Biomineralization and Properties of Guanine Crystals
Abstract
:1. Introduction
2. Physical and Chemical Properties of Guanine Crystals
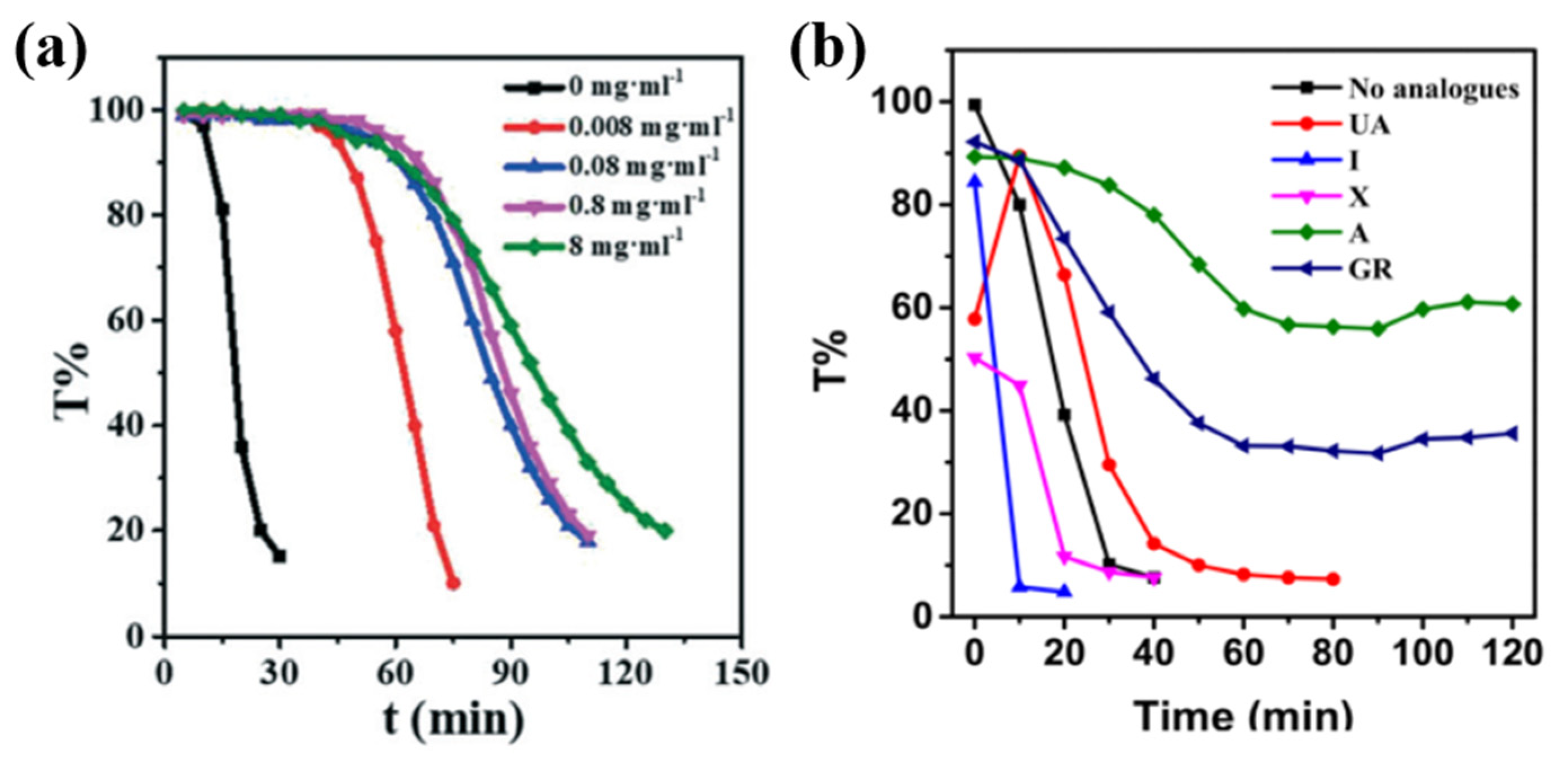
2.1. Solubility
2.2. Tautomers
2.3. Band Gap and Refractivity
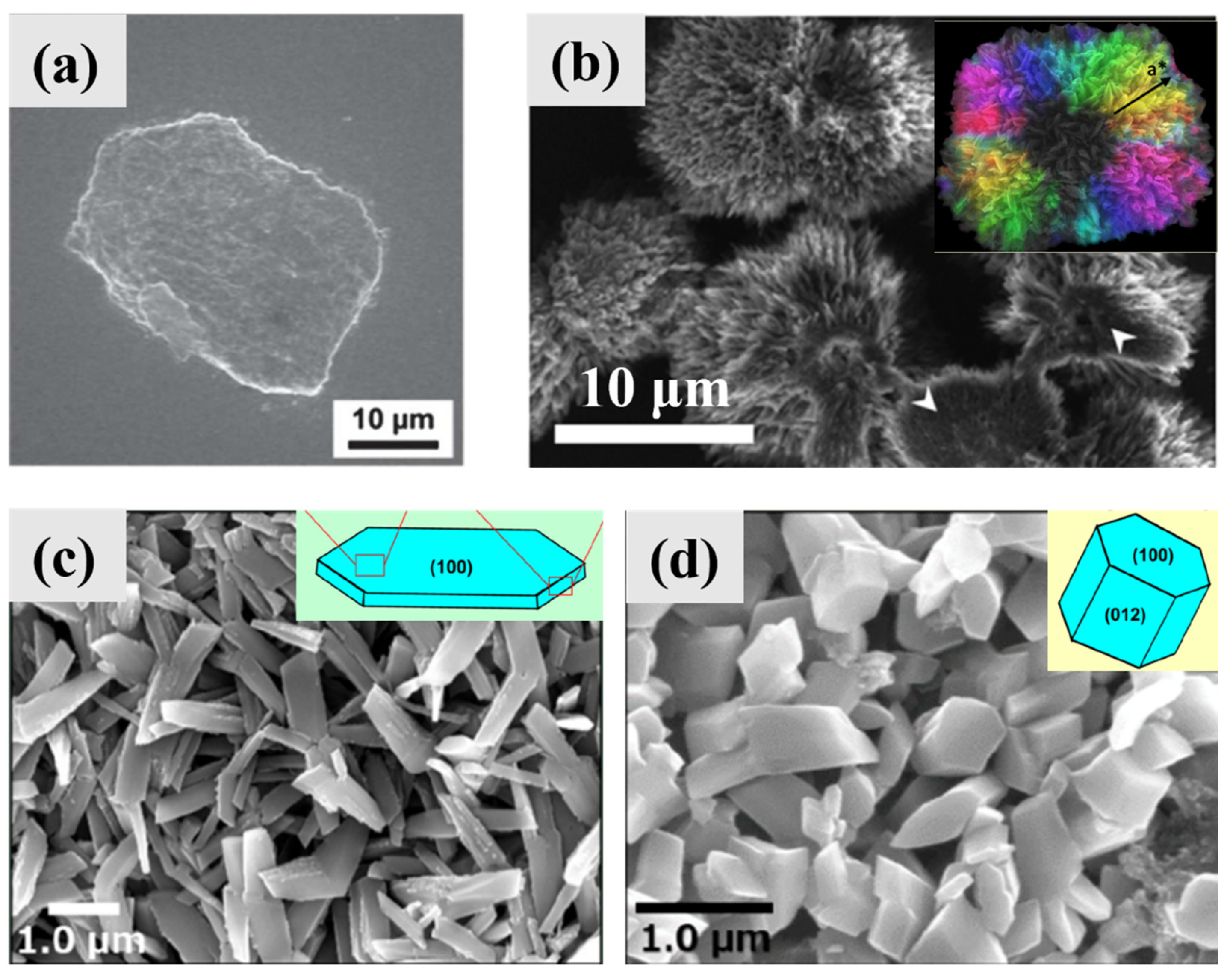
3. Guanine Polymorphs and Morphology
3.1. Guanine Monohydrate and Dehydrated Guanine Monohydrate
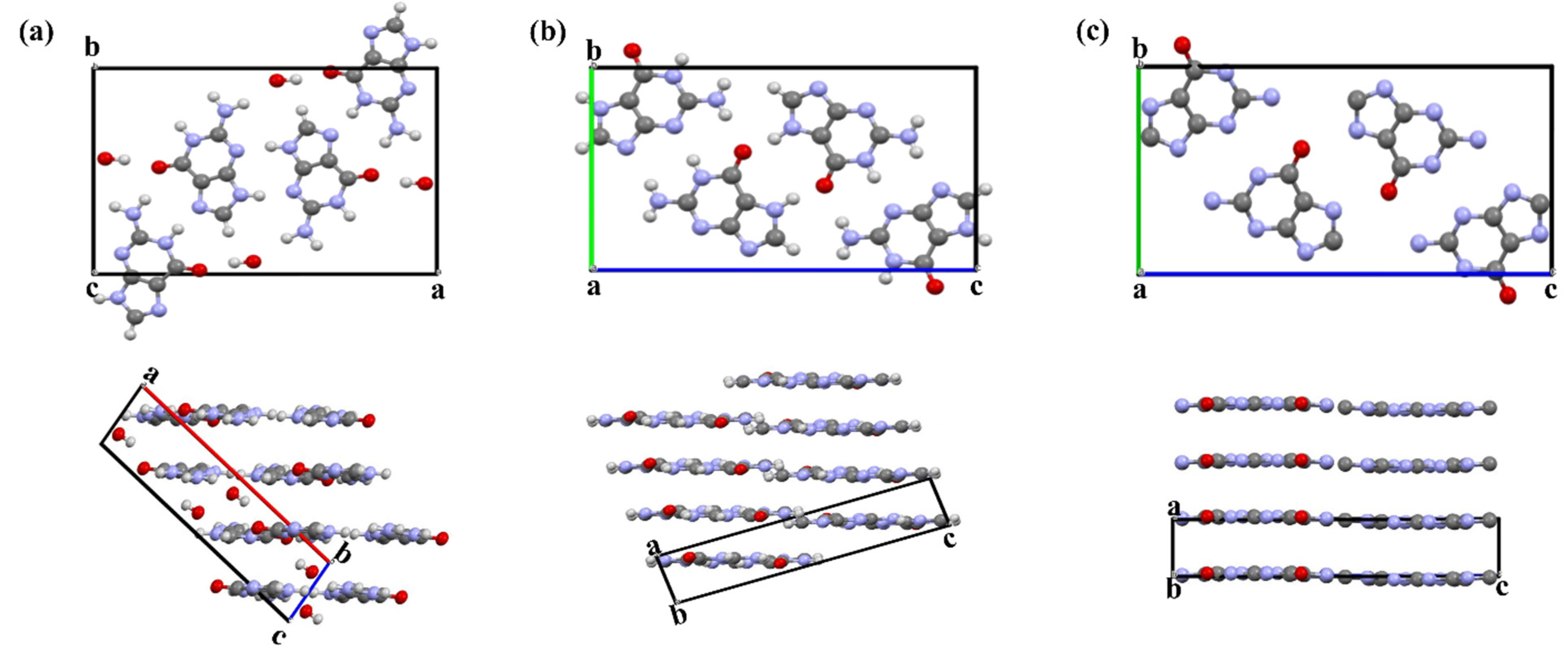
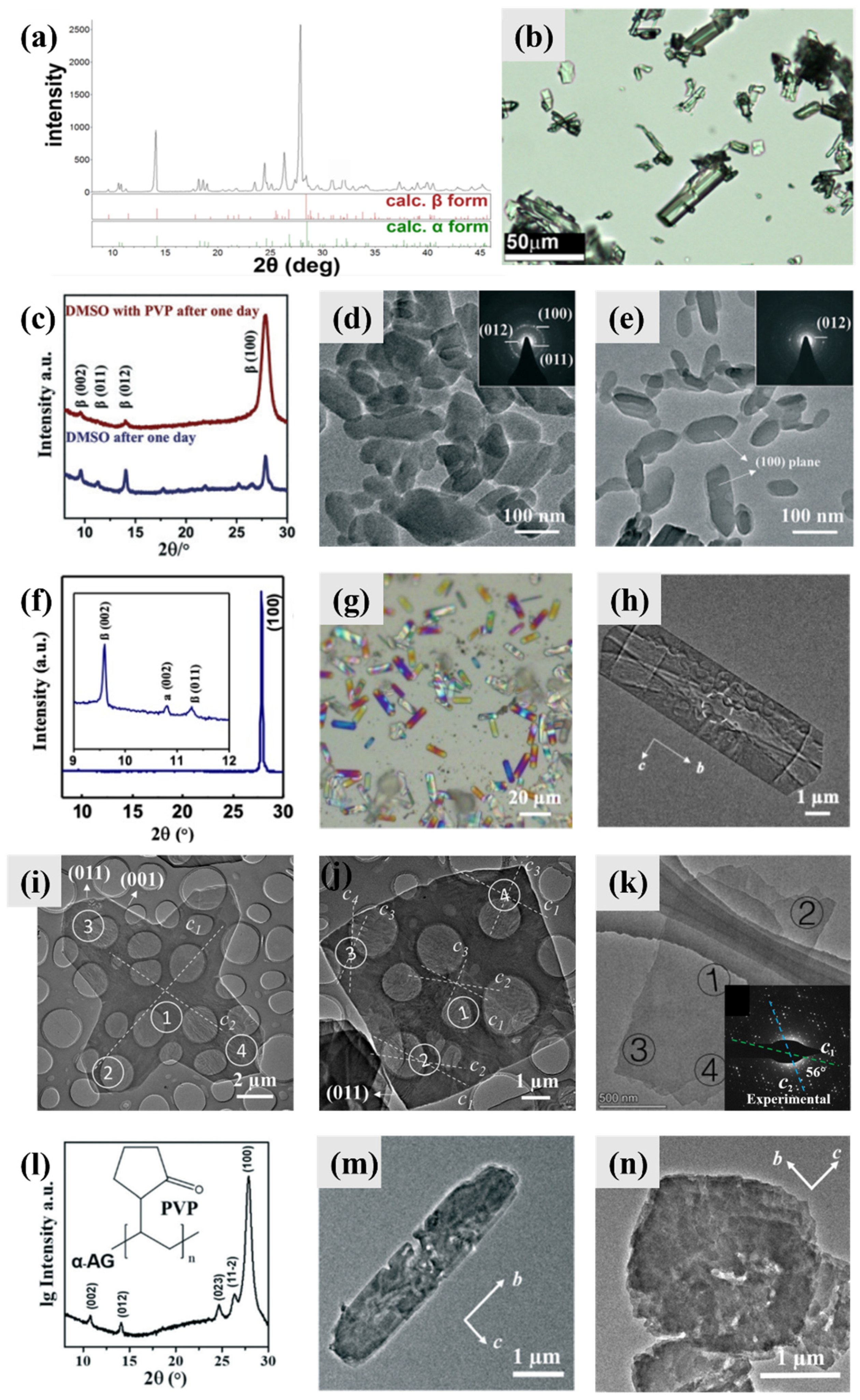
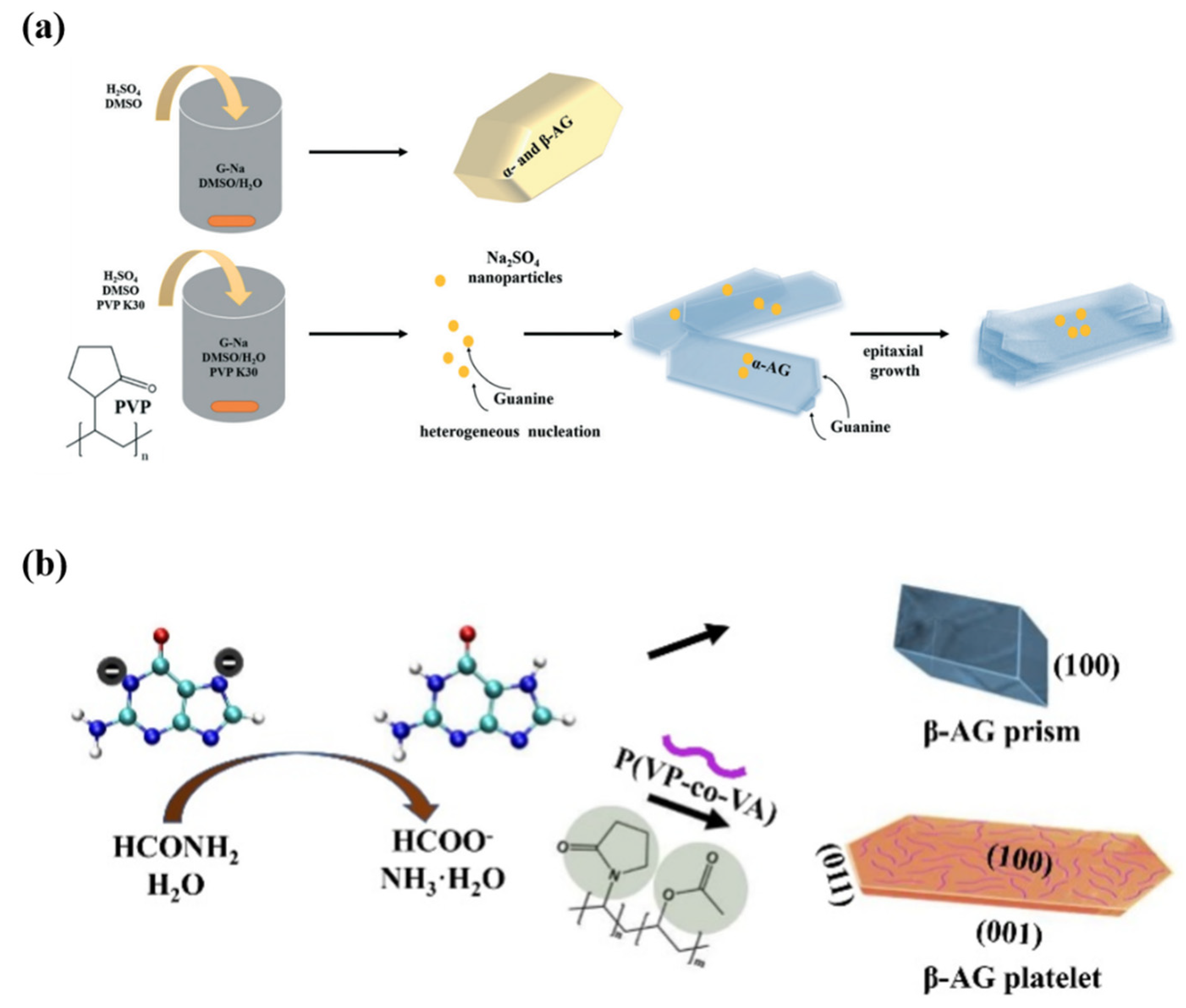
3.2. Anhydrous Guanine
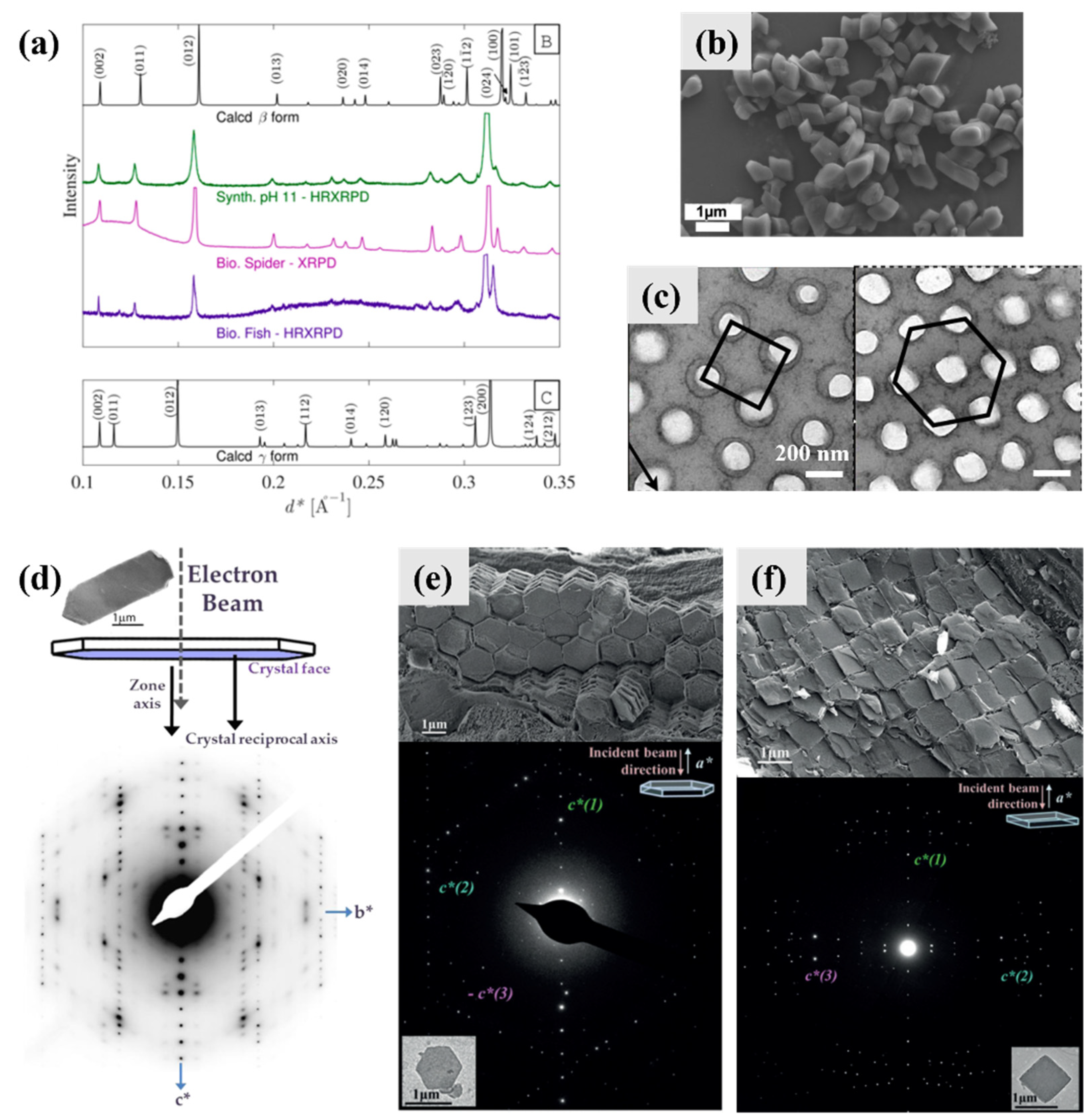
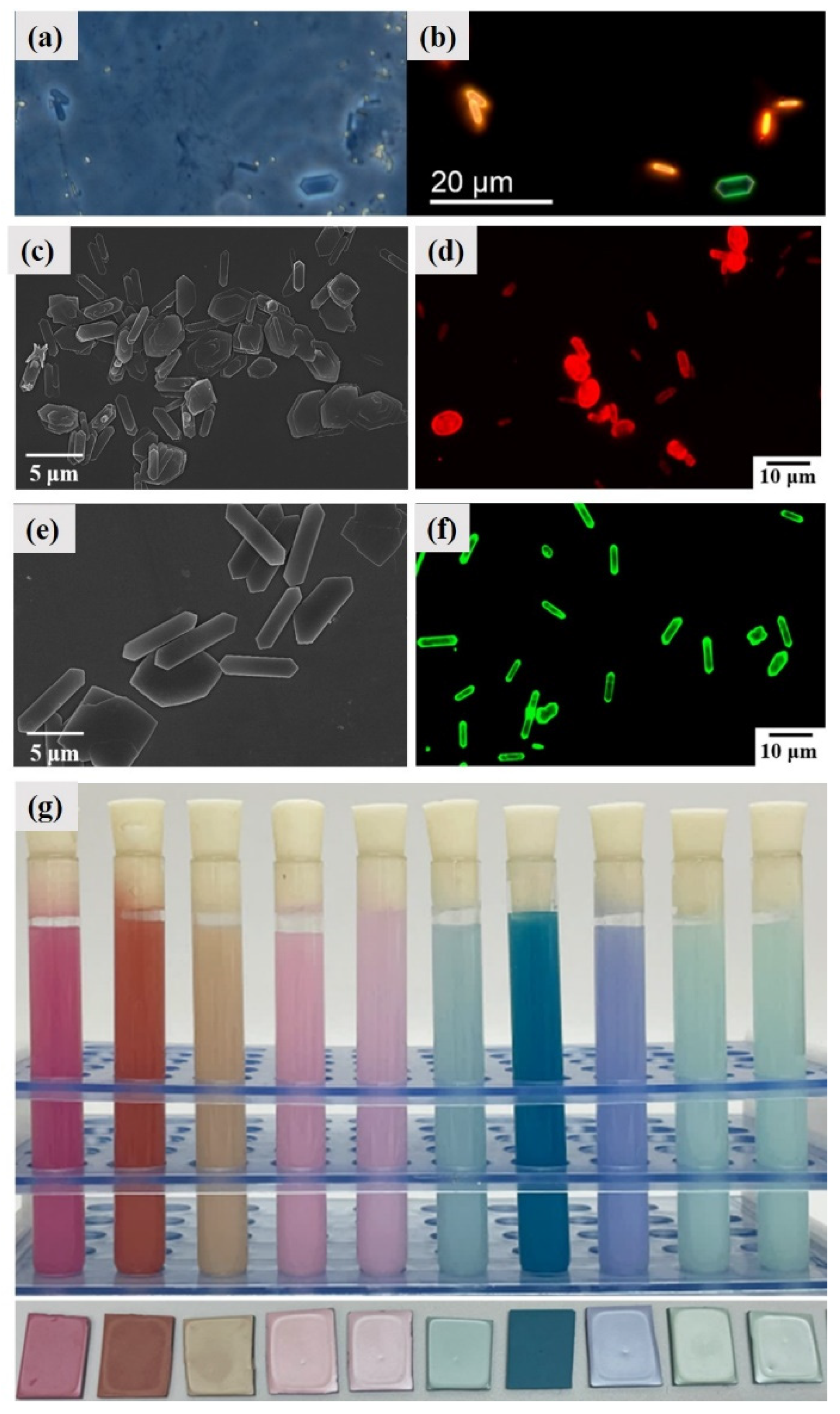
4. Biomineralization Principles of Guanine Crystals
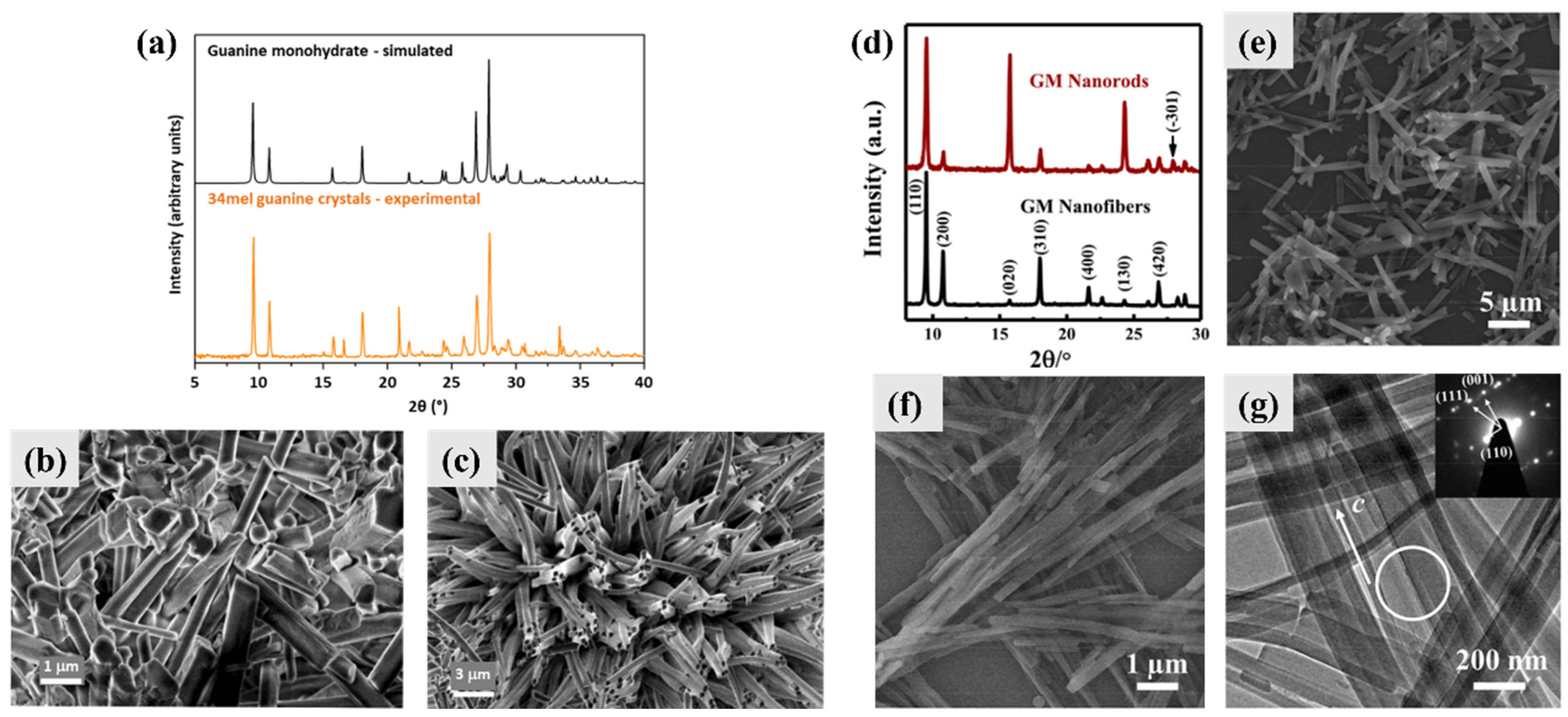
4.1. Intermediate Phase and Selective Recrystallization of Amorphous Guanine
4.2. Preassembled Scaffolds and Interface Control
4.3. Macromolecules and Small Organic Molecules as Additives
4.4. Twinning
4.5. Solid Solutions/Hypoxanthine Doping
4.6. Fluorescence
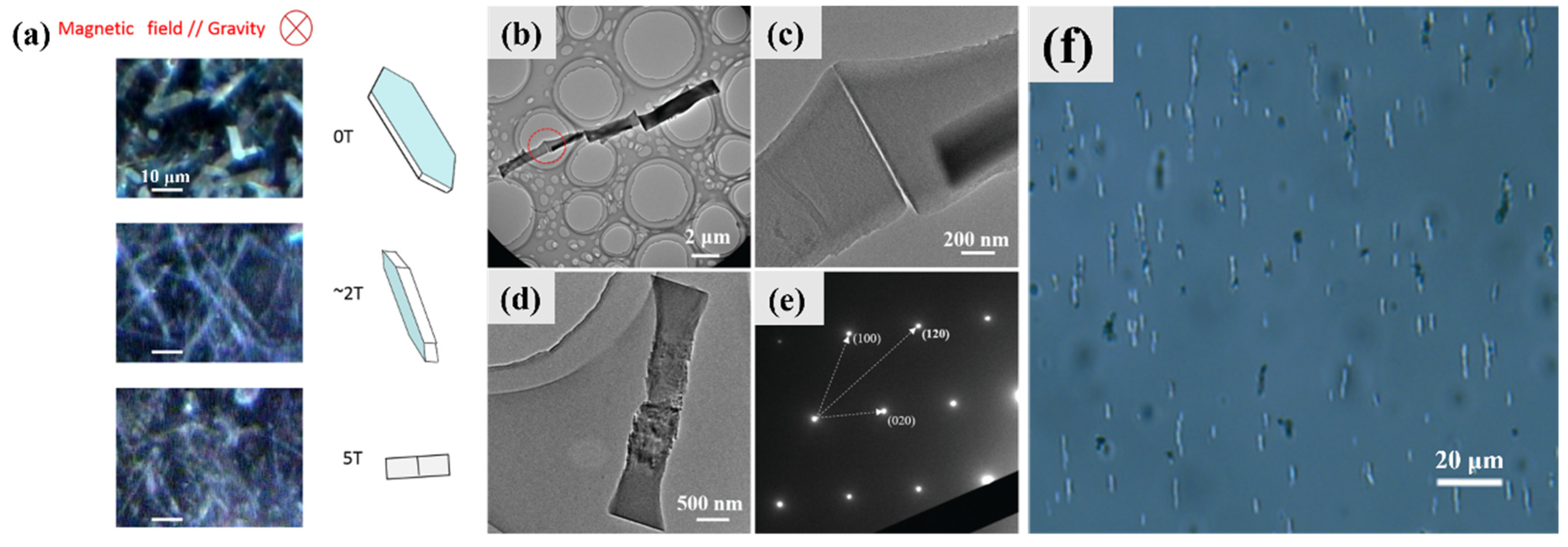
4.7. Orientation and Assembly
5. Challenges in Synthetic Guanine Crystals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From material tactics to biological strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef] [PubMed]
- Kahil, K.; Weiner, S.; Addadi, L.; Gal, A. Ion pathways in biomineralization: Perspectives on uptake, transport, and deposition of calcium, carbonate, and phosphate. J. Am. Chem. Soc. 2021, 143, 21100–21112. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wen, Q.; Pinsk, N.; Palmer, B.A. Functional molecular crystals in biology. Isr. J. Chem. 2021, 61, 668–678. [Google Scholar] [CrossRef]
- Gur, D.; Palmer, B.A.; Weiner, S.; Addadi, L. Light manipulation by guanine crystals in organisms: Biogenic scatterers, mirrors, multilayer reflectors and photonic crystals. Adv. Funct. Mater. 2017, 27, 1603514. [Google Scholar] [CrossRef]
- Hirsch, A.; Gur, D.; Polishchuk, I.; Levy, D.; Pokroy, B.; Cruz-Cabeza, A.J.; Addadi, L.; Kronik, L.; Leiserowitz, L. “Guanigma”: The revised structure of biogenic anhydrous guanine. Chem. Mater. 2015, 27, 8289–8297. [Google Scholar] [CrossRef]
- Pavan, M.E.; Movilla, F.; Pavan, E.E.; Di Salvo, F.; López, N.I.; Pettinari, M.J. Guanine crystals formation by bacteria. BMC Biol. 2023, 21, 66. [Google Scholar] [CrossRef]
- Gur, D.; Politi, Y.; Sivan, B.; Fratzl, P.; Weiner, S.; Addadi, L. Guanine-based photonic crystals in fish scales form from an amorphous precursor. Angew. Chem. Int. Ed. 2013, 52, 388–391. [Google Scholar] [CrossRef]
- Chen, F.; Guo, D.; Gao, J.; Ma, Y. Bioinspired Crystallization of Guanine. J. Phy. Chem. Lett. 2021, 12, 11695–11702. [Google Scholar] [CrossRef]
- Jordan, T.M.; Partridge, J.C.; Roberts, N.W. Non-polarizing broadband multilayer reflectors in fish. Nat. Photonics 2012, 6, 759–763. [Google Scholar] [CrossRef]
- Gur, D.; Leshem, B.; Oron, D.; Weiner, S.; Addadi, L. The structural basis for enhanced silver reflectance in Koi fish scale and skin. J. Am. Chem. Soc. 2014, 136, 17236–17242. [Google Scholar] [CrossRef]
- Gur, D.; Palmer, B.A.; Leshem, B.; Oron, D.; Fratzl, P.; Weiner, S.; Addadi, L. The mechanism of color change in the neon tetra fish: A light-induced tunable photonic crystal array. Angew. Chem. Int. Ed. 2015, 54, 12426–12430. [Google Scholar] [CrossRef] [PubMed]
- Devoe, H.; Wasik, S.P. Aqueous solubilities and enthalpies of solution of adenine and guanine. J. Solut. Chem. 1984, 13, 51–60. [Google Scholar] [CrossRef]
- Darvishzad, T.; Lubera, T.; Kurek, S.S. Puzzling aqueous solubility of guanine obscured by the formation of nanoparticles. J. Phys. Chem. B 2018, 122, 7497–7502. [Google Scholar] [CrossRef] [PubMed]
- Bennema, P.; van Eupen, J.; van der Wolf, B.M.A.; Los, J.H.; Meekes, H. Solubility of molecular crystals: Polymorphism in the light of solubility theory. Int. J. Pharm. 2008, 351, 74–91. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Y.; Wang, Y.; Qi, L. A novel tautomeric polymorph of anhydrous guanine and its reversible water harvesting property. Cryst. Growth Des. 2018, 18, 6497–6503. [Google Scholar] [CrossRef]
- Chen, F.; Wu, B.; Elad, N.; Gal, A.; Liu, Y.; Ma, Y.; Qi, L. Controlled crystallization of anhydrous guanine β nano-platelets via an amorphous precursor. CrystEngComm 2019, 21, 3586–3591. [Google Scholar] [CrossRef]
- Hirano, A.; Tokunaga, H.; Tokunaga, M.; Arakawa, T.; Shiraki, K. The solubility of nucleobases in aqueous arginine solutions. Arch. Biochem. Biophys. 2010, 497, 90–96. [Google Scholar] [CrossRef]
- Cong, Y.; Du, C.; Wang, M.; Jiang, Z.; Ye, T.; Zhang, Y.; Qiao, B.; Wang, M. Determination, construction, and evaluation of ternary and quaternary solid–liquid phase equilibrium of uric acid, adenine, and guanine in water. J. Chem. Eng. Data 2020, 65, 2133–2143. [Google Scholar] [CrossRef]
- Hirsch, A.; Palmer, B.A.; Elad, N.; Gur, D.; Weiner, S.; Addadi, L.; Kronik, L.; Leiserowitz, L. Biologically controlled morphology and twinning in guanine crystals. Angew. Chem. Int. Ed. 2017, 56, 9420–9424. [Google Scholar] [CrossRef]
- Gur, D.; Pierantoni, M.; Elool Dov, N.; Hirsh, A.; Feldman, Y.; Weiner, S.; Addadi, L. Guanine crystallization in aqueous solutions enables control over crystal size and polymorphism. Cryst. Growth Des. 2016, 16, 4975–4980. [Google Scholar] [CrossRef]
- Liang, W.; Li, H.; Hu, X.; Han, S. Systematic theoretical investigations on all of the tautomers of guanine: From both dynamics and thermodynamics viewpoint. Chem. Phys. 2006, 328, 93–102. [Google Scholar] [CrossRef]
- Yu, L.-J.; Pang, R.; Tao, S.; Yang, H.-T.; Wu, D.-Y.; Tian, Z.-Q. Solvent effect and hydrogen bond interaction on tautomerism, vibrational frequencies, and Raman spectra of guanine: A density functional theoretical study. J. Phys. Chem. A 2013, 117, 4286–4296. [Google Scholar] [CrossRef] [PubMed]
- Bharatam, P.V.; Valanju, O.R.; Wani, A.A.; Dhaked, D.K. Importance of tautomerism in drugs. Drug Discov. Today 2023, 28, 103494. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J. Polymorphism and desmotropy in heterocyclic crystal structures. Cryst. Growth Des. 2011, 11, 4731–4738. [Google Scholar] [CrossRef]
- Gomez, E.F.; Venkatraman, V.; Grote, J.G.; Steckl, A.J. Exploring the potential of nucleic acid bases in organic light emitting diodes. Adv. Mater. 2015, 27, 7552–7562. [Google Scholar] [CrossRef]
- Xue, R.; Liang, C.; Li, Y.; Chen, X.; Li, F.; Ren, S.; Chen, F. Solid-state separation of hypoxanthine tautomers through a doping strategy. CrystEngComm 2022, 24, 3448–3456. [Google Scholar] [CrossRef]
- Thewalt, U.; Bugg, C.E.; Marsh, R.E. The crystal structure of guanine monohydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 2358–2363. [Google Scholar] [CrossRef]
- Guille, K.; Clegg, W. Anhydrous guanine: A synchrotron study. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, o515–o517. [Google Scholar] [CrossRef]
- Levy-Lior, A.; Pokroy, B.; Levavi-Sivan, B.; Leiserowitz, L.; Weiner, S.; Addadi, L. Biogenic guanine crystals from the skin of fish may be designed to enhance light reflectance. Cryst. Growth Des. 2008, 8, 507–511. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Y.; Qi, L. Synthesis of porous microplatelets of α form anhydrous guanine in DMSO/water mixed solvents. CrystEngComm 2022, 24, 4215–4223. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Li, L.; Qi, L.; Ma, Y. Synthesis of bio-inspired guanine microplatelets: Morphological and crystallographic control. Chem. Eur. J. 2020, 26, 16228–16235. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, F.; Hu, Y.; Liu, Y.; Qi, L. Controlled crystallization of twinned crystalline guanine microplatelets. CrystEngComm 2019, 21, 6346–6353. [Google Scholar] [CrossRef]
- Oaki, Y.; Kaneko, S.; Imai, H. Morphology and orientation control of guanine crystals: A biogenic architecture and its structure mimetics. J. Mater. Chem. 2012, 22, 22686–22691. [Google Scholar] [CrossRef]
- Wittig, N.K.; Christensen, T.E.K.; Grünewald, T.A.; Birkedal, H. Vase-like β-polymorph guanine crystal aggregates formed at the air—Water interface. ACS Mater. Lett. 2020, 2, 446–452. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Lancaster, R.W.; Bernstein, J. Disappearing polymorphs revisited. Angew. Chem. Int. Ed. 2015, 54, 6972–6993. [Google Scholar] [CrossRef]
- Palmer, B.A.; Taylor, G.J.; Brumfeld, V.; Gur, D.; Shemesh, M.; Elad, N.; Osherov, A.; Oron, D.; Weiner, S.; Addadi, L. The image-forming mirror in the eye of the scallop. Science 2017, 358, 1172–1175. [Google Scholar] [CrossRef]
- Levy-Lior, A.; Shimoni, E.; Schwartz, O.; Gavish-Regev, E.; Oron, D.; Oxford, G.; Weiner, S.; Addadi, L. Guanine-based biogenic photonic-crystal arrays in fish and spiders. Adv. Funct. Mater. 2010, 20, 320–329. [Google Scholar] [CrossRef]
- Teyssier, J.; Saenko, S.V.; van der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef]
- Guo, D.; Hao, J.; Hou, X.; Ren, Y.; Zhang, Y.; Gao, J.; Ma, Y. Controlled synthesis of twinning β-form anhydrous guanine nanoplatelets in aqueous solution. CrystEngComm 2023, 25, 2052–2063. [Google Scholar] [CrossRef]
- Deng, Z.; Jia, Z.; Li, L. Biomineralized materials as model systems for structural composites: Intracrystalline structural features and their strengthening and toughening mechanisms. Adv. Sci. 2022, 9, 2103524. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y.; Hou, X.; Wang, X.; Fan, C.; Bao, L.; He, X.; Zhang, H.; Ma, Y. Formation mechanism of twinned β-form anhydrous guanine platelets in scallop eyes. CrystEngComm 2023, 25, 4521–4530. [Google Scholar] [CrossRef]
- Jantschke, A.; Pinkas, I.; Hirsch, A.; Elad, N.; Schertel, A.; Addadi, L.; Weiner, S. Anhydrous β-guanine crystals in a marine dinoflagellate: Structure and suggested function. J. Struct. Biol. 2019, 207, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takasaki, M.; Oaki, Y.; Imai, H. Biomimetic morphology-controlled anhydrous guanine via an amorphous intermediate. Cryst. Growth Des. 2020, 20, 3341–3346. [Google Scholar] [CrossRef]
- Mao, L.-B.; Gao, H.-L.; Yao, H.-B.; Liu, L.; Colfen, H.; Liu, G.; Chen, S.-M.; Li, S.-K.; Yan, Y.-X.; Liu, Y.-Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Eyal, Z.; Deis, R.; Varsano, N.; Dezorella, N.; Rechav, K.; Houben, L.; Gur, D. Plate-like guanine biocrystals form via templated nucleation of crystal leaflets on preassembled scaffolds. J. Am. Chem. Soc. 2022, 144, 22440–22445. [Google Scholar] [CrossRef]
- Wagner, A.; Upcher, A.; Maria, R.; Magnesen, T.; Zelinger, E.; Raposo, G.; Palmer, B.A. Macromolecular sheets direct the morphology and orientation of plate-like biogenic guanine crystals. Nat. Commun. 2023, 14, 589. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, L.; Wang, L.; Kong, H.; Tan, Q.; Xu, W. Atomic-scale insight into tautomeric recognition, separation, and interconversion of guanine molecular networks on Au(111). J. Am. Chem. Soc. 2015, 137, 11795–11800. [Google Scholar] [CrossRef]
- Pinsk, N.; Wagner, A.; Cohen, L.; Smalley, C.J.H.; Hughes, C.E.; Zhang, G.; Pavan, M.J.; Casati, N.; Jantschke, A.; Goobes, G.; et al. Biogenic guanine crystals are solid solutions of guanine and other purine metabolites. J. Am. Chem. Soc. 2022, 144, 5180–5189. [Google Scholar] [CrossRef]
- Wucherer, M.F.; Michiels, N.K. Regulation of red fluorescent light emission in a cryptic marine fish. Front. Zool. 2014, 11, 1. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Y.; Hao, J.; Ren, Y.; Hou, X.; Gao, J.; Ma, Y. Biomimetic synthesis of organic molecular-doped fluorescent guanine crystals with pearlescence. Chem. Eur. J. 2023, 29, e202300004. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, D. Synthesis Method of Guanine Based Colorful Pearlescent Pigments. China Patent CN115073937A, 20 September 2022. [Google Scholar]
- Chikashige, T.; Iwasaka, M. Magnetically-assembled micro/mesopixels exhibiting light intensity enhancement in the (012) planes of fish guanine crystals. AIP Adv. 2018, 8, 056704. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Guo, D.; Ma, Y. One-dimensional assembly of β-form anhydrous guanine microrods. Soft Matter 2021, 17, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Shavit, K.; Wagner, A.; Schertel, L.; Farstey, V.; Akkaynak, D.; Zhang, G.; Upcher, A.; Sagi, A.; Yallapragada, V.J.; Haataja, J.; et al. A tunable reflector enabling crustaceans to see but not be seen. Science 2023, 379, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Berger, O.; Yoskovitz, E.; Adler-Abramovich, L.; Gazit, E. Spectral transition in bio-inspired self-assembled peptide nucleic acid photonic crystals. Adv. Mater. 2016, 28, 2195–2200. [Google Scholar] [CrossRef] [PubMed]

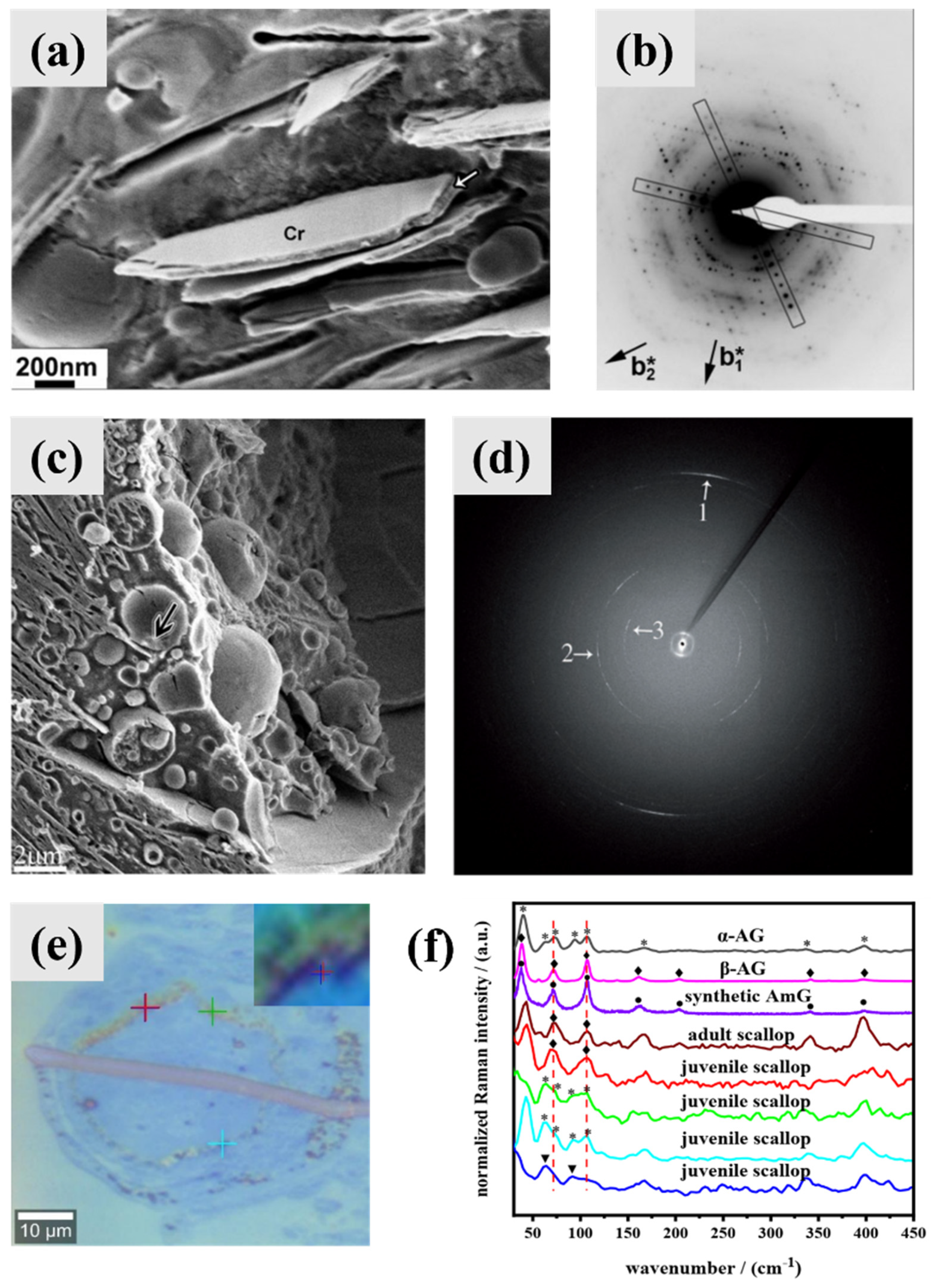
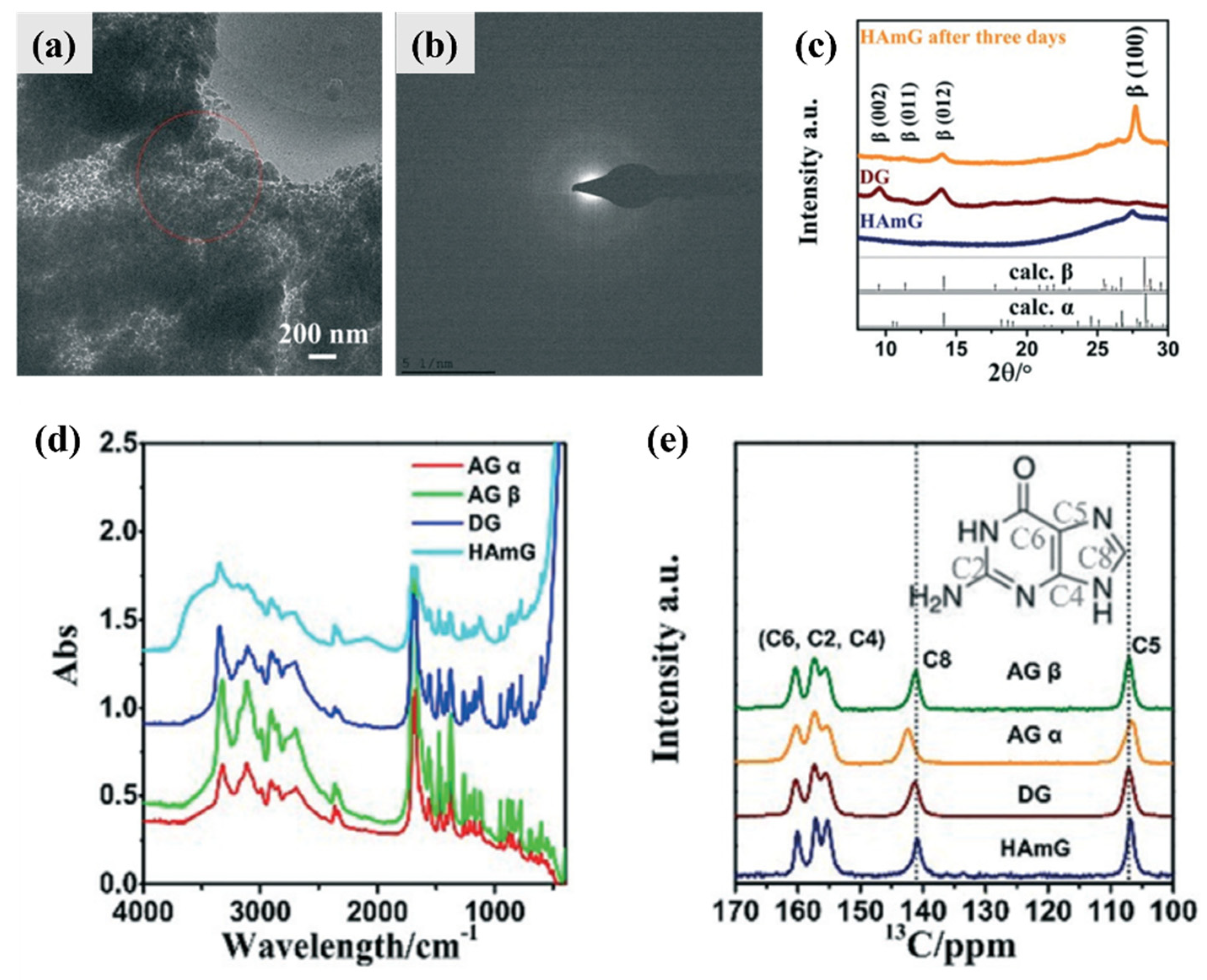
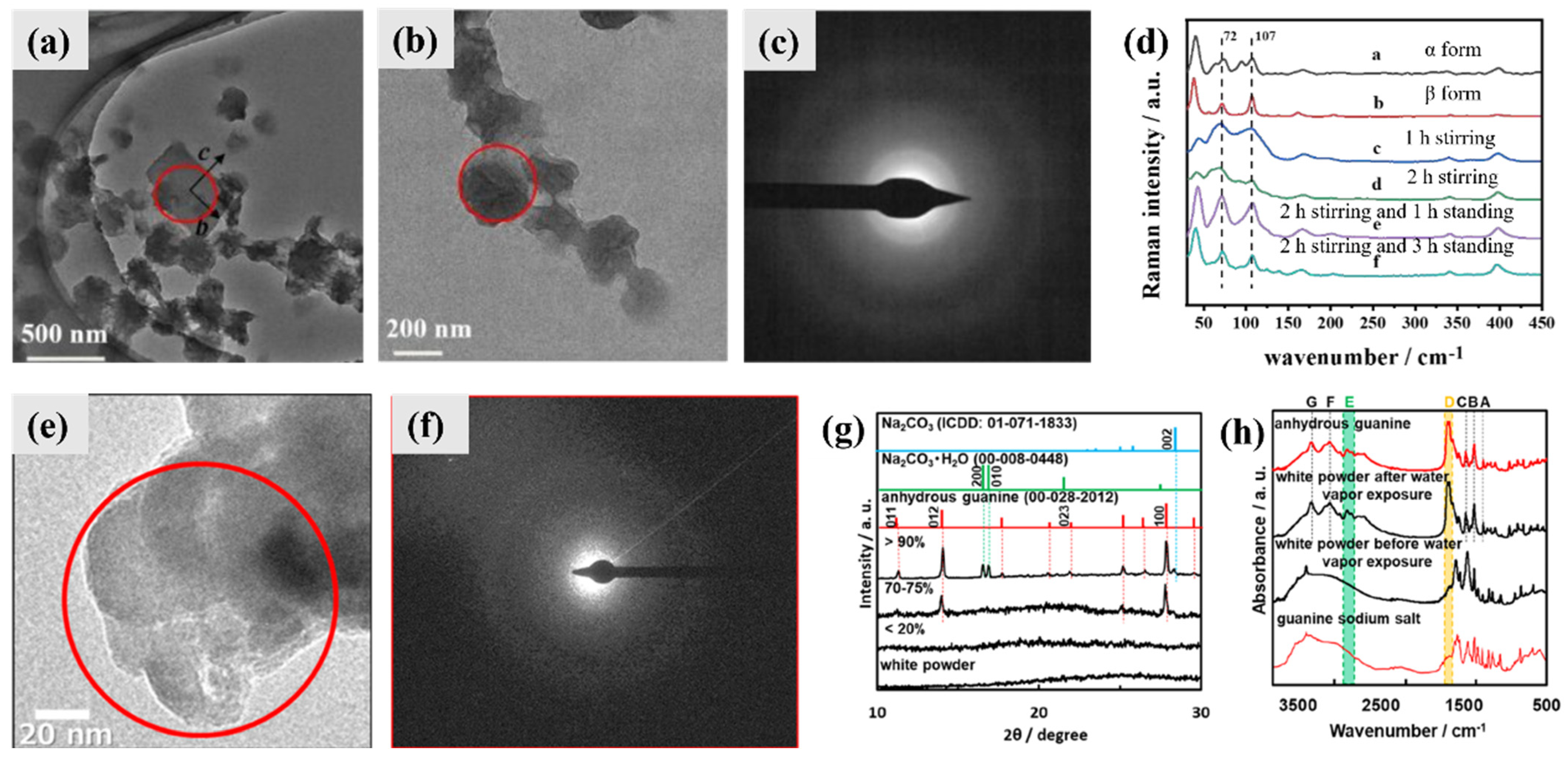
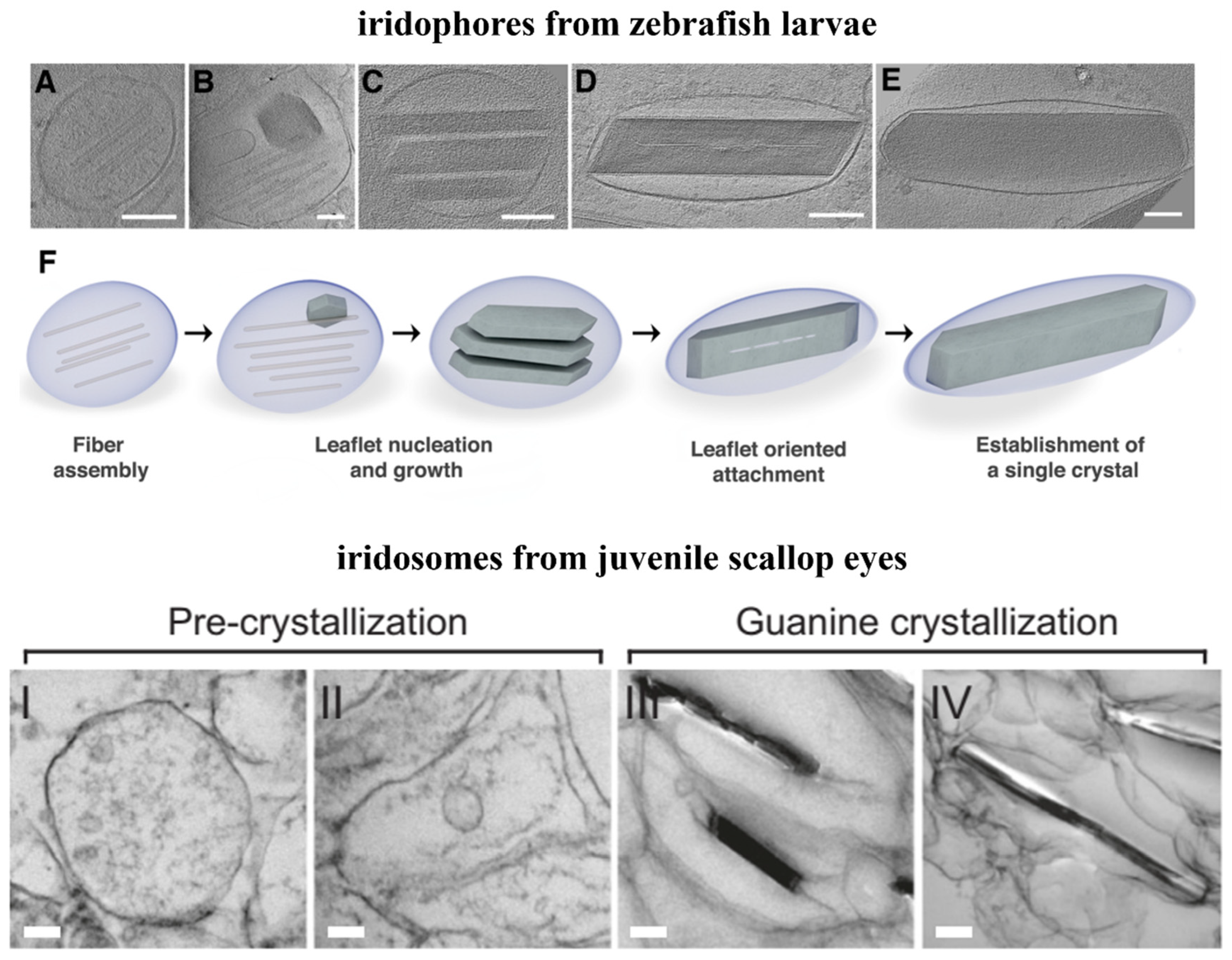


| Solubility (μM) | Solvent | Polymorphs | Method | Ref. |
|---|---|---|---|---|
| 39 ± 1 | water | commercial guanine | LC 1 | [12] |
| 111 ± 4 | citrate–phosphate buffer | commercial guanine | UV 2 | [17] |
| 25.4 | water | commercial guanine | UV 2 | [13] |
| 42.6 | water | commercial guanine | HPLC 3 | [18] |
| 13.9 ± 0.4 | water | AG α | UV 2 | unpublished data |
| 16.5 ± 0.2 | water | AG β | UV 2 | |
| 11.8 ± 0.2 | phosphate-buffered solution | AG α | UV 2 | |
| 13.0 ± 0.1 | phosphate-buffered solution | AG β | UV 2 |
| Hydrated Guanine | Anhydrous Guanine | ||||
|---|---|---|---|---|---|
| GM | Dehydrated GM | AG α | AG β | AG γ | |
| Year | 1971 | 2018 | 2006 | 2015 | 2015 |
| CCDC number | GUANMH10 | none | KEMDOW | KEMDOW01 | none |
| Tautomer | keto-N9H | keto-N9H | keto-N7H | keto-N7H | keto-N7H |
| Temperature (K) | - | 288 | |||
| Syngony | monoclinic | monoclinic | monoclinic | monoclinic | orthorhombic |
| Space | P21/n | - | P121/c1 | P1121/b | P21/m21/n21/b |
| a/Å | 16.51 | 16.20 | 3.56 | 3.59 | 6.38 |
| b/Å | 11.28 | 10.99 | 9.65 | 9.72 | 9.73 |
| c/Å | 3.65 | 3.60 | 18.45 | 18.34 | 18.39 |
| α | 90 | 90 | 90 | 90 | 90 |
| β | 96.8 | 96.1 | 118.5 | 90 | 90 |
| γ | 90 | 90 | 90 | 119.5 | 90 |
| Ref. | [27] | [15] | [5,28] | [5] | [5] |
| Polymorph | Method | Ref. |
|---|---|---|
| GM | a. evaporation of a dimethylamine/water solution | [27] |
| b. crystallization in an acidic aqueous solution | [15,20] | |
| c. crystallization in a neutral aqueous solution with surfactants | [15] | |
| Dehydrated GM | dehydration of GM at 150 °C | [15] |
| AG α | a. saturated DMSO solution stored at 4 °C overnight | [29] |
| b. neutralization precipitation in DMSO/water (4:1) with PVP 1 (guanine ~6 mM) | [30] | |
| c. neutralization precipitation in formamide with P(VP-co-VA) 2 and adenine or guanosine | [31] | |
| d. suspension in water at pH 2 and 90 °C | [6] | |
| AG β | a. recrystallization of amorphous guanine | [16] |
| b. neutralization precipitation in formamide with P(VP-co-VA) 2 | [31,32] | |
| c. volatilization of guanine ammonia solution | [32,33] | |
| d. ammonia-induced crystallization of guanine-HCl solution at the air–water interface | [34] | |
| e. aqueous crystallization at pH 10 | [6] | |
| HAmG | rapid aqueous neutralization precipitation with high concentration guanine (~50 mM) | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Xue, R.; Chen, F. Biomineralization and Properties of Guanine Crystals. Molecules 2023, 28, 6138. https://doi.org/10.3390/molecules28166138
Hu H, Xue R, Chen F. Biomineralization and Properties of Guanine Crystals. Molecules. 2023; 28(16):6138. https://doi.org/10.3390/molecules28166138
Chicago/Turabian StyleHu, Haoxin, Rongrong Xue, and Fenghua Chen. 2023. "Biomineralization and Properties of Guanine Crystals" Molecules 28, no. 16: 6138. https://doi.org/10.3390/molecules28166138
APA StyleHu, H., Xue, R., & Chen, F. (2023). Biomineralization and Properties of Guanine Crystals. Molecules, 28(16), 6138. https://doi.org/10.3390/molecules28166138





