The Phytochemical Screening and Biological Properties of Brassica napus L. var. napobrassica (Rutabaga) Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields
2.2. Reducing Sugar Content
2.3. Total Polyphenol Content (TPC)
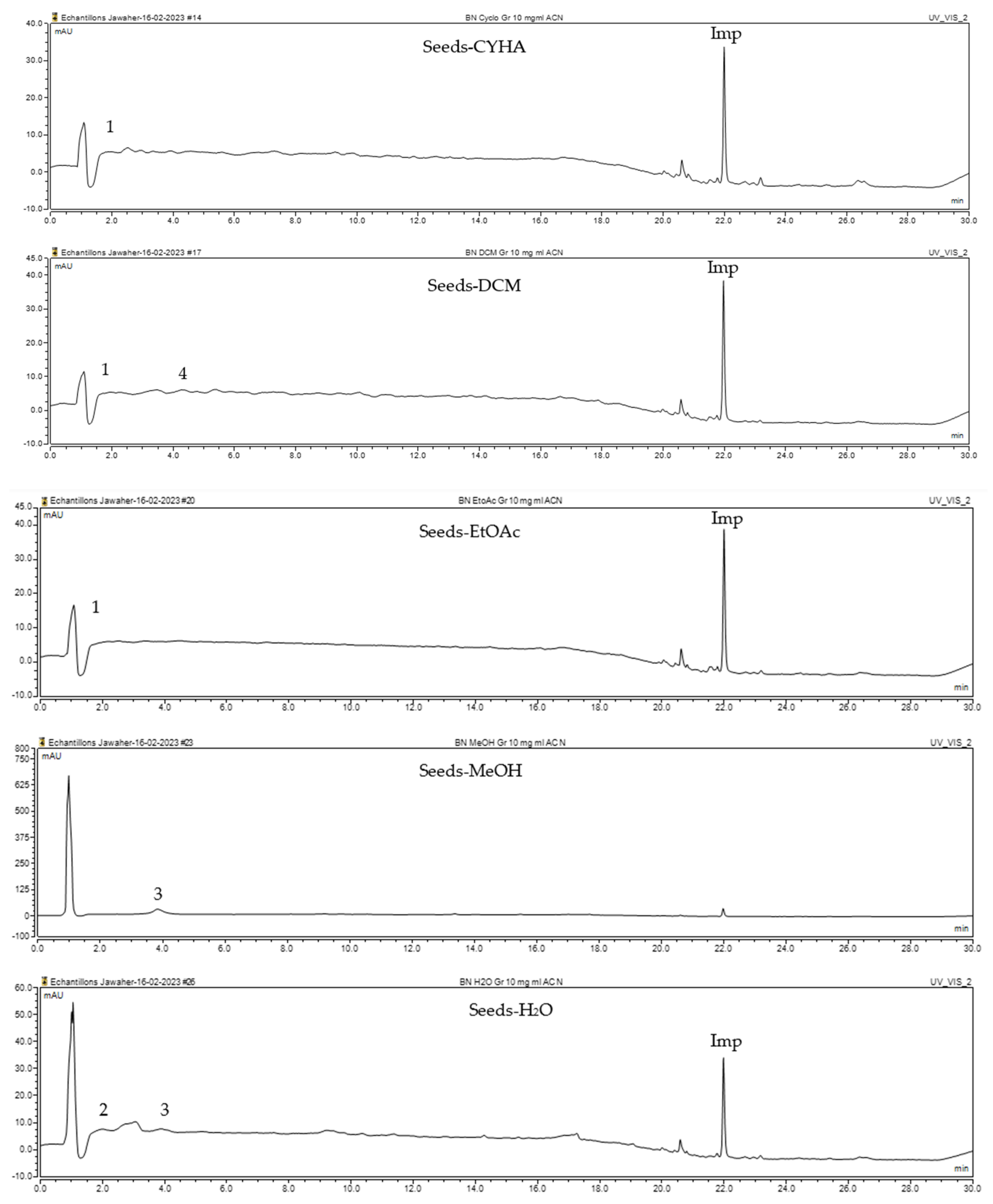
2.4. The Identification and Quantification of Phenolic Compounds Using HPLC-DAD
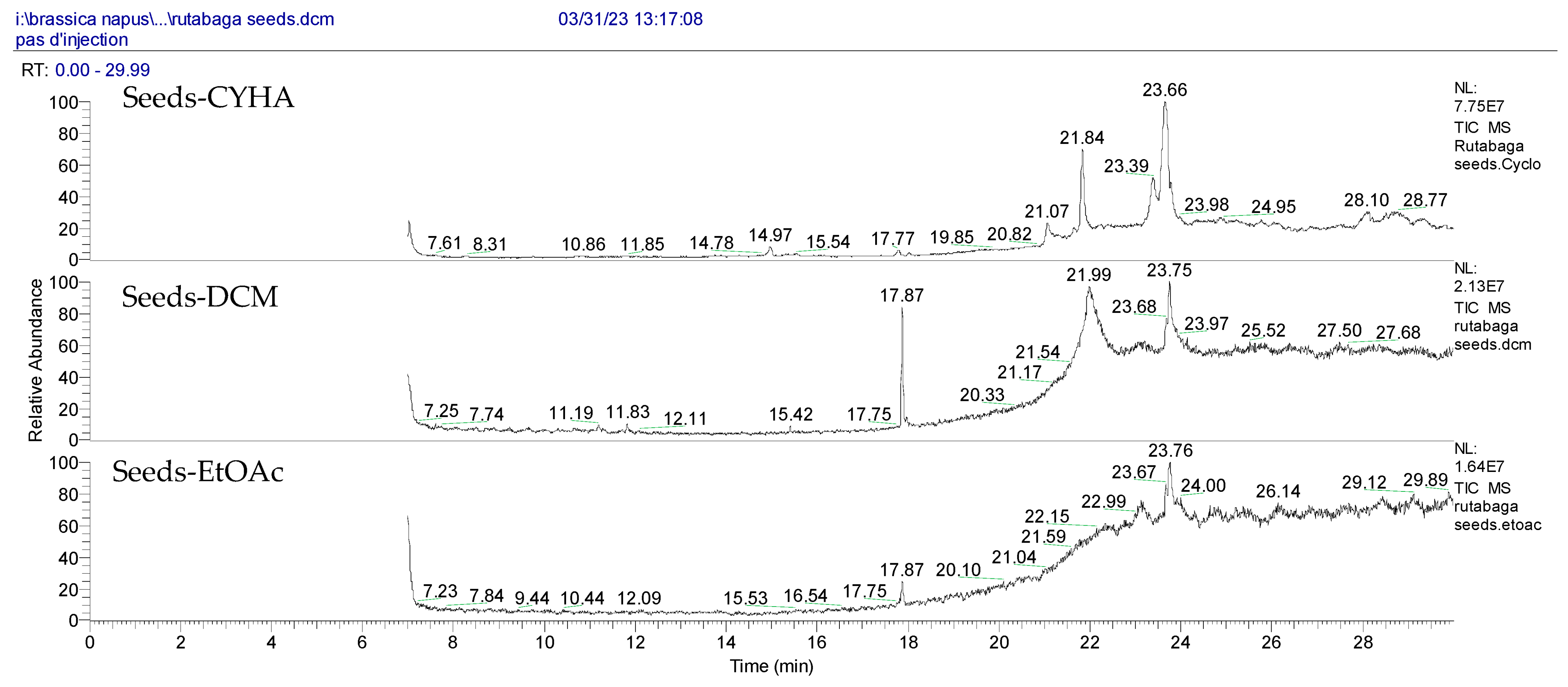
2.5. The Identification of Volatile Compounds in Rutabaga Seed Extracts Using GC-MS (before and after Derivatization)
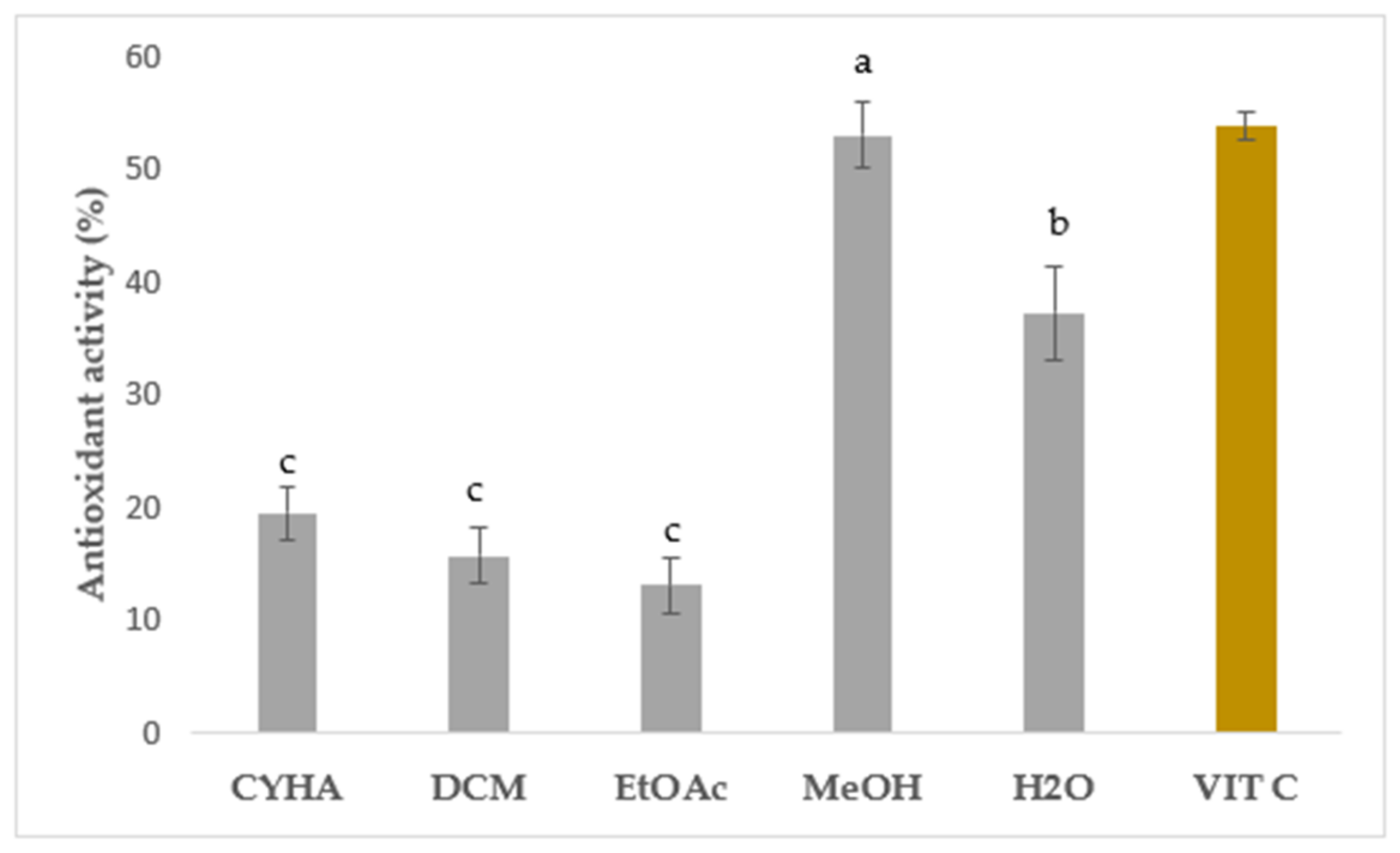
2.6. Antioxidant Activity with DPPH Assay
2.7. Biological Activities
2.7.1. Anti-Inflammatory Activity
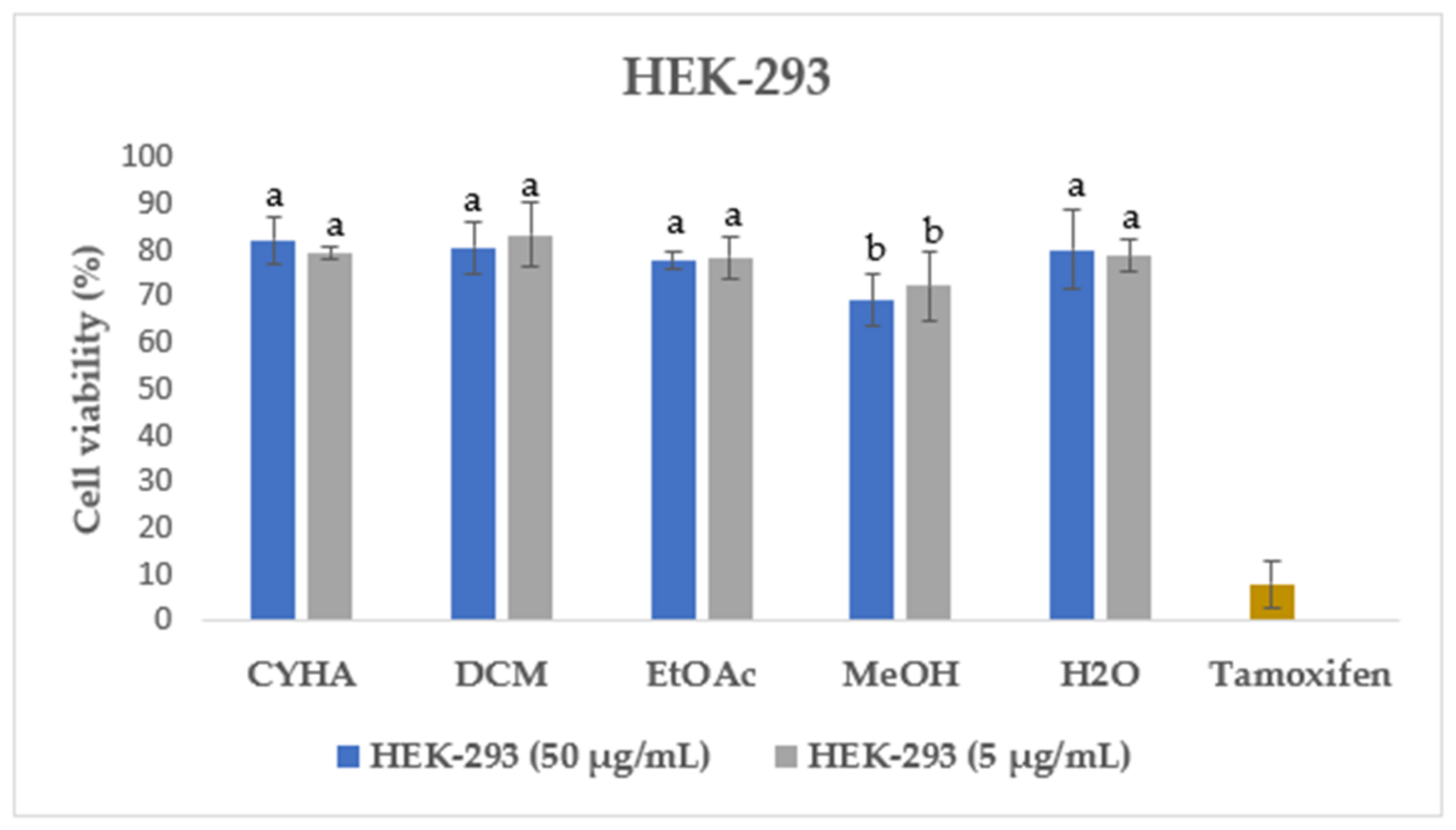
2.7.2. Cytotoxic Activity
2.8. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Extraction
3.3. Quantification of Reducing Sugar Content
3.4. Total Phenolic Content (TPC)
3.5. High-Performance Liquid Chromatography Analysis (HPLC-DAD)
3.6. Gas Chromatography Mass Spectrometry (GC-MS) Analysis
3.7. Free Radical Scavenging Activity DPPH Test
3.8. Biological Activities
3.8.1. Anti-Inflammatory Activity (15-LOX)
3.8.2. Cytotoxicity Evaluation
Cell Culture Growth and Maintenance
MTT Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chopra, B.; Dhingra, A.K. Natural Products: A Lead for Drug Discovery and Development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Butnariu, M.; Sarac, I.; Marmouzi, I.; Kamle, M.; Tripathi, V.; Kumar, P.; Bouyahya, A.; Capanoglu, E. Phytotherapy and Food Applications from Brassica Genus. Phytother. Res. 2021, 35, 3590–3609. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules 2022, 27, 6008. [Google Scholar] [CrossRef] [PubMed]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The Value of Bioactive Compounds of Cruciferous Vegetables (Brassica) as Antimicrobials and Antioxidants: A Review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Mattosinhos, P.d.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. Anti-Inflammatory, Antioxidant, and Skin Regenerative Potential of Secondary Metabolites from Plants of the Brassicaceae Family: A Systematic Review of In Vitro and In Vivo Preclinical Evidence (Biological Activities Brassicaceae Skin Diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef]
- Le, T.N.; Sakulsataporn, N.; Chiu, C.-H.; Hsieh, P.-C. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals 2020, 13, 82. [Google Scholar] [CrossRef]
- Peña, M.; Guzmán, A.; Martínez, R.; Mesas, C.; Prados, J.; Porres, J.M.; Melguizo, C. Preventive Effects of Brassicaceae Family for Colon Cancer Prevention: A Focus on in Vitro Studies. Biomed. Pharmacother. 2022, 151, 113145. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B. Early Allopolyploid Evolution in the Post-Neolithic Brassica napus Oilseed Genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Muzaffar, S. Phytochemical Composition and Antioxidant Potential of Brassica. In Brassica Germplasm—Characterization, Breeding and Utilization; IntechOpen: London, UK, 2018; Volume 1, pp. 7–26. [Google Scholar] [CrossRef]
- Pasko, P.; Bukowska-Strakova, K.; Gdula-Argasinska, J.; Tyszka-Czochara, M. Rutabaga (Brassica napus L. Var. Napobrassica) Seeds, Roots, and Sprouts: A Novel Kind of Food with Antioxidant Properties and Proapoptotic Potential in Hep G2 Hepatoma Cell Line. J. Med. Food 2013, 16, 749–759. [Google Scholar] [CrossRef]
- Lim, T.K.; Lim, T.K. Brassica napus Var. Napobrassica. In Edible Medicinal and Non Medicinal Plants: Volume 9, Modified Stems, Roots, Bulbs; Springer: Dordrecht, The Netherlands, 2015; pp. 761–767. [Google Scholar] [CrossRef]
- Lugast, A.; Hovari, J. Flavonoid Aglycons in Foods of Plant Origin I. Vegetables. Acta Aliment. 2000, 29, 345–352. [Google Scholar] [CrossRef]
- Carlson, D.G.; Daxenbichler, M.E.; VanEtten, C.H.; Tookey, H.L.; Williams, P.H. Glucosinolates in Crucifer Vegetables: Turnips and Rutabagas. J. Agric. Food Chem. 1981, 29, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Soengas, P.; Vilar, M.; Cartea, M.E.; del Rio, M. Comparison of Glucosinolate Profiles in Leaf and Seed Tissues of Different Brassica napus Crops. J. Am. Soc. Hortic. Sci. 2008, 133, 551–558. [Google Scholar] [CrossRef]
- Mullin, W.; COLLINS, M.; Proudfoot, K. Glucosinolate Content and Clubroot of Rutabaga and Turnip. Can. J. Plant Sci. 1980, 60, 605–612. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Danlami, U.; Orishadipe Abayomi, T.; Lawal, D.R. Phytochemical, Nutritional and Antimicrobial Evaluations of the Aqueous Extract of Brassica nigra (Brassicaceae) Seeds. Am. J. Appl. Chem. 2016, 4, 161. [Google Scholar] [CrossRef]
- Grygier, A. Mustard Seeds as a Bioactive Component of Food. Food Rev. Int. 2022, 1–14. [Google Scholar] [CrossRef]
- Stefanucci, A.; Zengin, G.; Llorent-Martinez, E.J.; Dimmito, M.P.; Della Valle, A.; Pieretti, S.; Ak, G.; Sinan, K.I.; Mollica, A. Chemical Characterization, Antioxidant Properties and Enzyme Inhibition of Rutabaga Root’s Pulp and Peel (Brassica napus L.). Arab. J. Chem. 2020, 13, 7078–7086. [Google Scholar] [CrossRef]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuña, C.L. Sulforaphane and Its Antioxidative Effects in Broccoli Seeds and Sprouts of Different Cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef]
- Krishnaveni, M.; Saranya, S. Secondary metabolites, antioxidant activity, phytonutrient analysis of Nigella sativa and Brassica hirta seeds. Int. J. Pharm. Biol. Sci. 2016, 6, 137–143. [Google Scholar]
- Ogidi, O.I.; Omu, O.; Ezeagba, P.A. Ethno Pharmacologically Active Components of Brassica juncea (Brown Mustard) Seeds. Int. J. Pharm. Res. Dev. 2019, 1, 9–13. [Google Scholar] [CrossRef]
- Jun, H.-I.; Wiesenborn, D.P.; Kim, Y.-S. Antioxidant Activity of Phenolic Compounds from Canola (Brassica napus) Seed. Food Sci. Biotechnol. 2014, 23, 1753–1760. [Google Scholar] [CrossRef]
- Oh, S.; Kim, K.; Choi, M. Antioxidant Activity of Different Parts of Dolsan Leaf Mustard. Food Sci. Biotechnol. 2016, 25, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of Different Light Conditions and Time of Sprouting on Harmful and Beneficial Aspects of Rutabaga Sprouts in Comparison to Their Roots and Seeds. J. Sci. Food Agric. 2019, 99, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Pagnotta, E.; Piragine, E.; Flori, L.; Citi, V.; Martelli, A.; Mannelli, L.D.C.; Ghelardini, C.; Matteo, R.; Suriano, S. Cardiovascular Benefits of Eruca Sativa Mill. Defatted Seed Meal Extract: Potential Role of Hydrogen Sulfide. Phytother. Res. 2022, 36, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Nicácio, A.E.; Rodrigues, C.A.; Visentainer, J.V.; Maldaner, L. Evaluation of the QuEChERS Method for the Determination of Phenolic Compounds in Yellow (Brassica alba), Brown (Brassica juncea), and Black (Brassica nigra) Mustard Seeds. Food Chem. 2021, 340, 128162. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Olech, M. Optimization of Ultrasound-Assisted Extraction and LC-ESI–MS/MS Analysis of Phenolic Acids from Brassica oleracea L. Var. Sabellica. Ind. Crops Prod. 2016, 83, 359–363. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, X.; Chen, B.; Chao, Y.; Hu, P.; Cai, Y.; Wu, B.; Wei, M. Identification and Determination of Chemical Constituents of Citrus Reticulata Semen through Ultra High Performance Liquid Chromatography Combined with Q Exactive Orbitrap Tandem Mass Spectrometry. J. Sep. Sci. 2020, 43, 438–451. [Google Scholar] [CrossRef]
- Hamed, M.A.; Aboul Naser, A.F.; Aboutabl, M.E.; Osman, A.F.; Hassan, E.E.; Aziz, W.M.; Khalil, W.K.; Farghaly, A.A.; El-Hagrassi, A.M. Bioactive Compounds and Therapeutic Role of Brassica oleracea L. Seeds in Rheumatoid Arthritis Rats via Regulating Inflammatory Signalling Pathways and Antagonizing Interleukin-1 Receptor Action. Biomarkers 2021, 26, 788–807. [Google Scholar] [CrossRef]
- Baky, M.H.; Shamma, S.N.; Xiao, J.; Farag, M.A. Comparative Aroma and Nutrients Profiling in Six Edible versus Nonedible Cruciferous Vegetables Using MS Based Metabolomics. Food Chem. 2022, 383, 132374. [Google Scholar] [CrossRef]
- Kafaltiya, M.; Lohani, H.; Bhandari, U.; Zafar Haider, S.; Chauhan, N.; Ahuja, T.M.; Pant, S.; Joshi, N. Chemical Composition, Antioxidant and Antimicrobial Potential of the Essential Oils from Aerial Parts of Tagetes patula L. at Different Phenological Stages. J. Essent. Oil Bear. Plants 2022, 25, 495–507. [Google Scholar] [CrossRef]
- Selvaraju, R.; Sakuntala, P.; Jaleeli, K. GC–MS and FTIR Analysis of Chemical Compounds in Ocimum gratissimum Plant. Biophysics 2021, 66, 401–408. [Google Scholar] [CrossRef]
- Shilpa, K.; Sangeetha, K.N.; Muthusamy, V.S.; Sujatha, S.; Lakshmi, B.S. Probing Key Targets in Insulin Signaling and Adipogenesis Using a Methanolic Extract of Costus Pictus and Its Bioactive Molecule, Methyl Tetracosanoate. Biotechnol. Lett. 2009, 31, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Amado, P.A.; Ferraz, V.; da Silva, D.B.; Carollo, C.A.; Castro, A.H.F.; Alves Rodrigues dos Santos lima, L. Chemical Composition, Antioxidant and Cytotoxic Activities of Extracts from the Leaves of Smilax Brasiliensis Sprengel (Smilacaceae). Nat. Prod. Res. 2018, 32, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Fu, X.; Wang, X.; Liao, Y.; Zeng, L.; Dong, F.; Yang, Z. Studies on the Biochemical Formation Pathway of the Amino Acid L-Theanine in Tea (Camellia sinensis) and Other Plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef]
- Salusjärvi, L.; Havukainen, S.; Koivistoinen, O.; Toivari, M. Biotechnological Production of Glycolic Acid and Ethylene Glycol: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, E.J.; Chang, J.Y.; Song, K.B.; Chun, H.H. Effect of High Hydrostatic Pressure (HHP) and Supercooling Storage in Leaf Mustard (Brassica juncea L.) Kimchi: Modelling of Microbial Activity and Preservation of Physicochemical Properties. LWT 2021, 145, 111325. [Google Scholar] [CrossRef]
- Hassan, M.; El-Badawy, S.; Draz, M.; Ibrahim, E. New Acaricidal Activities and Chemical Compositions of Orange Oil and Extracts of (Wild Mint and Henna) against Tetranychus Urticae Koch (Acari.: Tetranychidae). Arch. Phytopathol. Plant Prot. 2021, 54, 1848–1863. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. P-Coumaric Acid Induces Antioxidant Capacity and Defense Responses of Sweet Cherry Fruit to Fungal Pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Šamec, D.; Salopek-Sondi, B. Cruciferous (Brassicaceae) Vegetables. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Parikh, H.; Khanna, A. Pharmacognosy and Phytochemical Analysis of Brassica juncea Seeds. Pharmacogn. J. 2014, 6, 47–54. [Google Scholar] [CrossRef]
- Aziz, S.S.; El-Zayat, M.M.; El-Khateeb, A.Y. Phytochemical Characterization, Antioxidant and Antimicrobial Activities of Brassica juncea (L.) Mustard Seeds Aqueous and Ethanolic Extracts. J. Plant Prod. 2020, 11, 85–88. [Google Scholar] [CrossRef]
- Rahmani, R.; Bouajila, J.; Jouaidi, M.; Debouba, M. African Mustard (Brassica tournefortii) as Source of Nutrients and Nutraceuticals Properties. J. Food Sci. 2020, 85, 1856–1871. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.; Abdel-Wahab, B.A.; Ali, F.E.; Abd El-Ghafar, O.A.; Kozman, M.R.; Sharkawi, S.M. Trans-Ferulic Acid Ameliorates Cisplatin-Induced Testicular Damage via Suppression of TLR4, P38-MAPK, and ERK1/2 Signaling Pathways. Environ. Sci. Pollut. Res. 2021, 28, 41948–41964. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.V.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2, 4-Di-Tert-Butylphenol Isolated from Humboldtia unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Pérez, C.; Pouplana, R. QSAR Study of Dual Cyclooxygenase and 5-Lipoxygenase Inhibitors 2, 6-Di-Tert-Butylphenol Derivatives. Bioorg. Med. Chem. 2003, 11, 4207–4216. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Cavò, E.; Ragusa, M.; Cacciola, F.; Mondello, L.; Dugo, L.; Acquaviva, R.; Malfa, G.A.; Marino, A.; D’Arrigo, M. Brassica Incana Ten. (Brassicaceae): Phenolic Constituents, Antioxidant and Cytotoxic Properties of the Leaf and Flowering Top Extracts. Molecules 2020, 25, 1461. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Shakil, S.; Zeeshan, M.; Khan, M.S.; Shaikh, S.; Biswas, D.; Ahmad, A.; Kamal, M.A. An Enzoinformatics Study Targeting Polo-like Kinases-1 Enzyme: Comparative Assessment of Anticancer Potential of Compounds Isolated from Leaves of Ageratum Houstonianum. Pharmacogn. Mag. 2014, 10, S14. [Google Scholar] [CrossRef]
- Wiebe, J.; Dinsdale, C. Inhibition of Cell Proliferation by Glycerol. Life Sci. 1991, 48, 1511–1517. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential Transformation of Human Neuronal Cells by Human Adenoviruses and the Origin of HEK 293 Cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef]
- Pulix, M.; Lukashchuk, V.; Smith, D.C.; Dickson, A.J. Molecular Characterization of HEK293 Cells as Emerging Versatile Cell Factories. Curr. Opin. Biotechnol. 2021, 71, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Malfa, G.A.; De Leo, M.; Tundis, R.; Braca, A.; Loizzo, M.R.; Di Giacomo, C.; Raimondo, F.M.; Bucchini, A.E.A.; Acquaviva, R. Biological Investigation and Chemical Study of Brassica villosa subsp. Drepanensis (Brassicaeae) Leaves. Molecules 2022, 27, 8447. [Google Scholar] [CrossRef] [PubMed]
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.-A.; Cazaux, S.; Bouajila, J. Chemical Composition and Biological Activities of Extracts and Essential Oil of Boswellia Dalzielii Leaves. Pharm. Biol. 2017, 55, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis Vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]

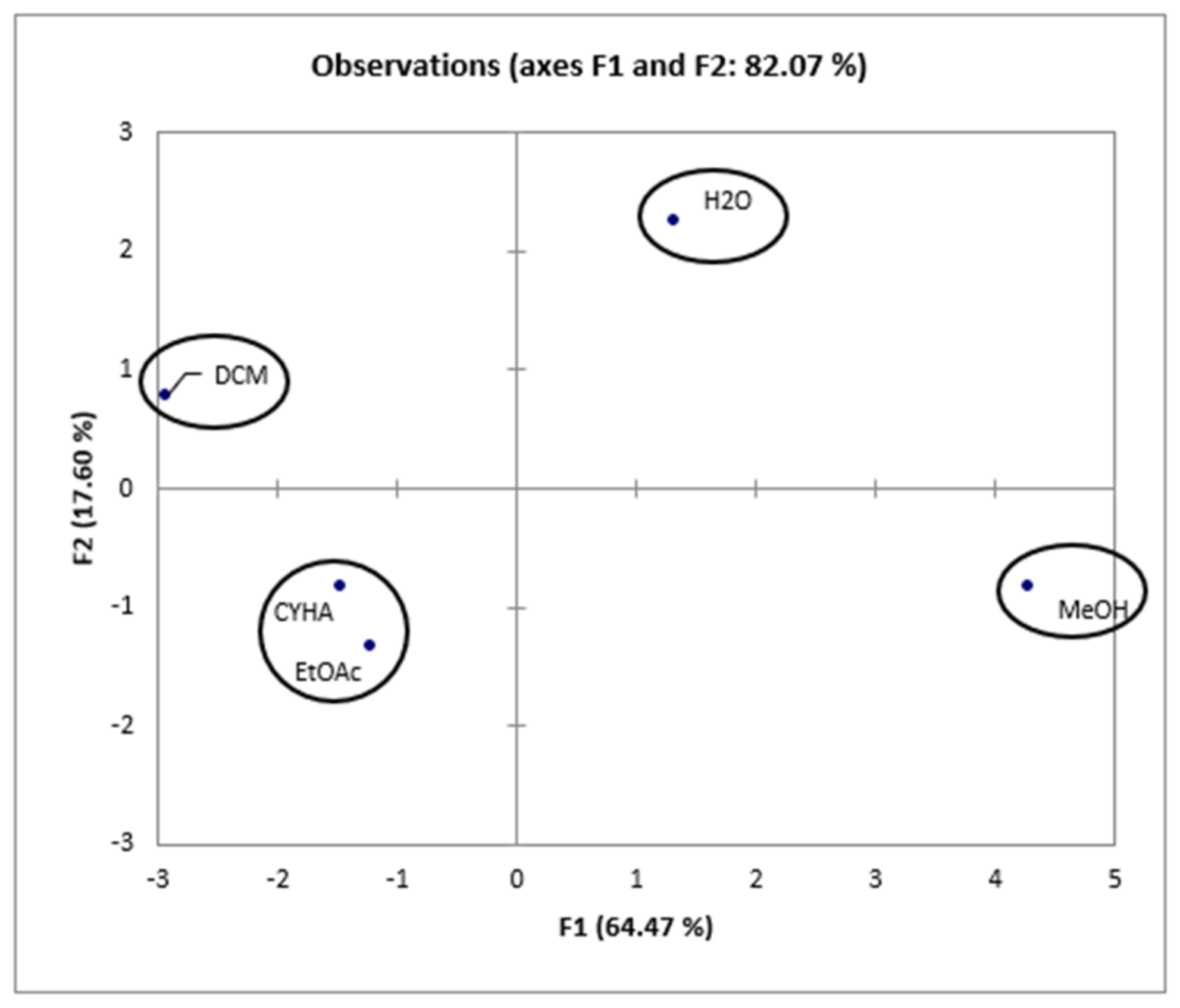
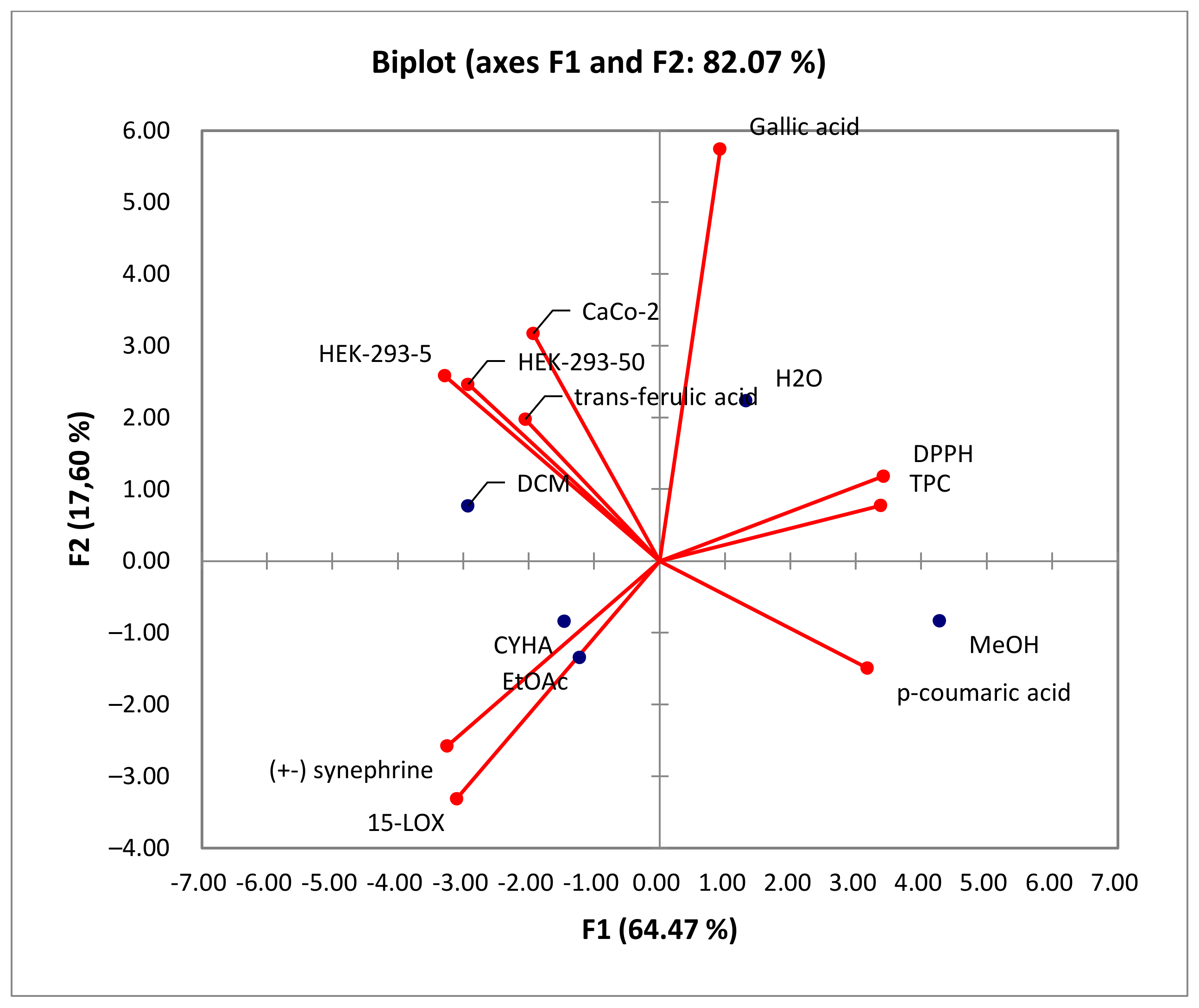
| Fractional Extraction | |||||
|---|---|---|---|---|---|
| CYHA | DCM | EtOAc | MeOH | H2O | |
| Yields (%) | 16.80 | 7.50 | 3.65 | 5.30 | 5.85 |
| Sugars (mg GE/g DW) | nd | nd | 327.40 ± 6.98 b | 473.75 ± 36.19 a | 189.87 ± 30.48 c |
| TPC (mg GAE/g DW) | 3.68 ± 0.65 d | 9.46 ± 2.37 c | 9.95 ± 1.06 c | 43.77 ± 1.48 a | 25.23 ± 3.33 b |
| N° | Compounds | RT (min) | Calibration Curves | Concentration (mg/g of Extract) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| CYHA | DCM | EtOAc | MeOH | H2O | |||||
| 1 | 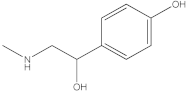 (±) synephrine | 1.08 | y = 0.385x + 0.5679 R2 = 0.9897 | 0.21 | 0.27 | 0.25 | - | - | [30] |
| 2 | 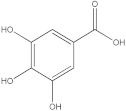 Gallic acid | 1.98 | y = 0.9282x − 4.35 R2 = 0.9939 | - | - | - | - | 0.46 | [27] |
| 3 | 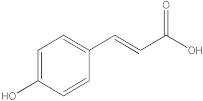 p-coumaric acid | 3.84 | y = 4.8392x + 4.714 R2 = 0.9906 | - | - | - | 5.64 | 0.66 | [28] |
| 4 | 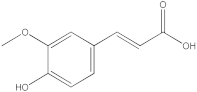 trans-ferulic acid | 4.26 | y = 2.1952x + 14.822 R2 = 0.9939 | - | 1.62 | - | - | - | [29] |
| N° | RT (min) | Volatile Compounds | Area (×107) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| CYHA | DCM | EtOAc | MeOH | H2O | ||||
| Before Derivatization | ||||||||
| 1 | 14.97 |  1,1′-Bicyclohexyl | 2.55 | - | - | - | - | [33] |
| 2 | 17.87 | 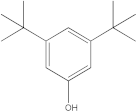 Phenol, 3,5-bis(1,1-dimethylethyl)- | - | 4.37 | - | - | - | [34] |
| 3 | 17.87 | 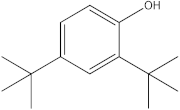 2,4-Di-tert-butylphenol | - | - | 1.13 | - | - | [31] |
| 4 | 21.07 |  Tetracosanoic acid, methyl ester | 8.11 | - | - | - | - | [35] |
| 5 | 21.84 |  Docosanoic acid, methyl ester | 19.47 | - | - | - | - | [36] |
| After Derivatization | ||||||||
| 1′ | 8.28 |  Ethanamine | 1.27 | - | 0.86 | - | - | [37] |
| 2′ | 8.71 |  Ethylene glycol | - | 0.56 | - | - | - | [38] |
| 3′ | 8.86 | 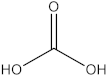 Carbonic acid | - | - | 4.44 | - | - | [39] |
| 4′ | 13.19 | 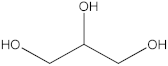 Glycerol | - | 0.91 | - | - | - | [32] |
| 5′ | 19.66 | 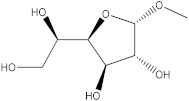 Methyl α-D-glucofuranoside | - | - | - | - | 1.51 | [40] |
| F1 | F2 | F3 | F4 | |
|---|---|---|---|---|
| Total phenolic content (TPC) | 14.000 | 0.735 | 4.401 | 9.667 |
| Antioxidant activity DPPH | 14.369 | 1.725 | 1.196 | 21.841 |
| Anti-15-lipoxygenase activity (15-LOX) | 11.785 | 13.439 | 0.192 | 0.474 |
| Cytotoxic activity (HEK-293) | 10.513 | 7.449 | 8.922 | 38.434 |
| Cytotoxic activity (HEK-293) | 13.220 | 8.189 | 0.197 | 0.260 |
| Cytotoxic activity (Caco-2) | 4.576 | 12.326 | 28.910 | 0.184 |
| (±) synephrine | 12.948 | 8.138 | 0.965 | 5.458 |
| Gallic acid | 1.051 | 40.469 | 11.994 | 16.711 |
| p-coumaric acid | 12.381 | 2.735 | 8.677 | 6.839 |
| trans-ferulic acid | 5.157 | 4.795 | 34.546 | 0.133 |
| F1 | F2 | F3 | |
|---|---|---|---|
| TPC | 0.950 | 0.114 | 0.273 |
| DPPH | 0.963 | 0.174 | 0.142 |
| 15-LOX | −0.872 | −0.486 | −0.057 |
| HEK-293 | −0.823 | 0.362 | −0.388 |
| HEK-293 | −0.923 | 0.380 | 0.058 |
| Caco-2 | −0.543 | 0.466 | 0.699 |
| (±) synephrine | −0.914 | −0.378 | 0.128 |
| Gallic acid | 0.260 | 0.844 | −0.450 |
| p-coumaric acid | 0.893 | −0.219 | 0.383 |
| trans-ferulic acid | −0.577 | 0.291 | 0.764 |
| Variables | TPC | DPPH | 15-LOX | HEK-293 | HEK-293 | Caco-2 | (±) Synephrine | Gallic Acid | p-Coumaric Acid | trans-Ferulic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.958 | −0.897 | −0.867 | −0.820 | −0.271 | −0.869 | 0.234 | 0.920 | −0.308 |
| DPPH | 0.958 | 1 | −0.935 | −0.754 | −0.812 | −0.345 | −0.939 | 0.313 | 0.889 | −0.394 |
| 15-LOX | −0.897 | −0.935 | 1 | 0.559 | 0.616 | 0.208 | 0.975 | −0.609 | −0.696 | 0.318 |
| HEK-293 | −0.867 | −0.754 | 0.559 | 1 | 0.878 | 0.342 | 0.550 | 0.239 | −0.946 | 0.286 |
| HEK-293 | −0.820 | −0.812 | 0.616 | 0.878 | 1 | 0.718 | 0.706 | 0.052 | −0.885 | 0.687 |
| Caco-2 | −0.271 | −0.345 | 0.208 | 0.342 | 0.718 | 1 | 0.410 | −0.061 | −0.321 | 0.982 |
| (±) synephrine | −0.869 | −0.939 | 0.975 | 0.550 | 0.706 | 0.410 | 1 | −0.605 | −0.691 | 0.513 |
| Gallic acid | 0.234 | 0.313 | −0.609 | 0.239 | 0.052 | −0.061 | −0.605 | 1 | −0.136 | −0.250 |
| p-coumaric acid | 0.920 | 0.889 | −0.696 | −0.946 | −0.885 | −0.321 | −0.691 | −0.136 | 1 | −0.286 |
| trans-ferulic acid | −0.308 | −0.394 | 0.318 | 0.286 | 0.687 | 0.982 | 0.513 | −0.250 | −0.286 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. The Phytochemical Screening and Biological Properties of Brassica napus L. var. napobrassica (Rutabaga) Seeds. Molecules 2023, 28, 6250. https://doi.org/10.3390/molecules28176250
Ayadi J, Debouba M, Rahmani R, Bouajila J. The Phytochemical Screening and Biological Properties of Brassica napus L. var. napobrassica (Rutabaga) Seeds. Molecules. 2023; 28(17):6250. https://doi.org/10.3390/molecules28176250
Chicago/Turabian StyleAyadi, Jawaher, Mohamed Debouba, Rami Rahmani, and Jalloul Bouajila. 2023. "The Phytochemical Screening and Biological Properties of Brassica napus L. var. napobrassica (Rutabaga) Seeds" Molecules 28, no. 17: 6250. https://doi.org/10.3390/molecules28176250
APA StyleAyadi, J., Debouba, M., Rahmani, R., & Bouajila, J. (2023). The Phytochemical Screening and Biological Properties of Brassica napus L. var. napobrassica (Rutabaga) Seeds. Molecules, 28(17), 6250. https://doi.org/10.3390/molecules28176250








