A Study of the Chemical Composition, Antioxidant Potential, and Acute Toxicity of Bulgarian Tanacetum vulgare L. Essential Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Organic Compounds and Antioxidant Capacity of Tanacetum vulgare L. Inflorescences Essential Oil
2.2. Evaluation of Single Dose Acute Toxicity (LD50)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Isolation of Essential Oil
3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.5. Lipophilic Oxygen Radical Absorbance Capacity (ORAC) Assay
3.6. Single Dose Acute Toxicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Todorova, M.N.; Evstatieva, L.N. Comparative Study of Tanacetum Species Growing in Bulgaria. Z. Naturforschung C 2001, 56, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative Studies on the Antimicrobial and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Methanol Extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Azhar, M.A.M.; Salleh, W.M.N.H.W. Chemical Composition and Biological Activities of Essential Oils of the Genus Litsea (Lauraceae)—A Review. Agric. Conspec. Sci. 2020, 85, 97–103. [Google Scholar]
- Tassorelli, C.; Greco, R.; Morazzoni, P.; Riva, A.; Sandrini, G.; Nappi, G. Parthenolide Is the Component of Tanacetum parthenium That Inhibits Nitroglycerin-Induced Fos Activation: Studies in an Animal Model of Migraine. Cephalalgia 2005, 25, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, A.M.; Iliev, I.A.; Lozanov, V.S.; Dimitrova, M.B.; Mitev, V.I.; Ivanov, I.P. In Vitro Study on the Antitumor Activity of Tanacetum vulgare L. Extracts. Bulg. Chem. Commun. 2019, 51, 249–255. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an Effective Bioresource with Promising Pharmacological Effects from Natural Arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Gospodinova, Z.; Antov, G.; Angelova, S.; Krasteva, M. In vitro antitumor potential of Bulgarian Tanacetum vulgare L. on human breast adenocarcinoma cells. Int. J. Pharm. Sci. 2014, 4, 468–472. [Google Scholar]
- Rosselli, S.; Bruno, M.; Raimondo, F.M.; Spadaro, V.; Varol, M.; Koparal, A.T.; Maggio, A. Cytotoxic Effect of Eudesmanolides Isolated from Flowers of Tanacetum vulgare ssp. Siculum. Molecules 2012, 17, 8186–8195. [Google Scholar] [CrossRef]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Mishchenko, O.; Kalko, K.; Zolotaikina, M.; Gontova, T.; Mashtaler, V.; Yurchenko, C.; Derymedvid, L.; Pozdniakova, A. Hepatoprotective and choleretic activity of dried extract of Tanacetum vulgare flowers. Thai J. Pharm. Sci. 2019, 43, 30–35. [Google Scholar]
- Larocque, N.; Vincent, C.; Bélanger, A.; Bourassa, J.-P. Effects of Tansy Essential Oil from Tanacetum Vulgare on Biology of Oblique-Banded Leafroller, Choristoneura Rosaceana. J. Chem. Ecol. 1999, 25, 1319–1330. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)—A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Bączek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Węglarz, Z. Antibacterial and Antioxidant Activity of Essential Oils and Extracts from Costmary (Tanacetum balsamita L.) and Tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Sharopov, F.; Braun, M.; Gulmurodov, I.; Khalifaev, D.; Isupov, S.; Wink, M. Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Essential Oils of Selected Aromatic Plants from Tajikistan. Foods 2015, 4, 645–653. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Aćimović, M.; Puvača, N. Tanacetum vulgare L.—A Systematic Review. Technol. Eng. Manag. J. Agron. Technol. Eng. Manag. 2020, 3, 416–422. [Google Scholar]
- Committee on Herbal Medicinal Products. Public Statement on the Use of Herbal Medicinal Products Containing Thujone; Revision 1; European Medicines Agency: London, UK, 2011; pp. 1–9.
- Mockute, D.; Judzentiene, A. Composition of the Essential Oils of Tanacetum vulgare L. Growing Wild in Vilnius District (Lithuania). J. Essent. Oil Res. 2004, 16, 550–553. [Google Scholar] [CrossRef]
- Mikulášová, M.; Vaverková, Š. Antimicrobial Effects of Essential Oils from Tanacetum vulgare L. and Salvia officinalis L., Growing in Slovakia. Nova Biotechnol. Chim. 2021, 9, 161–166. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bruno, M.; Rosselli, S.; Bellone, G.; Spadaro, V. Essential Oil Composition of Tanacetum vulgare subsp. siculum (Guss.) Raimondo et Spadaro (Asteraceae) from Sicily. Nat. Prod. Commun. 2009, 4, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Hausner, E.A.; Poppenga, R.H. Hazards Associated with the Use of Herbal and Other Natural Products. In Small Animal Toxicology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 335–356. ISBN 978-1-4557-0717-1. [Google Scholar]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Barnes, J.; Anderson, L.; Phillipson, D. Herbal Medicines; RPS Publishing: London, UK, 2007. [Google Scholar]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Israili, Z.H.; Lyoussi, B. Acute and Chronic Toxicity of a Lyophilised Aqueous Extract of Tanacetum vulgare Leaves in Rodents. J. Ethnopharmacol. 2008, 117, 221–227. [Google Scholar] [CrossRef]
- Von Cossel, M. How to Reintroduce Arable Crops after Growing Perennial Wild Plant Species Such as Common Tansy (Tanacetum vulgare L.) for Biogas Production. Energies 2022, 15, 4380. [Google Scholar] [CrossRef]
- Grossel, S.G.; Crowl, D.A. Handbook of Highly Toxic Materials; Marcel Dekkar Inc.: New York, NY, USA, 1994. [Google Scholar]
- Gheraissa, N.; Chemsa, A.E.; Cherrada, N.; Erol, E.; Elsharkawy, E.R.; Ghemam-Amara, D.; Zeghoud, S.; Rebiai, A.; Messaoudi, M.; Sawicka, B.; et al. Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules 2023, 28, 3580. [Google Scholar] [CrossRef]
- Benchikha, N.; Chelalba, I.; Debbeche, H.; Messaoudi, M.; Begaa, S.; Larkem, I.; Amara, D.G.; Rebiai, A.; Simal-Gandara, J.; Sawicka, B.; et al. Lobularia Libyca: Phytochemical Profiling, Antioxidant and Antimicrobial Activity Using In Vitro and In Silico Studies. Molecules 2022, 27, 3744. [Google Scholar] [CrossRef]
- Elshafie, H.S.; De Martino, L.; Formisano, C.; Caputo, L.; De Feo, V.; Camele, I. Chemical Identification of Secondary Metabolites from Rhizospheric Actinomycetes Using LC-MS Analysis: In Silico Antifungal Evaluation and GrowthPromoting Effects. Plants 2023, 12, 1869. [Google Scholar] [CrossRef]
- Amato, G.; Caputo, L.; Francolino, R.; Martino, M.; De Feo, V.; De Martino, L. Origanum heracleoticum Essential Oils: Chemical Composition, Phytotoxic and Alpha-Amylase Inhibitory Activities. Plants 2023, 12, 866. [Google Scholar] [CrossRef]
- Kozhuharov, S.; Anchev, M. Flora of the Republic of Bulgaria, 11th ed.; Academic Publishing House “Prof. Marin Drinov”: Sofia, Bulgaria, 2012. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; ISBN 978-0-9981557-2-2. [Google Scholar]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/chemistry (accessed on 8 August 2023).
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
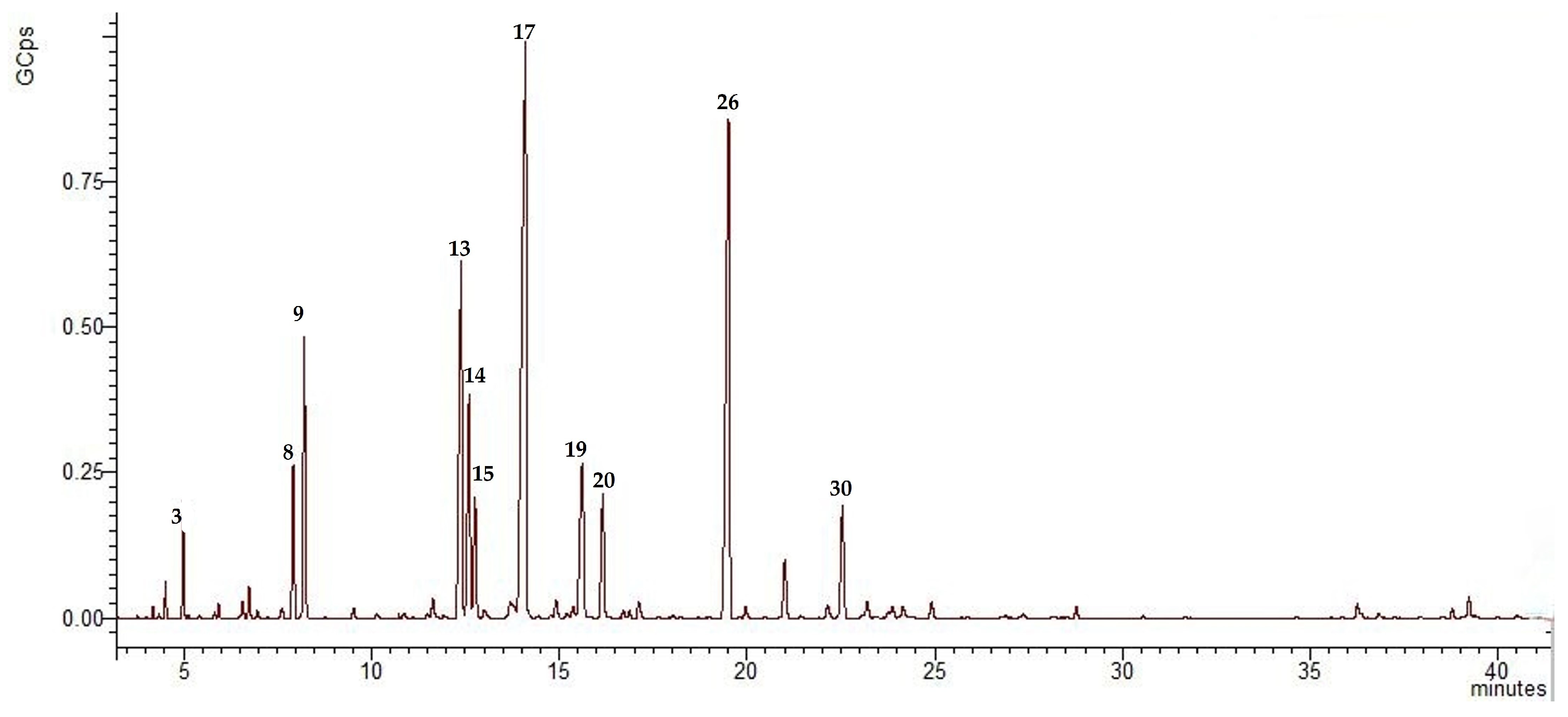
| No. a | Compound b | RI c | Molecular Formula | Class of Compound | % of Total d |
|---|---|---|---|---|---|
| 1 | α-Thujene | 932 | C10H16 | MH | tr |
| 2 | α-Pinene | 937 | C10H16 | MH | 0.52 |
| 3 | Camphene | 947 | C10H16 | MH | 1.84 |
| 4 | β-Pinene | 966 | C10H16 | MH | 0.25 |
| 5 | Yomogi alcohol (3,3,6-Trimethyl-1,4-heptadien-6-ol) | 990 | C10H18O | O | 0.14 |
| 6 | Mesitylene | 999 | C9H12 | O | 0.27 |
| 7 | β-Phellandrene | 1002 | C10H16 | MH | 0.11 |
| 8 | p-Cymene | 1008 | C10H14 | MH | 3.16 |
| 9 | Eucalyptol (1,8-cineole) | 1020 | C10H18O | MO | 5.99 |
| 10 | α-Terpinene | 1030 | C10H16 | MH | 0.24 |
| 11 | γ-Terpinene | 1049 | C10H18O | MO | 0.11 |
| 12 | Terpinolene | 1076 | C10H16 | MH | tr |
| 13 | cis-Verbenol | 1117 | C10H16O | MO | 10.85 |
| 14 | β-Thujone | 1118 | C10H16O | MO | 6.06 |
| 15 | Chrysantenone | 1120 | C10H14O | MO | 2.94 |
| 16 | p-Menth-2-en-1-ol | 1122 | C10H18O | MO | 0.28 |
| 17 | Camphor | 1128 | C10H16O | MO | 25.24 |
| 18 | Pinocarvone | 1159 | C10H14O | MO | 0.49 |
| 19 | α-Campholenal | 1135 | C10H16O | O | 5.98 |
| 20 | Terpinen-4-ol | 1156 | C10H18O | MO | 3.50 |
| 21 | p-Cymene-8-ol | 1180 | C10H14O | MO | 0.19 |
| 22 | Myrtenal | 1185 | C10H14O | MO | 0.21 |
| 23 | α-Terpineol | 1188 | C10H18O | MO | 0.51 |
| 24 | Myrtenol | 1190 | C10H16O | MO | 0.14 |
| 25 | Verbenone | 1203 | C10H14O | MO | tr |
| 26 | trans-Chrysantenyl acetate | 1236 | C12H18O2 | MO | 18.35 |
| 27 | Carvone | 1245 | C10H14O | MO | 0.32 |
| 28 | Isopiperitenone (p-mentha-1,8-dien-3-one) | 1274 | C10H16O | MO | tr |
| 29 | Verbenyl acetate (bicyclo [3.1.1] hept-2-en-4ol) | 1282 | C12H18O2 | MO | 0.47 |
| 30 | Bornyl acetate | 1296 | C12H20O2 | MO | 3.38 |
| 31 | p-Cymen-7-ol | 1300 | C10H14O | MO | tr |
| 32 | Carvacrol | 1306 | C10H14O | MO | 0.52 |
| 33 | Aromandrene oxide | 1435 | C15H24O | SO | tr |
| 34 | Spathulenol | 1569 | C15H24O | SO | 0.36 |
| 35 | Caryophyllene oxide | 1571 | C15H24O | SO | 0.11 |
| 36 | Longiverbenone | 1627 | C15H22O | SO | 0.23 |
| 37 | β-Eudesmol | 1640 | C15H26O | SO | 0.47 |
| Terpene classes | |||||
| Monoterpene hydrocarbons (MH) | 6.12 | ||||
| Oxygenated monoterpenes (MO) | 79.55 | ||||
| Sesquiterpene hydrocarbons (SH) | ND | ||||
| Oxygenated sesquiterpenes (SO) | 1.17 | ||||
| Others (O) | 6.39 | ||||
| Total identified | 93.23 | ||||
| Group | Dose (g/kg b.w.) | D/T | Dead Rats (%) | Symptoms |
|---|---|---|---|---|
| I | 5 | 0/6 | 0 | None |
| II | 10 | 0/6 | 0 | None |
| III | 20 | 0/6 | 0 | None |
| IV | 30 | 0/6 | 0 | Hypoactivity. Short twists of the body. |
| V | 50 | 0/6 | 0 | As above plus rapid breathing. No mortality and no symptoms of intoxication. |
| Group | Dose (g/kg b.w.) | D/T | Dead Rats (%) | Toxic Effect up to 24 h |
|---|---|---|---|---|
| VI | 1.0 | 0/6 | 0 | None |
| VII | 1.5 | 4/6 | 66.7 | Rapid breathing. Exitus of 4 animals, at the 12 h. |
| VIII | 1.6 | 3/6 | 50.0 | Asthenia. Rapid breathing. Trembling. Exitus of 3 rats at the 10 h. |
| IX | 1.8 | 5/6 | 83.3 | Behavior as dose of 1.6 g/kg b.w. Breathing difficulty. Exitus of 5 rats. |
| X | 2.0 | 5/6 | 83.3 | Rapid breathing. Difficulty breathing. Some animals emit a short scream. Exitus of 5 rats. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karcheva-Bahchevanska, D.; Benbassat, N.; Georgieva, Y.; Lechkova, B.; Ivanova, S.; Ivanov, K.; Todorova, V.; Peychev, L.; Peychev, Z.; Denev, P. A Study of the Chemical Composition, Antioxidant Potential, and Acute Toxicity of Bulgarian Tanacetum vulgare L. Essential Oil. Molecules 2023, 28, 6155. https://doi.org/10.3390/molecules28166155
Karcheva-Bahchevanska D, Benbassat N, Georgieva Y, Lechkova B, Ivanova S, Ivanov K, Todorova V, Peychev L, Peychev Z, Denev P. A Study of the Chemical Composition, Antioxidant Potential, and Acute Toxicity of Bulgarian Tanacetum vulgare L. Essential Oil. Molecules. 2023; 28(16):6155. https://doi.org/10.3390/molecules28166155
Chicago/Turabian StyleKarcheva-Bahchevanska, Diana, Niko Benbassat, Yoana Georgieva, Borislava Lechkova, Stanislava Ivanova, Kalin Ivanov, Velislava Todorova, Lyudmil Peychev, Zhivko Peychev, and Petko Denev. 2023. "A Study of the Chemical Composition, Antioxidant Potential, and Acute Toxicity of Bulgarian Tanacetum vulgare L. Essential Oil" Molecules 28, no. 16: 6155. https://doi.org/10.3390/molecules28166155
APA StyleKarcheva-Bahchevanska, D., Benbassat, N., Georgieva, Y., Lechkova, B., Ivanova, S., Ivanov, K., Todorova, V., Peychev, L., Peychev, Z., & Denev, P. (2023). A Study of the Chemical Composition, Antioxidant Potential, and Acute Toxicity of Bulgarian Tanacetum vulgare L. Essential Oil. Molecules, 28(16), 6155. https://doi.org/10.3390/molecules28166155









