Abstract
Salvia hispanica L., commonly known as chía, and its seeds have been used since ancient times to prepare different beverages. Due to its nutritional content, it is considered a dietary ingredient and has been reported with many health benefits. Chia seed components are helpful in cardiovascular disease (CVD) by reducing blood pressure, platelet aggregation, cholesterol, and oxidation. Still, its vasodilator effects on the vascular system were not reported yet. The hexanic (HESh), dichloromethanic (DESh), and methanolic (MESh) extracts obtained from chía seeds were evaluated on an aortic ring ex-vivo experimental model. The vasorelaxant efficacy and mechanism of action were determined. Also, phytochemical data was obtained through 13C NMR-based dereplication. The MESh extract showed the highest efficacy (Emax = 87%), and its effect was partially endothelium-dependent. The mechanism of action was determined experimentally, and the vasorelaxant curves were modified in the presence of L-NAME, ODQ, and potassium channel blockers. MESh caused a relaxing effect on KCl 80 mM-induced contraction and was less potent than nifedipine. The CaCl2-induced contraction was significantly decreased compared with the control curve. Phytochemical analysis of MESh suggests the presence of mannitol, previously reported as a vasodilator on aortic rings. Our findings suggest NO-cGMP pathway participation as a vasodilator mechanism of action of S. hispanica seeds; this effect can be attributed, in part, to the mannitol presence. S. hispanica could be used in future research focused on antihypertensive therapies.
1. Introduction
Salvia L. genus is the great abundant taxonomic group of the Lamiaceae family with ca. 1000 species [1]. The Salvia spp., have a wide worldwide distribution [2] and has been used in ancient traditional medicine, as food, and even in the production of cosmetics in different parts of the world [3,4,5], e.g., Salvia hispanica L., commonly known as chía, is an essential Mesoamerican plant. Since ancient times, chía seeds have been used to prepare different beverages such as “agua fresca de chía”, a drink mixed with lemon and sugar, or “atole”, a drink mixed with toasted maize [6,7], and foods such as “chiapinolli” flour, roasted and grinding seeds [8].
Chía seeds are considered in the European Union as a food ingredient for their nutritional content [9]. It has been employed in the food industry in bakery, dairy, meat, and fish products [10]. The nutritional components of chía comprise high content of soluble and insoluble fiber, protein, minerals, vitamins, and saturated fatty acids, principally from the ω-3 group, such as α-linolenic acid [11,12]. Also, different secondary metabolites such as caffeic acid, chlorogenic acid, quercetin [8], myricetin, oleacein [13] and rosmarinic acid [14] have been reported as principal components. Currently, research on chía seeds on the antioxidant [15,16], anti-diabetic [14,17] weight loss [18] and anti-hypertensive [19,20] biological activities are of great interest considering the clinical benefits on cardiovascular risk. These characteristics target to chía seeds as a potential functional food, principally by health-promoting based on the in vitro and in vivo reports to have cardiovascular protection effects [15]. Some functional foods, such as chía have antioxidant properties and thus are helpful in prevents of cardiovascular disease (CVD) by reducing blood pressure, platelet aggregation, cholesterol, and oxidation [21]. Chia seeds contain omega-3 fatty acids and antioxidants that can be used as a functional component to aid in reducing the risk of CVD, thus, considering that the Mexican population has a prevalence of cardiovascular disease and that chía is one of our country’s pseudo cereals of high consumption, the current study aimed to explore the potential of Mexican chía seeds as a functional food helpful vascular disease.
Nitric oxide (NO) is a small gas molecule that has been found in the vascular endothelium layer. It plays a critical physiological role in maintaining the homeostasis of blood vessels, including the regulation of vascular tone [22]. The mechanisms underlying the regulation of vascular tone by NO are well understood. In the vascular endothelium, the amino acid L-arginine is used constitutively by the endothelial type of NO synthase (eNOS) to produce NO, which diffuses into adjacent smooth muscle cells to promote cGMP formation and subsequently, cGMP leads to vasodilation [23].
In the current work, we present for the first time the vasorelaxant effect of S. hispanica seeds in an ex vivo experimental model to identify its cardiovascular benefits. In addition, an approximation of the mechanism of action and a phytochemical analysis are presented.
2. Results
2.1. Extraction Yield
Following the maceration extraction of S. hispanica seeds, the percentage of major yield was obtained from the hexanic extract (HESh, 16.75%) followed by dichloromethanic (DESh, 2.5%) and methanolic (MESh, 1.5%) extracts, respectively. The three extracts were evaluated using an aortic ring pre–contracted with norepinephrine (NE) to determine their efficacy as important vasorelaxant agents in cardiovascular diseases.
2.2. Pharmacological Evaluation
2.2.1. Vasorelaxant Effect
The MESh extract was the most effective and potent test sample in endothelium–intact aortic rings (E+) (Emax = 87.69%; CE50 = 124 μg/mL) compared with the HESh (Emax = 28%; CE50 = >1000 μg/mL) and DESh (Emax = 14%; CE50 = >1000 μg/mL) (Figure 1a). Whereas without endothelium the vasorelaxant effect was not significant (Figure 1b). The MESh vasorelaxant effect was concentration and partially endothelium–dependent, while HESh and DESh did not showed significant efficacy. In both cases, any extract was more active than positive controls (carbachol E+: Emax = 85% or nifedipine E–: 100%) (Figure 1a,b). Table 1 shows pharmacological data of Emax and EC50 values, calculated using extracts and positive controls.
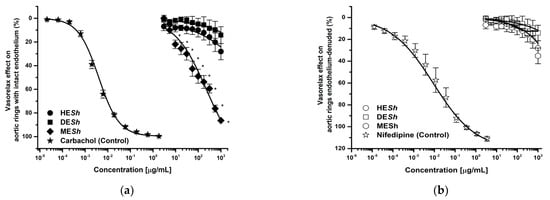
Figure 1.
Concentration–response curves of vasorelaxant effect of S. hispanica extracts on intact–endothelium (a) and denuded–endothelium aortic rings (b). Results are expressed as the means ± SEM of six experiments, * p < 0.05 represents a statistically significant difference between extracts. HESh: hexane extract DESh: Dichloromethane extract, MESh: methanol extract. Carbachol: positive control in endothelium–intact aortic rings, Nifedipine: positive control in endothelium–denuded aortic rings.

Table 1.
Pharmacological data of the vasorelaxant effect of S. hispanica extracts and control drugs.
2.2.2. Mechanism of Action
The MESh showed the major vasorelaxant effect, so its mechanism of action was assessed. The MESh effect was partially endothelium dependent. This suggests that endothelium–derived relaxant factors (EDRF) such as nitric oxide (NO), prostacyclin (PGI2), or Endothelium–Derived Hyperpolarizing Factor (EDHF) and direct mechanisms on vascular smooth muscle cells as antagonism of the adrenergic receptor or calcium channel blocking are involved in the MESh–induced relaxation [24]. Based on earlier data, the participation of EDRF as NO and PGI2 was first investigated.
The MESh extract showed the major vasorelaxant effect, so its mechanism of action was assessed. A MESh relaxation curve was shifted to the right in the presence of L–NAME (Emax = 18.48%; EC50 = >1000 μg/mL) and ODQ (Emax = 28.32%; EC50 = >1000 μg/mL) and efficacy was significantly lowered (Figure 2a), while relaxation curve in the presence of indomethacin or atropine were not altered respect to the control (MESh: Emax = 87.69%; CE50 = 124 μg/mL) (Figure 2b). The curve in the presence of potassium channel blockers such as 2–aminopyridine, glibenclamide, and tetraethylammonium were significantly modified (Figure 3a). The same shows the relaxant curve of MESh in aortic rings contracted with high potassium solution (KCl; 80 mM) (Figure 3b). The MESh extract was efficient (Emax = 70%) compared with a calcium channel blocker (nifedipine, Emax = 99%). While Figure 3c shows the CaCl2–induced contraction curve. The MESh significantly caused inhibition of calcium contraction as well as nifedipine. The efficacy of norepinephrine in the presence of MESh was not modified.
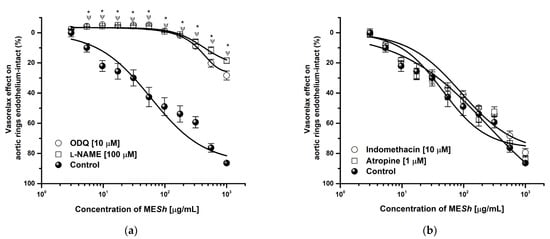
Figure 2.
Concentration–response curves of vasorelaxant effect of MESh in the presence of (a) L-NAME (100 μM) an ODQ (10 μM), (b) Indomethacin (10 μM) and Atropine (1 μM) on intact-endothelium. Results are expressed as the means ± SEM of six experiments, * p < 0.05 represents a significant difference compared with the control.
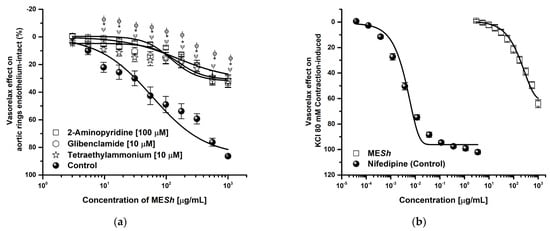
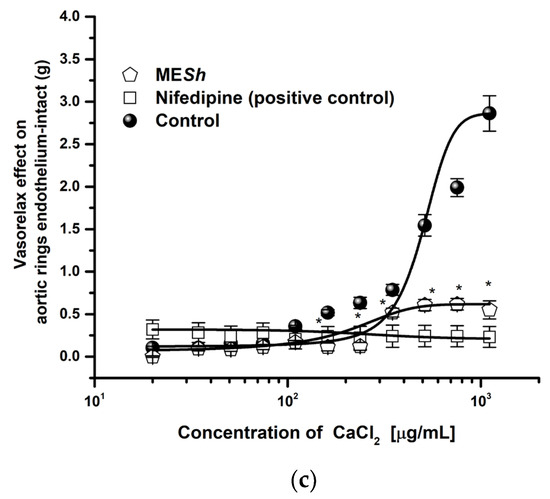
Figure 3.
Concentration–response curves of (a) vasorelaxant effect of MESh in presence of potassium channel blockers: 2–Aminopyridine (100 μM), glibenclamide and tetraethylammonium (10 μM), (b) vasorelaxant effect of MESh On high potassium KCl (80 mM) –induced contraction and (c) effect of MESh on CaCl2–induced contraction. Results are expressed as the means ± SEM of six experiments, * p < 0.05 represents significant difference compared with control group.
2.2.3. Phytochemical Analysis
The 13C NMR–based dereplication was performed by comparing the chemical shift (δC) experimental values from the crude extracts (Figures S1–S3) with those predicted chemical shifts contained in a dedicated database Salvia/DB. Based on the score obtained from comparing the experimental vs. predicted chemical shift, the dereplication analysis performed with HESh and DESh extract suggested the presence of alcohols, saturated and unsaturated fatty acids in the first 20 results (Table 2 and Table 3), of which linoleic acid (18/18 matching carbons, score 1) was confirmed in HESh by comparing their experimental chemical shift vs. reported chemical shift [25] (Table S1). Similarly, the results of the MESh extract show the presence of sugars, glycosides, and polyalcohols (Table 4); of these, mannitol (6/6 matching carbons, score 1) was confirmed [26] (Table S1). The presence of fatty acids in DESh was not confirmed.

Table 2.
First 20 NPs predicted from the results of the 13C NMR–based dereplication analysis, of the HESh extract.

Table 3.
First 20 NPs predicted from the results of the 13C NMR–based dereplication analysis, of the DESh extract.

Table 4.
First 20 NPs predicted from the results of the 13C NMR–based dereplication process, of the MESh extract.
3. Discussion
Many research groups are investigated the nutraceutical properties and pharmacology efficacy of S. hispanica as an antioxidant [16], anti–diabetic [14,17], a weight loss adjuvant [18], and hypertensive prevent [19,20]. Thus, this work was focused to obtained evidence of the vasorelaxant direct effect and phytochemistry composition of S. hispanica seed. Three extracts of S. hispanica seeds were prepared: HESh, DESh, and MESh, and their preclinical pharmacology efficacy were tested. The MESh extract showed more efficiency, and vasorelaxant efficacy was partially endothelium–dependent, so its mechanism of action was attributed to a dual mechanism correspondent to endothelium factors’ presence and targets in smooth muscle cells [24,27].
The role of the vascular endothelium is affected by the presence of membrane-bound receptors like cholinergic, mainly M3 receptors, which when activated by an agonist induces nitric oxide production and release [28]. The MESh curve in the presence of atropine (an M3 antagonist) was not significantly modified. Thus, behavior discarded M3 receptor activation as the first trigger of the extract vasorelaxant effect. The major endothelium–derived releasing factor (EDRF) is nitric oxide (NO), which diffuses to smooth muscle cells where the guanylyl cyclase enzyme is activated, thus catalyzing GMP to cGMP conversion. To identify the participation of nitric oxide pathway activation, endothelium–intact aortic rings were pre-incubated with L–NAME (an NO synthetase inhibitor) or ODQ (a guanylyl cyclase inhibitor), respectively, and after that, the MESh relaxant curve was obtained. Both curves were lowered in the presence of these inhibitors, thus suggesting that the NO–cGMP pathway could be involved in MESh–induced relaxation [29]. On the other hand, it is well known that PGI2 endothelium releases can evoke a vasorelaxant effect [30]. Thus, with the purpose to identified prostaglandins–participation in the MESh effect, aortic rings were pre–incubated with indomethacin (an unspecific prostaglandins inhibitor); however, the MESh efficacy and potency were not modified, thus discarding PGI2–participation in MESh vasorelaxant effect [30].
As described, EDRF can increase the second’s messengers as cGMP or cAMP and thus cause PKG or PKA activation, which participates in the opening of KATP, potassium channel calcium–activation (KCa), and repolarization. In consequence, the relaxation process is evoked [31]. In this context, the relaxant effect of MESh, in the presence of tetraethylammonium (KCa channels blocker), glibenclamide (an ATP–sensitive potassium channel blocker), and or 2–aminopyridine (an inhibitor of KV), was significantly decreased by a half percent (Figure 3a), and the curve was shifted to the right respect to control (indicating a loss in potency), suggesting potassium channels opening because of NO-production MESh induced. The opening of potassium channels will cause cell repolarization, and consequently, calcium channel blocking occurs [32].
To assess if Ca2+ channel blockade was involved in the vasorelaxant effect of the MESh, the assay was carried out in Ca2+–free of RKH solution, and a curve for contraction with CaCl2 was obtained (control curve). The contractile effect induced by CaCl2 was compared in the absence and presence of the MESh (EC50 = 124.7 μg/mL). The CaCl2-induced contraction was significantly decreased by MESh-like nifedipine used as the positive control (Figure 3c). Moreover, the MESh (3.03 to 1000 μg/mL) produced a moderate vasorelaxant effect on the contraction induced with high potassium Krebs solution (Emax = 65%) (Figure 3b). These behaviors suggest a half blocker of L–type Ca2+ channels in the membrane or due to the repolarization process [33]. Changes in the intracellular Ca2+ concentration and membrane depolarization stimulate large–conductance Ca2+–activated K+ (BKCa2+) channels, which are thought to play an essential role in maintaining the membrane potential of vascular smooth muscle cells [34].
Meanwhile, phytochemical analyses were performed through the 13C–NMR dereplication method as a spectroscopic strategy that has proven to help identify NPs in complex mixtures, even without purification [35]. This technique offers advantages over the dereplication analyses using mass spectrometry (MS), liquid chromatography (LC), gas chromatography (GC), or combined instrumentation such as LC–UV, LC–MS, or GC–MS [36]. Here, we have used 13C–NMR chemical shift values obtained from crude extracts for the dereplication process to obtain a phytochemical approximation of S. hipanica seeds obtained in Jalisco, México. Based on the comparison of experimental vs. reported chemical shifts (Table S1), the results showed the presence of linoleic acid (Table 2), previously reported in aerial parts of Salvia triloba L. f., [37]; and mannitol (Table 4), reported in leaves of S. officinalis [38]. This is the first report of mannitol on S. hispanica seeds. Also, the endothelium–dependent vasorelaxant effect of mannitol on mousse mesenteric arterioles was reported and attributed to hyperosmotic action. Therefore, this compound could be responsible for MESh vasorelaxant effect [38]. However, further studies are needed to determine the presence of mannitol and its relationship with the reported biological activity.
4. Materials and Methods
4.1. Plant Material and Extraction
Seeds of Salvia hispanica L. (ID: SF2G/90/2022) were purchased with certified supplies (SuperFoods2Go) in Jalisco, México, in June 2022. Seeds were dried and ground at room temperature (25 °C) for 3 days. Powdered seeds (400 g) were submitted to successive maceration processes (three times, 24 h each) using 100% of: n–hexane, dichloromethane, and methanol (100 g/L), correspondingly. After filtration through a Whatman grade 1 filter, organic extracts were concentrated under reduced pressure using a rotary evaporator (BUCHI R-300) coupled to a vacuum pump (Lab Companion VE-11) and lyophilized using CentriVap FriZowe 6 (LABCONCO) to give the correspondent low (HESh, 67 g), medium (DESh, 10 g) and high (MESh, 6 g) polarity extracts.
4.2. Chemicals and Solution Preparation
(+/-)-norepinephrine bitartrate hydrate (NE), carbamylcholine chloride (carbachol), indomethacin, L-NG-Nitroarginine methyl ester (L-NAME), 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), potassium chloride (KCl), calcium chloride (CaCl2), and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Krebs Henseleit Solution; composition, mM: NaCl, 119; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 1.5; NaHCO3, 20; EDTA, 0.026 and glucose, 11.4.
All other reagents and solvents were analytical grade. Stock solutions of all the chemicals were made in distilled water, except for the extracts, which were dissolved in DMSO (10%). Fresh dilutions were made at the day of the experiment.
4.3. Experimental Subjects
Adult male Wistar rats (250–300 g bodyweight) were obtained from the Universidad Anáhuac–Mayab animal house in Mérida, Yucatan, México. Animals were housed in polycarbonate cages and maintained under standard laboratory conditions (12–h light/dark cycle, 25 ± 2 °C and humidity 45–65%) and were fed with a standard rodent diet and water ad libitum. All animal procedures were conducted by Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación of México (SAGARPA, 1999) [39] and approved by the Institutional Animal Care and Use Committee (UJAT-0001-2017). All experiments were carried out using six animals per group. All study animals were sacrificed by cervical dislocation after deep anesthesia with phenobarbital (65 mg/kg, i.p.).
4.4. Pharmacology Assay
4.4.1. General Experimental Procedures
The Adult male Wistar rats were sacrificed accordingly to the method described by Sánchez-Recillas et al. (2020) [40]. Cervical dislocation and thoracic dissection were carried out to extract the thoracic aorta. It was cleaned from adjacent and connective tissue and then cut into strips 3 mm long (we use a Vernier measurement instrument). In addition, for some aortic rings, the endothelium layer was gently removed by cotton rub manual procedures. The aortic rings were assembled in chambers at 37 °C containing Krebs-Henseleit solution (KHS) at pH 7.4 using stainless steel hooks under an optimal tension of 3 g. After that, aortic rings were submitted to stabilize period for 20 and were constantly bubbled with an O2:CO2 (95:5%) mixture. Changes in tension were recorded by force transducers Grass-FT03 (Astromed, West Warwick, RI, USA) connected to an analyzer MP-150 (BIOPAC 4.1 Instruments, Santa Barbara, CA, USA).
A sensitization process was carried out after the stabilization period. The tissues were stimulated with noradrenaline (NE, 0.1 μM) for 15 min after washed with fresh KHS, and allowed to stabilize for 15 min, this procedure was repeat three times. The absence of endothelium layer was confirmed by the lack of the relaxant response (>50%) induced by carbachol (CCH; 1 μM) in the last contraction with NE before washing with fresh KHS to assess viability.
4.4.2. Ex Vivo Vasorelaxant Evaluation
After sensitization period, the tissues were allowed to stabilize for 20 min and then, contracted with NE (0.1 μM). Extracts (3.03 to 1000 μg/mL), vehicle (100% final concentration) or the positive controls CCH for endothelium–intact aortic rings (E+; 0.303 to 100 μg/mL) and nifedipine for endothelium–denuded aortic rings (E-; 3.89 × 10−5 to 3.46 μg/mL) were added to the chamber in quarter–logarithm dilutions and cumulative concentration–response curves (CRC). The relaxant effect of the samples was determined by their ability to reduce the maximal vascular contraction (Emax) and potency (EC50) effects induced by NE comparing the tissue tension before and after their addition.
4.4.3. Mechanism of Action Approach
In order to establish the mechanism of action of methanol extract of S. hispanica (MESh), the following experiments were conducted.
(a) To establish a possible antagonism of adrenergic receptors or disruption of the NE pathway, the following procedures were performed on E– aortic rings. A cumulative NE-induced contraction (4.15 × 10−11 to 3.6 × 10−5 M) of CRC was made as the positive control (control CRC). In another experiment, aortic rings were pre–incubated with MESh (EC50 = 125 μg/mL) for 15 min. Then the CRC to NE-induced contraction was performed to compare the contraction induced by NE in the absence and presence of MESh.
(b) To know the role of endothelium-derived relaxing factors as nitric oxide (NO) or prostacyclin (PGI2), the E+ aortic rings were pre-incubated with NG-nitro-L-arginine methyl ester (L–NAME, nitric oxide synthase inhibitor (100 μM) or indomethacin and cyclooxygenases inhibitor (10 μM), respectively for 15 min, before the contraction with NE (0.1 μM). The relaxation CRC of MESh (3.03 to 1000 μg/mL) was built as described in the vasorelaxant set experiments. The maximal relaxing effect of the MESh was compared in the absence and presence of L–NAME or indomethacin, respectively.
(c) To establish the possible inhibition of soluble guanylyl cyclase enzyme (sGC), the E+ aortic rings were pre–incubated with 1-H-[1,2,4]-oxadiazolo-[4,3a]-quinoxalin-1-one (ODQ an sGC inhibitor (10 μM) for 15 min, previous to the contraction with NE (0.1 μM). The relaxation CRC of MESh (3.03 to 1000 µg/mL) was built as described before. The maximal relaxing effect of the MESh was compared in the absence and presence of ODQ.
(d) To know the role of K+ channels on extract–induced vasorelaxant effect, the E+ aortic rings were pre–incubated with tetraethylammonium (TEA, non–selective KCa channels blocker (10 μM), 2–Aminopyridine (2AP; 100 μM) an inhibitor of voltage-gated potassium channels (KV) or glibenclamide (10 μM) an ATP–sensitive potassium channel blocker (KATP) for 15 min, previous to the contraction with NE (1 µM). The relaxation CRC of MESh (3.03 to 1000 µg/mL) was built as described before. The maximal relaxing effect of the MESh was compared in the absence and presence of KATP.
(e) To determine whether inhibition of extracellular Ca2+ influx was involved in the extract-induced vasorelaxation, the experiments were carried out in Ca2+–free KHS. After sensitization, endothelium–intact aortic rings were washed with Ca2+–free KHS containing KCl (80 mM) and stabilized for 15 min. Then, a CRC for CaCl2–induced contraction was obtained without the MESh (control group). Once the maximal contraction was reached, tissue was washed with Ca2+–free, KCl (80 mM), and KHS, and allowed to stabilize for 20 min. Finally, after 15 min incubation with the ∫ (EC50 = 125 μg/mL), another CRC for CaCl2-induced contraction was obtained. The contractile effect induced by CaCl2 was compared in the absence and presence of the MESh.
4.5. Phytochemical Analysis
4.5.1. Natural Products Databases for 13C NMR Dereplication
A database (DB) of natural products (NPs) for 13C NMR–based dereplication process was prepared with some modifications to the method described by Bruguière et al. (2021) [41]. Briefly, a search was carried out for previously NPs reported in Salvia spp., using LOTUS: Natural Products Online, available at https://lotus.naturalproducts.net/ (accessed on 20 March 2023). The structures resulting from the LOTUS research were exported in .sdf format, containing 2392 NPs. The 13C NMR chemical shifts (δC) were predicted for each NPs’ methyl, methylene, methine, and quaternary carbons using the algorithm described by Nuzillard (2021) [42] and the ACD NMR predictor (ACD/Labs) to obtain Salvia/DB in the required format for MixONat software (v.1.0.1., SONAS, France).
4.5.2. 13C NMR–Based Dereplication
Dereplication analyses were carried out using MixONat software available at https://sourceforge.net/projects/mixonat/ (accessed on 24 April 2023) [43]. Previously, 50 mg of the crude plant extracts were dissolved in chloroform–d4 or methanol–d4 (600 µL). Carbon spectra (13C–NMR, 150 MHz) were obtained on a Varian-Agilent AR Premium Compact spectrometer (Santa Clara, CA, USA). The spectra were acquired with 6000 scans, and the spectral width was 230 ppm. Phase and baseline correction of spectra was performed automatically using MestReNova software (v. 12.0.0, Mestrelab Research, Santiago de Compostela, Spain). A minimum intensity threshold was then used to collect positive 13C–NMR signals. Each spectrum’s experimental δC list and intensities data were exported to a .csv file using Excel Microsoft Office (Microsoft, Redmond, WA, USA) as an input file in MixONat. The software ranked the putative NPs contained in the mixture with a range score between 1 and 0, according to the number of matching experimental δC obtained from extracts vs. the predicted δC-SDF values of NPs in the Salvia/DB. The predicted NPs were then confirmed by comparing the experimental vs. with that δC reported in the literature analyzed in the same deuterated solvent, with a displacement tolerance range of ±0.5 ppm.
4.6. Statistical Analysis
The results are expressed as the standard error of the mean (n = 5) ± SEM. Concentration-response curves (CRC) were plotted, and the experimental data from the CRC were adjusted by the nonlinear Hill equation with a curve-fitting program (ORIGIN 8.0 MICROCAL). The statistical significance of differences between means was assessed by a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test; p-values lower less than 0.05 (* p < 0.05) were statistically significant [44,45].
5. Conclusions
The vasorelaxant effect of S. hispanica seems to be characterized, and our results suggest NO–cGMP pathway participation as a vasodilator mechanism of action. Also, the preliminary phytochemical report was presented. This work is the first report on ex vivo pharmacology analysis of S. hipanica species, which could be used in future research focused on antihypertensive therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176225/s1, Figure S1: 13C–NMR (150 MHz, CDCl3) spectrum of hexanic extract (HESh) from S. hispanica seeds; Figure S2: 13C–NMR (150 MHz, CDCl3) spectrum of chloroform extract (DESh) from S. hispanica seeds; Figure S3: 13C–NMR (150 MHz, CD3OD) spectrum of methanolic extract (MESh) from S. hispanica seeds; Table S1: Experimental and reported (in the same deuterated solvent; CD3OD) 13C–NMR chemical shift values of mannitol predicted in dereplication analyses results of methanol extract (MESh).
Author Contributions
Conceptualization, A.S.-R., L.A.H.-D. and R.O.-A.; Methodology, A.S.-R., L.A.H.-D. and S.E.-S.; Formal Analysis, A.S.-R., L.A.H.-D., S.E.-S., E.H.-N., T.I.C.-M. and M.R.S.C.; Investigation, M.R.S.C., N.N.C.-C., J.A.A.-L. and R.O.-A.; Resource, T.I.C.-M., N.N.C.-C. and M.R.S.C.; Data curation and Formal Analysis, A.S.-R., J.A.A.-L. and R.O.-A.; Writing—Review and Editing, A.S.-R., J.A.A.-L. and R.O.-A.; Supervision and Project Administration, R.O.-A. and E.H.-N.; Visualization, R.O.-A., J.A.A.-L. and N.N.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by CYTED-Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo, under the project number 119RT0567.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Universidad Juárez Autónoma de Tabasco (protocol code UJAT-0001-2017, with date of July 2017 approval).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Acknowledgments
Authors thanks to Facultad de Química-UADY, CONVESTAV-IPN and SONAS Laboratory, University Angers (France) for the institutional support, scientific equipment and hardware/software supports. Sánchez-Recillas A. and Herbert-Doctor L.A., thanks to CONAHCYT for postdoctoral fellowship references CVU: 298553 and CVU: 515581, respectively. Authors thanks to Hugo Pilotzi Xahuentitla, Priscila Vazquez Garcia and Gloria Ivonne Hernández Bolio (CINVESTAV-IPN) for support in the 13C-NMR analyses, and also to Heidy Quiñones Díaz and Álvaro Yam Galaz (Facultad de Química, UADY) and Victor Ceja Moreno (CINVESTAV-IPN) for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walker, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Kriebel, R.; Drew, B.T.; Claßen-Bockhoff, R.; Sytsma, K.J. Evolution of anther connective teeth in sages (Salvia, Lamiaceae) under bee and hummingbird pollination. Flora 2023, 298, 152199. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants—from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Xiao-Dan, M.; Yan-Feng, C.; Yan-Yun, C.; LI, J.; Zhan-Peng, S.; Wen-Jing, Z.; Yan-Jiang, Q.; Jia-Yu, Z. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar]
- Cahil, J.P. Ethnobotany of Chia, Salvia hispanica L. (Lamiaceae). Econ. Bot. 2003, 57, 604–618. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seed (Salvia hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary fiber content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- EC. Commission Implementing Decision (EU) 2017/2354 of 14 December 2017 Authorising an Extension of use of Chia Seeds (Salvia hispanica) as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C(2017) 8470). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017D2354&qid=1684778533012 (accessed on 31 May 2023).
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Anunciação, P.C.; Matyelka, J.C.S.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The Chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Marineli, R.S.; Moraes, É.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- De Souza Ferreira, C.; de Sousa Fomes, L.F.; da Silva, G.E.S.; Rosa, G. Effect of chia seed (Salvia hispanica L.) consumption on cardiovascular risk factors in humans: A systematic review. Nutr. Hosp. 2015, 32, 1909–1918. [Google Scholar] [PubMed]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Vuksan, V.; Whitham, D.; Sievenpiper, J.L.; Jenkins, A.L.; Rogovik, A.L.; Bazinet, R.P.; Vidgen, E.; Hanna, A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: Results of a randomized controlled trial. Diabetes Care 2007, 30, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Toscano, L.T.; Toscano, L.T.; Tavares, R.L.; da Silva, C.S.O.; Silva, A.S. Chia induces clinically discrete weight loss and improves lipid profile only in altered previous values. Nutr. Hosp. 2014, 31, 1176–1182. [Google Scholar]
- Alwosais, E.Z.M.; Al-Ozairi, E.; Zafar, T.A.; Alkandari, S. Chia seed (Salvia hispanica L.) supplementation to the diet of adults with type 2 diabetes improved systolic blood pressure: A randomized controlled trial. Nutr. Health 2021, 27, 181–189. [Google Scholar] [CrossRef]
- Toscano, L.T.; da Silva, C.S.O.; Toscano, L.T.; de Almeida, A.E.M.; da Cruz Santos, A.; Silva, A.S. Chia flour supplementation reduces blood pressure in hypertensive subjects. Plant Foods Hum. Nutr. 2014, 69, 392–398. [Google Scholar] [CrossRef]
- Khalid, W.; Arshad, M.S.; Aziz, A.; Rahim, M.A.; Qaisrani, T.B.; Afzal, F.; Ali, A.; Ranjha, M.M.A.N.; Khalid, M.Z.; Anjum, F.M. Chia seeds (Salvia hispanica L.): A therapeutic weapon in metabolic disorders. Food Sci. Nutr. 2022, 11, 3–16. [Google Scholar] [CrossRef]
- Raddino, R.; Caretta, G.; Teli, M.; Bonadei, I.; Robba, D.; Zanini, G.; Madureri, A.; Nodari, S.; Dei Cas, L. Nitric oxide and cardiovascular risk factors. Heart Int. 2007, 3, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–140. [Google Scholar] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Marwah, R.G.; Fatope, M.O.; Deadman, M.L.; Al-Maqbali, Y.M.; Husband, J. Musanahol: A New Aureonitol-Related Metabolite from a Chaetomium Sp. Tetrahedron 2007, 63, 8174–8180. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.C.; Shim, S.H.; Lee, E.J.; Jin, W.; Bae, K.; Son, K.H.; Kim, H.P.; Kang, S.S.; Chang, H.W. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch. Pharm. Res. 2006, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Smooth muscle cell calcium activation mechanisms. J. Physiol. 2008, 586, 5047–5061. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.M.; Chitaley, K.C.; Webb, R.C. Vascular smooth muscle contraction and relaxation. In Hypertension Primer: The Essentials of High Blood Pressure; Izzo, J.L., Black, H.R., Eds.; American Heart Association: Dallas, TX, USA, 2003; pp. 97–99. [Google Scholar]
- Bian, K.; Doursout, M.F.; Murad, F. Vascular system: Role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. 2008, 10, 304–310. [Google Scholar] [CrossRef]
- Fukuo, K.; Morimoto, S.; Koh, E.; Yukawa, S.; Tsuchiya, H.; Imanaka, S.; Yamamoto, H.; Onishi, T.; Kumahara, Y. Effects of prostaglandins on the cytosolic free calcium concentration in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1986, 136, 247–252. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Sobey, C. Inwardly rectifying potassium channels in the regulation of vascular tone. Curr. Drug Targets 2003, 4, 281–289. [Google Scholar] [CrossRef]
- Milesi, V.; Aiello, E.A.; Rebolledo, A.; Gomez Alvis, A.; Grassi De Gende, A.O. Role of a Ca2+-activated K+ current in the maintenance of resting membrane potential of isolated, human, saphenous vein smooth muscle cells. Pflugers Arch. 1999, 437, 455–461. [Google Scholar]
- Hilgers, R.H.P.; Webb, R.C. Molecular aspects of arterial smooth muscle contraction: Focus on Rho. Exp. Biol. Med. 2005, 230, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Bruguière, A.; Derbré, S.; Coste, C.; Le Bot, M.; Siegler, B.; Leong, S.T.; Sulaiman, S.N.; Awang, K.; Richomme, P. 13C-NMR dereplication of Garcinia extracts: Predicted chemical shifts as reliable databases. Fitoterapia 2018, 131, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Herbert, L.A.; Bruguière, A.; Derbré, S.; Richomme, P.; Peña-Rodríguez, L.M. 13C NMR dereplication-assisted isolation of bioactive polyphenolic metabolites from Clusia flava Jacq. Nat. Prod. Res. 2022, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.; Butina, D.; Dunn, A.J.; Green, R.H.; Hajek, M.; Jones, M.M.; Lindon, J.C.; Sidebottom, P.J. A rapid and facile method for the dereplication of purified natural products. J. Nat. Prod. 2001, 64, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.H.; Khalifa, T.I.; Ibrahim, M.T.; Mabry, T.J. Constituents from Salvia triloba. Fitoterapia 2001, 72, 850–853. [Google Scholar] [CrossRef] [PubMed]
- SAGARPA. Normas Oficiales Mexicanas en Materia de Salud Animal. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Gobierno | gob.mx. Available online: https://www.gob.mx/senasica/documentos/normatividad-en-materia-de-salud-animal (accessed on 20 December 2022).
- Sánchez-Recillas, A.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Bonilla-Hernández, M.; Ortiz-Andrade, R.; Ibarra-Barajas, M.; Yáñez-Pérez, V.; Sánchez-Salgado, J.C. Pharmacological characterization of the cardiovascular effect of Nibethione: ex vivo, in vivo and in silico studies. J. Pharm. Pharmacol. 2020, 72, 1186–1198. [Google Scholar] [CrossRef]
- Bruguière, A.; Derbré, S.; Breárd, D.; Tomi, F.; Nuzillard, J.M.; Richomme, P. 13C NMR Dereplication Using MixONat Software: A Practical Guide to Decipher Natural Products Mixtures. Planta Med. 2021, 87, 1061–1068. [Google Scholar] [CrossRef]
- Nuzillard, J.M. Taxonomy-Focused Natural Product Databases for Carbon-13 NMR-Based Dereplication. Analytica 2021, 2, 50–56. [Google Scholar] [CrossRef]
- Available online: https://sourceforge.net/projects/mixonat/ (accessed on 27 June 2023).
- Bailey, N.T.J. Statistical Methods in Biology, 3rd ed.; Cambridge University Press: Cambridge, UK, 1995; Volume 75. [Google Scholar]
- Daniel, W.W.; Cross, C.L. Biostatistics: Basic Concepts and Methodology for the Health Sciences, 10th ed.; International Student Version; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).