Fabrication of Ag-CaCO3 Nanocomposites for SERS Detection of Forchlorfenuron
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CaCO3 NPs
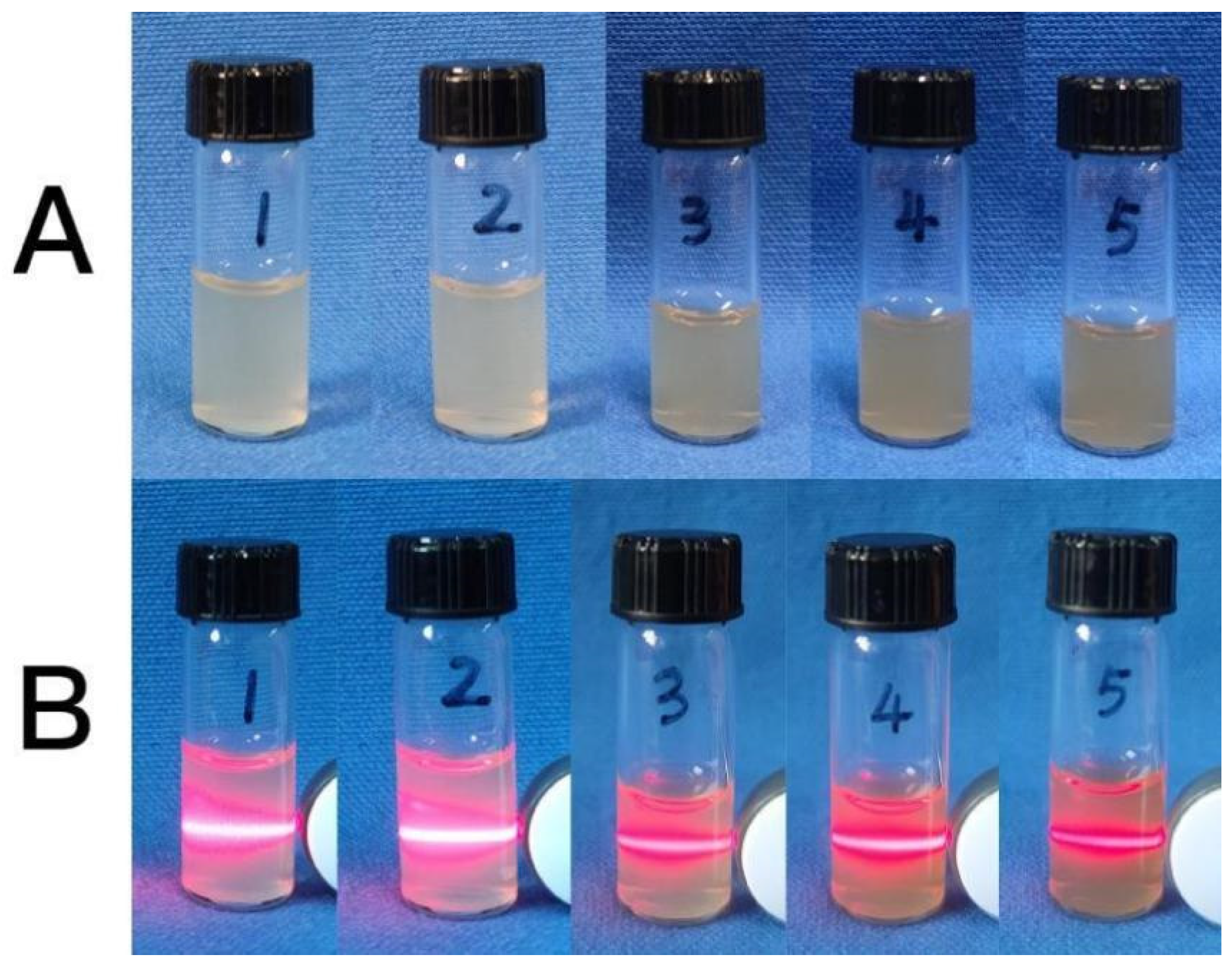
2.1.1. Effects of Different Concentrations of Calcium Lignosulfonate on the Synthesized CaCO3 NPs
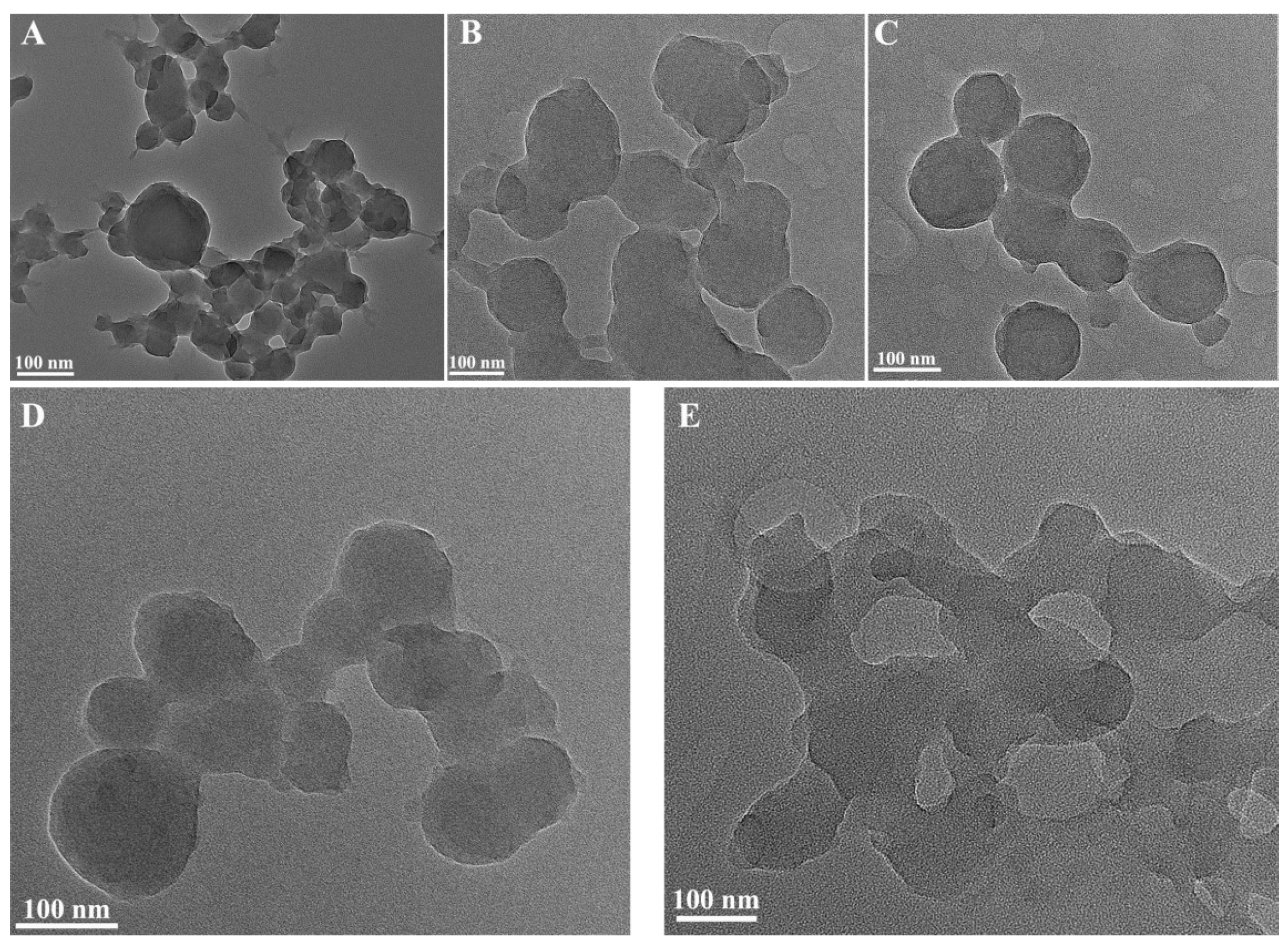
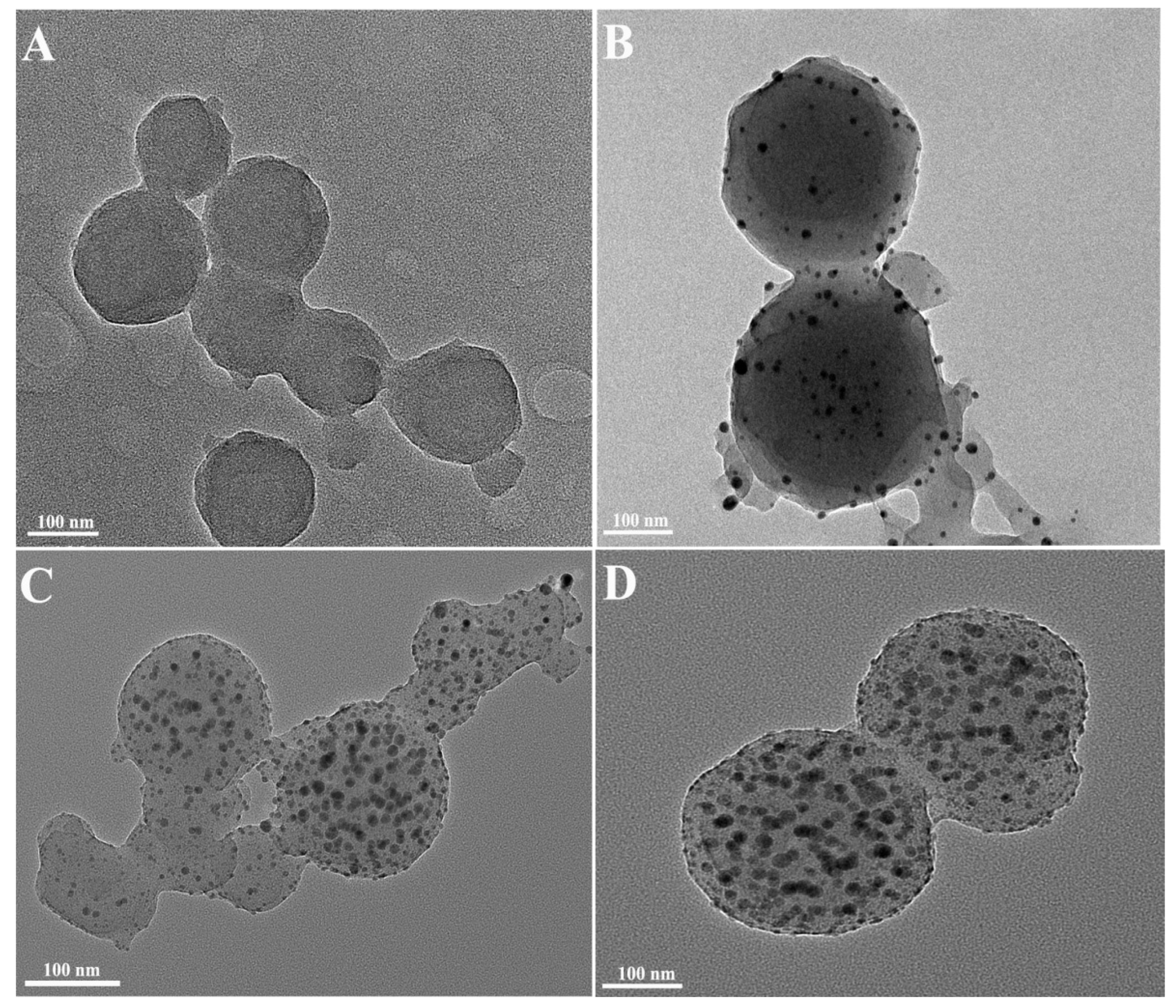
2.1.2. TEM Images of CaCO3 NPs
2.1.3. Infrared Spectra of CaCO3 NPs
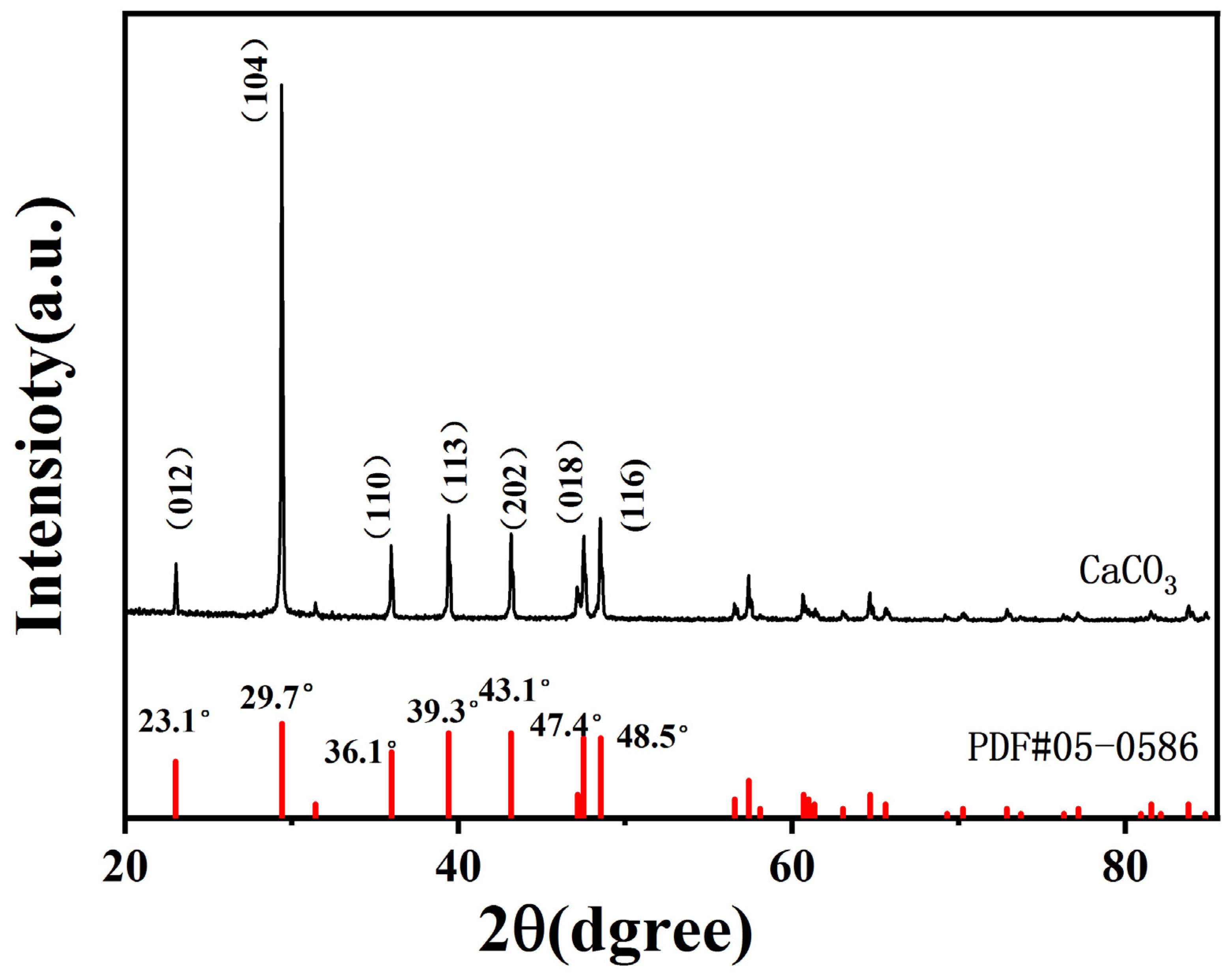
2.1.4. X-ray Diffraction (XRD) Spectra of CaCO3 NPs
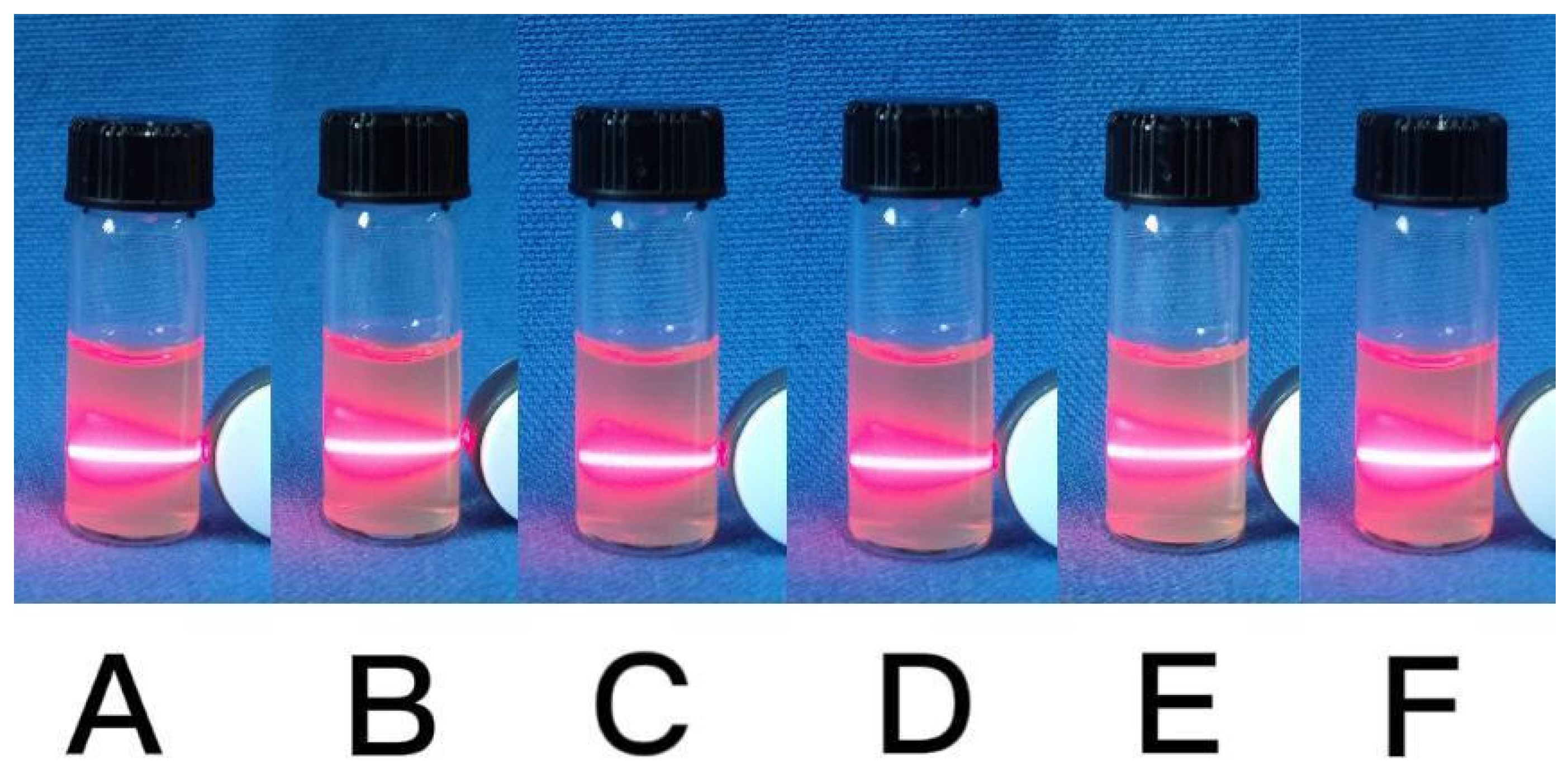
2.1.5. Solubility Research of CaCO3 NPs
2.2. Characterization of the Ag-CaCO3 Nanocomposites
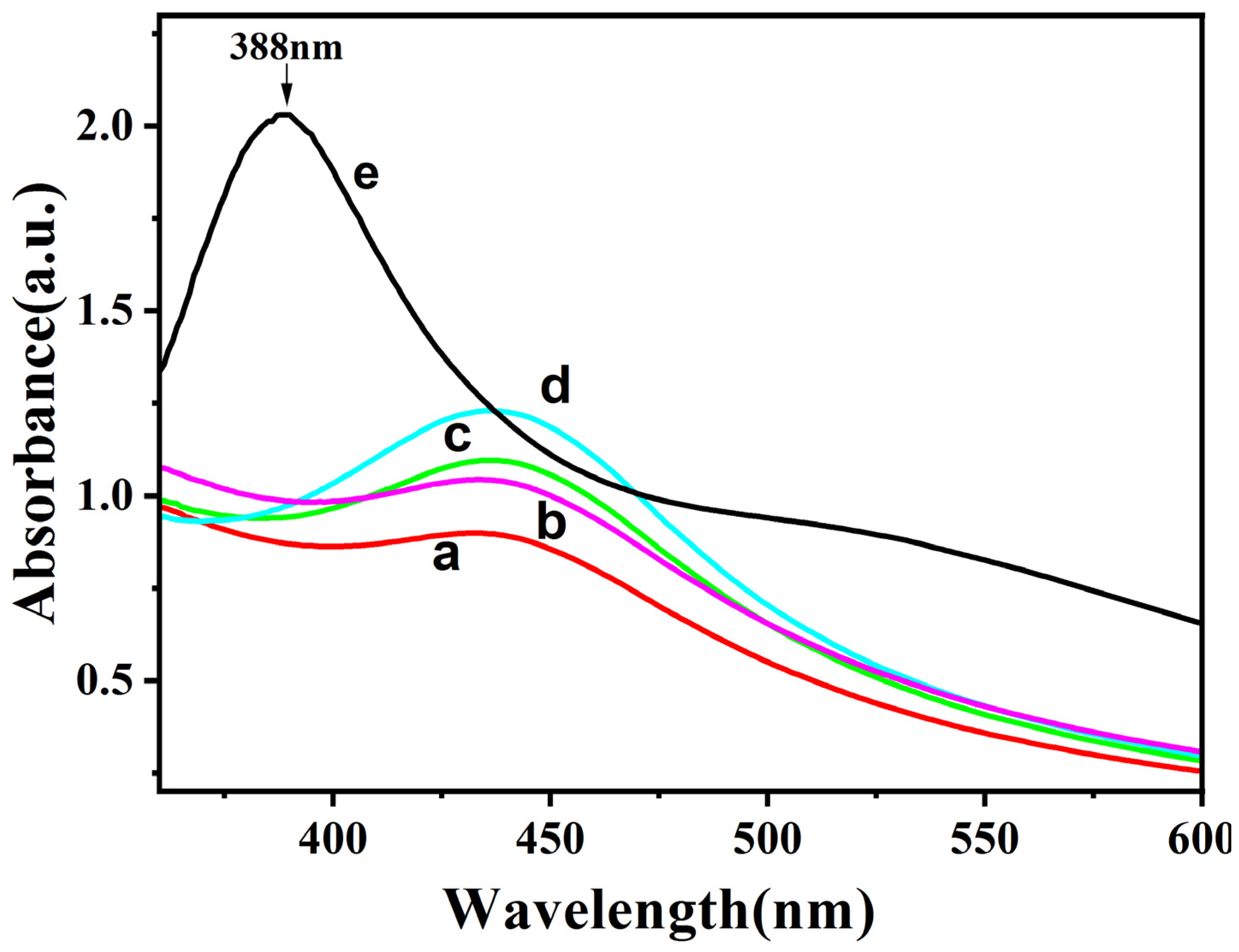
2.2.1. Effects of Different Concentrations of AgNO3 on the Synthesized Ag-CaCO3 Nanocomposites
2.2.2. SERS Activity Study of Ag-CaCO3 Nanocomposites
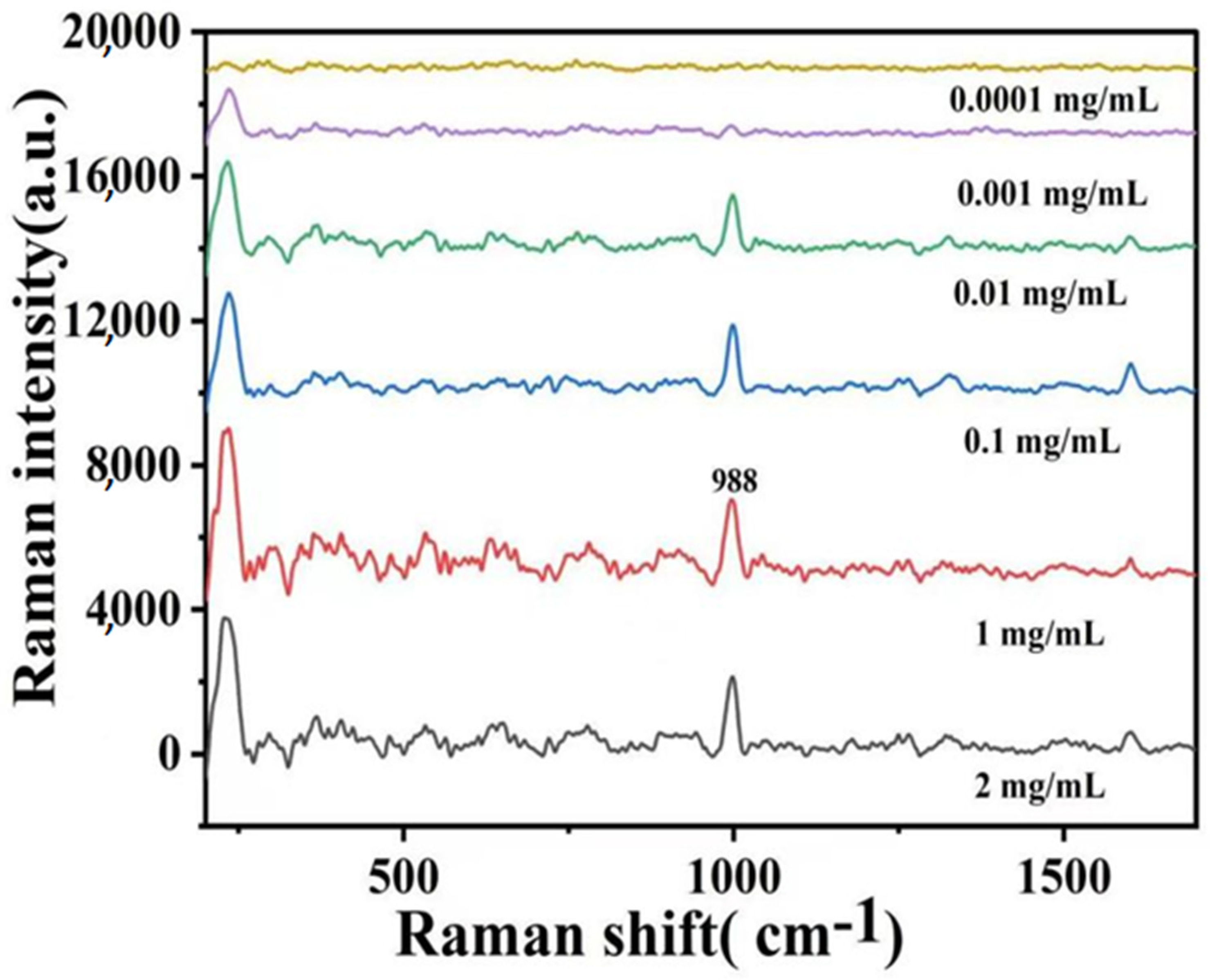
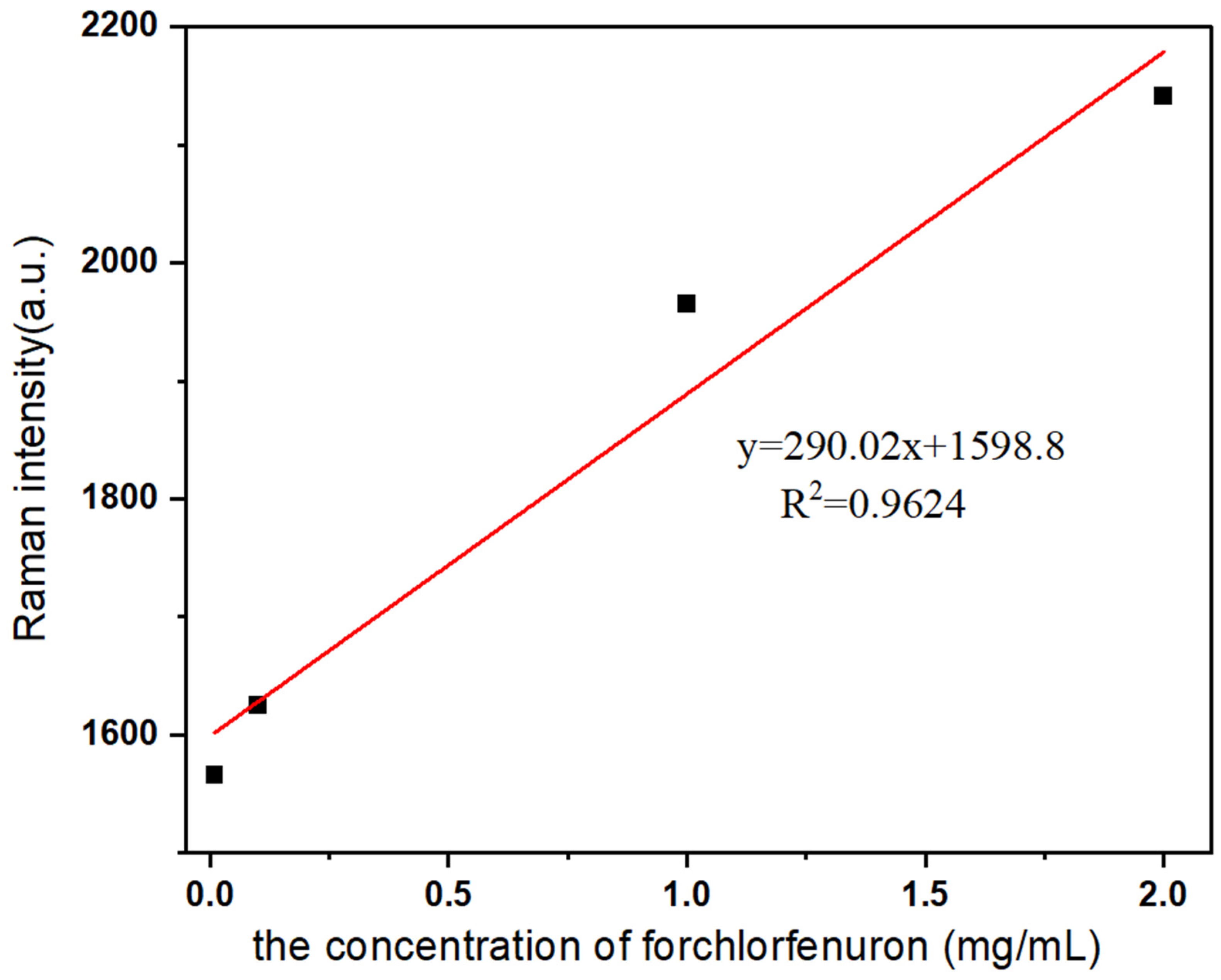
2.3. SERS Detection of Forchlorfenuron
3. Experiment
3.1. Experiment Reagents
3.2. Experimental Apparatus
3.3. Synthesis of CaCO3 NPs
3.4. Synthesis of Ag-CaCO3 Nanocomposites
3.5. SERS Detection of Forchlorfenuron
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zou, Z.; Habraken, W.J.E.M.; Matveeva, G.; Jensen, A.C.S.; Bertinetti, L.; Hood, M.A.; Sun, C.; Gilbert, P.U.P.A.; Polishchuk, I.; Pokroy, B. A hydrated crystalline calcium carbonate phase: Calcium carbonate hemihydrate. Science 2019, 363, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, S.; Wang, Y.; Wang, J.; Li, B.; Liu, X.; Liu, X.; Wang, Q.; Cheng, J. Polymorphic crystallization and diversified growth of CaCO3 in HPAM-HABS-Na2SiO3 hybrid solutions. ChemistrySelect 2018, 3, 6050–6055. [Google Scholar] [CrossRef]
- Yang, A.; Huang, Z.; Zhu, Y.; Han, Y.; Yang, A. Preparation of nano-sized calcium carbonate in solution mixing process. J. Cryst. Growth 2021, 571, 126247. [Google Scholar] [CrossRef]
- Yang, T.; Fu, J.; Ma, L.; Du, H.; Yue, X.; Zhao, B.; Wang, C. Biomimetic synthesis of calcium carbonate under phenylalanine: Control of polymorph and morphology. Mater. Sci. Eng. C 2020, 114, 111019. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef]
- Dizaj, S.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Hao, Y.; Li, Q.; Chen, M.; Yang, Z.; Zhao, H.; Liu, Z. Synthesis of CaCO3-based nanomedicine for enhanced sonodynamic therapy via amplification of tumor oxidative stress. Chem 2020, 6, 1391–1407. [Google Scholar] [CrossRef]
- Dong, Z.; Hao, Y.; Li, Q.; Yang, Z.; Zhu, Y.; Liu, Z.; Feng, L. Metal-polyphenol-network coated CaCO3 as pH-responsive nanocarriers to enable effective intratumoral penetration and reversal of multidrug resistance for augmented cancer treatments. Nano Res. 2020, 13, 3057–3067. [Google Scholar] [CrossRef]
- Xu, C.; Yan, Y.; Tan, J.; Yang, D.; Jia, X.; Wang, L.; Xu, Y.; Cao, S.; Sun, S. Biodegradable nanoparticles of polyacrylic acid–stabilized amorphous CaCO3 for Tunable pH-Responsive Drug Delivery and Enhanced Tumor Inhibition. Adv. Funct. Mater. 2019, 29, 1808146. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Shen, F.; Dong, Z.; Hao, Y.; Chen, Y.; Liu, Z.; Feng, L. Lipid-coated CaCO3 nanoparticles as a versatile ph-responsive drug delivery platform to enable combined chemotherapy of breast cancer. ACS Appl. Bio Mater. 2022, 5, 1194–1201. [Google Scholar] [CrossRef]
- Wang, C.; Dong, Z.; Hao, Y.; Zhu, Y.; Ni, J.; Li, Q.; Liu, B.; Han, Y.; Yang, Z.; Wan, J.; et al. Coordination Polymer-Coated CaCO3 reinforces radiotherapy by reprogramming the immunosuppressive metabolic microenvironment. Adv. Mater. 2022, 34, 2106520. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Walkenfort, B.; Konig, M.; Salehi, M.; Schlucker, S. Rapid, quantitative, and ultrasensitive Point-of-Care testing: A portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. Int. Ed. 2019, 58, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Zou, L.; Li, Q. Recent developments in rurface-enhanced Raman spectroscopy and its application in food analysis: Alcoholic beverages as an example. Foods 2022, 11, 2165. [Google Scholar] [CrossRef]
- Lin, T.; Song, Y.; Liao, J.; Liu, F.; Zeng, T. Applications of surface-enhanced Raman spectroscopy in detection fields. Nanomedicine 2020, 15, 2971–2989. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Huang, F.; Ji, X.; Dai, H.; Wu, W. Surface enhanced Raman scattering substrate for the detection of explosives: Construction strategy and dimensional effect. J. Hazard. Mater. 2020, 387, 121714. [Google Scholar] [CrossRef]
- Rousaki, A.; Vandenabeele, P. In situ Raman spectroscopy for cultural heritage studies. Raman Spectrosc. 2021, 52, 2178–2189. [Google Scholar] [CrossRef]
- Chazhengina, S.; Kovalevski, V. Raman spectroscopy of weathered shungites. Raman Spectrosc. 2017, 48, 1590–1596. [Google Scholar] [CrossRef]
- Pozzi, F.; Leona, M. Surface-enhanced Raman spectroscopy in art and archaeology. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Niu, W.; Yan, H.; Lu, X.; Liu, B. Tip-selective growth of silver on gold nanostars for surface-enhanced Raman scattering. ACS Appl. Mater. Interfaces 2018, 10, 14850–14856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Zhang, J.; Liu, Y.; Wu, L.; Shen, J.; Zhang, Y.; Hu, Y.; Fan, Q.; Huang, W.; et al. Individual Au-nanocube based plasmonic nanoprobe for cancer relevant microRNA biomarker detection. ACS Sens. 2017, 2, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Lu, J.; Zhu, Z.; Shi, Z. Template-free synthesis of porous ZnO/Ag microspheres as recyclable and ultra-sensitive SERS substrates. Appl. Surf. Sci. 2018, 427, 830–836. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.; Wei, T.; Ma, X.; Zheng, X.; Hu, R.; Ren, B. Surface-enhanced raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Barhoum, A.; Rehan, M.; Rahier, H.; Bechelany, M.; Assche, G. Seed-mediated hot-injection synthesis of tiny Ag nanocrystals on nanoscale solid supports and reaction mechanism. ACS Appl. Mater. Interfaces 2016, 8, 10551–10561. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Yokota, T.; Honda, M.; Lim, P.; Osaka, N.; Makita, M.; Nishikawa, Y.; Kasuga, T.; Aizawa, M. Regulating size of silver nanoparticles on calcium carbonate via ultrasonic spray for effective antibacterial efficacy and sustained release. Mater. Sci. Eng. C 2021, 125, 112083. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Jeong, G.; Lee, Y.; Kim, M.; Han, S. High-yield synthesis of multi-branched gold nanoparticles and their surface-enhanced raman scattering properties. J. Colloid. Interface Sci. 2009, 329, 97–102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Liu, R.; Wu, Q.; Wang, S.; Liu, F.; Wei, Q.; Xu, J.; Luo, Z. Fabrication of Ag-CaCO3 Nanocomposites for SERS Detection of Forchlorfenuron. Molecules 2023, 28, 6194. https://doi.org/10.3390/molecules28176194
Qin F, Liu R, Wu Q, Wang S, Liu F, Wei Q, Xu J, Luo Z. Fabrication of Ag-CaCO3 Nanocomposites for SERS Detection of Forchlorfenuron. Molecules. 2023; 28(17):6194. https://doi.org/10.3390/molecules28176194
Chicago/Turabian StyleQin, Fangyi, Rongjun Liu, Qiong Wu, Shulong Wang, Fa Liu, Qingmin Wei, Jiayao Xu, and Zhihui Luo. 2023. "Fabrication of Ag-CaCO3 Nanocomposites for SERS Detection of Forchlorfenuron" Molecules 28, no. 17: 6194. https://doi.org/10.3390/molecules28176194
APA StyleQin, F., Liu, R., Wu, Q., Wang, S., Liu, F., Wei, Q., Xu, J., & Luo, Z. (2023). Fabrication of Ag-CaCO3 Nanocomposites for SERS Detection of Forchlorfenuron. Molecules, 28(17), 6194. https://doi.org/10.3390/molecules28176194







