Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Docking Study
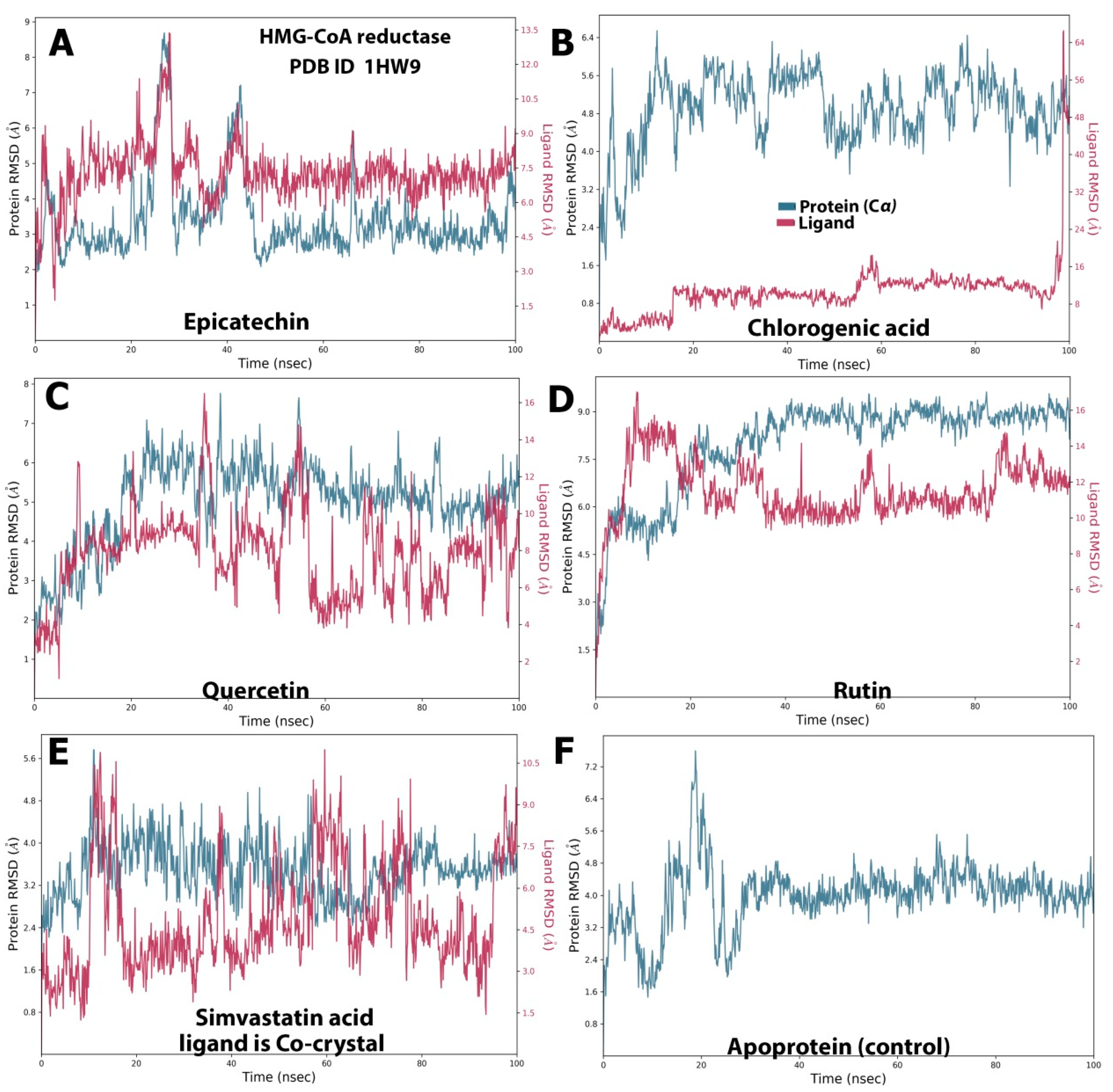
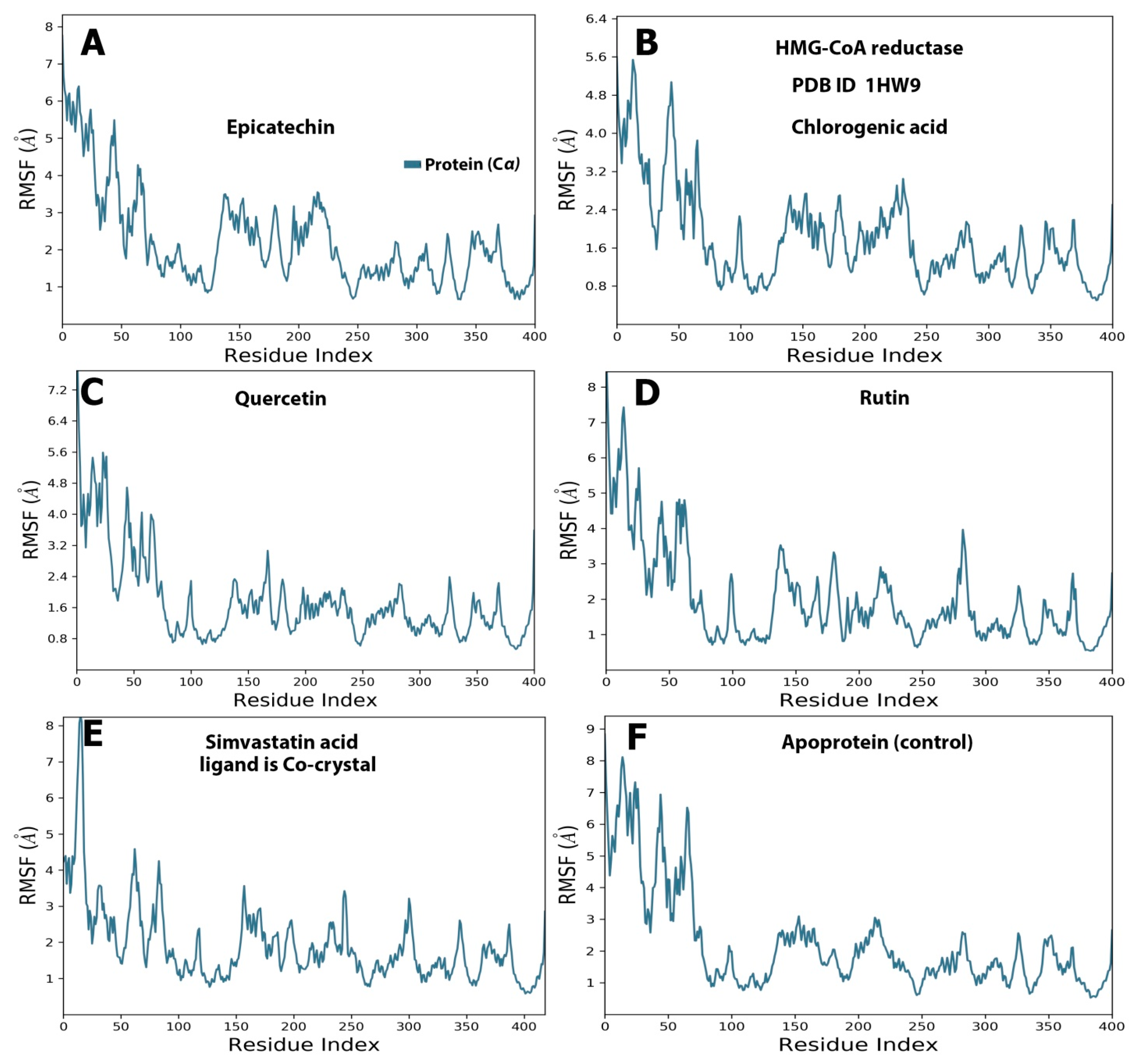
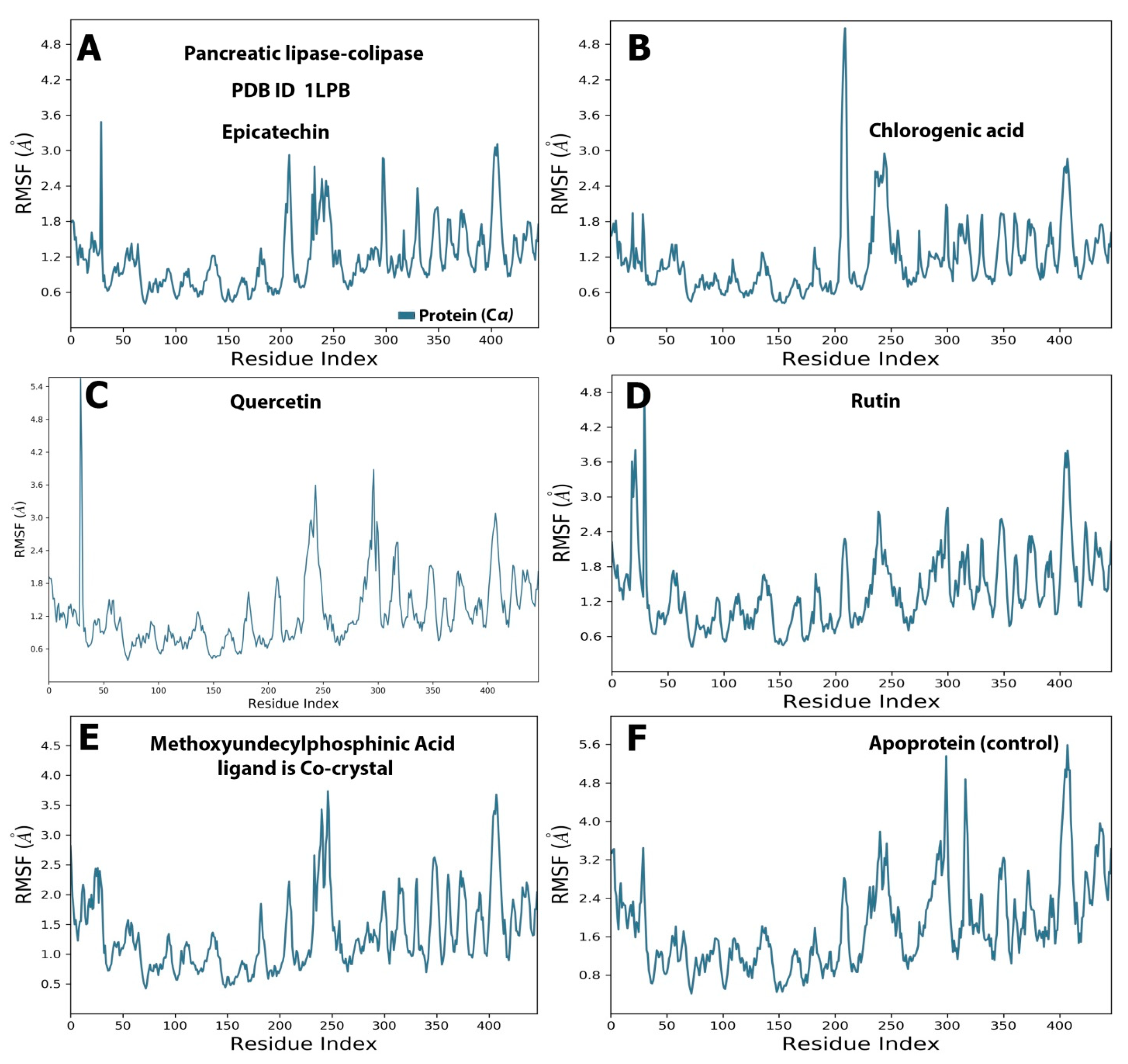
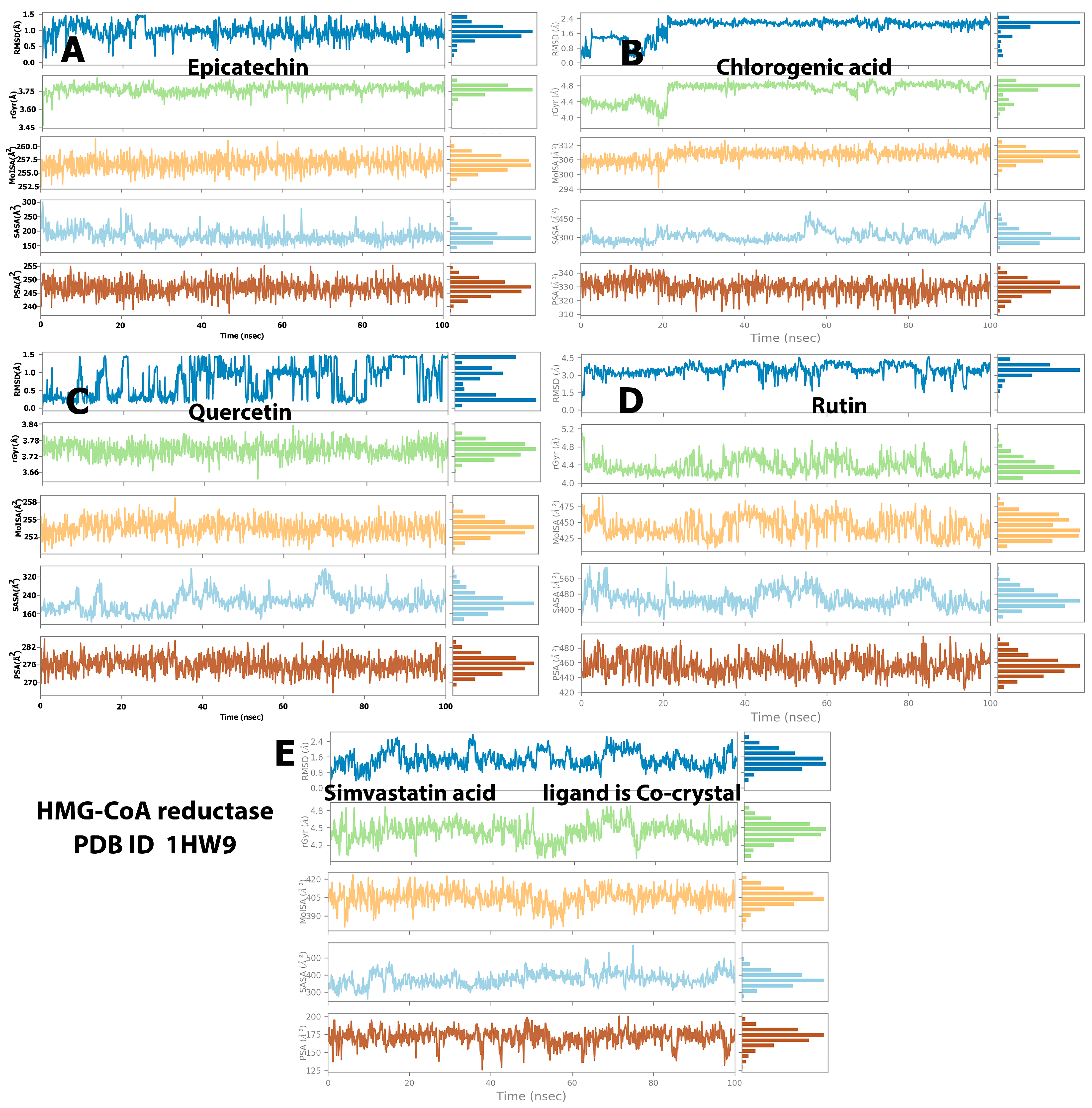
2.2. MD Simulation Study
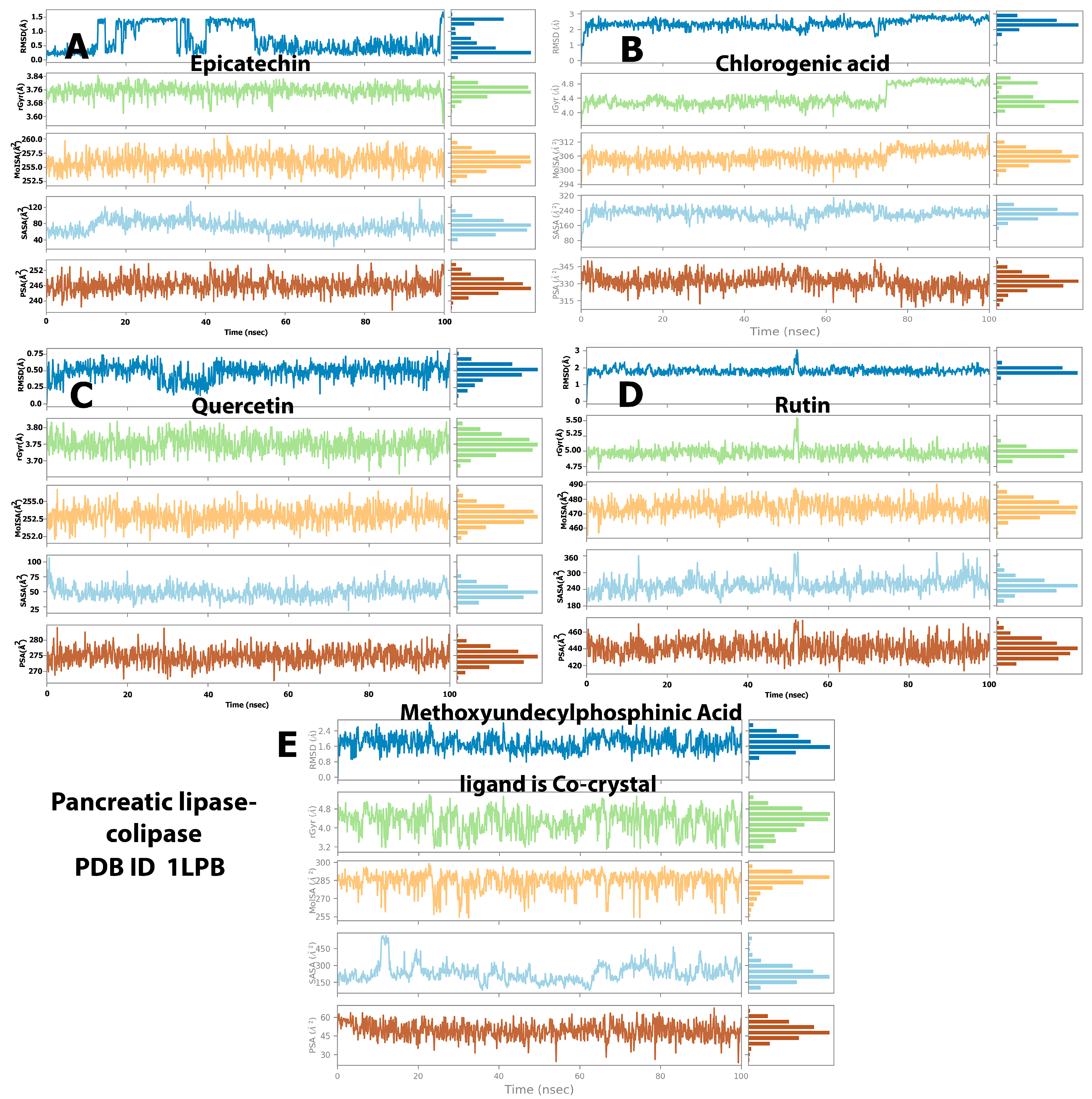
2.3. Stability of Protein–Ligand Complexes
2.4. Protein and Ligand Properties from MD Simulation Analysis
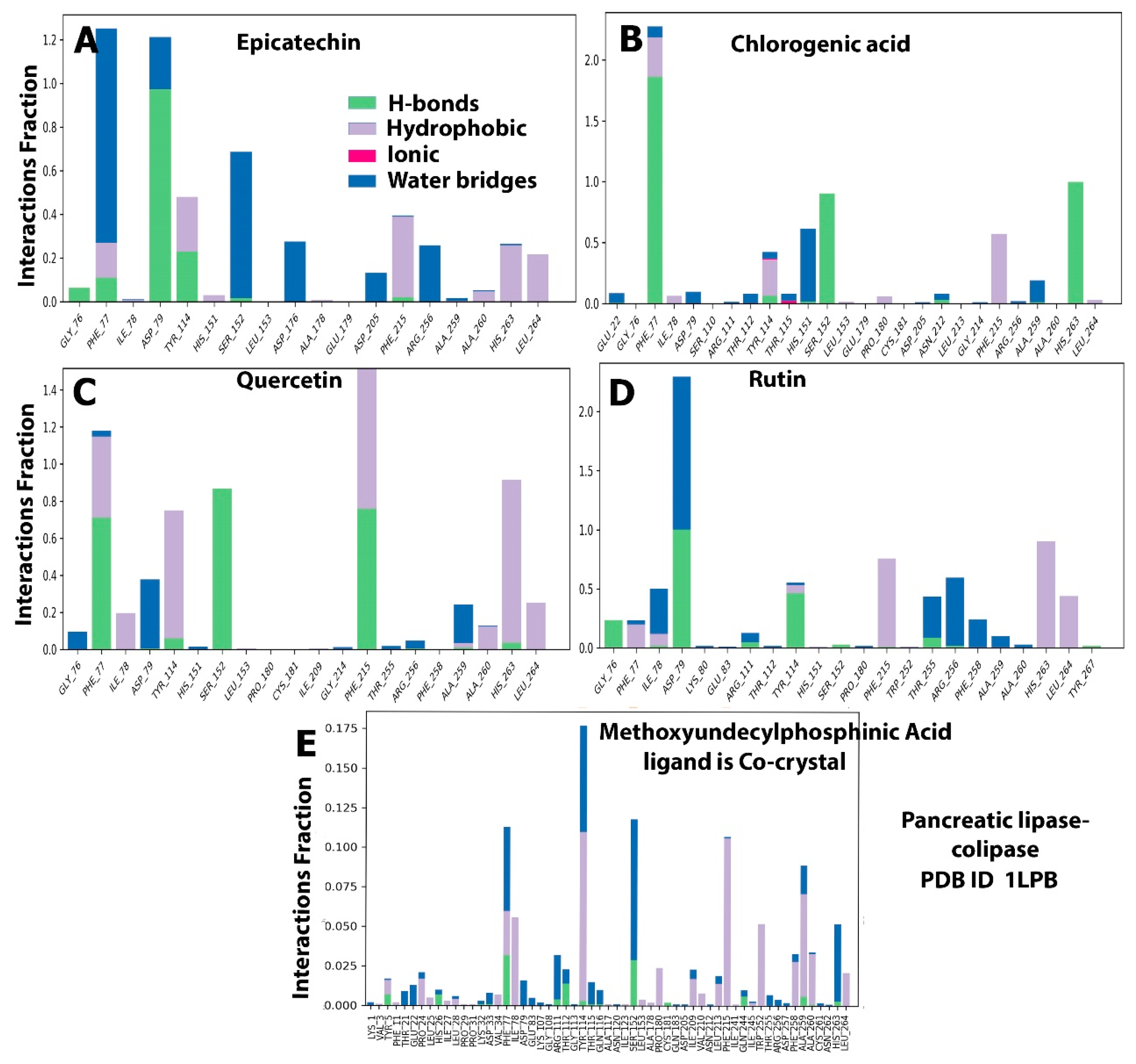
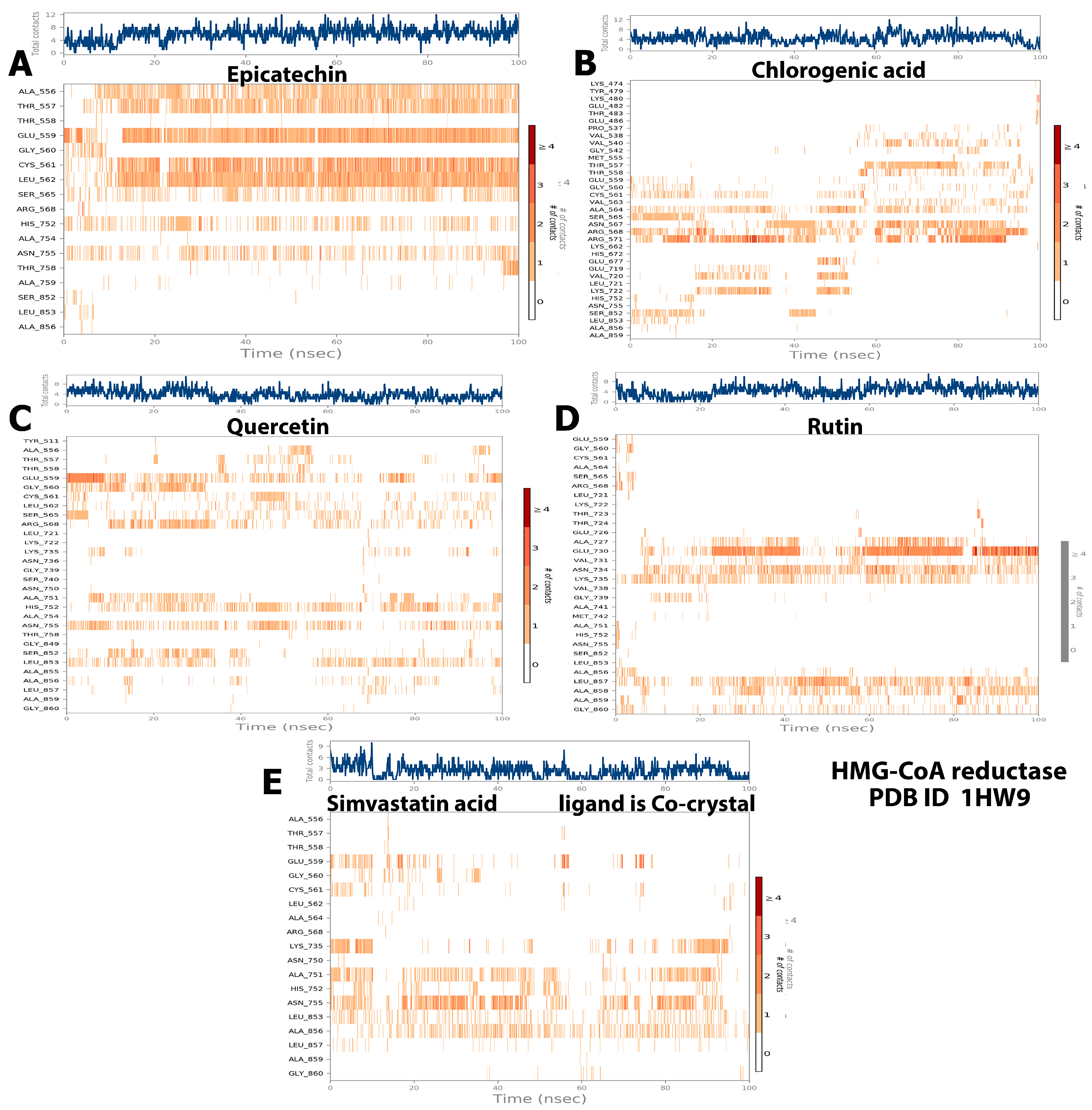
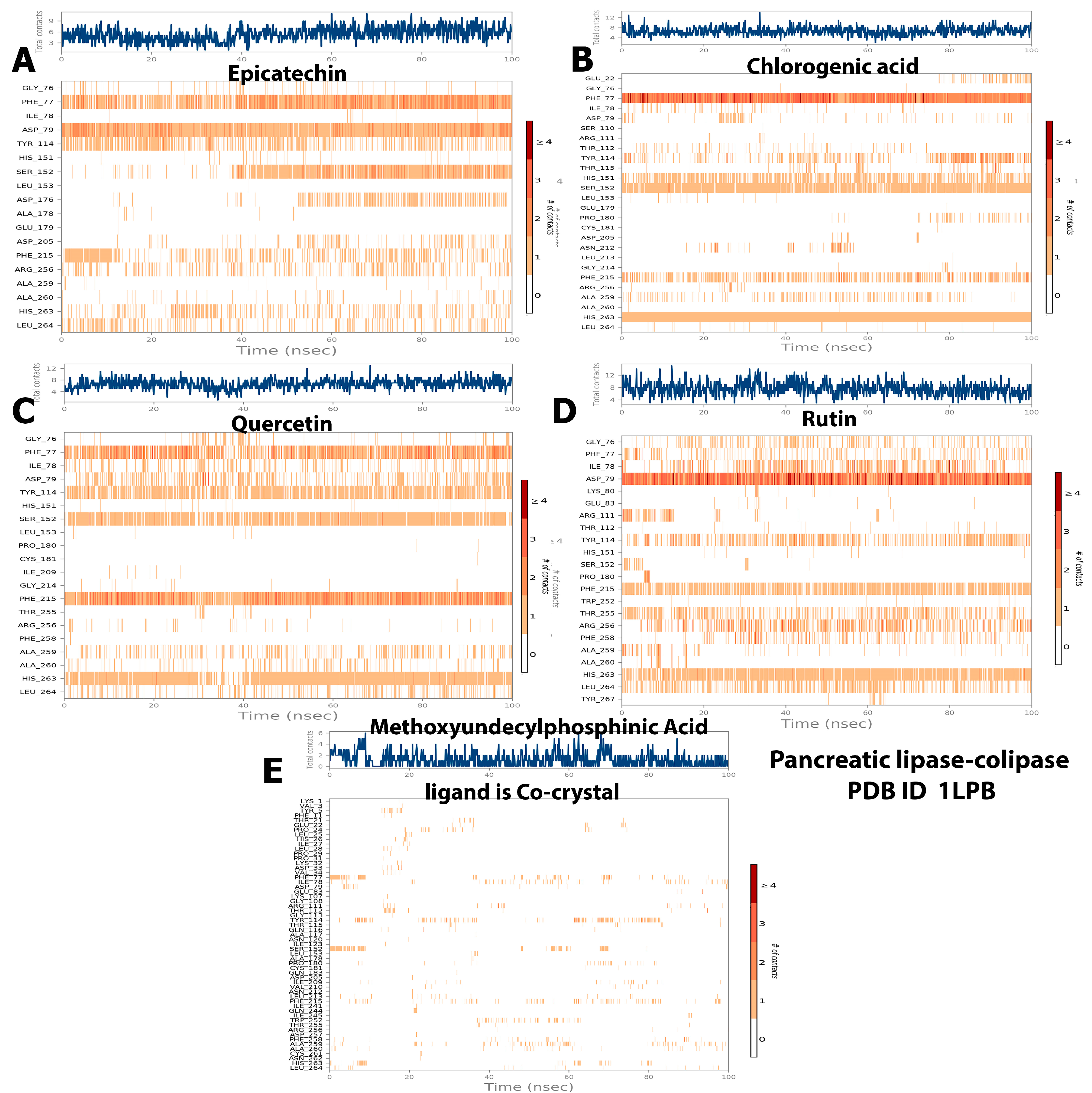
2.5. Protein–Ligand Interaction and Bond Formation
3. Methodology
3.1. Protein and Ligand Preparation and Docking
3.2. MD Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Bukhari, S.M.A.; Herrera, E.C.; Awuah, W.A.; Lawrence, J.; de Andrade, H.; Patel, N.; Shah, R.; Shaikh, R.; Capriles, C.A.A.; et al. Lipid Lowering Therapy: An Era Beyond Statins. Curr. Probl. Cardiol. 2022, 47, 101342. [Google Scholar] [CrossRef]
- Salhi, A.; Carriere, F.; Grundy, M.M.L.; Aloulou, A. Enzymes involved in lipid digestion. In Bioaccessibility and Digestibility of Lipids from Food; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–28. [Google Scholar]
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.M. Managing hyperlipidemia: Current and future roles of HMG-CoA reductase inhibitors. Am. J. Health Syst. Pharm 2002, 59, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Veeramachaneni, G.K.; Raj, K.K.; Chalasani, L.M.; Annamraju, S.K.; Js, B.; Talluri, V.R. Shape based virtual screening and molecular docking towards designing novel pancreatic lipase inhibitors. Bioinformation 2015, 11, 535–542. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Vande Griend, J.P.; Linnebur, S.A. FDA drug safety communications: A narrative review and clinical considerations for older adults. Am. J. Geriatr. Pharmacother 2012, 10, 264–271. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, L.; Zhang, N.; Zhou, J.; Zhang, L.; Wu, W.; Ji, B.; Zhou, F. Bioactivity of dietary polyphenols: The role in LDL-C lowering. Foods 2021, 10, 2666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Cheng, Q.; Ji, B.; Yang, M.; Sanidad, K.Z.; Wang, C.; Zhou, F. Structurally Different Flavonoid Subclasses Attenuate High-Fat and High-Fructose Diet Induced Metabolic Syndrome in Rats. J. Agric. Food Chem. 2018, 66, 12412–12420. [Google Scholar] [CrossRef]
- Islam, B.; Sharma, C.; Adem, A.; Aburawi, E.; Ojha, S. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug Des. Devel. Ther. 2015, 9, 4943–4951. [Google Scholar]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical Profile, Antioxidant and Antidiabetic Activities of Adansonia digitata L. (Baobab) from Mali, as a Source of Health-Promoting Compounds. Molecules 2018, 23, 3104. [Google Scholar] [CrossRef]
- El Yahyaoui, O.; Bouabid, B.; Ait Ouaaziz, N.; El Bakkali, M.; El Harche, H.; Lrhorfi, L.A.; Nakari, K. The antibacterial activity and biochemical composition of Adansonia Digitata edible parts. Arab. Gulf. J. Sci. Res. 2023, 41, 91–106. [Google Scholar] [CrossRef]
- Fatema, S.; Rode, P.; Jadhav, S.B.; Farooqui, M. Anti-inflammatory and analgesic study of fibrous part of Adansonia digitatafruit using microwave extraction techniques. Der. Pharm. Lett. 2015, 7, 341–347. [Google Scholar]
- Rana, H.; Kumar, R.; Chopra, A.; Pundir, S.; Kumar Gautam, G.; Kumar, G. The Various Pharmacological Activity of Adansonia digitata. Res. J. Pharmacol. Pharmacodyn. 2022, 5, 53–59. [Google Scholar] [CrossRef]
- Arbianti, R.; Larasati, A.; Utami, T.S.; Muharam, Y.; Slamet, S. Molecular docking bioactive compound from Strobilanthes crispus to decrease cholesterol levels. AIP Conf. Proc. 2021, 23, 2376. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Krupanidhi, S.; Abraham Peele, K.; Venkateswarulu, T.C.; Ayyagari, V.S.; Nazneen Bobby, M.; John Babu, D.; Venkata Narayana, A.; Aishwarya, G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2: An in silico study. J. Biomol. Struct. Dyn. 2020, 39, 5799–5803. [Google Scholar] [CrossRef]
- Samad, A.; Huq, M.A.; Rahman, M.S. Bioinformatics approaches identified dasatinib and bortezomib inhibit the activity of MCM7 protein as a potential treatment against human cancer. Sci. Rep. 2022, 12, 1539. [Google Scholar] [CrossRef]
- Ononamadu, C.J.; Abdalla, M.; Ihegboro, G.O.; Li, J.; Owolarafe, T.A.; John, T.D.; Tian, Q. In silico identification and study of potential anti-mosquito juvenile hormone binding protein (MJHBP) compounds as candidates for dengue virus—Vector insecticides. Biochem. Bioph. Rep. 2021, 28, 101178. [Google Scholar] [CrossRef]
- Riyad, P.; Purohit, A.; Sen, K.; Panwar, A.; Ram, H. HMG–CoA reductase inhibition mediated hypocholesterolemic potential of myricetin and quercetin: In-silico and in-vivo studies. CYTA–J. Food 2023, 21, 115–125. [Google Scholar] [CrossRef]
- Abdalla, M.; Eltayb, W.A.; El-Arabey, A.A.; Singh, K.; Jiang, X. Molecular dynamic study of SARS-CoV-2 with various S protein mutations and their effect on thermodynamic properties. Comput. Biol. Med. 2022, 141, 105025. [Google Scholar] [CrossRef]
- Radwan, A.; Mahrous, G.M. Docking studies and molecular dynamics simulations of the binding characteristics of waldiomycin and its methyl ester analog to Staphylococcus aureus histidine kinase. PLoS ONE 2020, 15, 234215. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Alam, S.; Rajib, T.K.; Islam, S.S.; Araf, Y.; Ullah, M.A. Identification of the most potent acetylcholinesterase inhibitors from plants for possible treatment of Alzheimer’s disease: A computational approach. Egypt J. Med. Hum. Genet. 2021, 22, 10. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Nandagopal, B.; Anbarasu, A. Natural Inhibitors of HMG-CoA Reductase—An Insilico Approach Through Molecular Docking and Simulation Studies. J. Cell Biochem. 2017, 118, 52–57. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference Supercomput SC’06, 2006, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
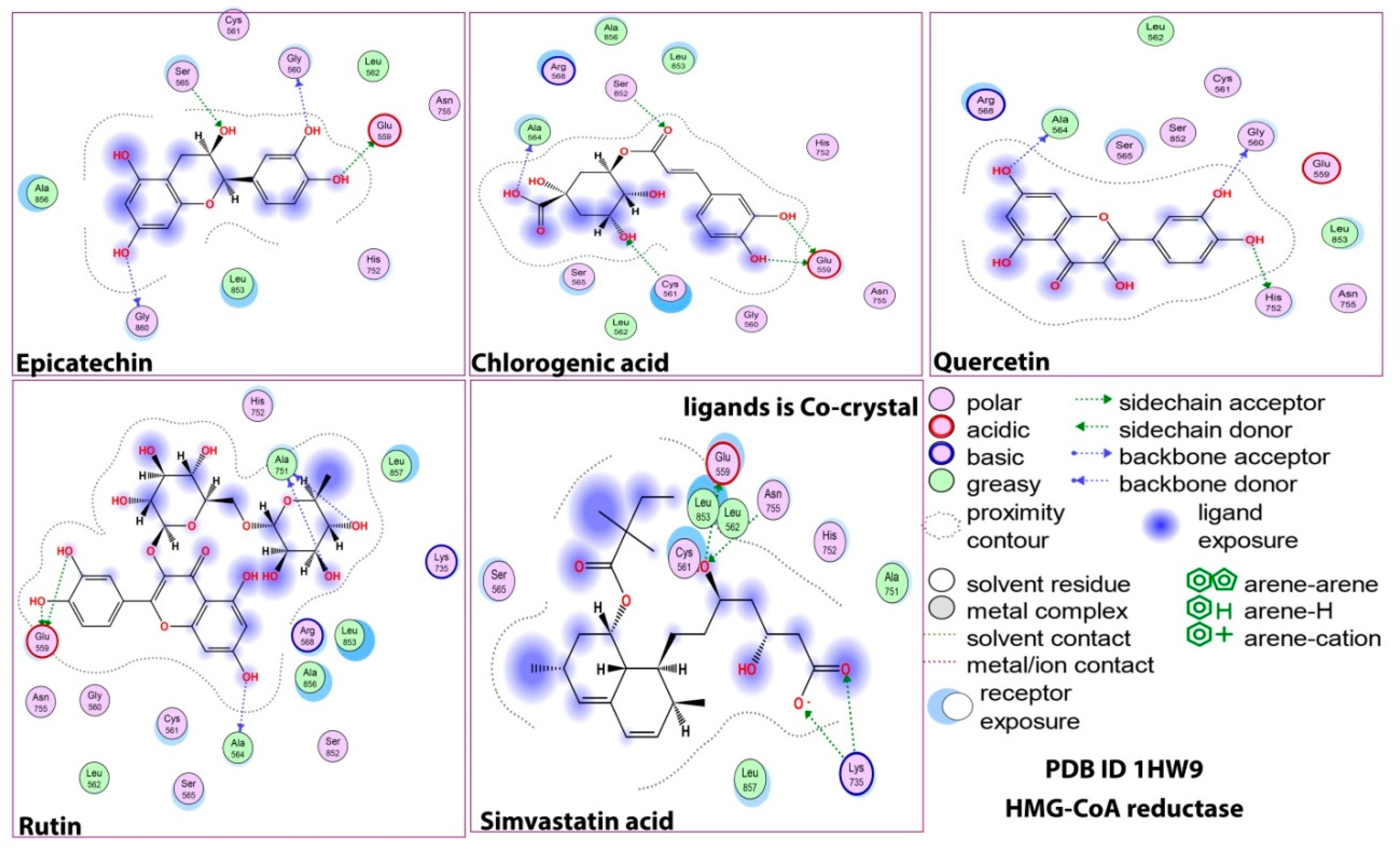
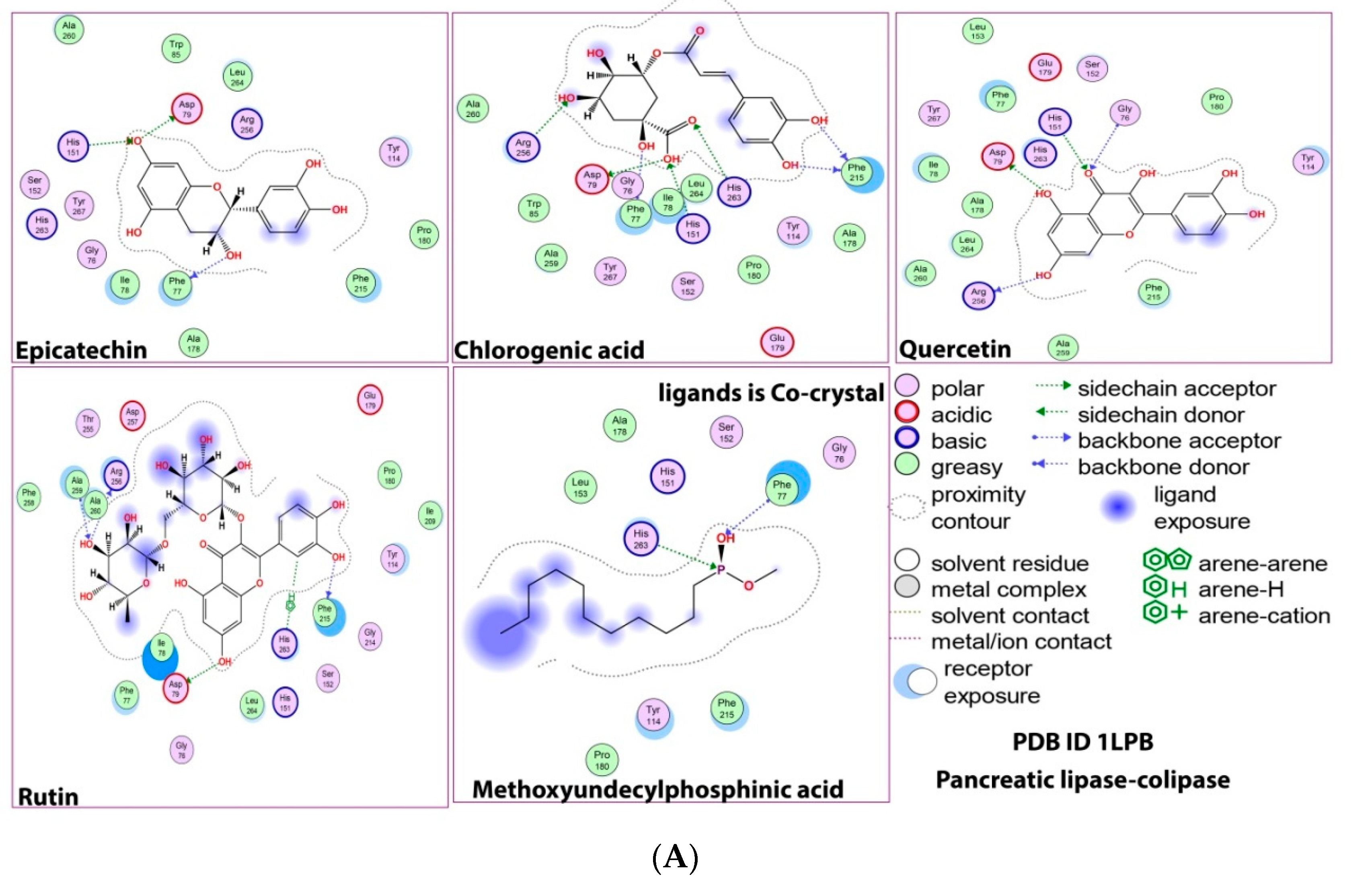
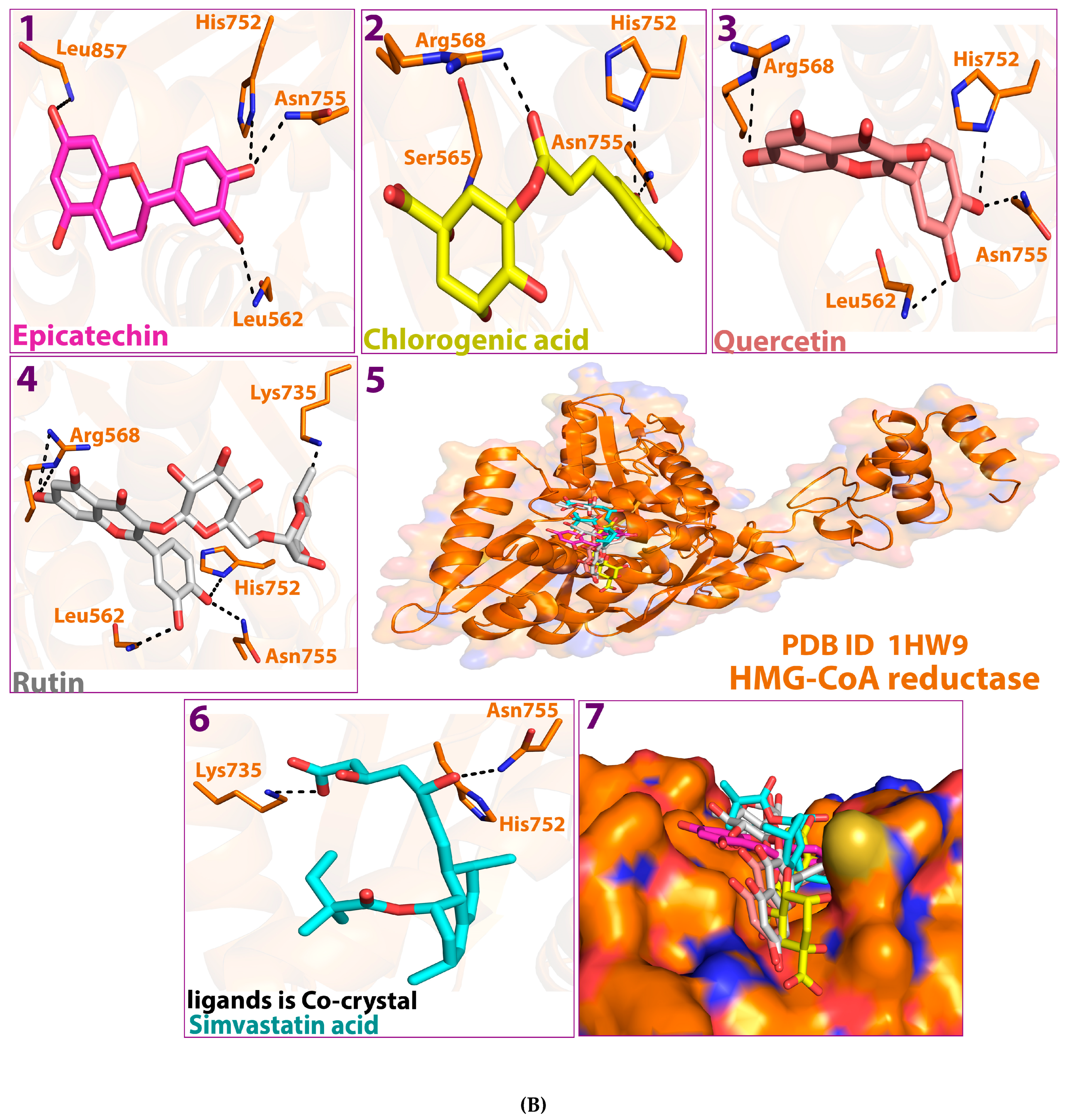
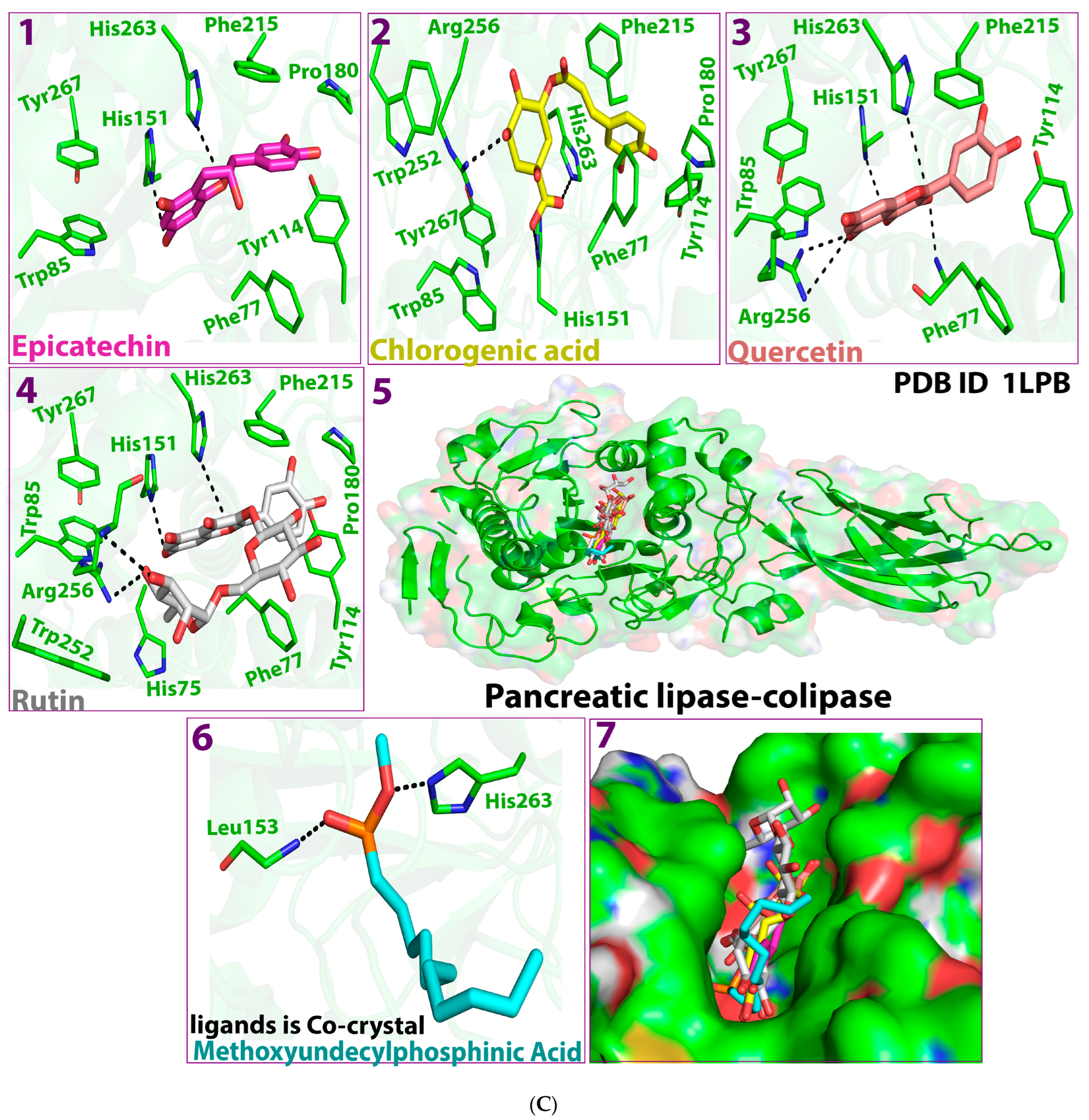


| Ligands | Affinity of the Binding (ΔG) kcal/mol against HMG-CoA Reductase | Interacting Amino Acid Residue(s) in HMG-CoA Reductase | Affinity of Binding (ΔG) kcal/mol against Pancreatic Lipase | Residue(s) of the Interacting Amino Acid in Pancreatic Lipase |
|---|---|---|---|---|
| Epicatechin | −5.5 | GLY560, GLU559, GLY860, SER565 | −9.4 | PHE77, ASP79, HIS151 |
| Chlorogenic acid | −5.7 | GLU559, ALA564, SER852, CYS561 | −8.6 | PHE215, PHE77, ASP79, HIS263, HIS151 ARG256 |
| Quercetin | −6.2 | GLY560, HIS752, ALA564 | −9.8 | ASP79, ARG256, GLY76, HIS151 |
| Rutin | −6.5 | GLU559, ALA564, ALA751 | −8.7 | PHE215, ASP 9, ARG256, ALA259, HIS263 |
| HMG-CoA cocrystal control | −5.2 | GLU559, LYS735, ASN755 | - | - |
| Pancreatic lipase cocrystal control MUP | - | −5.5 | HIS263, PHE77 |
| (A) | ||||||||
| Compounds | MMGBSA dG Bind (Kcal/ mol) | MMGBSA dG Bind Coulomb (kcal/mol) | MMGBSA dG Bind Covalent (kcal/mol) | MMGBSA dG Bind H-bond (kcal/mol) | MMGBSA dG Bind Lipo (kcal/mol) | MMGBSA dG Bind Packing (kcal/mol) | MMGBSA dG Bind Solv GB (kcal/mol) | MMGBSA dG Bind vdW (kcal/mol) |
| Epicatechin | −41.241 | −32.0133 | 0.917075 | −2.49725 | −10.038 | −1.6385 | 28.47324 | −24.445 |
| Chlorogenic acid | −14.084 | −11.7143 | 2.027484 | −1.28119 | −5.7528 | 24.95107 | −22.3151 | −14.0848 |
| Quercetin | −25.014 | −11.6902 | 1.259447 | −1.39654 | −4.0198 | −2.91924 | 15.03979 | −21.2879 |
| Rutin | −42.749 | −24.3292 | 2.892599 | −2.17707 | −8.19377 | −2.98948 | 23.46127 | −31.4134 |
| Simvastatin | −31.62 | 26.47115 | 2.267489 | −0.53712 | −10.942 | −20.7549 | −28.132 | |
| (B) | ||||||||
| Compounds | MMGBSA dG Bind (kcal/mol) | MMGBSA dG Bind Coulomb (kcal/mol) | MMGBSA dG Bind Covalent (kcal/mol) | MMGBSA dG Bind H-bond (kcal/mol) | MMGBSA dG Bind Lipo (kcal/mol) | MMGBSA dG Bind Packing (kcal/mol) | MMGBSA dG Bind Solv GB (kcal/mol) | MMGBSA dG Bind vdW (kcal/mol) |
| Epicatechin | −34.773 | −10.016 | 3.09688 | −1.77298 | −17.455 | −1.51529 | 27.2685 | −34.3795 |
| Chlorogenic acid | −43.4258 | −14.151 | 1.08367 | 18.8239 | −27.5483 | |||
| Quercetin | −57.9291 | −18.634 | 1.8993 | −1.599 | −17.0719 | −6.32072 | 28.23726 | −42.5352 |
| Rutin | −52.1559 | −7.02153 | 0.030699 | −2.05604 | −18.4954 | −3.51286 | 28.33565 | −49.4365 |
| MUP | −21.108 | −0.5151 | 4.030 | −16.112 | 17.85074 | −21.09654 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alameen, A.A.; Alothman, M.R.; Al Wahibi, M.S.; Abdullah, E.M.; Ali, R.; Abdalla, M.; Fattiny, S.Z.A.; Elsayim, R. Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study. Molecules 2023, 28, 6112. https://doi.org/10.3390/molecules28166112
Alameen AA, Alothman MR, Al Wahibi MS, Abdullah EM, Ali R, Abdalla M, Fattiny SZA, Elsayim R. Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study. Molecules. 2023; 28(16):6112. https://doi.org/10.3390/molecules28166112
Chicago/Turabian StyleAlameen, Alaa Alnoor, Monerah R. Alothman, Mona S. Al Wahibi, Ejlal Mohamed Abdullah, Rehab Ali, Mohnad Abdalla, Sndos Z. A. Fattiny, and Rasha Elsayim. 2023. "Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study" Molecules 28, no. 16: 6112. https://doi.org/10.3390/molecules28166112
APA StyleAlameen, A. A., Alothman, M. R., Al Wahibi, M. S., Abdullah, E. M., Ali, R., Abdalla, M., Fattiny, S. Z. A., & Elsayim, R. (2023). Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study. Molecules, 28(16), 6112. https://doi.org/10.3390/molecules28166112







