Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion
Abstract
:1. Introduction
2. Result and Discussion
2.1. Production Yield of Food Waste-Based Biochar
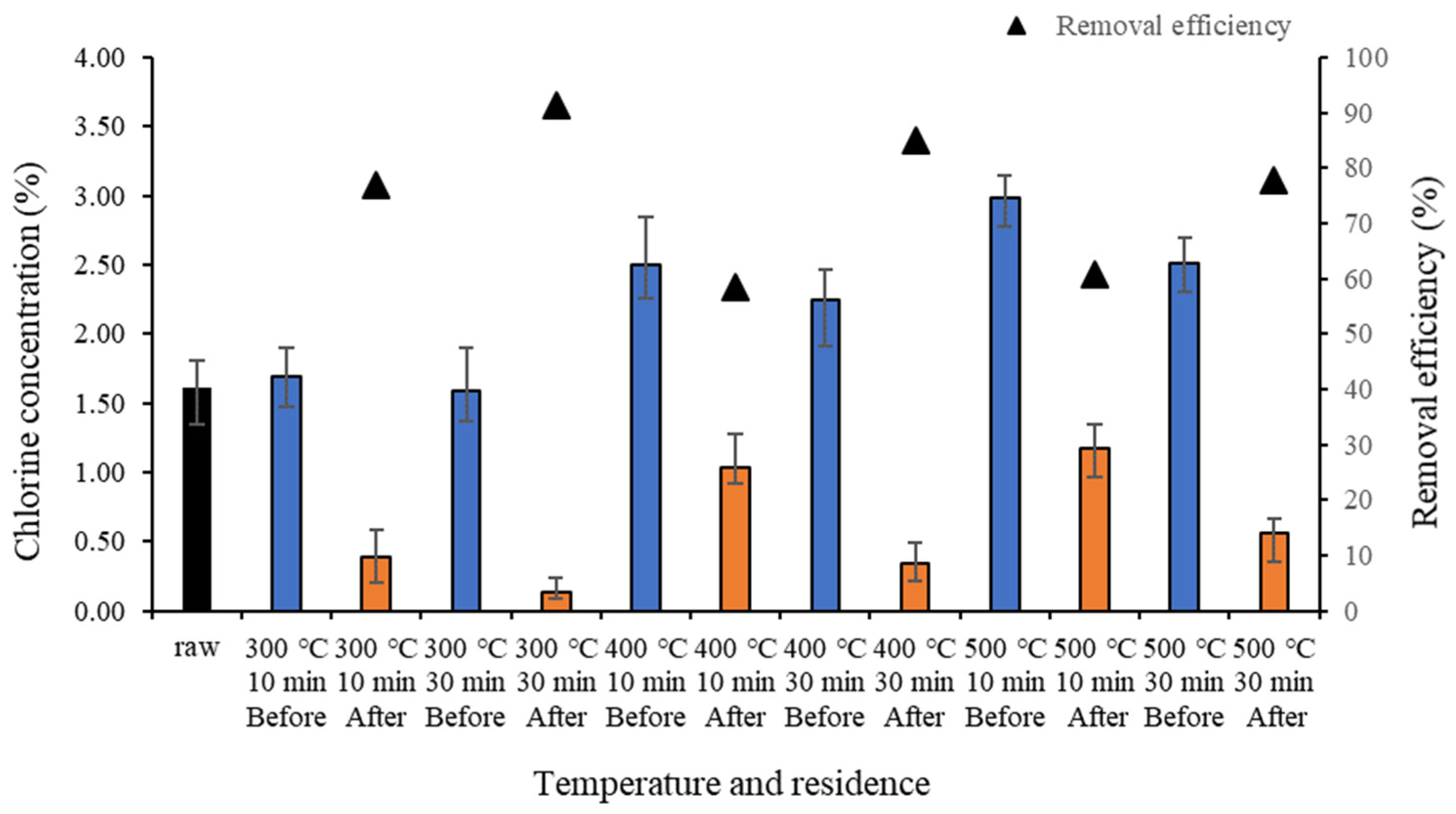
2.2. Heating Value and Chloride Ions of Food Waste-Based Biochar before and after Demineralization
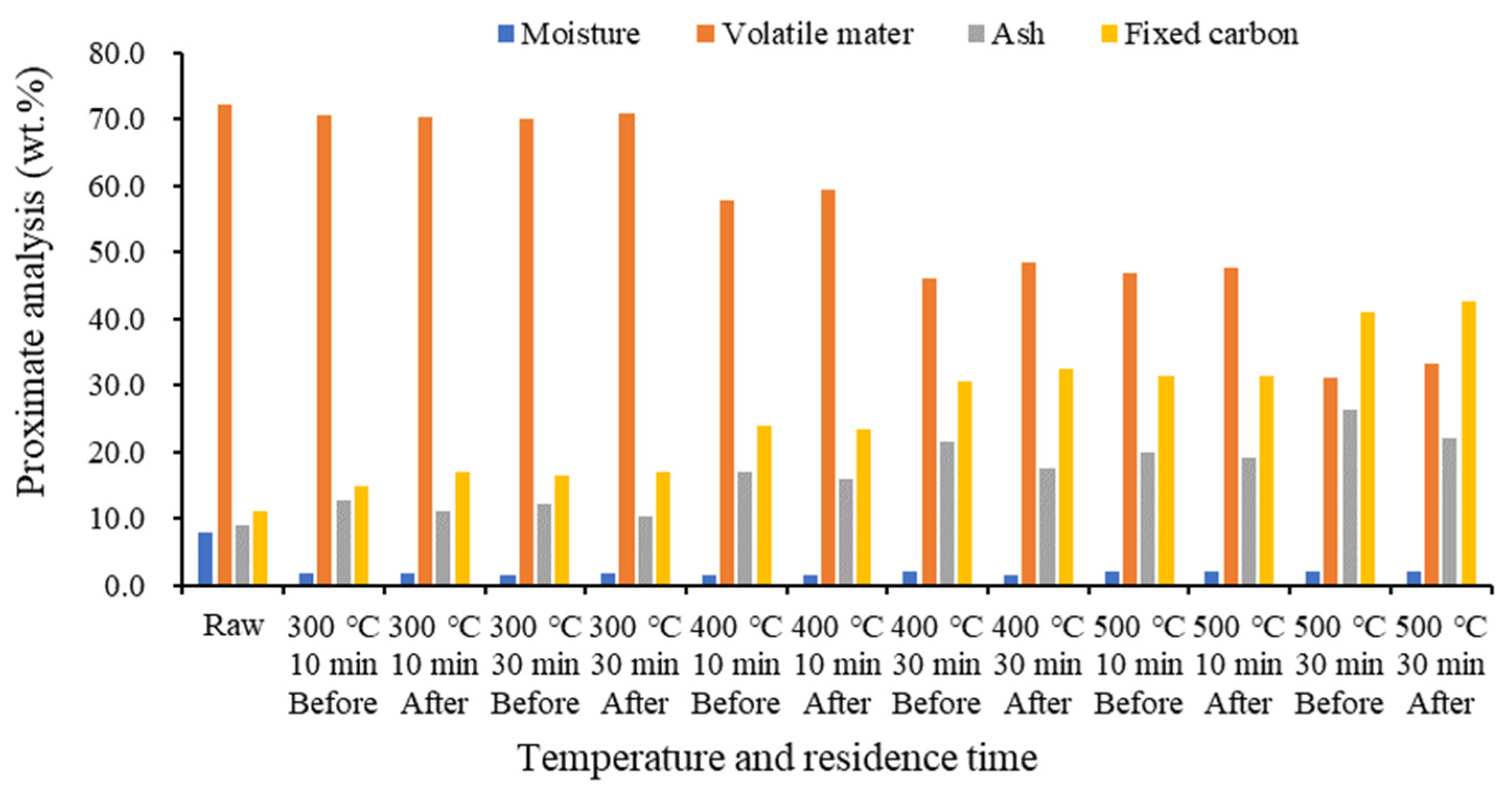
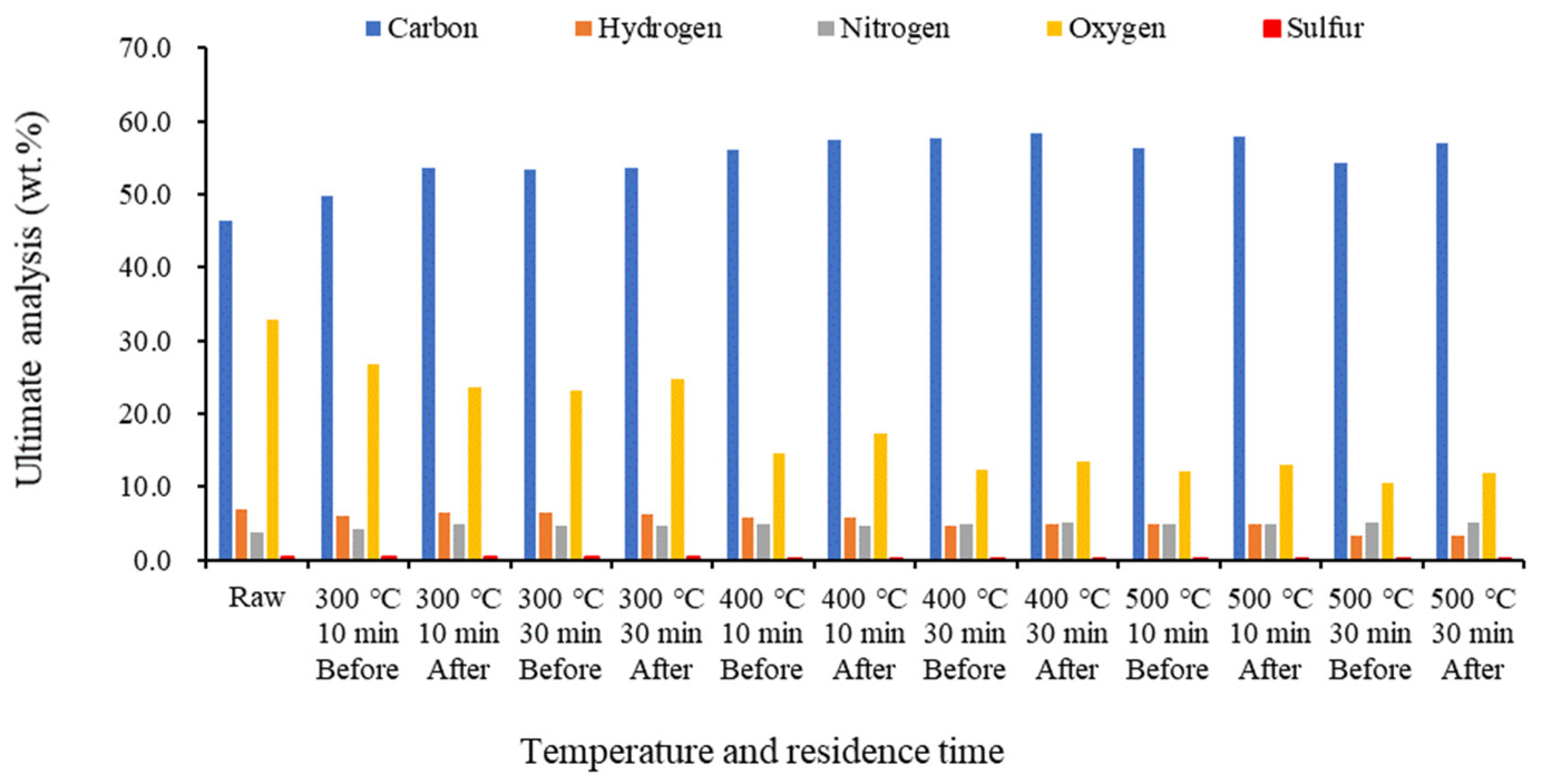
2.3. Proximate and Ultimate Analyses of Biochar before and after Demineralization at Different Temperatures
2.4. Component Analysis According to the Bio-SRF Standard
2.5. Saltwater Quality Characteristics
3. Materials and Methods
3.1. Materials
3.2. Kiln Description
3.2.1. Kiln
3.2.2. Demineralization System
3.3. Sample Analysis
3.4. Experimental Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fabi, C.; Cachia, F.; Conforti, P.; English, A.; Rosero Moncayo, J. Improving data on food losses and waste: From theory to practice. Food Policy 2021, 98, 101934. [Google Scholar] [CrossRef]
- FAO. Global Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2015; Volume 25, Available online: https://www.fao.org/3/i4068e/i4068e.pdf (accessed on 1 July 2020).
- Schanes, K.; Dobernig, K.; Gözet, B. Food waste matters—A systematic review of household food waste practices and their policy implications. J. Clean. Prod. 2018, 182, 978–991. [Google Scholar] [CrossRef]
- Lopez Barrera, E.L.; Hertel, T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy 2021, 98, 101874. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- FAO (Ed.) Moving forward on food loss and waste reduction. In The State of Food and Agriculture; FAO: Rome, Italy, 2019; Volume 2019, Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 10 April 2020).
- Reitemeier, M.; Aheeyar, M.; Drechsel, P. Perceptions of food waste reduction in Sri Lanka’s commercial capital, Colombo. Sustainability 2021, 13, 838. [Google Scholar] [CrossRef]
- El Bilali, H.; Ben Hassen, T. Food waste in the countries of the gulf cooperation council: A systematic review. Foods 2020, 9, 463. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Badgett, A.; Milbrandt, A. Food waste disposal and utilization in the United States: A spatial cost benefit analysis. J. Clean. Prod. 2021, 314, 128057. [Google Scholar] [CrossRef]
- You, S.; Wang, W.; Dai, Y.; Tong, Y.W.; Wang, C.H. Comparison of the co-gasification of sewage sludge and food wastes and cost-benefit analysis of gasification- and incineration-based waste treatment schemes. Bioresour. Technol. 2016, 218, 595–605. [Google Scholar] [CrossRef]
- Ong, K.; Kaur, G.; Pensupa, N.; Uisan, K.; Lin, C.S.K. Trends in food waste valorization for the production of chemicals, materials and fuels: Case study South and Southeast Asia. Bioresour. Technol. 2018, 248, 100–112. [Google Scholar] [CrossRef]
- Quested, T.E.; Palmer, G.; Moreno, L.C.; McDermott, C.; Schumacher, K. Comparing diaries and waste compositional analysis for measuring food waste in the home. J. Clean. Prod. 2020, 262, 121263. [Google Scholar] [CrossRef]
- Grasso, A.C.; Olthof, M.R.; Boevé, A.J.; van Dooren, C.; Lähteenmäki, L.; Brouwer, I.A. Socio-demographic predictors of food waste behavior in Denmark and Spain. Sustainability 2019, 11, 3244. [Google Scholar] [CrossRef]
- Lebersorger, S.; Schneider, F. Discussion on the methodology for determining food waste in household waste composition studies. Waste Manag. 2011, 31, 1924–1933. [Google Scholar] [CrossRef]
- Ministry of the Environment. Status of Food Waste Treatment Facilities in 2021. Republic of Korea. Available online: http://www.me.go.kr/home/web/policy_data/read.do?pagerOffset=0&maxPageItems=10&maxIndexPages=10&searchKey=&searc-Value=&menuId=10264&orgCd=&condition.toInpYmd=null&condition.fromInpYmd=null&condition.orderSeqId=6689&condition.rnSeq=1007&condition.deleteYn=N&condition.deptNm=null&seq=7916 (accessed on 14 February 2023).
- Al-Rumaihi, A.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Environmental impact assessment of food waste management using two composting techniques. Sustainability 2020, 12, 1595. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Gao, A.; Tian, Z.; Wang, Z.; Wennersten, R.; Sun, Q. Comparison between the technologies for food waste treatment. Energy Procedia 2017, 105, 3915–3921. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Andrew Lin, K.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Lee, C.-G.; Hong, S.-H.; Hong, S.-G.; Choi, J.-W.; Park, S.-J. Production of biochar from food waste and its application for phenol removal from aqueous solution. Water Air Soil Pollut. 2019, 230, 70. [Google Scholar] [CrossRef]
- Rago, Y.P.; Surroop, D.; Mohee, R. Assessing the potential of biofuel (biochar) production from food wastes through thermal treatment. Bioresour. Technol. 2018, 248, 258–264. [Google Scholar] [CrossRef]
- Lee, Y.E.; Jo, J.; Kim, I.; Yoo, Y. Influence of NaCl concentration on food-waste biochar structure and templating effects. Energies 2018, 11, 2341. [Google Scholar] [CrossRef]
- Samad, N.A.F.A.; Jamin, N.A.; Saleh, S. Torrefaction of municipal solid waste in Malaysia. Energy Procedia 2017, 138, 313–318. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of slagging and fouling tendency during biomass co-firing with coal in a fluidized bed. Biomass Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- Kassman, H.; Pettersson, J.; Steenari, B.-M.; Åmand, L.-E. Two strategies to reduce gaseous KCl and chlorine in deposits during biomass combustion—Injection of ammonium sulphate and co-combustion with peat. Fuel Process. Technol. 2013, 105, 170–180. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic assessment of pyrolyzed food waste digestate for sandy soil management. J. Environ. Manag. 2017, 187, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.I.; Oh, S.C. A study on torrefaction of food waste. Fuel 2015, 140, 275–281. [Google Scholar] [CrossRef]
- Iacovidou, E.; Hahladakis, J.; Deans, I.; Velis, C.; Purnell, P. Technical properties of biomass and solid recovered fuel (SRF) co-fired with coal: Impact on multi-dimensional resource recovery value. Waste Manag. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterization of renewable fuels’ torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Mathabatha, T.I.K.; Matheri, A.N.; Belaid, M. Peanut shell-derived biochar as a low-cost adsorbent to extract cadmium, chromium, lead, copper, and zinc (heavy metals) from wastewater: Circular economy approach. Circ. Econ. Sustain. 2023, 3, 1045–1064. [Google Scholar] [CrossRef]
- Lee, Y.E.; Jo, J.; Kim, I.; Yoo, Y. Value-added performance and thermal decomposition characteristics of dumped food waste compost by pyrolysis. Energies 2018, 11, 1061. [Google Scholar] [CrossRef]
- Lee, Y.E.; Jeong, Y.; Shin, D.C.; Yoo, Y.S.; Ahn, K.H.; Jung, J.; Kim, I.T. Effects of demineralization on food waste biochar for co-firing: Behaviors of alkali and alkaline earth metals and chlorine. Waste Manag. 2022, 137, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment. Enforcement Rule of the Act on the Promotion of Saving and Recycling of Resources; Ministry of the Environment: Sejong City, Republic of Korea, 2020. Available online: https://elaw.klri.re.kr/kor_service/lawView.do?hseq=46235&lang=ENG (accessed on 28 November 2017).
- BS EN 15359; Solid Recovered Fuels—Specifications and Classes. BSI Standards Publication: London, UK, 2011.
- Yasuhara, A.; Katami, T.; Okuda, T.; Ohno, N.; Shibamoto, T. Formation of dioxins during the combustion of newspapers in the presence of sodium chloride and poly(vinyl chloride). Environ. Sci. Technol. 2001, 35, 1373–1378. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Capunitan, J.A.; Capareda, S.C. Assessing the potential for biofuel production of corn stover pyrolysis using a pressurized batch reactor. Fuel 2012, 95, 563–572. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Yadama, V.; Garcia-Perez, M.; Lowell, E.; McDonald, A.G. Effect of temperature during wood torrefaction on the formation of lignin liquid intermediates. J. Anal. Appl. Pyrol. 2014, 109, 222–233. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Diao, R.; Zhu, X.; Wang, C.; Zhu, X. Synergistic effect of physicochemical properties and reaction temperature on gasification reactivity of walnut shell chars. Energy Convers. Manag. 2020, 204, 112313. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, Y.E.; Kim, I. Characterization of sewage sludge and food waste-based biochar for co-firing in a coal-fired power plant: A case study in Korea. Sustainability 2020, 12, 9411. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L.) biochar and feasibility for carbon capture and energy balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Ghiasi, B.; Soelberg, N.R.; Sokhansanj, S. Biomass torrefaction process, product properties, reactor types, and moving bed reactor design concepts. Front. Energy Res. 2021, 9, 728140. [Google Scholar] [CrossRef]
- Bessi, C.; Lombardi, L.; Meoni, R.; Canovai, A.; Corti, A. Solid recovered fuel: An experiment on classification and potential applications. Waste Manag. 2016, 47, 184–194. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland–Case study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Fan, X.; Wang, W.; Huang, W. Quantitative evaluation of heavy metals’ pollution hazards and estimation of heavy metals’ environmental costs in leachate during food waste composting. Waste Manag. 2019, 84, 119–128. [Google Scholar] [CrossRef]
- Vassalini, I.; Litvinava, M.; Alessandri, I. All food waste-based membranes for chromium(VI) removal. Environ. Sustain. 2021, 4, 429–435. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Shin, D.; Jung, J.; Jeong, Y.; Lee, Y.; Kim, I. Physicochemical properties of torrefied and pyrolyzed food waste biochars as fuel: A pilot-scale study. Energies 2022, 15, 333. [Google Scholar] [CrossRef]
- Ministry of the Environment. Emission Standards of Water Pollutants in the Enforcement Rules of the Water Environment Conservation Act, Republic of Korea. 2021. Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=62518&type=new&key= (accessed on 5 January 2023).
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.S. Methane production potential of leachate generated from Korean food waste recycling facilities: A lab-scale study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, L.M.; Belitz, K.; Fram, M.S.; Lindsey, B.D. Volatile organic compounds in groundwater used for public supply across the United States: Occurrence, explanatory factors, and human-health context. Sci. Total Environ. 2022, 827, 154313. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.J.; Kim, H.Y.; Choi, H.N. The operation characteristics of advanced sewage treatment process using aerobic granular sludge in pilot plant. J. Korean Soc. Environ. Eng. 2019, 41, 61–68. [Google Scholar] [CrossRef]
- APHA AWWA. W.P.C.F. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association; American Water Work Association; Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]


| Temperature (°C), Residence Time (min) | Before Pyrolysis (kg) | After Pyrolysis (kg) | Yield (%) |
|---|---|---|---|
| 300, 10 | 10.0 ± 0.4 | 7.66 ± 0.4 | 76.6 |
| 300, 30 | 10.0 ± 0.3 | 7.22 ± 0.2 | 72.2 |
| 400, 10 | 10.0 ± 0.4 | 5.87 ± 0.5 | 58.7 |
| 400, 30 | 10.0 ± 0.2 | 5.46 ± 0.4 | 54.6 |
| 500, 10 | 10.0 ± 0.4 | 5.38 ± 0.3 | 53.8 |
| 500, 30 | 10.0 ± 0.4 | 4.32 ± 0.4 | 43.2 |
| Component | Unit | 300 °C 30 Min before | 300 °C 30 Min after | 400 °C 30 Min before | 400 °C 30 Min after | 500 °C 30 Min before | 500 °C 30 Min after | Bio-SRF (Non-Pellet) | SRF (Non-Pellet) |
|---|---|---|---|---|---|---|---|---|---|
| Moisture | wt.% | 1.54 | 1.84 | 2.00 | 1.53 | 1.96 | 2.05 | ≤25 | ≤25 |
| Ash | wt.% | 12.14 | 10.33 | 21.35 | 17.6 | 26.25 | 22.1 | ≤15 | ≤20 |
| Chlorine | wt.% | 1.59 | 0.14 | 2.24 | 0.34 | 2.51 | 0.56 | ≤0.5 | ≤2.0 |
| Sulfur | wt.% | 0.23 | 0.21 | 0.16 | 0.2 | 0.12 | 0.2 | ≤0.6 | ≤0.6 |
| Biomass | wt.% | >99.5 | >99.5 | >99.5 | >99.5 | >99.5 | >99.5 | ≥95 | |
| Mercury | (mg·kg−1) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ≤0.6 | ≤1.0 |
| Cadmium | (mg·kg−1) | 0.45 | 0.51 | 0.62 | 0.75 | 0.48 | 0.53 | ≤5.0 | ≤5.0 |
| Lead | (mg·kg−1) | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 | ≤100 | ≤150.0 |
| Arsenic | (mg·kg−1) | 0.76 | 0.37 | 0.32 | 0.22 | 0.35 | 0.31 | ≤5.0 | ≤13.0 |
| Chromium | (mg·kg−1) | 56.1 | 48.3 | 15.8 | 20.5 | 35.2 | 44.1 | ≤70.0 |
| Analysis Item | Unit | 300 °C 30 Min | 400 °C 30 Min | 500 °C 30 Min | Clean Area Standard | |
|---|---|---|---|---|---|---|
| Organic matter | Biochemical oxygen demand | mg/L | 999.00 | 141.00 | 118.00 | ≤30 |
| Total organic carbon | mg/L | 859.00 | 64.20 | 43.20 | ≤25 | |
| Suspended solids | mg/L | 75.00 | 23.50 | 11.60 | ≤30 | |
| Total nitrogen | mg/L | 69.40 | 13.00 | 8.83 | ≤30 | |
| Total phosphorus | mg/L | 13.40 | 1.92 | 0.73 | ≤4.0 | |
| VOCs | Fluorine | mg/L | ND | ND | ND | ≤3 |
| Trichloroethylene | mg/L | ND | ND | ND | ≤0.06 | |
| Tetrachloroethylene | mg/L | ND | ND | ND | ≤0.02 | |
| Dichloromethane | mg/L | ND | ND | ND | ≤0.02 | |
| Benzene | mg/L | ND | ND | ND | ≤0.01 | |
| Carbon tetrachloride | mg/L | ND | ND | ND | ≤0.004 | |
| 1,1-dichloroethylene | mg/L | ND | ND | ND | ≤0.03 | |
| 1,2-dichloroethane | mg/L | ND | ND | ND | ≤0.03 | |
| Chloroform | mg/L | ND | ND | ND | ≤0.08 | |
| 1,4-dioxane | mg/L | ND | ND | ND | ≤0.05 | |
| Vinyl chloride | mg/L | ND | ND | ND | ≤0.01 | |
| Acrylonitrile | mg/L | ND | ND | ND | ≤0.01 | |
| Bromoform | mg/L | ND | ND | ND | ≤0.03 | |
| Naphthalene | mg/L | ND | ND | ND | ≤0.05 | |
| Formaldehyde | mg/L | 0.10 | 0.03 | 0.04 | ≤0.5 | |
| Toluene | mg/L | ND | ND | ND | ≤0.7 | |
| Xylene | mg/L | ND | ND | ND | ≤0.5 | |
| Styrene | mg/L | ND | ND | ND | ≤0.02 | |
| Ionic substances | Chloride ion, Cl− | mg/L | 374.00 | 422.00 | 621.00 | - |
| Sulfate ion, SO42− | mg/L | 44.50 | 16.10 | 14.50 | - | |
| Phosphate ion, PO43− | mg/L | 33.40 | 4.70 | 1.70 | ||
| Proximate Analysis (wt. %) | Ultimate Analysis (wt. %) | Others | HHV (MJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile Matter | Ash | Fixed Carbon | C | H | O | N | S | Cl | ||
| 7.80 | 72.21 | 8.89 | 11.10 | 46.43 | 6.92 | 3.18 | 32.89 | 0.31 | 1.61 | 6.16 | 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, K.-H.; Shin, D.-C.; Lee, Y.-E.; Jeong, Y.; Jung, J.; Kim, I.-T. Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion. Molecules 2023, 28, 6114. https://doi.org/10.3390/molecules28166114
Ahn K-H, Shin D-C, Lee Y-E, Jeong Y, Jung J, Kim I-T. Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion. Molecules. 2023; 28(16):6114. https://doi.org/10.3390/molecules28166114
Chicago/Turabian StyleAhn, Kwang-Ho, Dong-Chul Shin, Ye-Eun Lee, Yoonah Jeong, Jinhong Jung, and I-Tae Kim. 2023. "Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion" Molecules 28, no. 16: 6114. https://doi.org/10.3390/molecules28166114
APA StyleAhn, K.-H., Shin, D.-C., Lee, Y.-E., Jeong, Y., Jung, J., & Kim, I.-T. (2023). Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion. Molecules, 28(16), 6114. https://doi.org/10.3390/molecules28166114






