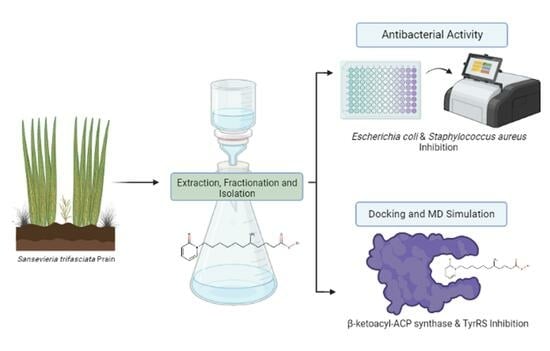
Antibacterial Potency of an Active Compound from Sansevieria trifasciata Prain: An Integrated In Vitro and In Silico Study
Abstract
:1. Introduction
2. Results
2.1. Maceration and Isolation
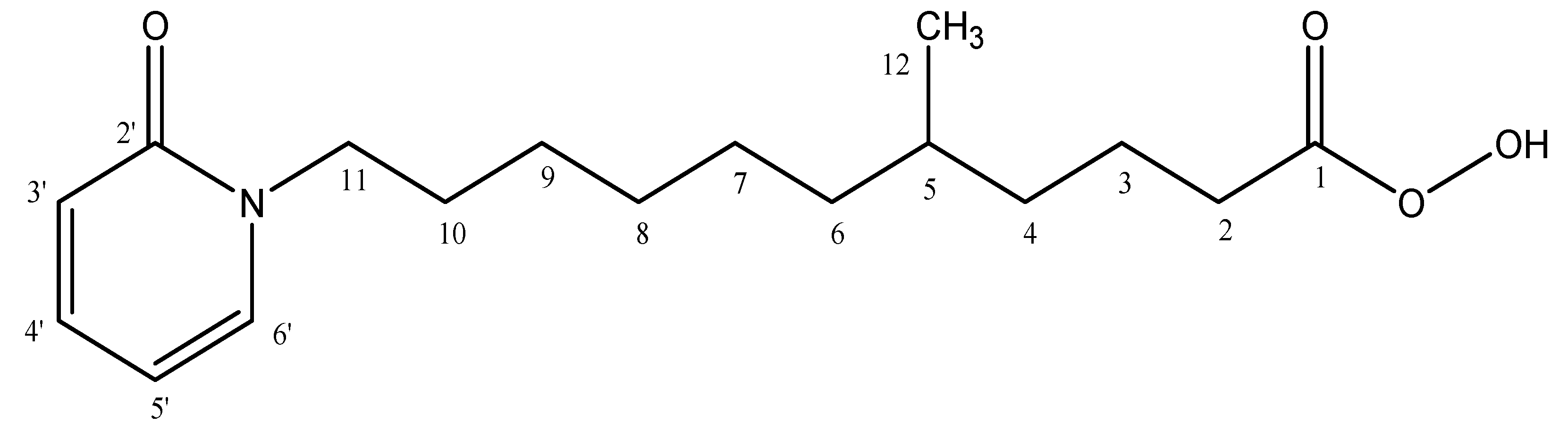
2.2. Characterization of 5-Methyl-11-(2-oxopyridin-1(2H)-yl)undecaneperoxoicacid Compound
2.3. Computational Study
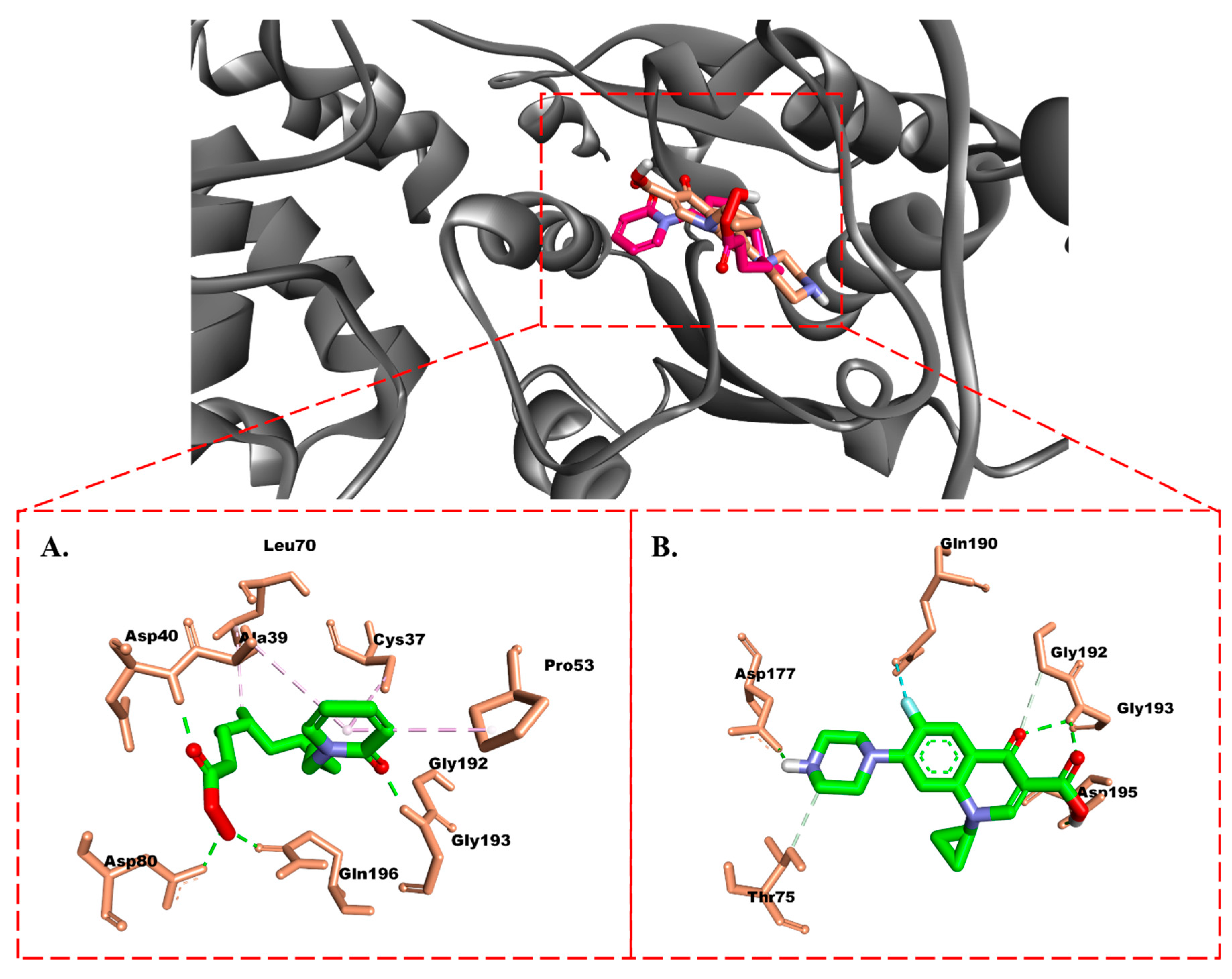
2.3.1. Molecular Docking
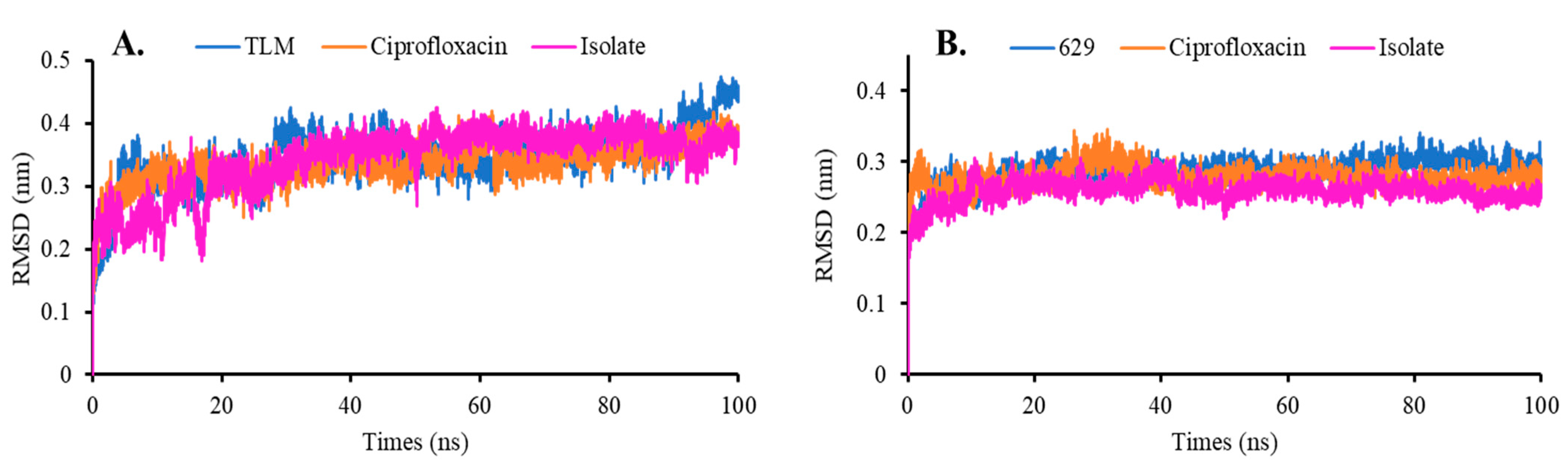
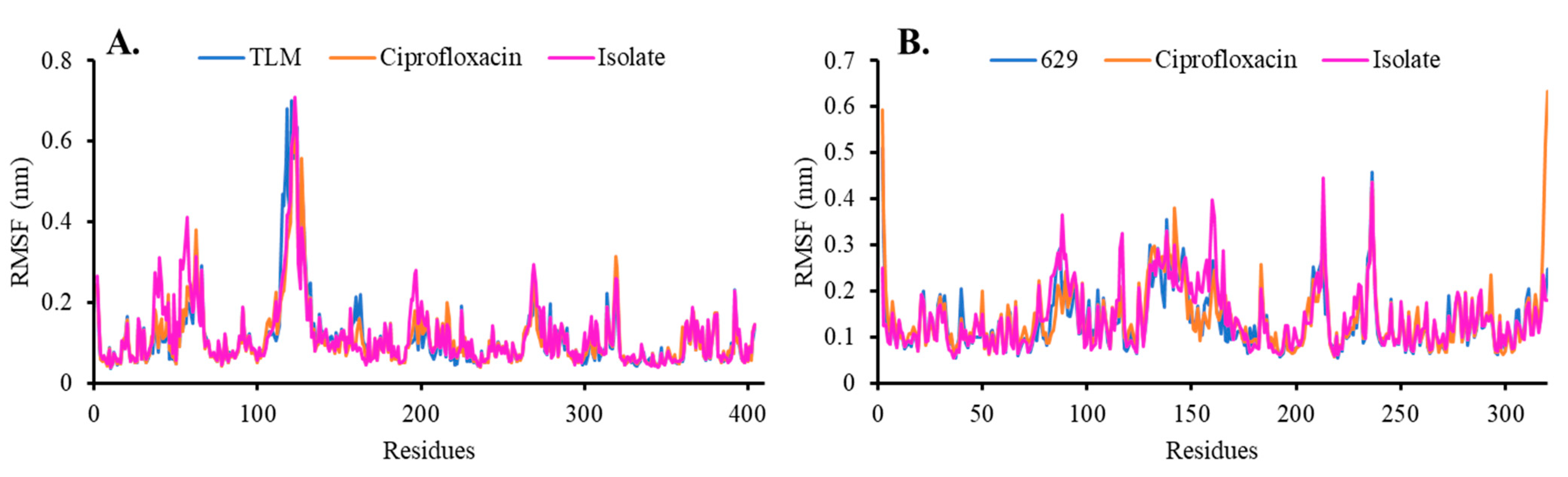
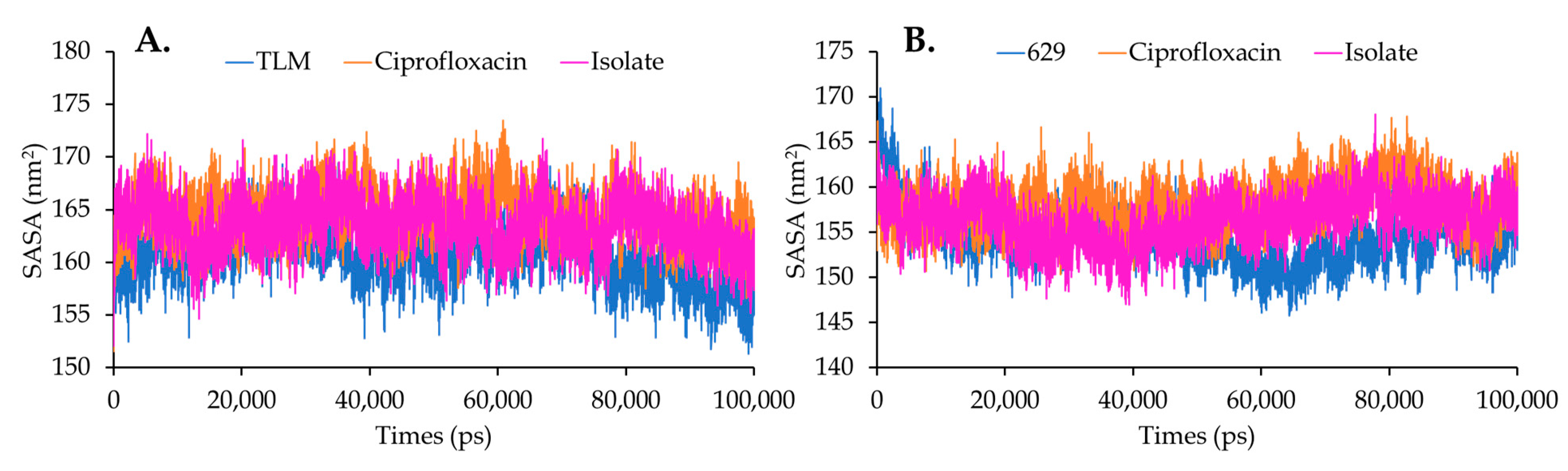
2.3.2. Molecular Dynamics Simulation
Root Mean Square Deviation and Fluctuation (RMSD and RMSF)
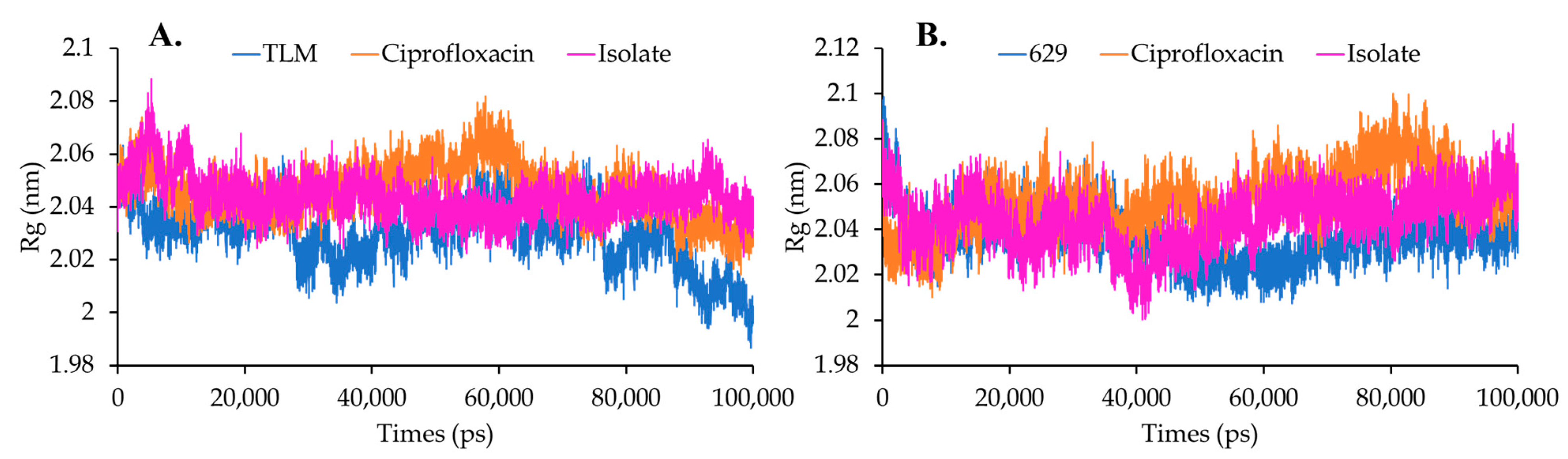
Solvent Accessible Surface Area (SASA) and Radius Gyration (Rg)
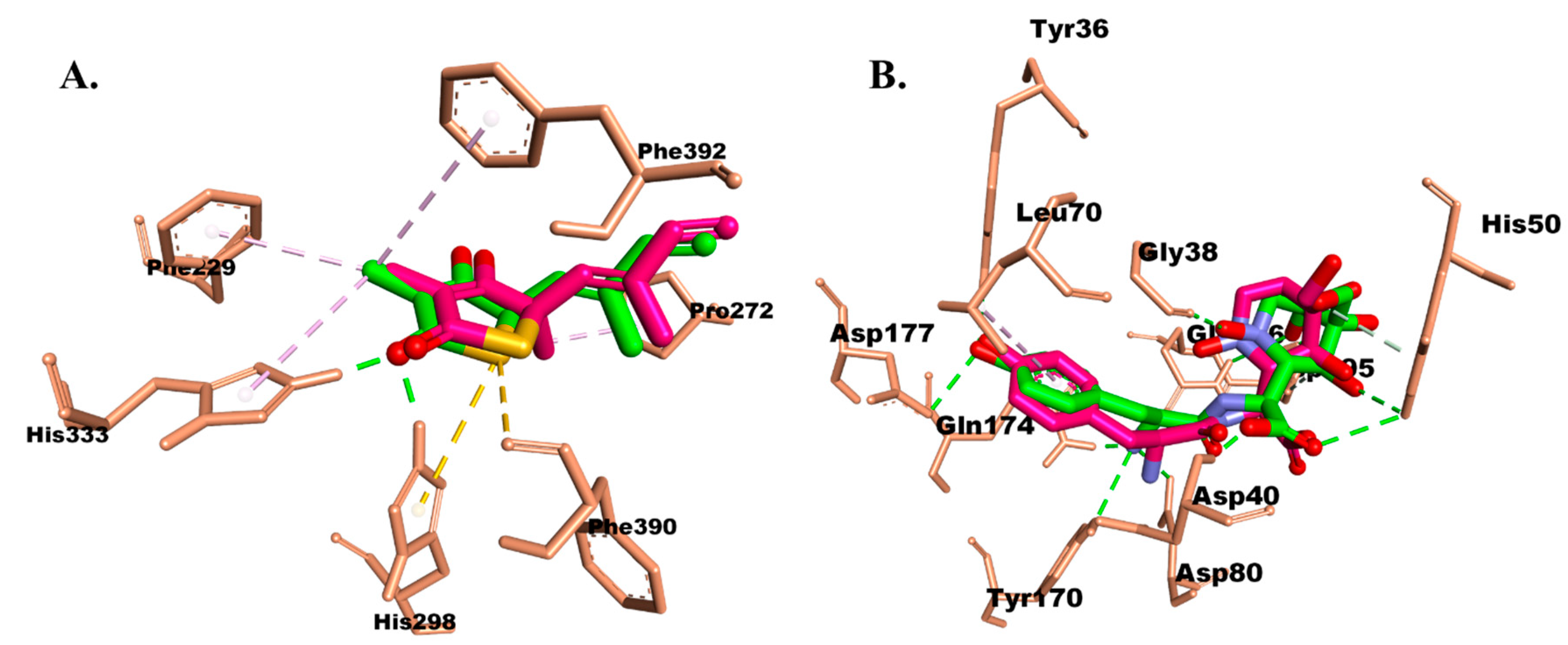
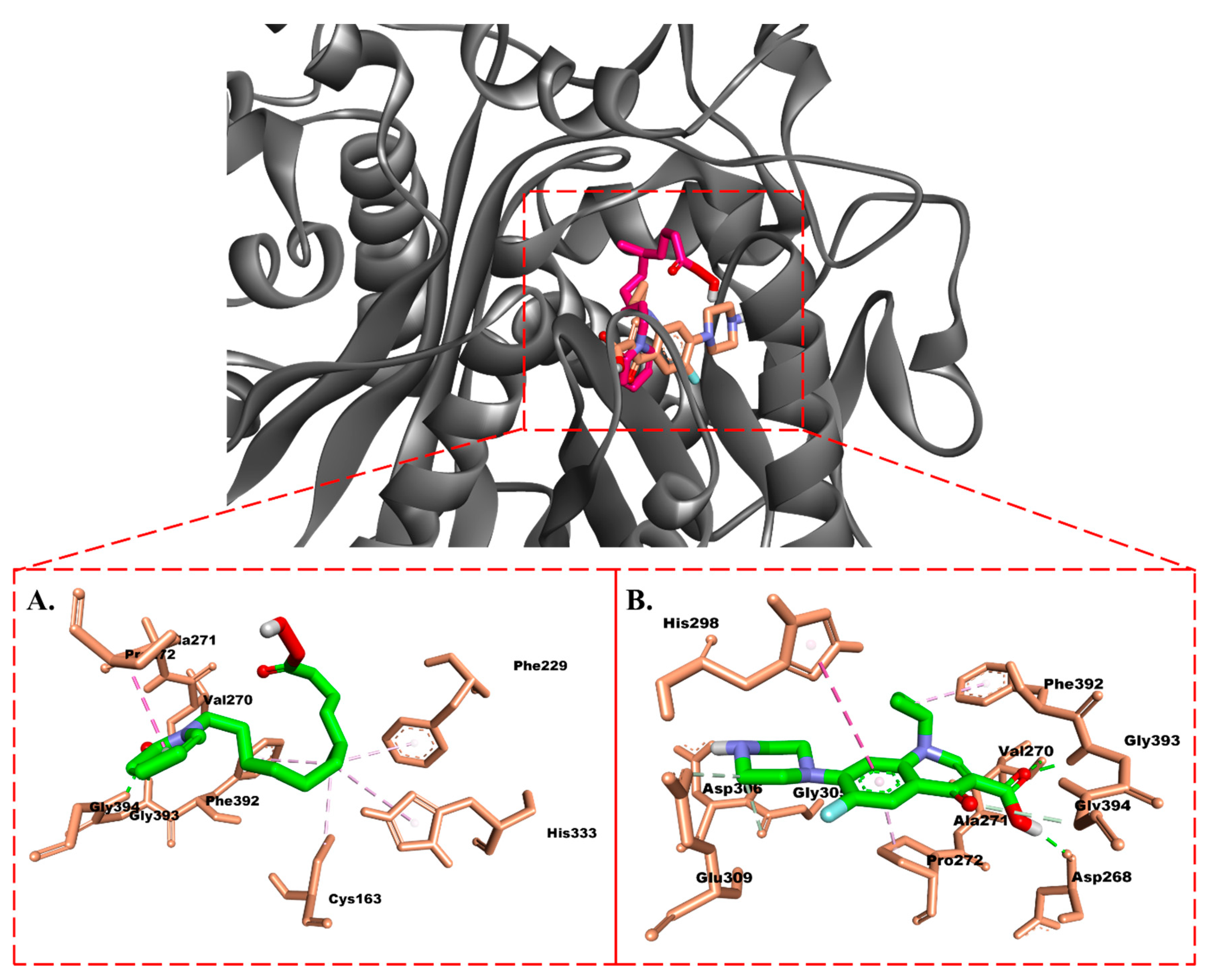
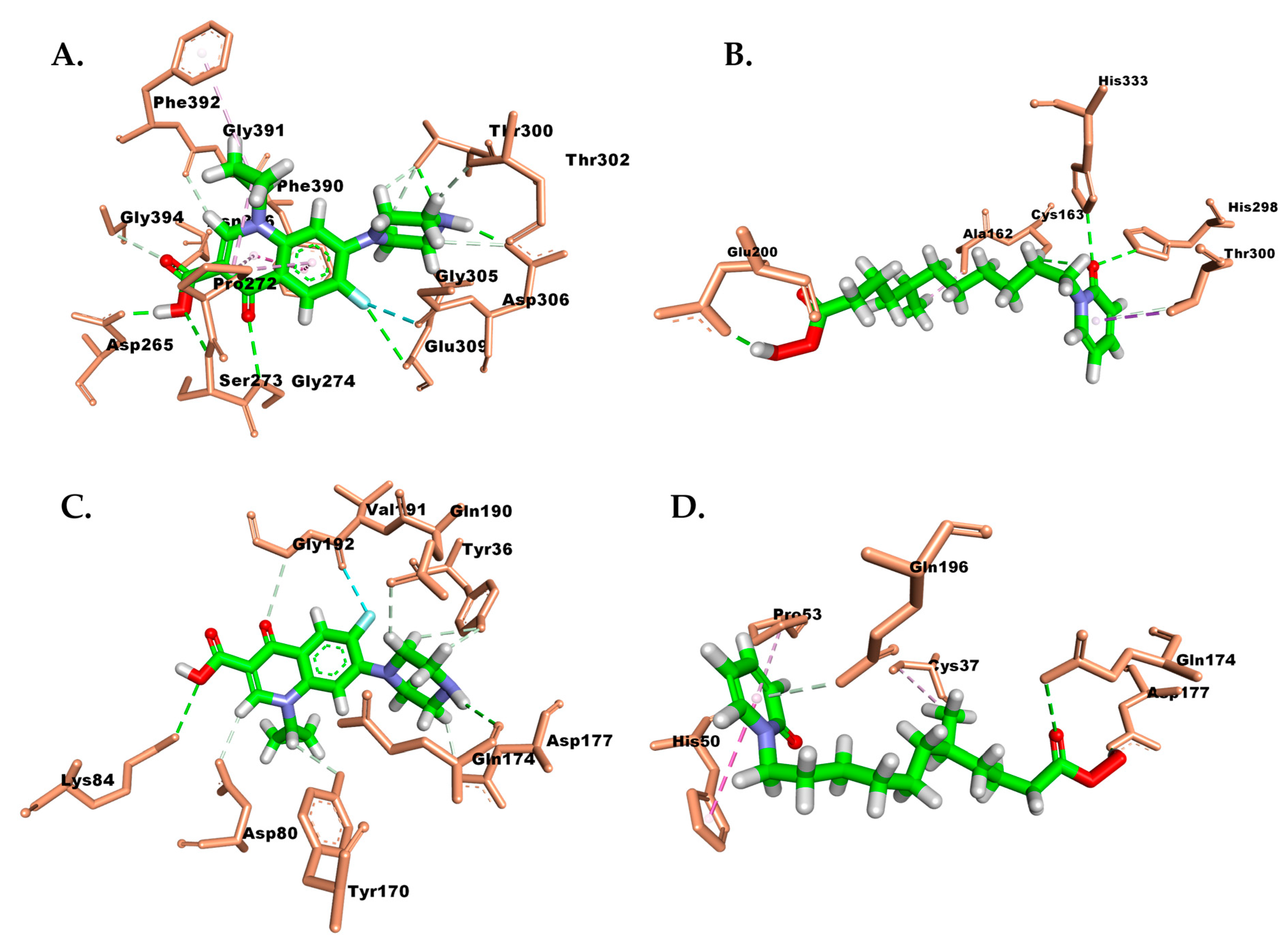
Protein–Ligand Interaction
Binding Free-Energy Enzyme Compounds
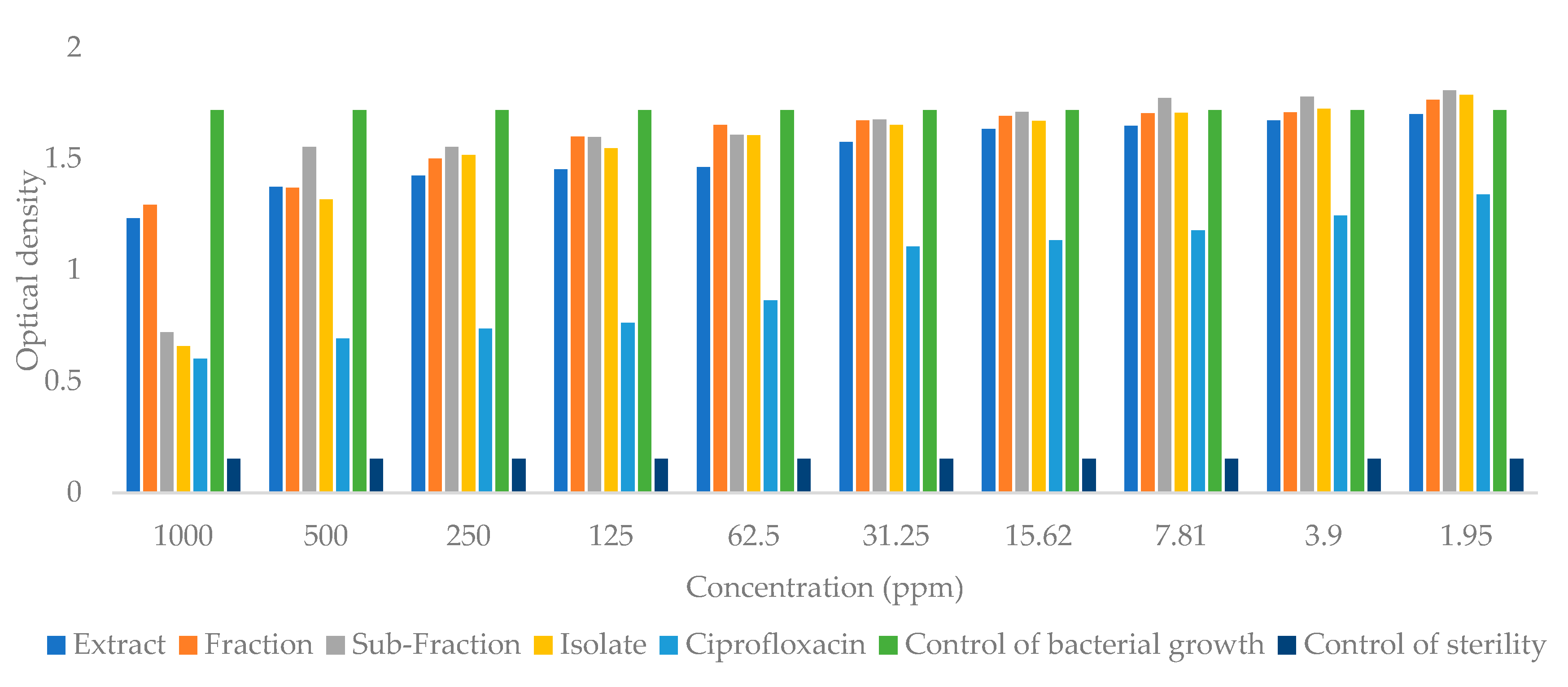
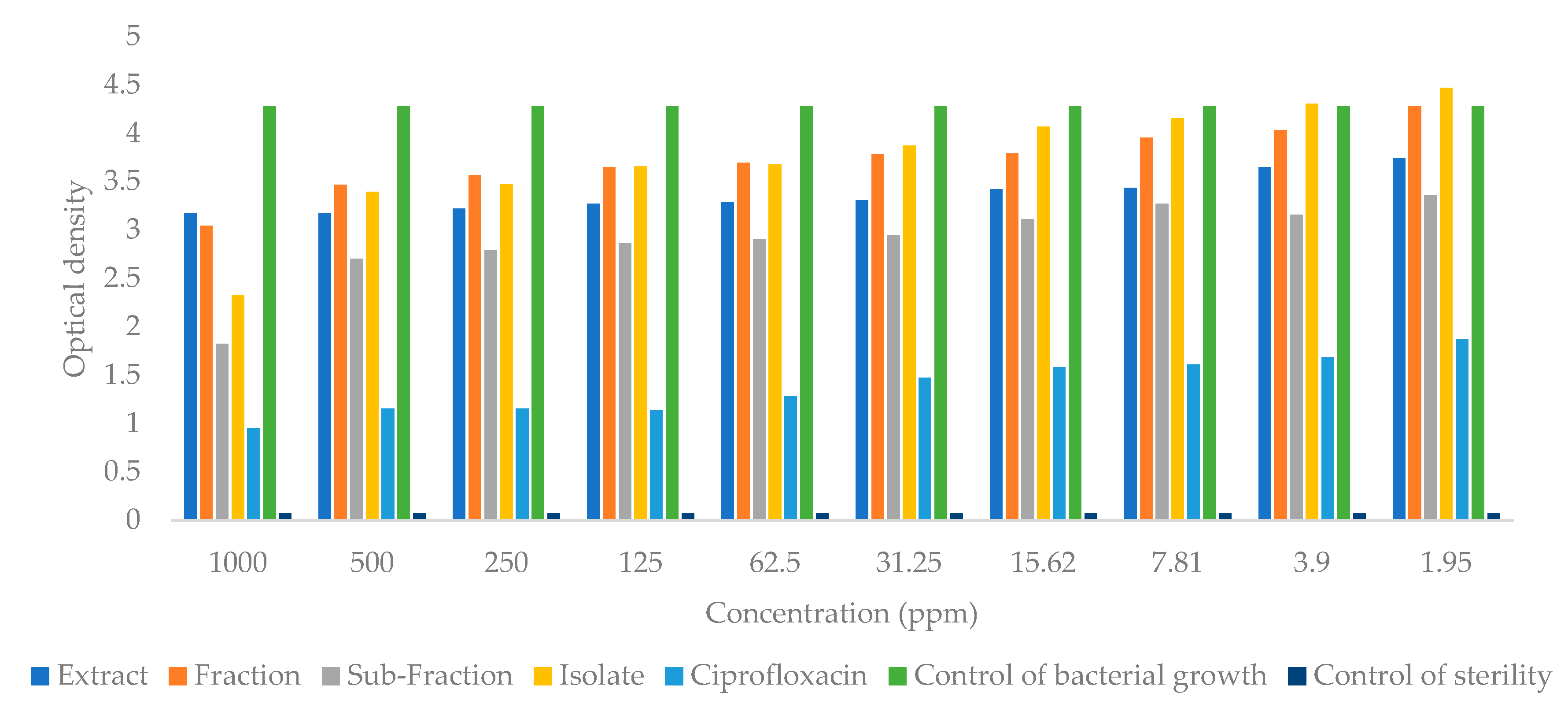
2.4. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Maceration and Isolation
4.2. Isolate Characterization
5-Methyl-11-(2-oxopyridin-1(2H)-yl)undecaneperoxoicacid: Yellow Oil Form
4.3. Computational Study
4.3.1. Molecular Docking Study
4.3.2. Molecular Dynamics Simulation
4.4. Antibacterial Activity
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewatisari, W.F.; Nugroho, L.H.; Retnaningrum, E.; Purwestri, Y.A. The Potency of Sansevieria trifasciata and S. Cylindrica Leaves Extracts as an Antibacterial against Pseudomonas aeruginosa. Biodiversitas J. Biol. Divers. 2021, 22, 408–415. [Google Scholar] [CrossRef]
- Yumna, M.; Arbianti, R.; Utami, T.S.; Hermansyah, H. Effect of Mother-in-Law’s Tongue Leaves (Sansevieria trifasciata) Extract’s Solvent Polarity on Anti-Diabetic Activity through in Vitro α-Glucosidase Enzyme Inhibition Test. E3S Web Conf. 2018, 67, 03003. [Google Scholar] [CrossRef]
- Kasmawati, H.; Mustarichie, R.; Halimah, E.; Ruslin, R.; Arfan, A.; Sida, N.A. Unrevealing the Potential of Sansevieria trifasciata Prain Fraction for the Treatment of Androgenetic alopecia by Inhibiting Androgen Receptors Based on LC-MS/MS Analysis, and In-Silico Studies. Molecules 2022, 27, 4358. [Google Scholar] [CrossRef] [PubMed]
- Tchegnitegni, B.T.; Teponno, R.B.; Jenett-Siems, K.; Melzig, M.F.; Miyamoto, T.; Tapondjou, L.A. A Dihydrochalcone Derivative and Further Steroidal Saponins from Sansevieria Trifasciata Prain. Zeitschrift fur Naturforsch.-Sect. C J. Biosci. 2017, 72, 477–482. [Google Scholar] [CrossRef]
- Teponno, R.B.; Tanaka, C.; Jie, B.; Tapondjou, L.A.; Miyamoto, T. Trifasciatosides A-J, Steroidal Saponins from Sansevieria Trifasciata. Chem. Pharm. Bull. 2016, 64, 1347–1355. [Google Scholar] [CrossRef]
- González, A.G.; Freire, R.; García-Estrada, M.G.; Salazar, J.A.; Suárez, E. New Sources of Steroid Sapogenins-XIV. 25S-Ruscogenin and Sansevierigenin, Two New Spirostan Sapogenins from Sansevieria Trifasciata. Tetrahedron 1972, 28, 1289–1297. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Tanaka, C.; Gabriel, A.F.; Tapondjou, L.A.; Miyamoto, T. Sappanin-Type Homoisoflavonoids from Sansevieria Trifasciata Prain. Phytochem. Lett. 2015, 12, 262–266. [Google Scholar] [CrossRef]
- Mimaki, Y.; Imoue, T.; Kuroda, M.; Sashida, Y. Pregnane Glycosides from Sansevieria. Phytochemistry 1997, 44, 107–111. [Google Scholar] [CrossRef]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities. Molecules 2020, 25, 2608. [Google Scholar] [CrossRef]
- Dewatisari, W.F.; Nugroho, L.H.; Retnaningrum, E.; Purwestri, Y.A. Antibacterial and Anti-Biofilm-Forming Activity of Secondary Metabolites from Sansevieria Trifasciata Leaves Against Pseudomonas Aeruginosa. Indones. J. Pharm. 2022, 33, 100–109. [Google Scholar] [CrossRef]
- Febriani, Y.; Mierza, V.; Handayani, N.P.; Surismayanti, S.; Ginting, I. Antibacterial Activity of Lidah Mertua (Sansevieria trifasciata Prain.) Leaves Extract on Escherichia coli and Staphylococcus aureus. Open Access Maced. J. Med. Sci. 2019, 7, 1857–9655. [Google Scholar] [CrossRef]
- Desi, S.; Siti, H.A.; Mery, S. Potensi Lidah Mertua (Sansevierria Trifasciata Prain) Dalam Menghambat Pertumbuhan Bakteri Salmonella Sp Dan Staphylococcus aureus. Ris. Inf. Kesehat. 2018, 7, 129–133. [Google Scholar] [CrossRef]
- Mani, J.; Johnson, J.; Hosking, H.; Hoyos, B.E.; Walsh, K.B.; Neilsen, P.; Naiker, M. Bioassay Guided Fractionation Protocol for Determining Novel Active Compounds in Selected Australian Flora. Plants 2022, 11, 2886. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.A.; Imtiaz, M.; Parvez, A.; Rai, P.K.; Jaremko, M.; Emwas, A.H.; Bholay, A.D.; Fatmi, M.Q. In Vitro and In Silico Approaches for the Evaluation of Antimicrobial Activity, Time-Kill Kinetics, and Anti-Biofilm Potential of Thymoquinone (2-Methyl-5-Propan-2-Ylcyclohexa-2, 5-Diene-1,4-Dione) against Selected Human Pathogens. Antibiotics 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Qin, J.; Verderosa, A.D.; Hawas, S.; Zhang, B.; Blaskovich, M.A.T.; Cronan, J.E.; Totsika, M. Loss of β-Ketoacyl Acyl Carrier Protein Synthase III Activity Restores Multidrug-Resistant Escherichia Coli Sensitivity to Previously Ineffective Antibiotics. mSphere 2022, 7, e00117-22. [Google Scholar] [CrossRef]
- Sanyal, R.; Singh, V.; Harinarayanan, R. A Novel Gene Contributing to the Initiation of Fatty Acid Biosynthesis in Escherichia coli. J. Bacteriol. 2019, 201, 354–373. [Google Scholar] [CrossRef]
- Mohedano, M.L.; Overweg, K.; De La Fuente, A.; Reuter, M.; Altabe, S.; Mulholland, F.; De Mendoza, D.; López, P.; Wells, J.M. Evidence That the Essential Response Regulator YycF in Streptococcus Pneumoniae Modulates Expression of Fatty Acid Biosynthesis Genes and Alters Membrane Composition. J. Bacteriol. 2005, 187, 2357–2367. [Google Scholar] [CrossRef]
- Sun, J.; Lv, P.C.; Zhu, H.L. Tyrosyl-TRNA Synthetase Inhibitors: A Patent Review. Expert Opin. Ther. Pat. 2017, 27, 557–564. [Google Scholar] [CrossRef]
- Muensaen, S.; Ruangwises, N.; Jarikasem, S.; Sithisarn, P. Isolation, Structure Elucidation and Quantitative Analysis of Callicarpone and Oleanolic Acid in the Leaves of Callicarpa Candicans Collected from Different Provinces in Thailand. Agric. Nat. Resour. 2019, 53, 251–259. [Google Scholar] [CrossRef]
- Bruguière, A.; Derbré, S.; Breárd, D.; Tomi, F.; Nuzillard, J.M.; Richomme, P. 13C NMR Dereplication Using MixONat Software: A Practical Guide to Decipher Natural Products Mixtures. Planta Med. 2021, 87, 1061–1068. [Google Scholar] [CrossRef]
- Gartika, M.; Pramesti, H.T.; Kurnia, D.; Satari, M.H. A Terpenoid Isolated from Sarang Semut (Myrmecodia Pendans) Bulb and Its Potential for the Inhibition and Eradication of Streptococcus Mutans Biofilm. BMC Complement. Altern. Med. 2018, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Pitaloka, D.A.E.; Arfan, A.; Alfarafisa, N.M.; Chaidir, L.; Supratman, U. Discovery of Potent Triterpenoid and Limonoid Compounds Targeting Mycobacterium Tuberculosis Enoyl Acyl Carrier Protein Reductase (InhA): An in Silico Investigation. Inform. Med. Unlocked 2023, 40, 101299. [Google Scholar] [CrossRef]
- Sargsyan, K.; Grauffel, C.; Lim, C. How Molecular Size Impacts RMSD Applications in Molecular Dynamics Simulations. J. Chem. Theory Comput. 2017, 13, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhang, Q.; Xu, P.; Jiang, Z. Effects of the Temperature and Salt Concentration on the Structural Characteristics of the Protein (PDB Code 1BBL). Polymers 2022, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Manfredi, M.; Martelli, P.L.; Casadio, R. Solvent Accessibility of Residues Undergoing Pathogenic Variations in Humans: From Protein Structures to Protein Sequences. Front. Mol. Biosci. 2021, 7, 626363. [Google Scholar] [CrossRef] [PubMed]
- Sneha, P.; Priya Doss, C.G. Molecular Dynamics: New Frontier in Personalized Medicine. Adv. Protein Chem. Struct. Biol. 2016, 102, 181–224. [Google Scholar] [CrossRef]
- Kasmawati, H.; Mustarichie, R.; Halimah, E. The Identification Of Molecular Mechanisms From Bioactive Compounds In Sansevieria Trifasciata Plant As Anti-Alopecia: In-Silico Approach. Rasayan J. Chem. 2022, 15, 925–932. [Google Scholar] [CrossRef]
- Raslan, M.A.; Abdel-Rahman, R.F.; Fayed, H.M.; Ogaly, H.A.; Tahere, R.F. Metabolomic Profiling of Sansevieria Trifasciata Hort Ex. Prain Leaves and Roots by HPLC-PAD-ESI/MS and Its Hepatoprotective Effect via Activation of the NRF2/ARE Signaling Pathway in an Experimentally Induced Liver Fibrosis Rat Model. Egypt. J. Chem. 2021, 64, 6647–6671. [Google Scholar] [CrossRef]
- Kasmawati, H.; Mustarichie, R.; Halimah, E.; Ruslin, R.; Sida, N.A. Antialopecia Activity and IR-Spectrometry Characterization of Bioactive Compounds From Sansevieria trifasciata P. Egypt. J. Chem. 2022, 65, 19–24. [Google Scholar] [CrossRef]
- Morrison, R.D.; Murphy, B. Environmental Forensics: Contaminant Specific Guide; Elsevier Academic Press: Massachusetts, MA, USA, 2006; ISBN 9780080494784. [Google Scholar]
- Caesar, L.K.; Cech, N.B.; Kubanek, J.; Linington, R.; Luesch, H. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Adv. Protein Chem. Struct. Biol. 2019, 36, 845–936. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, D.; Wu, D.; He, W.; Zuo, M.; Zhu, W.; Xu, Y.; Wang, L. Two New 4-Hydroxy-2-Pyridone Alkaloids with Antimicrobial and Cytotoxic Activities from Arthrinium Sp. GZWMJZ-606 Endophytic with Houttuynia Cordata Thunb. Molecules 2023, 28, 2192. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Chaieb, K.; Parveen, S.; Ahmed, H.A.; Alnoman, R.B. N-Alkyl 2-Pyridone versus O-Alkyl 2-Pyridol: Ultrasonic Synthesis, DFT, Docking Studies and Their Antimicrobial Evaluation. J. Mol. Struct. 2020, 1199, 126926. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. Synthesis and Antitumour Activity of 4-Hydroxy-2-Pyridone Derivatives. Eur. J. Med. Chem. 2000, 35, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Wang, L.; Cheng, Y.N.; Cao, Z.Q.; Zhang, X.K.; Guo, X.L. Discovery and Characterization of 4-Hydroxy-2-Pyridone Derivative Sambutoxin as a Potent and Promising Anticancer Drug Candidate: Activity and Molecular Mechanism. Mol. Pharm. 2018, 15, 4898–4911. [Google Scholar] [CrossRef]
- Price, A.C.; Choi, K.H.; Heath, R.J.; Li, Z.; White, S.W.; Rock, C.O. Inhibition of Beta-Ketoacyl-Acyl Carrier Protein Synthases by Thiolactomycin and Cerulenin. Structure and Mechanism. J. Biol. Chem. 2001, 276, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Janson, C.A.; Smith, W.W.; Green, S.M.; McDevitt, P.; Johanson, K.; Carter, P.; Hibbs, M.; Lewis, C.; Chalker, A.; et al. Crystal Structure of Staphylococcus aureus Tyrosyl-TRNA Synthetase in Complex with a Class of Potent and Specific Inhibitors. Protein Sci. 2001, 10, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Arba, M.; Aslikah, N.; Arfan, A.; Ruslin, R.; Yanuar, A. Insight on Estrogen Receptor Alpha Modulator from Indonesian Herbal Database: An in-Silico Analysis. J. Farm. Indones. Pharm. J. Indones. 2020, 17, 343–351. [Google Scholar] [CrossRef]
- Arfan, A.; Muliadi, R.; Malina, R.; Trinovitasari, N.; Asnawi, A. Docking and Dynamics Studies: Identifying the Binding Ability of Quercetin Analogs to the ADP-Ribose Phosphatase of SARS-CoV-2. J. Kartika Kim. 2022, 5, 145–151. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Petrov, D.; Zagrovic, B. Are Current Atomistic Force Fields Accurate Enough to Study Proteins in Crowded Environments? PLoS Comput. Biol. 2014, 10, e1003638. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Vranken, B.F. ACPYPE-AnteChamber PYthon Parser Interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Arba, M.; Arfan, A.; Yamin, Y.; Zubair, M.S. The Potential of Clerodendrum Paniculatum Leaves Fraction as a 3-Chymotrypsin-Like (3CL) Protease Inhibitor of SARS-CoV-2. Indones. J. Chem. 2023, 23, 770–781. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Fang, J. Multiple Staggered Mesh Ewald: Boosting the Accuracy of the Smooth Particle Mesh Ewald Method. J. Chem. Theory Comput. 2016, 12, 5596–5608. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Lynn, A. G-Mmpbsa -A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Ari, R.M.; Yahya, H.; Rehan, M.M.; Yahya, H.N. Antibacterial Properties of Ethanolic Extract of Mushrooms Sold in Malaysian Local Market. East Afr. Sch. J. Agric. Life Sci. 2019, 2, 516–523. [Google Scholar]
| β-Ketoacyl-ACP Synthase from Escherichia coli | TyrRS from Streptococcus aureus | ||
|---|---|---|---|
| Compounds | Binding Energy (kcal/mol) | Compounds | Binding Energy (kcal/mol) |
| TLM | −7.96 | 629 | −9.35 |
| Ciprofloxacin | −6.81 | Ciprofloxacin | −6.69 |
| Isolate | −6.72 | Isolate | −6.15 |
| β-Ketoacyl-ACP Synthase from Escherichia coli (All Energies Are in kJ/mol) | |||||
|---|---|---|---|---|---|
| Compounds | van der Waal | Electrostatic | Polar Solvation | SASA | Binding Energy |
| TLM | −116.620 | −41.128 | 86.225 | −13.079 | −84.603 |
| Ciprofloxacin | −179.729 | −102.199 | 192.537 | −16.739 | −106.131 |
| Isolate | −153.888 | −46.728 | 114.871 | −18.356 | −104.101 |
| TyrRS from Streptococcus aureus (All Energies Are in kJ/mol) | |||||
| Compounds | van der Waal | Electrostatic | Polar Solvation | SASA | Binding Energy |
| 629 | −207.705 | −116.709 | 257.610 | −19.976 | −86.780 |
| Ciprofloxacin | −205.931 | −86.473 | 228.331 | −18.094 | −82.167 |
| Isolate | −193.642 | −49.507 | 182.081 | −19.992 | −81.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasmawati, H.; Ruslin, R.; Arfan, A.; Sida, N.A.; Saputra, D.I.; Halimah, E.; Mustarichie, R. Antibacterial Potency of an Active Compound from Sansevieria trifasciata Prain: An Integrated In Vitro and In Silico Study. Molecules 2023, 28, 6096. https://doi.org/10.3390/molecules28166096
Kasmawati H, Ruslin R, Arfan A, Sida NA, Saputra DI, Halimah E, Mustarichie R. Antibacterial Potency of an Active Compound from Sansevieria trifasciata Prain: An Integrated In Vitro and In Silico Study. Molecules. 2023; 28(16):6096. https://doi.org/10.3390/molecules28166096
Chicago/Turabian StyleKasmawati, Henny, Ruslin Ruslin, Arfan Arfan, Nurramadhani A. Sida, Dimas Isnu Saputra, Eli Halimah, and Resmi Mustarichie. 2023. "Antibacterial Potency of an Active Compound from Sansevieria trifasciata Prain: An Integrated In Vitro and In Silico Study" Molecules 28, no. 16: 6096. https://doi.org/10.3390/molecules28166096
APA StyleKasmawati, H., Ruslin, R., Arfan, A., Sida, N. A., Saputra, D. I., Halimah, E., & Mustarichie, R. (2023). Antibacterial Potency of an Active Compound from Sansevieria trifasciata Prain: An Integrated In Vitro and In Silico Study. Molecules, 28(16), 6096. https://doi.org/10.3390/molecules28166096