Abstract
Since Hydrogen Sulfide (H2S) was recognized as a gas transmitter, its detection and quantification have become a hot research topic among chemists and biologists. In this area, fluorescent probes have shown great advantages: fast and strong response, low detection limit and easy manipulation. Here we developed a new fluorescent probe that detected H2S selectively among various bioactive and inorganic salts. This probe was based on the core structure of fluorescein and reacted with H2S through azide-reduction. Great linearity was achieved correlating fluorescence intensity and H2S concentrations in solution. The detection of H2S in cancer cells was also achieved.
1. Introduction
Since being recognized as an important cell-signaling gasotransmitter [1,2], hydrogen sulfide (H2S) has attracted great interest from research areas of both biology [3,4] and chemistry [5,6]. H2S has been reported playing important roles in many physiological and pathological conditions, such as inflammation regulation [7,8], cardio protection [9], neuromodulation [1], hypertension [10], pain perception [11,12] and even cancer [13]. It has been found that the amount of endogenously generated H2S varies in different tissues. The H2S amount in healthy cells is also different from that in abnormal cells. As a result, detection and quantification of H2S are of great importance in understanding its biological effects. Various technologies and methods have been developed for H2S detection [14,15]. Among these technologies, fluorescent probes show great potential both in vitro and in vivo, due to fast response, excellent selectivity, high sensitivity and real time imaging [16,17,18]. Several strategies have been developed for the design of fluorescent probes of H2S [19], including the reduction of aryl azide [20] and aryl nitro compounds [21,22], H2S-specific reactions based on its nucleophilicity [23], an H2S-induced metal displacement approach [24], and a disulfide exchange reaction [25]. Among these strategies, azide-reduction has attracted extensive attention due to its fast reaction rate, high selectivity and harmless byproduct N2. In 2011, the Chang group reported a series of rhodamine-based fluorescent probes (SF1 and SF2), which successfully and selectively detect H2S via azide reduction in both water and living cells [26]. In the same year, the Wang group developed a fluorescent probe DNS–Az, which reached the maximum fluorescent intensity within 30 s upon mixing with sulfide anion [27]. The extremely fast reaction was believed to favor the real time detection of H2S in vivo. At the same time, various fluorophores were modified with the azide group for the design of H2S probes, such as Cyanines–N3 (Cy–N3) [28], Phenanthroimidazole–N3 (PI–N3) [29], 7-nitrobenz-2-oxa-[1,3]diazole–N3 (NBD–N3) [30], Pyrene-1,3,6-trisulfonate–N3 [31], Coumarin–N3 [32,33], Naphthalimide–N3 [34] and so on. In all these probes, an azide group was attached to an aromatic ring or a conjugate system.
Recently, the mechanism for a sulfide-induced azide reduction was studied experimentally and computationally in detail by Henthorn [35]. The active species HS−, which is the dominant form of H2S in aqueous solution, firstly attacks the tailed N atom of the azide group. Then another molecule of HS− comes in to attack the sulfur atom, forming HS2− and the release of one molecule of N2 (Figure S6).
Azidomethyl has been used to protect amine or alcohol in the synthesis of carbohydrates [36,37], nucleosides [38], triazoles [39] and peptides [37]. Nowadays, the most important application of azidomethyl is as a reversible terminator in DNA sequencing by synthesis (SBS) [40]. The azidomethyl capped 3′–OH can be easily and quickly deprotected with reducing agents, and thus, the SBS process can be continued. Because DNA sequencing is such a big project and it takes a long time, the chemical reaction of each step is required to be as fast as possible. Azidomethyl reduction meets all requirements perfectly. Inspired by these works, we developed a turn-on fluorescent probe (FL–N3) for H2S detection by protecting fluorescein with azidomethyl, and tested the probe in aqueous solution and cancer cells.
2. Results
2.1. Synthesis
The probe FL–N3 was based on fluorescein with one of the hydroxyl groups protected by a polyethylene glycol (PEG) side chain, which was expected to increase the probe’s hydrophilicity [41]. The other hydroxyl group was caged with azidomethyl, which reacts with sulfide to release free hydroxyl and turn fluorescence on (Scheme 1, FL–N3 to FL–ONa). We synthesized this probe starting from commercially available fluorescein sodium (compound A) in five steps including two processes of two–step in one–pot. Fluorescein sodium and tosylated hexaethylene glycol monomethyl ether were heated together in DMF. The free carboxylic acid and hydroxyl were both protected with hexaethylene glycol monomethyl ether by forming an ester and an ether, respectively. Without purification of the intermediate, hydrolyzation of the newly formed ester with sodium hydroxide and subsequent acidification with HCl led to the formation of compound B. The phenolic hydroxyl was protected with chloromethyl methyl thioether (as in compound C), which was then converted to chloromethyl by reacting with NCS and TMSCl. The probe (FL–N3) was achieved after nucleophilic substitution of the chloride with azide. Although five steps were required, two intermediates were not necessarily to be separated, which made the synthesis quite straight forward.
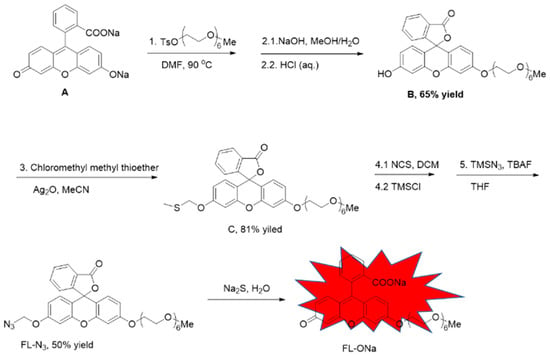
Scheme 1.
Synthetic scheme for the probe FL–N3 and its reaction with sulfide.
2.2. Fluorescence Response
We first tested the UV absorbance of the probe in solution with and without Na2S. The probe alone showed no absorbance in the range from 300 to 600 nm. After being incubated with sodium sulfide for 30 min, a strong absorbance peak appeared at 455 nm (Supporting Information, Figure S1), which indicated the formation of fluorescent product. The fluorescence properties of this probe were then tested at an excitation wavelength of 455 nm. Although a PEG side chain has been attached, the solubility of the probe is still not good enough in pure water. As a result, tests were carried out in water and a methanol mixture. When the probe FL–N3 was mixed with Na2S at a concentration of 1 mM, the fluorescence intensity increased by 32-fold at λem of 515 nM after 20 min (Figure 1a). The fluorescence response over time was also examined (Figure 1b). FL–N3 and Na2S were mixed at concentrations of 5 mM and 10 mM, respectively. The fluorescence intensity increased quickly in the first twenty minutes. It reached the max after a slow increase during the period from 20 to 60 min. The maximum fluorescence intensity kept almost consistent for one hour from 60 to 120 min. The long-lasting fluorescence of the probe could be important in many applications [42]. HPLC analysis demonstrated the formation of a major product (Supporting Information, Figure S2) and MS analysis indicated that the fluorescence increase was due to the formation of FL–ONa (Scheme 1, mass spectra data in Supporting Information, Figure S3).
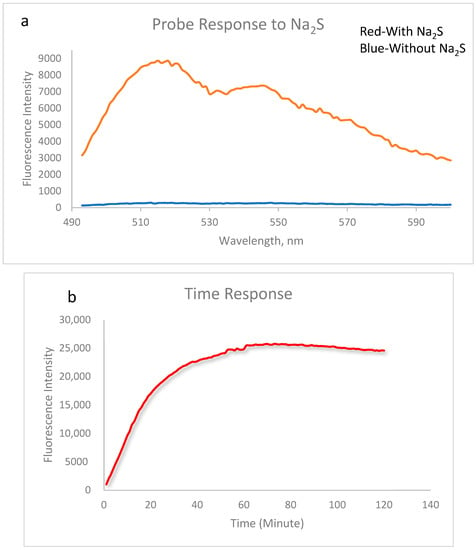
Figure 1.
Probe response to Na2S: (a) Fluorescence response of 1 mM FL–N3 to 20 mM Na2S. Data were acquired after 20 min of reaction at 25 °C in water/MeOH (1/1, v/v) with excitation at λex = 455 nm, emission was collected between 490 and 600 nm; (b) Fluorescence response over time of 5 mM FL–N3 to 10 mM Na2S. Data were acquired at 25 °C in water/MeOH (1/1, v/v) with excitation at λex = 455 nm, emission was collected at λem = 515 nm.
2.3. Selectivity
To test the probe’s selectivity, we selected a series of biothiols and bio-relevant sulfur-containing anions. Only HS− induced a significant increase of fluorescence (Figure 2). Biothiols including cysteine, glutathione and dithiothreitol led to negligible fluorescence intensity change. Bio-relevant reducing salts such as sodium L–ascorbate, sodium thiocyanate and sodium thiosulfate also failed to turn the probe on. Other inorganic salts that were commonly used in buffer were also tested (Supporting Information, Figure S4). Ammonium acetate, sodium citrate, lithium chloride and dipotassium phosphate did not increase the fluorescence intensity. However, basic salts like potassium carbonate (pH 10.3) and lithium hydroxide (pH 10.7) led to about three- and five-fold fluorescence increases, respectively, compared with the blank. This is probably due to the acidic property of the C–H bond on the carbon of the azidomethyl group, which was deprotonated by hydroxide. One molecule of nitrogen and formamide were released to liberate the free hydroxyl group. Under basic conditions (pH 10.3~10.7), the lacton was transformed into monoaion to turn on the fluorescence. A possible mechanism was predicted, as in Supporting Information (Figure S5).
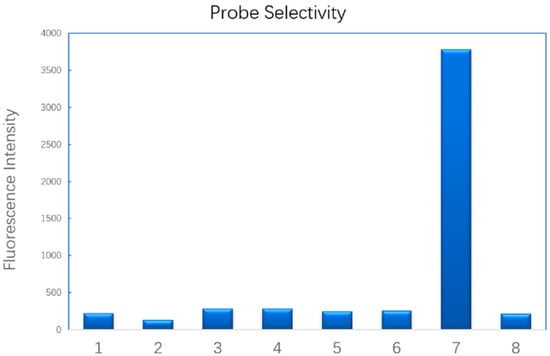
Figure 2.
Fluorescence intensity of the probe (0.1 mM probe, 30 min after mixing) in the presence of sulfide or other species in water and methanol (10% MeOH, v/v). 1. Glutathione, 2. L-Cysteine, 3. Dithiothreitol, 4. Sodium L-Ascorbate, 5. Sodium Thiocyanate, 6. Sodium Thiosulfate, 7. Sodium sulfide, 8. Blank, (1–7: 1 mM).
2.4. Linearity
It has been found that the bioactivity of H2S is concentration-dependent [43], which means the quantification of H2S in solution or cells is of great importance in H2S study. When FL-N3 was exposed to Na2S of different concentrations, a linear correlation was observed between fluorescence responses of the probe and Na2S concentrations in the range of 0 to 0.8 mM, enabling a calibration equation to be established and applicable to the analysis of H2S concentrations (Figure 3). The linear equation was found to be Y = 4584.3X + 204.66. R2 = 0.9952. The detection limit was calculated to be 3.99 μM based on the signal-to-noise ratio (S/N = 3).
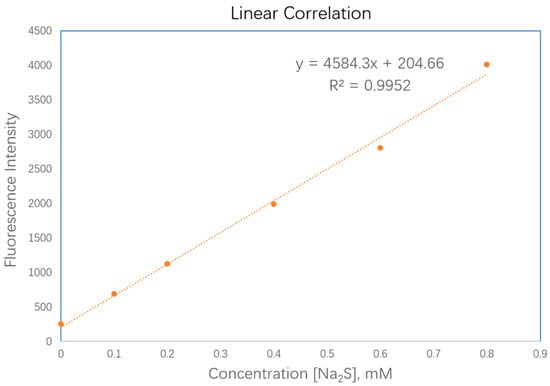
Figure 3.
Linear correlation between fluorescence responses of FL–N3 and Na2S concentrations in water and methanol (10% MeOH, v/v). The probe concentration was 1 mM and data were acquired 30 min after mixing with Na2S.
2.5. Detection in Cells
- Since the probe FL–N3 has well-behaved fluorescent properties and selectivity for H2S, it is probably suitable for the detection of H2S in cultured cells. Confocal imaging to visualize H2S inside cells was carried out. MCF–7 cells were incubated together with FL–N3; after washing free molecules of the probe away, Na2S solution was added. Confocal images were taken after another 60-min incubation and results are shown in Figure 4. The probe successfully entered cells and reacted with sulfide to show red fluorescence (Figure 4a). In contrast, the sample of control (Figure 4b) showed no fluorescence at all. We also tested compound D, which contains a methyl group instead of a PEG side chain. Surprisingly, compound D failed to enter cells and little fluorescence was observed after treatment with Na2S (Figure 4c). This result proved the importance of the PEG side chain for the probe’s hydrophilicity and cell permeability, which are key factors in bio-applications.
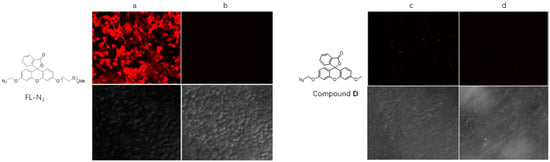 Figure 4. Confocal images showing signals in MCF–7 cells exposed to Na2S. MCF–7 cells were incubated with 20 μM probe FL–N3 or comparing compound D for 2 h at 37 °C, washed, and then incubated with or without Na2S for 1 h before imaging: (a) FL-N3 with 100 μM Na2S; (b) FL-N3 without Na2S; (c) Compound D with 100 μM Na2S; (d) Compound D without Na2S.
Figure 4. Confocal images showing signals in MCF–7 cells exposed to Na2S. MCF–7 cells were incubated with 20 μM probe FL–N3 or comparing compound D for 2 h at 37 °C, washed, and then incubated with or without Na2S for 1 h before imaging: (a) FL-N3 with 100 μM Na2S; (b) FL-N3 without Na2S; (c) Compound D with 100 μM Na2S; (d) Compound D without Na2S.
3. Discussion
We designed and synthesized a turning-on probe for the detection of H2S in solution and in cells. The probe works through the reduction of the azidomethyl group by H2S. It also inspired us that the azidomethyl group could be an ideal choice in the modification of turning-on dyes. When fluorescence is turned off by capping different dyes with azidomethyl, it can be turned on easily by adding certain reductant, which could have potential in biology research.
4. Materials and Methods
Procedure for probe response to sodium sulfide test: The probe FL-N3 was dissolved in methanol at a concentration of 2 mM. Sodium sulfide was dissolved in water at a concentration of 40 mM. Two wells of a corning 96-well plate were selected and to each well was added 100 μL probe solution. Then, 100 μL Na2S solution was added to one of the wells and to the other was added 100 μL water. The plate was incubated at RT for 20 min before reading fluorescence on a plate reader. The fluorescence was read at excitation wavelength λex = 455 nm with emission wavelength λem set from 500 nm to 600 nm.
Procedure for fluorescence response over time test: The probe FL-N3 was dissolved in methanol at a concentration of 10 mM. Sodium sulfide was dissolved in water at a concentration of 20 mM. To a corning 96-well plate was added 100 μL probe solution and 100 μL Na2S solution. The fluorescence was read on a plate reader every minute in 2 h with excitation wavelength λex = 455 nm and emission wavelength λem = 515 nm.
Procedure for probe selectivity test: The probe FL-N3 was dissolved in methanol at a concentration of 1 mM. Other tested compounds or salts were dissolved in water at a concentration of 10 mM. To a corning 96-well plate was added 160 μL water and 20 μL probe solution. Then 20 μL aqueous solution of one tested compound or salt was added to one well. To the well of the blank, 20 μL water was added. After 30-min incubation at RT, fluorescence was read on a plate reader (brand) with excitation wavelength λex = 455 nm and emission wavelength λem = 515 nm.
Procedure for linear correlation test: The probe FL-N3 was dissolved in methanol at a concentration of 10 mM. To 6 wells of a corning 96-well plate was added 20 μL of the probe solution. Following this, to the 6 wells was added 180 μL, 160 μL, 140 μL, 100 μL, 60 μL, 20 μL water in order. Then 0 μL, 20 μL, 40 μL, 80 μL, 120 μL, 160 μL of sodium sulfide solution (1 mM) was added to the 6 wells in order. After incubation at RT for 20 min, the fluorescence was read on a plate reader with excitation wavelength λex = 455 nm and emission wavelength λem = 515 nm.
Procedure for confocal imaging: Human breast cancer (MCF7) cells were purchased from Biobw Pte Ltd. (ATCC, HTB22). MCF7 cancer cells were grown in RPMI Media (Merck, CN), which was supplemented with 10% fetal bovine serum, 50 μg/mL penicillin and 50 μg/mL streptomycin at 37 °C, 5% CO2. The cells were subcultured to 80–90% confluency and used within 15–20 passages for the assay. MCF7 cells were seeded at 20,000 cells/well in an 8-chamber plate (0.8 cm2). Probe FL-N3 (20 μM in DMSO) was added and cells were incubated for 2 h at 37 °C. Cells were then washed with PBS (3×). Then Na2S solution (0.1 mM) was added and incubated for 5 h. Fluorescent images of the live cells were obtained using a confocal fluorescence microscope FV 1000 and processed using Olympus Fluoview Ver.3.1. Viewer (Olympus, Tokyo, Japan).
Procedure for the synthesis of compound B: Fluorescein sodium salt (135 mg, 0.36 mmol) was added to a two necked rbf; after evacuation, the rbf was recharged with N2, and anhydrous DMF (3 mL) was added, followed by the addition of 2,5,8,11,14,17-hexaoxanonadecan-19-yl 4-methylbenzenesulfonate (450 mg, 1 mmol). The mixture was heated to 90 degrees centigrade and stirred for 36 h. The mixture was then cooled to room temperature, and DMF was removed by evaporation and the residual was re-dissolved in 10 mL 10% NaHCO3 solution. The residual was extracted with EA for three times and the organic layer was combined, washed with brine, dried over Na2SO4 and concentrated. The residual was re-dissolved in 5 mL methanol, 2 M NaOH (2 mL) was added, and stirred at room temperature for 2 h. Then 1 M HCl was added slowly to adjust pH to 2, and the mixture was stirred for 30 min. Methanol was removed via rotary evaporation and the residual was extracted with EA for three times. The combined organic layer was washed with brine, dried over Na2SO4 and concentrated. The product was isolated via silica gel chromatography (DCM/MeOH = 100/1) with a yield of 65%.
Procedure for the synthesis of compound C: Compound B (122 mg, 0.2 mmol) was dissolved in 10 mL anhydrous MeCN under Ar. Silver oxide (92 mg, 0.4 mmol) was added, followed by the addition of chloromethyl methyl thioether (167 μL, 2 mmol) and one drop of pyridine. The mixture was heated to 50 degrees centigrade and stirred for 48 h. The mixture was cooled to room temperature and solvent was removed via rotary evaporation. The product was isolated via silica gel chromatography (DCM/MeOH = 100/1) with a yield of 81%.
Procedure for the synthesis of probe FL-N3: Compound C (67 mg, 0.1 mmol) was dissolved in 5 mL anhydrous DCM under Ar. N-chlorosuccinimide (16 mg, 0.12 mmol) was added and the mixture was stirred at room temperature for 3 h. Then trimethyl silane chloride (13 mg, 0.12 mmol) was added and the mixture was stirred at room temperature further for 6 h. After removal of solvent via rotary evaporation, the residual was dried under vacuum and re-dissolved in dry THF under Ar; following this, TMSN3 (0.2 mmol) and TBAF (0.2 mmol, 1 M in THF) were added and the mixture was stirred at room temperature overnight. The solvent was removed via rotary evaporation and the product was isolated via silica gel chromatography (DCM/MeOH = 100/1) as a yellow oil. The product was further purified via prep HPLC in case it was impure.
5. Conclusions
In summary, we have developed a new strategy to construct a fluorescein-based probe for the detection of H2S in solution and in cells. The probe showed fast response and excellent selectivity to sulfide over a series of bio-relevant sulfur-containing compounds and inorganic salts. The linear correlation of Na2S concentration and probe fluorescent response made the quantification of H2S in solution possible. Its successful application in cell confocal imaging may provide a new tool in the study of the biological effects of hydrogen sulfide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176195/s1, Figure S1: UV spectra analysis of the reaction mixture of FL-N3 with Na2S; Figure S2: HPLC analysis of the reaction between FL-N3 and Na2S; Figure S3: Mass analysis of the reaction mixture of FL-N3 with Na2S; Figure S4: Fluorescence intensity of the probe (0.05 mM probe, 30 min after mixing) in the presence of sulfide or other species in water and methanol (10% MeOH, v/v); Figure S5: Proposed mechanism for base-induced fluorescence turn on; Figure S6: Possible mechanism for sulfide induced azide reduction.
Author Contributions
W.F. and Q.X. made equal contribution to this work. Conceptualization, W.F. and Y.Y.; methodology, W.F. and Q.X.; investigation, Q.X. and L.W.; writing—original draft preparation, W.F.; writing—review and editing, Y.Y.; supervision, Y.Y.; funding acquisition, W.F. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21807120, 22061012 and the Department of Science & Technology of Guizhou Province ([2020]4Y208).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Information.
Acknowledgments
We acknowledge the support from Sun Yat-sen University and BGI-Shenzhen.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine 2018, 97, e13065. [Google Scholar] [CrossRef]
- Cao, X.; Ding, L.; Xie, Z.-Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Song, Z.J.; Ng, M.Y.; Lee, Z.-W.; Dai, W.; Hagen, T.; Moore, P.K.; Huang, D.; Deng, L.-W.; Tan, C.-H. Hydrogen sulfide donors in research and drug development. MedChemComm 2014, 5, 557–570. [Google Scholar] [CrossRef]
- Jose, D.A.; Sakla, R.; Sharma, N.; Gadiyaram, S.; Kaushik, R.; Ghosh, A. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. ACS Sens. 2020, 5, 3365–3391. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Fiorucci, S. Hydrogen sulfide: From physiology to pharmacology. Inflamm. Allergy Drug Targets 2011, 10, 77–84. [Google Scholar] [CrossRef]
- Pan, T.-T.; Feng, Z.-N.; Lee, S.W.; Moore, P.K.; Bian, J.-S. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J. Mol. Cell. Cardiol. 2006, 40, 119–130. [Google Scholar] [CrossRef]
- Zhong, G.Z.; Chen, F.R.; Cheng, Y.Q.; Tang, C.S.; Du, J.B. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003, 21, 1879–1885. [Google Scholar] [CrossRef]
- Kawabata, A.; Ishiki, T.; Nagasawa, K.; Yoshida, S.; Maeda, Y.; Takahashi, T.; Sekiguchi, F.; Wada, T.; Ichida, S.; Nishikawa, H. Hydrogen sulfide as a novel nociceptive messenger. Pain 2007, 132, 74–81. [Google Scholar] [CrossRef]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Antonelli, E.; Roviezzo, F.; Morelli, A.; Cirino, G.; Wallace, J.L. Evidence That Hydrogen Sulfide Exerts Antinociceptive Effects in the Gastrointestinal Tract by Activating KATP Channels. J. Pharmacol. Exp. Ther. 2006, 316, 325–335. [Google Scholar] [CrossRef]
- Feng, W.; Teo, X.-Y.; Novera, W.; Ramanujulu, P.M.; Liang, D.; Huang, D.; Moore, P.K.; Deng, L.-W.; Dymock, B.W. Discovery of New H2S Releasing Phosphordithioates and 2,3-Dihydro-2-phenyl-2-sulfanylenebenzo[d][1,3,2]oxazaphospholes with Improved Antiproliferative Activity. J. Med. Chem. 2015, 58, 6456–6480. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef]
- Li, J.; Yin, C.; Huo, F. Chromogenic and fluorogenic chemosensors for hydrogen sulfide: Review of detection mechanisms since the year 2009. Rsc Adv. 2015, 5, 2191–2206. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, S.A.; Cha, Y.; Lee, M.H. Recent advances in fluorescent probes for cellular antioxidants: Detection of NADH, hNQO1, H2S, and other redox biomolecules. Coord. Chem. Rev. 2021, 428, 213613. [Google Scholar] [CrossRef]
- Li, H.; Fang, Y.; Yan, J.; Ren, X.; Zheng, C.; Wu, B.; Wang, S.; Li, Z.H.; Hua, H.; Wang, P.; et al. Small-molecule fluorescent probes for H2S detection: Advances and perspectives. TraAC Trends Anal. Chem. 2020, 134, 116117. [Google Scholar] [CrossRef]
- Feng, W.; Dymock, B.W. Fluorescent Probes for H2S Detection and Quantification. In Chemistry, Biochemistry Pharmacology of Hydrogen Sulfide; Springer: Berlin, Germany, 2015; pp. 291–323. [Google Scholar] [CrossRef]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Chapter Four. Azide-Based Fluorescent Probes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 554, pp. 63–80. [Google Scholar] [CrossRef]
- Bae, J.; Gil Choi, M.; Choi, J.; Chang, S.-K. Colorimetric signaling of hydrogen sulfide by reduction of a phenylseleno-nitrobenzoxadiazole derivative. Dye. Pigment. 2013, 99, 748–752. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Zhang, Y.; Gui, G.; Zhang, X.; Hou, S. A novel xanthene-based colorimetric and fluorescence probe for detection of H2S in living cells. J. Lumin. 2018, 204, 480–484. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Shi, W.; Peng, B.; Zhao, Y.; Ma, H.; Xian, M. Rational Design and Bioimaging Applications of Highly Selective Fluorescence Probes for Hydrogen Polysulfides. J. Am. Chem. Soc. 2014, 136, 7257–7260. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, C.; Chen, H.; Mack, J.; Guo, Z.; Shen, Z. A red fluorescent turn-on probe for hydrogen sulfide and its application in living cells. Chem. Commun. 2013, 49, 7510–7512. [Google Scholar] [CrossRef]
- Liu, C.; Pan, J.; Li, S.; Zhao, Y.; Wu, L.Y.; Berkman, C.E.; Whorton, A.R.; Xian, M. Capture and Visualization of Hydrogen Sulfide by a Fluorescent Probe. Angew. Chem. 2011, 123, 10511–10513. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. 2011, 123, 9846–9849. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Song, P.; Wang, B.; Zhao, J.; Han, K. Facilitative functionalization of cyanine dye by an on–off–on fluorescent switch for imaging of H2O2 oxidative stress and thiols reducing repair in cells and tissues. Chem. Commun. 2012, 48, 2852–2854. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, W.; Tan, L. A phenanthroimidazole-based fluorescent chemosensor for imaging hydrogen sulfide in living cells. Org. Biomol. Chem. 2012, 10, 9683–9688. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, H.; Yang, M.; Chen, X. An NBD fluorophore-based colorimetric and fluorescent chemosensor for hydrogen sulfide and its application for bioimaging. Tetrahedron 2013, 69, 867–870. [Google Scholar] [CrossRef]
- Hartman, M.C.T.; Dcona, M.M. Chemodosimeter for direct measurement of hydrogen sulfide in biological fluids. Analyst 2012, 137, 4910–4912. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Yu, X.; Du, L.; Li, M. Coumarin-based Fluorescent Probes for H2S Detection. J. Fluoresc. 2013, 23, 181–186. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.; Lv, C.; Zhao, M.; Jin, H.; Jin, H.; Du, J.; Zhang, L.; Tang, X. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 2012, 138, 946–951. [Google Scholar] [CrossRef]
- Montoya, L.A.; Pluth, M.D. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 4767–4769. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, H.A.; Pluth, M.D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z. (2-Azidomethyl)phenylacetyl as a new, reductively cleavable protecting group for hydroxyl groups in carbohydrate synthesis. Carbohydr. Res. 2002, 337, 87–91. [Google Scholar] [CrossRef]
- Pothukanuri, S.; Winssinger, N. A Highly Efficient Azide-Based Protecting Group for Amines and Alcohols. Org. Lett. 2007, 9, 2223–2225. [Google Scholar] [CrossRef]
- Wada, T.; Ohkubo, A.; Mochizuki, A.; Sekine, M. 2-(Azidomethyl)benzoyl as a new protecting group in nucleosides. Tetrahedron Lett. 2001, 42, 1069–1072. [Google Scholar] [CrossRef]
- Loren, J.C.; Krasiński, A.; Fokin, V.V.; Sharpless, K.B. NH-1,2,3-Triazoles from Azidomethyl Pivalate and Carbamates: Base-Labile N-Protecting Groups. Synlett 2005, 2005, 2847–2850. [Google Scholar] [CrossRef]
- Guo, J.; Xu, N.; Li, Z.; Zhang, S.; Wu, J.; Kim, D.H.; Marma, M.S.; Meng, Q.; Cao, H.; Li, X.; et al. Four-color DNA sequencing with 3′-O-modified nucleotide reversible terminators and chemically cleavable fluorescent dideoxynucleotides. Proc. Natl. Acad. Sci. USA 2008, 105, 9145–9150. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Mousavi, S.M.; Shahtahmassebi, N.; Saljoughi, E. Hydrophilicity improvement in polyphenylsulfone nanofibrous filtration membranes through addition of polyethylene glycol. Appl. Surf. Sci. 2015, 359, 252–258. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lakowicz, J.R. Reduced Blinking and Long-Lasting Fluorescence of Single Fluorophores Coupling to Silver Nanoparticles. Langmuir 2008, 24, 3429–3433. [Google Scholar] [CrossRef]
- Lee, Z.-W.; Teo, X.-Y.; Tay, E.Y.-W.; Tan, C.-H.; Hagen, T.; Moore, P.K.; Deng, L.-W. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br. J. Pharmacol. 2014, 171, 4322–4336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).