Seasonal Variation in Plant Polyphenols and Related Bioactivities across Three Years in Ten Tree Species as Visualized by Mass Spectrometric Fingerprint Mapping
Abstract
:1. Introduction
2. Results and Discussion
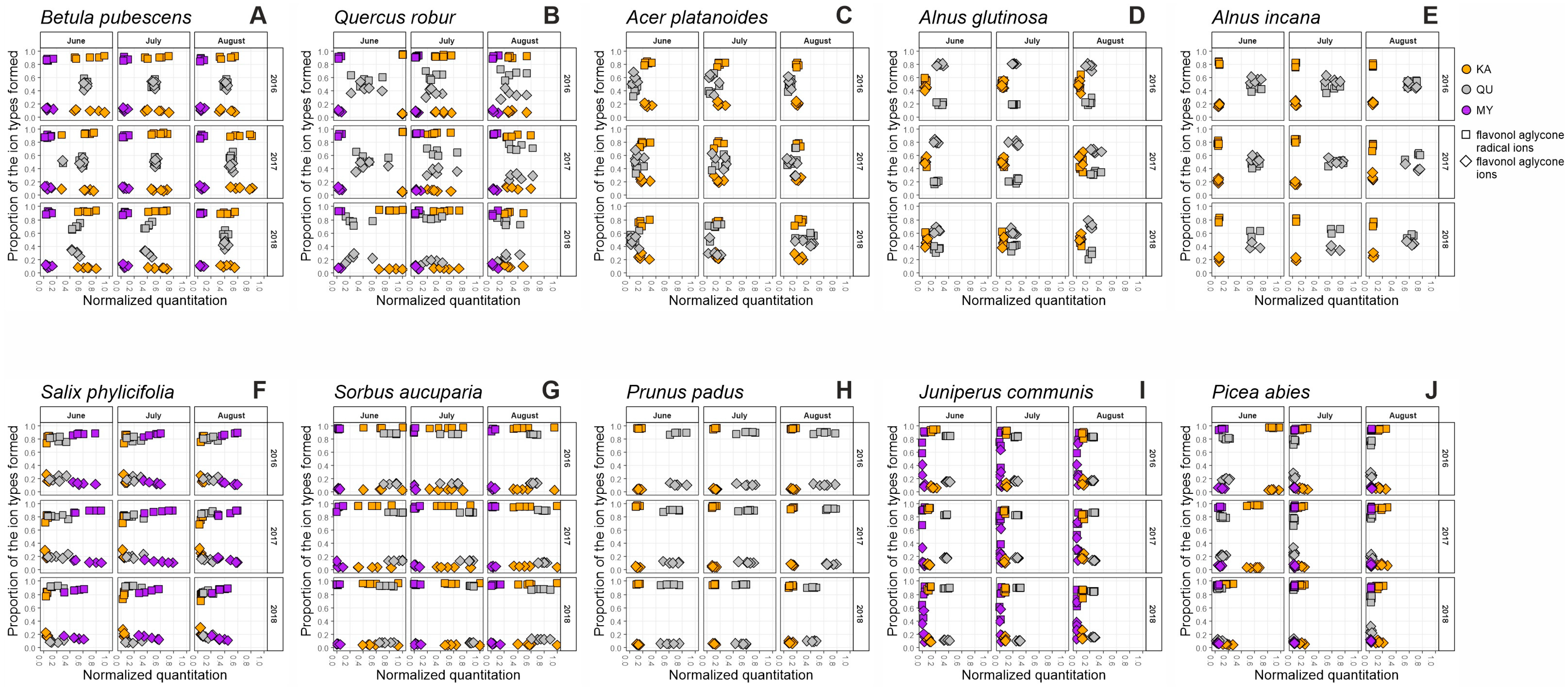
2.1. Seasonal Patterns in the Foliage of Betula pubescens
2.2. Seasonal Patterns in the Foliage of Quercus robur
2.3. Seasonal Patterns in the Foliage of Acer platanoides
2.4. Seasonal Patterns in the Foliage of Alnus glutinosa
2.5. Seasonal Patterns in the Foliage of Alnus incana
2.6. Seasonal Patterns in the Foliage of Salix phylicifolia
2.7. Seasonal Patterns in the Foliage of Sorbus aucuparia
2.8. Seasonal Patterns in the Foliage of Prunus padus
2.9. Seasonal Patterns in the Needles of Juniperus communis
2.10. Seasonal Patterns in the Needles of Picea abies
3. Materials and Methods
3.1. Studied Plant Species
3.2. Plant Sampling and Extraction
3.3. Chemical Analyses
3.4. Mass Spectrometric Fingerprint Mapping
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.J.; Maron, J.L.; Salminen, J.-P. Insect Herbivores Drive Real-Time Ecological and Evolutionary Change in Plant Populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef]
- McArt, S.H.; Halitschke, R.; Salminen, J.-P.; Thaler, J.S. Leaf Herbivory Increases Plant Fitness via Induced Resistance to Seed Predators. Ecology 2013, 94, 966–975. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Doublet, D.; Salminen, J.-P.; Burkepile, D.E.; Parker, J.D. Responses of Plant Phenology, Growth, Defense, and Reproduction to Interactive Effects of Warming and Insect Herbivory. Ecology 2017, 98, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Ives, A.R.; Ahern, J.; Salminen, J.-P. Macroevolution of Plant Defenses against Herbivores in the Evening Primroses. New Phytol. 2014, 203, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and Recent Climate Change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef] [PubMed]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and Climate Change Are Reshaping Insect Biodiversity Worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Seifert, C.L.; Jorge, L.R.; Volf, M.; Wagner, D.L.; Lamarre, G.P.A.; Miller, S.E.; Gonzalez-Akre, E.; Anderson-Teixeira, K.J.; Novotný, V. Seasonality Affects Specialisation of a Temperate Forest Herbivore Community. Oikos 2021, 130, 1450–1461. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal Variation in the Content of Hydrolysable Tannins in Leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal Variation in the Content of Hydrolyzable Tannins, Flavonoid Glycosides, and Proanthocyanidins in Oak Leaves. J. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef]
- Valkama, E.; Salminen, J.-P.; Koricheva, J.; Kalevi, P. Changes in Leaf Trichomes and Epicuticular Flavonoids during Leaf Development in Three Birch Taxa. Ann. Bot. 2004, 94, 233–242. [Google Scholar] [CrossRef]
- Zidorn, C. Seasonal Variation of Natural Products in European Trees. Phytochem. Rev. 2018, 17, 923–935. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Young, R.A. Conifer Seed Cone Proanthocyanidin Polymers: Characterization by 13C NMR Spectroscopy and Determination of Antifungal Activities. J. Agric. Food Chem. 1994, 42, 1704–1708. [Google Scholar] [CrossRef]
- Virjamo, V.; Julkunen-Tiitto, R. Shoot Development of Norway Spruce (Picea abies) Involves Changes in Piperidine Alkaloids and Condensed Tannins. Trees 2014, 28, 427–437. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Hagerman, A.E.; Karonen, M.; Salminen, J.-P. Ellagitannins Have Greater Oxidative Activities than Condensed Tannins and Galloyl Glucoses at High pH: Potential Impact on Caterpillars. J. Chem. Ecol. 2006, 32, 2253–2267. [Google Scholar] [CrossRef]
- Moilanen, J.; Salminen, J.-P. Ecologically Neglected Tannins and Their Biologically Relevant Activity: Chemical Structures of Plant Ellagitannins Reveal Their in vitro Oxidative Activity at High pH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.-P. Biological Activity of Ellagitannins: Effects as Anti-Oxidants, pro-Oxidants and Metal Chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pälijärvi, M.; Karonen, M.; Salminen, J.-P. Oxidatively Active Plant Phenolics Detected by UHPLC-DAD-MS after Enzymatic and Alkaline Oxidation. J. Chem. Ecol. 2018, 44, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pälijärvi, M.; Karonen, M.; Salminen, J.-P. Distribution of Enzymatic and Alkaline Oxidative Activities of Phenolic Compounds in Plants. Phytochemistry 2020, 179, 112501. [Google Scholar] [CrossRef]
- Dobreva, M.A.; Green, R.J.; Mueller-Harvey, I.; Salminen, J.-P.; Howlin, B.J.; Frazier, R.A. Size and Molecular Flexibility Affect the Binding of Ellagitannins to Bovine Serum Albumin. J. Agric. Food Chem. 2014, 62, 9186–9194. [Google Scholar] [CrossRef]
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.-P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Ellagitannins with Glucopyranose Cores Have Higher Affinities to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Leppä, M.M.; Laitila, J.E.; Salminen, J.-P. Distribution of Protein Precipitation Capacity within Variable Proanthocyanidin Fingerprints. Molecules 2020, 25, 5002. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Virtanen, V.; Salminen, J.-P. Influence of the Hydrolyzable Tannin Structure on the Characteristics of Insoluble Hydrolyzable Tannin-Protein Complexes. J. Agric. Food Chem. 2022, 70, 13036–13048. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Sun, X.; Suber, M.P.; Li, M.; Salminen, J.-P.; Hagerman, A.E. The Oxidative Activity of Ellagitannins Dictates Their Tendency to Form Highly Stabilized Complexes with Bovine Serum Albumin at Increased pH. J. Agric. Food Chem. 2016, 64, 8994–9003. [Google Scholar] [CrossRef]
- Baert, N.; Pellikaan, W.F.; Karonen, M.; Salminen, J.-P. A Study of the Structure-Activity Relationship of Oligomeric Ellagitannins on Ruminal Fermentation in vitro. J. Dairy Sci. 2016, 99, 8041–8052. [Google Scholar] [CrossRef]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.-P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in Vitro Activity against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef]
- Vanhakylä, S.; Salminen, J.-P. Mass Spectrometric Fingerprint Mapping Reveals Species-Specific Differences in Plant Polyphenols and Related Bioactivities. Molecules 2023. submitted. [Google Scholar]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.-P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef]
- Engström, M.T.; Pälijärvi, M.; Salminen, J.-P. Rapid Fingerprint Analysis of Plant Extracts for Ellagitannins, Gallic Acid, and Quinic Acid Derivatives and Quercetin-, Kaempferol- and Myricetin-Based Flavonol Glycosides by UPLC-QqQ-MS/MS. J. Agric. Food Chem. 2015, 63, 4068–4079. [Google Scholar] [CrossRef]
- Salminen, J.-P. Two-Dimensional Tannin Fingerprints by Liquid Chromatography Tandem Mass Spectrometry Offer a New Dimension to Plant Tannin Analyses and Help to Visualize the Tannin Diversity in Plants. J. Agric. Food Chem. 2018, 66, 9162–9171. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, V.; Salminen, J.-P.; Ossipova, S.; Haukioja, E.; Pihlaja, K. Gallic Acid and Hydrolysable Tannins Are Formed in Birch Leaves from an Intermediate Compound of the Shikimate Pathway. Biochem. Syst. Ecol. 2003, 31, 3–16. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Karonen, M. Chemical Ecology of Tannins and Other Phenolics: We Need a Change in Approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Riipi, M.; Haukioja, E.; Lempa, K.; Ossipov, V.; Ossipova, S.; Pihlaja, K. Ranking of Individual Mountain Birch Trees in Terms of Leaf Chemistry: Seasonal and Annual Variation. Chemoecology 2004, 14, 31–43. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Ossipov, V.; Pihlaja, K. Distribution of Hydrolysable Tannins in the Foliage of Finnish Birch Species. Z. Naturforsch. C J. Biosci. 2002, 57, 248–256. [Google Scholar] [CrossRef]
- Riipi, M.; Ossipov, V.; Lempa, K.; Haukioja, E.; Koricheva, J.; Ossipova, S.; Pihlaja, K. Seasonal Changes in Birch Leaf Chemistry: Are There Trade-Offs between Leaf Growth and Accumulation of Phenolics? Oecologia 2002, 130, 380–390. [Google Scholar] [CrossRef]
- Ossipova, S.V.; Ossipov, V.; Haukioja, E.; Loponen, J.; Pihlaja, K. Proanthocyanidins of Mountain Birch Leaves: Quantification and Properties. Phytochem. Anal. 2001, 12, 128–133. [Google Scholar] [CrossRef]
- James, A.M.; Ma, D.; Mellway, R.; Gesell, A.; Yoshida, K.; Walker, V.; Tran, L.; Stewart, D.; Reichelt, M.; Suvanto, J.; et al. Poplar MYB115 and MYB134 Transcription Factors Regulate Proanthocyanidin Synthesis and Structure. Plant Physiol. 2017, 174, 154–171. [Google Scholar] [CrossRef]
- Raal, A.; Boikova, T.; Püssa, T. Content and Dynamics of Polyphenols in Betula spp. Leaves Naturally Growing in Estonia. Rec. Nat. Prod. 2015, 9, 41–48. [Google Scholar]
- Visakorpi, K.; Riutta, T.; Malhi, Y.; Salminen, J.-P.; Salinas, N.; Gripenberg, S. Changes in Oak (Quercus robur) Photosynthesis after Winter Moth (Operophtera brumata) Herbivory Are Not Explained by Changes in Chemical or Structural Leaf Traits. PLoS ONE 2020, 15, e0228157. [Google Scholar] [CrossRef]
- Tálos-Nebehaj, E.; Hofmann, T.; Albert, L. Seasonal Changes of Natural Antioxidant Content in the Leaves of Hungarian Forest Trees. Ind. Crops Prod. 2017, 98, 53–59. [Google Scholar] [CrossRef]
- Veselá, H.; Lhotáková, Z.; Albrechtová, J.; Frouz, J. Seasonal Changes in Tree Foliage and Litterfall Composition at Reclaimed and Unreclaimed Post-Mining Sites. Ecol. Eng. 2021, 173, 106424. [Google Scholar] [CrossRef]
- Moilanen, J.; Koskinen, P.; Salminen, J.-P. Distribution and Content of Ellagitannins in Finnish Plant Species. Phytochemistry 2015, 116, 188–197. [Google Scholar] [CrossRef]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.; Lindfors, A.; O’Hara, R.B.; Aphalo, P.J. Seasonal Fluctuations in Leaf Phenolic Composition under UV Manipulations Reflect Contrasting Strategies of Alder and Birch Trees. Physiol. Plant 2010, 140, 297–309. [Google Scholar] [CrossRef]
- Tegelberg, R.; Veteli, T.; Aphalo, P.J.; Julkunen-Tiitto, R. Clonal Differences in Growth and Phenolics of Willows Exposed to Elevated Ultraviolet-B Radiation. Basic Appl. Ecol. 2003, 4, 219–228. [Google Scholar] [CrossRef]
- Nissinen, K.; Virjamo, V.; Mehtätalo, L.; Lavola, A.; Valtonen, A.; Nybakken, L.; Julkunen-Tiitto, R. A Seven-Year Study of Phenolic Concentrations of the Dioecious Salix myrsinifolia. J. Chem. Ecol. 2018, 44, 416–430. [Google Scholar] [CrossRef]
- Obeso, J.R. The Costs of Reproduction in Plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Olszewska, M. Separation of Quercetin, Sexangularetin, Kaempferol and Isorhamnetin for Simultaneous HPLC Determination of Flavonoid Aglycones in Inflorescences, Leaves and Fruits of Three Sorbus Species. J. Pharm. Biomed. Anal. 2008, 48, 629–635. [Google Scholar] [CrossRef]
- Turumtay, H.; Midilli, A.; Turumtay, E.A.; Demir, A.; Selvi, E.K.; Budak, E.E.; Er, H.; Kocaimamoglu, F.; Baykal, H.; Belduz, A.O.; et al. Gram (−) Microorganisms DNA Polymerase Inhibition, Antibacterial and Chemical Properties of Fruit and Leaf Extracts of Sorbus acuparia and Sorbus caucasica var. yaltirikii. Biomed. Chromatogr. 2017, 31, e3901. [Google Scholar] [CrossRef]
- Olszewska, M.A. Variation in the Phenolic Content and in Vitro Antioxidant Activity of Sorbus aucuparia Leaf Extracts during Vegetation. Acta Pol. Pharm. 2011, 68, 937–944. [Google Scholar]
- Ravetto Enri, S.; Probo, M.; Renna, M.; Caro, E.; Lussiana, C.; Battaglini, L.M.; Lombardi, G.; Lonati, M. Temporal Variations in Leaf Traits, Chemical Composition and in vitro True Digestibility of Four Temperate Fodder Tree Species. Anim. Prod. Sci. 2020, 60, 643–658. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Kwapisz, A. Metabolite Profiling and Antioxidant Activity of Prunus padus L. Flowers and Leaves. Nat. Prod. Res. 2011, 25, 1115–1131. [Google Scholar] [CrossRef]
- Mattila, H.; Valev, D.; Havurinne, V.; Khorobrykh, S.; Virtanen, O.; Antinluoma, M.; Mishra, K.B.; Tyystjärvi, E. Degradation of Chlorophyll and Synthesis of Flavonols during Autumn Senescence-the Story Told by Individual Leaves. AoB Plants 2018, 10, ply028. [Google Scholar] [CrossRef] [PubMed]
- Artemkina, N.A.; Orlova, M.A.; Lukina, N.V. Chemical Composition of Juniperus sibirica Needles (Cupressaceae) in the Forest-Tundra Ecotone, the Khibiny Mountains. Russ. J. Ecol. 2016, 47, 243–250. [Google Scholar] [CrossRef]
- Naumann, H.D.; Stewart, W.C.; Whitney, T.R. The Effect of Maturity on Concentration and Biological Activity of Protein Precipitating Polyphenolics in Ground Juniper Is Dependent upon Species. Anim. Feed Sci. Technol. 2018, 246, 11–17. [Google Scholar] [CrossRef]
- Slimestad, R.; Hostettmann, K. Characterisation of Phenolic Constituents from Juvenile and Mature Needles of Norway Spruce by Means of High Performance Liquid Chromatography-Mass Spectrometry. Phytochem. Anal. 1996, 7, 42–48. [Google Scholar] [CrossRef]
- Martz, F.-O.; Peltola, R.; Fontanay, S.P.; Raphae, R.; Duval, R.E.; Julkunen-Tiitto, R.; Stark, S. Effect of Latitude and Altitude on the Terpenoid and Soluble Phenolic Composition of Juniper (Juniperus communis) Needles and Evaluation of Their Antibacterial Activity in the Boreal Zone. J. Agric. Food Chem. 2009, 57, 9575–9584. [Google Scholar] [CrossRef]
- Slimestad, R. Amount of Flavonols and Stilbenes during Needle Development of Picea abies; Variations between Provenances. Biochem. Syst. Ecol. 1998, 26, 225–238. [Google Scholar] [CrossRef]
- Ganthaler, A.; Stöggl, W.; Kranner, I.; Mayr, S. Foliar Phenolic Compounds in Norway Spruce with Varying Susceptibility to Chrysomyxa rhododendri: Analyses of Seasonal and Infection-Induced Accumulation Patterns. Front. Plant Sci. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Christenhusz, M.; Reveal, J.L.; Farjon, A.; Gardner, M.; Mill, R.; Chase, M. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional Uses, Phytochemistry, and Pharmacology of the Genus Acer (Maple): A Review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Arshanitsa, A.; Ponomarenko, J.; Lauberte, L.; Jurkjane, V.; Pals, M.; Akishin, Y.; Lauberts, M.; Jashina, L.; Bikovens, O.; Telysheva, G. Advantages of MW-Assisted Water Extraction, Combined with Steam Explosion, of Black Alder Bark in Terms of Isolating Valuable Compounds and Energy Efficiency. Ind. Crops Prod. 2022, 181, 114832. [Google Scholar] [CrossRef]
- Lauberts, M.; Pals, M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants 2021, 10, 2531. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Zaczyńska, E.; Czarny, A.; Kolniak-Ostek, J. Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties. Molecules 2022, 27, 2804. [Google Scholar] [CrossRef]
- Mahdi, J.G.; Mahdi, A.J.; Bowen, I.D. The Historical Analysis of Aspirin Discovery, Its Relation to the Willow Tree and Antiproliferative and Anticancer Potential. Cell Prolif. 2006, 39, 147–155. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-ostek, J.; Kalinowska-lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Rank, N.E.; Köpf, A.; Julkunen-Tiitto, R.; Tahvanainen, J. Host Preference and Larval Performance of the Salicylate-Using Leaf Beetle Phratora vitellinae. Ecology 1998, 79, 618–631. [Google Scholar] [CrossRef]
- Ruuhola, T.; Nybakken, L.; Julkunen-Tiitto, R. Sex-Related Differences of Two Ecologically Divergent Salix Species in the Responses of Enzyme Activities to Atmospheric CO2 Enrichment. Biol. Plant 2013, 57, 732–738. [Google Scholar] [CrossRef]
- Sile, I.; Videja, M.; Makrecka-Kuka, M.; Tirzite, D.; Pajuste, K.; Shubin, K.; Krizhanovska, V.; Grinberga, S.; Pugovics, O.; Dambrova, M. Chemical Composition of Prunus padus L. Flower Extract and Its Anti-Inflammatory Activities in Primary Bone Marrow-Derived Macrophages. J. Ethnopharmacol. 2021, 268, 113678. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E. Radial Diffusion Method for Determining Tannin in Plant Extracts. J. Chem. Ecol. 1987, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar]
- Malisch, C.S.; Lüscher, A.; Baert, N.; Engström, M.T.; Studer, B.; Fryganas, C.; Suter, D.; Mueller-Harvey, I.; Salminen, J.-P. Large Variability of Proanthocyanidin Content and Composition in Sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2015, 63, 10234–10242. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing Herbivory as You Grow up: The Ontogeny of Resistance in Plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef]
- Koricheva, J. Interpreting Phenotypic Variation in Plant Allelochemistry: Problems with the Use of Concentrations. Oecologia 1999, 119, 467–473. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhakylä, S.; Salminen, J.-P. Seasonal Variation in Plant Polyphenols and Related Bioactivities across Three Years in Ten Tree Species as Visualized by Mass Spectrometric Fingerprint Mapping. Molecules 2023, 28, 6093. https://doi.org/10.3390/molecules28166093
Vanhakylä S, Salminen J-P. Seasonal Variation in Plant Polyphenols and Related Bioactivities across Three Years in Ten Tree Species as Visualized by Mass Spectrometric Fingerprint Mapping. Molecules. 2023; 28(16):6093. https://doi.org/10.3390/molecules28166093
Chicago/Turabian StyleVanhakylä, Suvi, and Juha-Pekka Salminen. 2023. "Seasonal Variation in Plant Polyphenols and Related Bioactivities across Three Years in Ten Tree Species as Visualized by Mass Spectrometric Fingerprint Mapping" Molecules 28, no. 16: 6093. https://doi.org/10.3390/molecules28166093
APA StyleVanhakylä, S., & Salminen, J.-P. (2023). Seasonal Variation in Plant Polyphenols and Related Bioactivities across Three Years in Ten Tree Species as Visualized by Mass Spectrometric Fingerprint Mapping. Molecules, 28(16), 6093. https://doi.org/10.3390/molecules28166093






