; Macallum, G.E. Preclinical toxicology of the anticonvulsant calcium valproate. Toxicology 1990, 63, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm. Weekbl. Sci. 1992, 14, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yazid, M.F.H.A.; Ta, G.C.; Mokhtar, M. Classified chemicals in accordance with the Globally Harmonized System of Classification and Labelling of Chemicals: Comparison of Lists of the European Union, Japan, Malaysia and New Zealand. Saf. Health Work 2020, 11, 152–158. [Google Scholar] [CrossRef]
- Moore, N.P.; Boogaard, P.J.; Bremer, S.; Buesen, R.; Edwards, J.; Fraysse, B.; Hallmark, N.; Hemming, H.; Langrand-Lerche, C.; McKee, R.H.; et al. Guidance on classification for reproductive toxicity under the Globally Harmonized System of Classification and Labelling of chemicals (GHS). Crit. Rev. Toxicol. 2013, 43, 850–891. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, T.; Ito, M.; Seki, T.; Fukuhara, T.; Bolati, K.; Arai, H.; Suzuki, T. Prenatal exposure to valproic acid is associated with altered neurocognitive function and neurogenesis in the dentate gyrus of male offspring rats. Brain Res. 2019, 1723, 146403. [Google Scholar] [CrossRef]
- Tong, V.; Teng, X.W.; Chang, T.K.; Abbott, F.S. Valproic acid I: Time course of lipid peroxidation biomarkers, liver toxicity, and valproic acid metabolite levels in rats. Toxicol. Sci. 2005, 86, 427–435. [Google Scholar] [CrossRef]
- Noai, M.; Soraoka, H.; Kajiwara, A.; Tanamachi, Y.; Oniki, K.; Nakagawa, K.; Ishitsu, T.; Saruwatari, J. Cytochrome P450 2C19 polymorphisms and valproic acid-induced weight gain. Acta Neurol. Scand. 2016, 133, 216–223. [Google Scholar] [CrossRef]
- Dinesen, H.; Gram, L.; Andersen, T.; Dam, M. Weight gain during treatment with valproate. Acta Neurol. Scand. 1984, 70, 65–69. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; p. 29494096. [Google Scholar]
- Šmíd, V. Liver tests. Cas. Lek. Cesk. 2022, 161, 52–56. [Google Scholar]
- Santos, N.A.; Medina, W.S.; Martins, N.M.; Rodrigues, M.A.; Curti, C.; Santos, A.C. Involvement of oxidative stress in the hepatotoxicity induced by aromatic antiepileptic drugs. Toxicol. In Vitro 2008, 22, 1820–1824. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Spasojević, I. Free radicals and antioxidants at a glance using EPR spectroscopy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 114–142. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef]
- Fujita, T. Formation and removal of reactive oxygen species, lipid peroxides and free radicals, and their biological effects. Yakugaku Zasshi. 2002, 122, 203–218. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Abdel-Dayem, M.A.; Abdel-Aziz, A.; El-Azab, M.F.; Said, S.A. Eicosapentaenoic acid ablates valproate-induced liver oxidative stress and cellular derangement without altering its clearance rate: Dynamic synergy and therapeutic utility. Biochim. Biophys. Acta 2011, 1811, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Otuguor, E.; Singh, D.; Ota, D.; Benebo, A.S. Hypoglycemic, antilipidemic and antioxidant effects of valproic acid in alloxan-induced diabetic rats. Eur. J. Pharmacol. 2015, 762, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Espandiari, P.; Zhang, J.; Schnackenberg, L.K.; Miller, T.J.; Knapton, A.; Herman, E.H.; Beger, R.D.; Hanig, J.P. Age-related differences in susceptibility to toxic effects of valproic acid in rats. J. Appl. Toxicol. 2008, 28, 628–637. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; De Caro, C.; Citraro, R.; Russo, E.; Tallarico, M.; Iannone, M.; Ferrante, M.C.; et al. Butyrate prevents valproate-induced liver injury: In vitro and in vivo evidence. Faseb. J. 2020, 34, 676–690. [Google Scholar] [CrossRef]
- Surendradoss, J.; Chang, T.K.; Abbott, F.S. Assessment of the role of in situ generated (E)-2,4-diene-valproic acid in the toxicity of valproic acid and (E)-2-ene-valproic acid in sandwich-cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 2012, 264, 413–422. [Google Scholar] [CrossRef]
- Löscher, W.; Nau, H.; Marescaux, C.; Vergnes, M. Comparative evaluation of anticonvulsant and toxic potencies of valproic acid and 2-en-valproic acid in different animal models of epilepsy. Eur. J. Pharmacol. 1984, 99, 211–218. [Google Scholar] [CrossRef]
- Sugimoto, T.; Woo, M.; Nishida, N.; Takeuchi, T.; Sakane, Y.; Kobayashi, Y. Hepatotoxicity in rat following administration of valproic acid. Epilepsia 1987, 28, 142–146. [Google Scholar] [CrossRef]
- Ferdowsian, H.R.; Beck, N. Ethical and scientific considerations regarding animal testing and research. PLoS ONE 2011, 6, e24059. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Fatemi, I.; Esmaeilizadeh, M.; Ghaznavi, H.; Kalantar, H.; Goudarzi, M. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed. Pharmacother. 2018, 97, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yanaşoğlu, E.; Büyükavcı, M.; Çetinkaya, A.; Turan, G.; Köroğlu, M.; Yazar, H.; Büyükokuroğlu, M.E. Silibinin effect on methotrexate-induced hepatotoxicity in rats. Eurasian J. Med. 2022, 54, 264–269. [Google Scholar] [CrossRef]
- Nur Ekincï-Akdemïr, F.; Bïngöl, Ç.; Yıldırım, S.; Kandemi, R.F.M.; Küçükler, S.; Sağlam, Y.S. The investigation of the effect of fraxin on hepatotoxicity induced by cisplatin in rats. Iran. J. Basic. Med. Sci. 2020, 23, 1382–1387. [Google Scholar]
- Coşkun, Ö.; Öztopuz, Ö.; Büyük, B. Possible protective activity of n-acetyl cysteine against cisplatin-induced hepatotoxicity in rats. Mol. Biol. Rep. 2021, 48, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Cüre, M.C.; Cüre, E.; Kalkan, Y.; Kırbaş, A.; Tümkaya, L.; Yılmaz, A.; Türkyılmaz, A.K.; Şehitoğlu, İ.; Yüce, S. Infliximab modulates cisplatin-induced hepatotoxicity in rats. Balkan Med. J. 2016, 33, 504–511. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.R.; Arafa, E.-S.A.; Shehata, B.A.; El Sherbiny, G.A.; Arab, H.H.; Elgendy, A.N. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol Rep. 2016, 68, 349–356. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. SAGARPA: Mexico City, Mexico, 1999.
- Norma Oficial Exicana NOM-033-ZOO-1995; Sacrificio Humanitario de los Animales Domésticos y Silvestres. Dirección General de Normas (DGN): Mexico City, Mexico, 1995.
- Morton, D.B. Humane endpoints in Animal Experimentation for Biomedical Research: Ethical, Legal and Practical Aspects. Humane Endpoints in Animal Experiments for Biomedical Research; The Royal Society Medical Press: London, UK, 1999; pp. 5–12. [Google Scholar]
- Tunali, S. The effects of vitamin B6 on lens antioxidant system in valproic acid-administered rats. Human. Exp. Toxicol. 2014, 33, 623–628. [Google Scholar] [CrossRef]
- Löscher, W. Serum protein binding and pharmacokinetics of valproate in man, dog, rat and mouse. J. Pharmacol. Exp. Ther. 1978, 204, 255–261. [Google Scholar]
- Aguilar Diaz-De Leon, J.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.W.; Belcher, R.V. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965, 94, 70. [Google Scholar] [CrossRef] [PubMed]





| Treatment Dose (mg/kg) | Weight (g) | |||
|---|---|---|---|---|
| 0 Day | 7 Days | 14 Days | ||
| Female | 175 | 193 | 220 | 226 |
| 550 | 202 | 229 | 231 | |
| 1750 | 199 | 221 | 231.5 | |
| 2000 | 203 | 230 | 271 | |
| 205 | 225 | 273 | ||
| 217 | 234 | 285 | ||
| 239 | 272 | 324 | ||
| Control | 209 | 227 | 231.50 | |
| Vehicle | 195 | 216 | 222 | |
| Male | 2000 | 189.2 | 237.5 | 291 |
| 199.2 | 248 | 301 | ||
| 195.2 | 241 | 292 | ||
| 193.2 | 241 | 291 | ||
| 193.7 | 242 | 299.5 | ||
| 198.7 | 246 | 300 | ||
| Control | 203 | 243 | 293.5 | |
| Vehicle | 198.7 | 241 | 277 | |
| Treatment Dose (mg/kg) | Weight (g) | |||
|---|---|---|---|---|
| 0 Day | 7 Days | 14 Days | ||
| Female | 2000 | 22.7 | 29 | 40.5 |
| 23.7 | 29.5 | 32 | ||
| 24.7 | 28 | 31 | ||
| 24 | 26 | 27 | ||
| 27.57 | 29 | 32 | ||
| 23 | 24 | 27 | ||
| Control | 26 | 28.5 | 31 | |
| Vehicle | 24.5 | 27 | 29.5 | |
| Male | 2000 | 33 | 35 | 36.5 |
| 34.2 | 39.5 | 42 | ||
| 32 | 34 | 36 | ||
| 34 | 35 | 38 | ||
| 30.5 | 31 | 32.5 | ||
| 29.5 | 31.5 | 34 | ||
| Control | 33 | 35 | 36.5 | |
| Vehicle | 29.5 | 30.5 | 32 | |
| Treatment | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|
| Control | 25.43 ± 4.69 | 54.93 ± 6.47 | 63.87 ± 16.97 |
| VPA (500 mg/kg) | 41.90 ± 12.46 | 63.05 ± 4.58 | 82.59 ± 12.06 |
| HO-AAVPA (708 mg/kg) | 23.78 ± 4.96 | 57.86 ± 8.28 | 75.25 ± 8.58 |
| Group | Treatment |
|---|---|
| G1: Control | without treatment |
| G2: Vehicle | propylene glycol40%, Tween 80 5%, and y Saline solution 55% (1 mL/kg) |
| G3: VPA | 500 mg/kg |
| G4: HO-AAVPA | 708 mg/kg (Equimolar relationship with respect to VPA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa Basurto, A.M.; Tamay Cach, F.; Jarillo Luna, R.A.; Cabrera Pérez, L.C.; Correa Basurto, J.; García Dolores, F.; Mendieta Wejebe, J.E. Hepatotoxic Evaluation of N-(2-Hydroxyphenyl)-2-Propylpentanamide: A Novel Derivative of Valproic Acid for the Treatment of Cancer. Molecules 2023, 28, 6282. https://doi.org/10.3390/molecules28176282
Correa Basurto AM, Tamay Cach F, Jarillo Luna RA, Cabrera Pérez LC, Correa Basurto J, García Dolores F, Mendieta Wejebe JE. Hepatotoxic Evaluation of N-(2-Hydroxyphenyl)-2-Propylpentanamide: A Novel Derivative of Valproic Acid for the Treatment of Cancer. Molecules. 2023; 28(17):6282. https://doi.org/10.3390/molecules28176282
Chicago/Turabian StyleCorrea Basurto, Ana María, Feliciano Tamay Cach, Rosa Adriana Jarillo Luna, Laura Cristina Cabrera Pérez, José Correa Basurto, Fernando García Dolores, and Jessica Elena Mendieta Wejebe. 2023. "Hepatotoxic Evaluation of N-(2-Hydroxyphenyl)-2-Propylpentanamide: A Novel Derivative of Valproic Acid for the Treatment of Cancer" Molecules 28, no. 17: 6282. https://doi.org/10.3390/molecules28176282
APA StyleCorrea Basurto, A. M., Tamay Cach, F., Jarillo Luna, R. A., Cabrera Pérez, L. C., Correa Basurto, J., García Dolores, F., & Mendieta Wejebe, J. E. (2023). Hepatotoxic Evaluation of N-(2-Hydroxyphenyl)-2-Propylpentanamide: A Novel Derivative of Valproic Acid for the Treatment of Cancer. Molecules, 28(17), 6282. https://doi.org/10.3390/molecules28176282







