Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance
Abstract
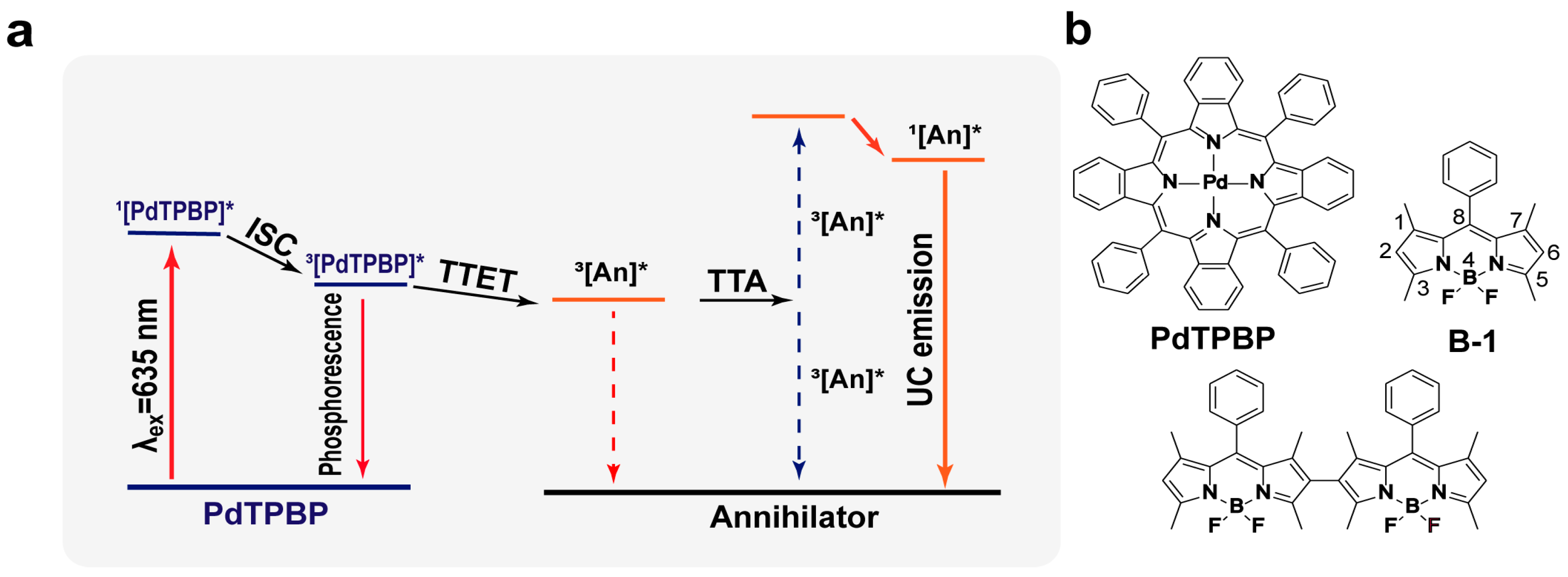
1. Introduction
2. Results and Discussion
2.1. Characterization of Photophysical Properties of Annihilators
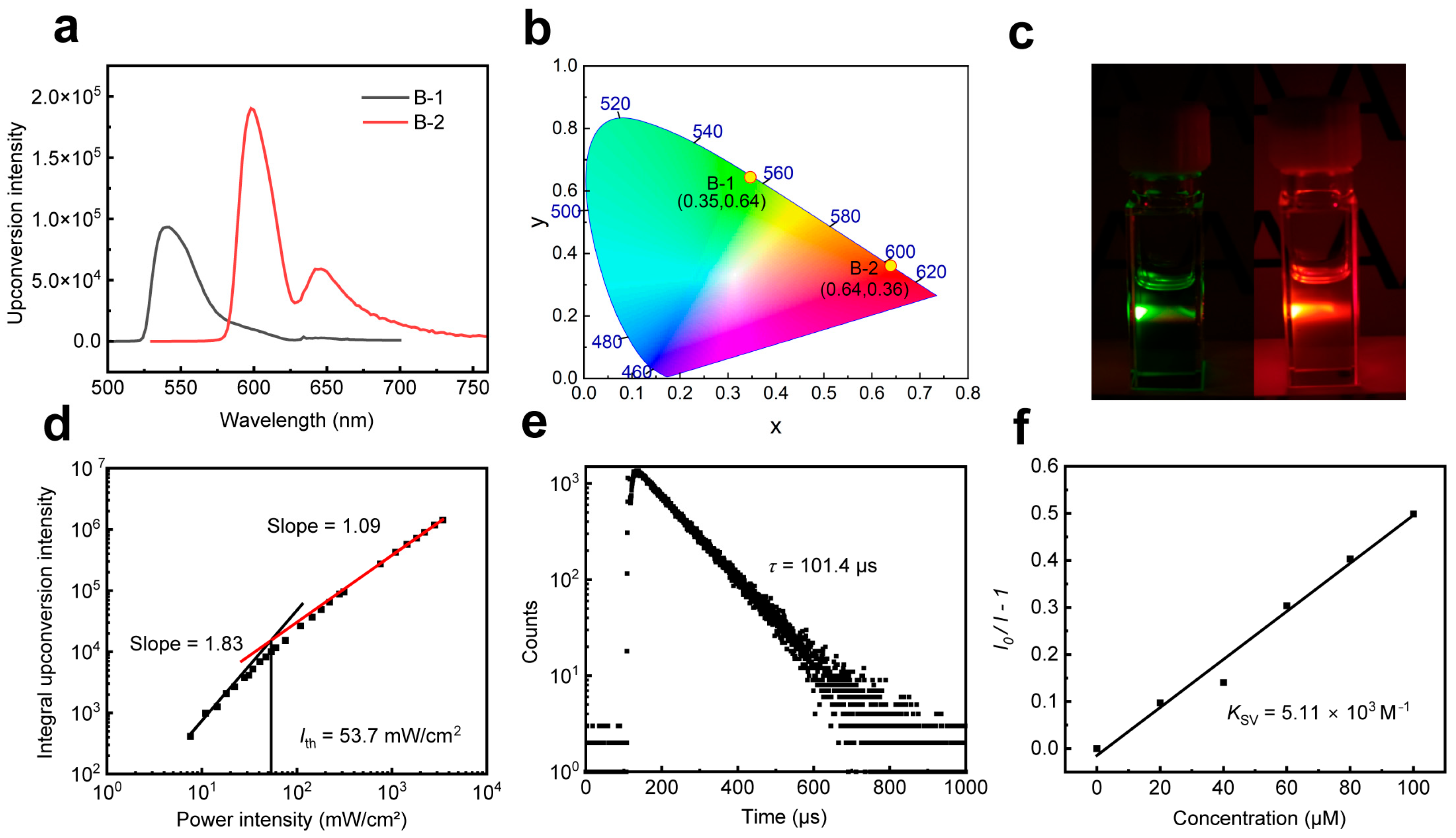
2.2. TTA-UC Properties of Annihilators
3. Materials and Methods
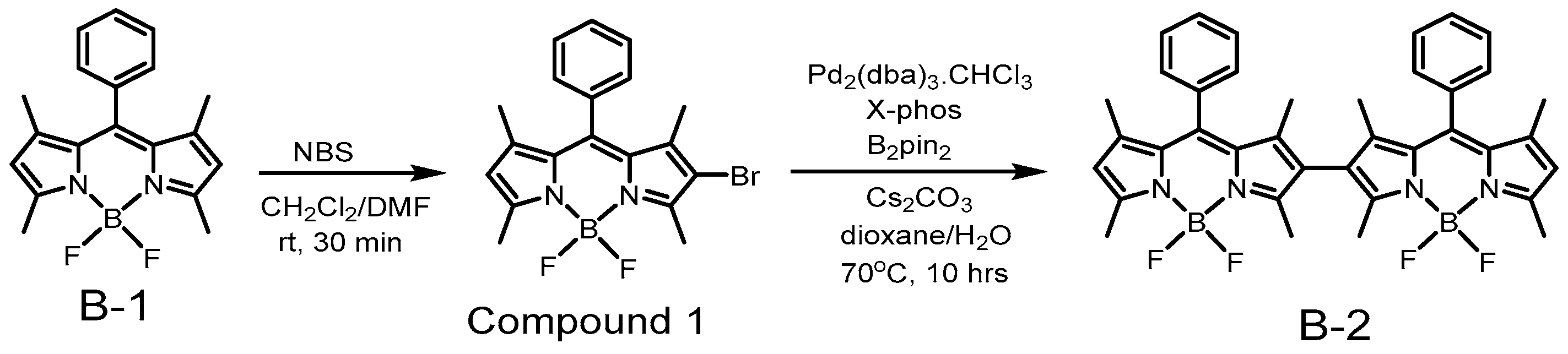
3.1. Preparation Process for B-2 [35]
3.2. Measurement of the Fluorescence Quantum Yields (Φf) of B-1 and B-2
3.3. Measurement of Upconversion Efficiency (ηUC)
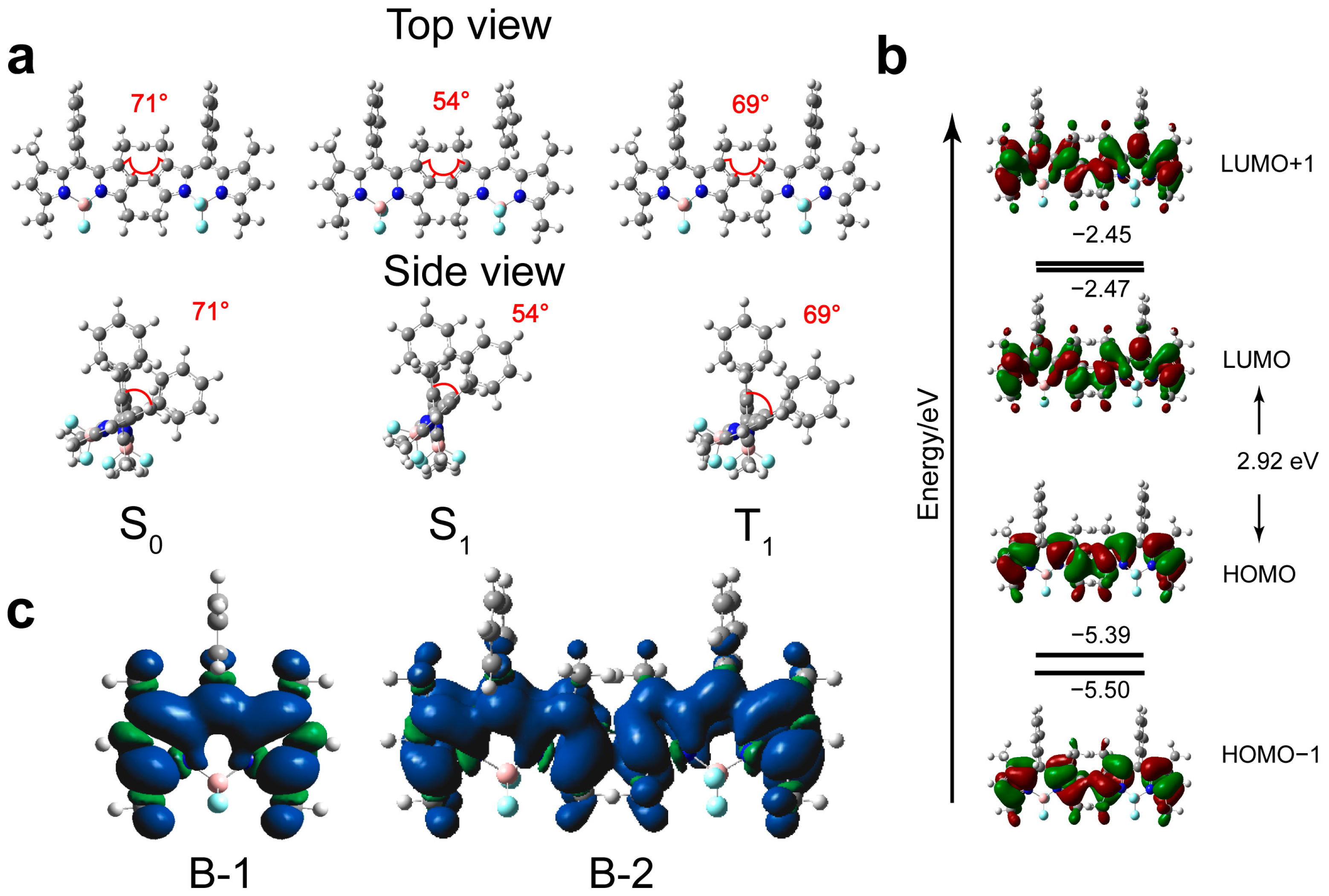
3.4. Theoretical Chemical Calculation [50]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924–936. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Schuck, P.J.; Suh, Y.D.; Schmidt, T.W.; Jin, D. Future and challenges for hybrid upconversion nanosystems. Nat. Photonics 2019, 13, 828–838. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.H.; Capobianco, J.A. Photon upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1299–1301. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.D.; Yan, C.H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.R.; Zheng, X.Y.; Lyu, G.M.; Sun, L.D.; Li, L.D.; Zhang, P.Z.; Zhang, C.; Yan, C.H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Huang, L.; Kakadiaris, E.; Vaneckova, T.; Huang, K.; Vaculovicova, M.; Han, G. Designing next generation of photon upconversion: Recent advances in organic triplet-triplet annihilation upconversion nanoparticles. Biomaterials 2019, 201, 77–86. [Google Scholar] [CrossRef]
- Sanders, S.N.; Schloemer, T.H.; Gangishetty, M.K.; Anderson, D.; Seitz, M.; Gallegos, A.O.; Stokes, R.C.; Congreve, D.N. Triplet fusion upconversion nanocapsules for volumetric 3D printing. Nature 2022, 604, 474–478. [Google Scholar] [CrossRef]
- Ravetz, B.D.; Pun, A.B.; Churchill, E.M.; Congreve, D.N.; Rovis, T.; Campos, L.M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. [Google Scholar] [CrossRef]
- Gholizadeh, E.M.; Prasad, S.K.K.; Teh, Z.L.; Ishwara, T.; Norman, S.; Petty, A.J., II; Cole, J.H.; Cheong, S.; Tilley, R.D.; Anthony, J.E.; et al. Photochemical upconversion of near-infrared light from below the silicon bandgap. Nat. Photonics 2020, 14, 585–590. [Google Scholar] [CrossRef]
- Richards, B.S.; Hudry, D.; Busko, D.; Turshatov, A.; Howard, I.A. Photon Upconversion for Photovoltaics and Photocatalysis: A Critical Review. Chem. Rev. 2021, 121, 9165–9195. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Gao, C.; Wong, W.W.H.; Qin, Z.; Lo, S.C.; Namdas, E.B.; Dong, H.; Hu, W. Application of Triplet-Triplet Annihilation Upconversion in Organic Optoelectronic Devices: Advances and Perspectives. Adv. Mater. 2021, 33, e2100704. [Google Scholar] [CrossRef]
- Yanai, N.; Kimizuka, N. Stimuli-Responsive Molecular Photon Upconversion. Angew. Chem. Int. Ed. 2020, 59, 10252–10264. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, L.; Han, J.; Han, G. Enhancing Triplet-Triplet Annihilation Upconversion: From Molecular Design to Present Applications. Acc. Chem. Res. 2022, 55, 2604–2615. [Google Scholar] [CrossRef]
- Ji, S.; Wu, W.; Wu, W.; Guo, H.; Zhao, J. Ruthenium(II) polyimine complexes with a long-lived 3IL excited state or a 3MLCT/3 IL equilibrium: Efficient triplet sensitizers for low-power upconversion. Angew. Chem. Int. Ed. 2011, 50, 1626–1629. [Google Scholar] [CrossRef]
- Huang, L.; Wu, W.; Li, Y.; Huang, K.; Zeng, L.; Lin, W.; Han, G. Highly Effective Near-Infrared Activating Triplet-Triplet Annihilation Upconversion for Photoredox Catalysis. J. Am. Chem. Soc. 2020, 142, 18460–18470. [Google Scholar] [CrossRef]
- Harada, N.; Sasaki, Y.; Hosoyamada, M.; Kimizuka, N.; Yanai, N. Discovery of Key TIPS-Naphthalene for Efficient Visible-to-UV Photon Upconversion under Sunlight and Room Light. Angew. Chem. Int. Ed. 2021, 60, 142–147. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Guo, H. Triplet–triplet annihilation based upconversion: From triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 2011, 1, 937–950. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The triplet excited state of Bodipy: Formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef]
- Pristash, S.R.; Corp, K.L.; Rabe, E.J.; Schlenker, C.W. Heavy-Atom-Free Red-to-Yellow Photon Upconversion in a Thiosquaraine Composite. ACS Appl. Energy Mater. 2019, 3, 19–28. [Google Scholar] [CrossRef]
- Borisov, S.M.; Saf, R.; Fischer, R.; Klimant, I. Synthesis and properties of new phosphorescent red light-excitable platinum(II) and palladium(II) complexes with Schiff bases for oxygen sensing and triplet-triplet annihilation-based upconversion. Inorg. Chem. 2013, 52, 1206–1216. [Google Scholar] [CrossRef]
- Pun, A.B.; Campos, L.M.; Congreve, D.N. Tunable Emission from Triplet Fusion Upconversion in Diketopyrrolopyrroles. J. Am. Chem. Soc. 2019, 141, 3777–3781. [Google Scholar] [CrossRef]
- Fallon, K.J.; Churchill, E.M.; Sanders, S.N.; Shee, J.; Weber, J.L.; Meir, R.; Jockusch, S.; Reichman, D.R.; Sfeir, M.Y.; Congreve, D.N.; et al. Molecular Engineering of Chromophores to Enable Triplet-Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2020, 142, 19917–19925. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2019, 18, 10–27. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Ziessel, R.; Harriman, A. Artificial light-harvesting antennae: Electronic energy transfer by way of molecular funnels. Chem. Commun. 2011, 47, 611–631. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Haefele, A.; Ziessel, R.; Castellano, F.N. Boron Dipyrromethene Chromophores: Next Generation Triplet Acceptors/Annihilators for Low Power Upconversion Schemes. J. Am. Chem. Soc. 2008, 130, 16164–16165. [Google Scholar] [CrossRef]
- Turshatov, A.; Busko, D.; Avlasevich, Y.; Miteva, T.; Landfester, K.; Baluschev, S. Synergetic effect in triplet-triplet annihilation upconversion: Highly efficient multi-chromophore emitter. Chemphyschem 2012, 13, 3112–3115. [Google Scholar] [CrossRef]
- Xu, M.; Zou, X.; Su, Q.; Yuan, W.; Cao, C.; Wang, Q.; Zhu, X.; Feng, W.; Li, F. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Commun. 2018, 9, 2698. [Google Scholar] [CrossRef]
- Sun, W.; Ronchi, A.; Zhao, T.; Han, J.; Monguzzi, A.; Duan, P. Highly efficient photon upconversion based on triplet–triplet annihilation from bichromophoric annihilators. J. Mater. Chem. C 2021, 9, 14201–14208. [Google Scholar] [CrossRef]
- Yang, M.; Sheykhi, S.; Zhang, Y.; Milsmann, C.; Castellano, F.N. Low power threshold photochemical upconversion using a zirconium(iv) LMCT photosensitizer. Chem. Sci. 2021, 12, 9069–9077. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, S.; Cha, W.Y.; Kim, D.; Shinokubo, H. Synthesis of Directly Connected BODIPY Oligomers through Suzuki–Miyaura Coupling. Org. Lett. 2011, 13, 2992–2995. [Google Scholar] [CrossRef]
- Zanini, G.P.; Montejano, H.A.; Previtali, C.M. Specific solvent effects on the charge separation efficiency in photoinduced electron transfer processes. J. Photoch. Photobio. A 2000, 132, 161–166. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Tan, J.; Zhang, X. Unique fluorescence of boronic acid derived salicylidenehydrazone complexes with two perpendicular ICT: Solvent effect on PET process. Dyes Pigm. 2018, 155, 186–193. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Vegesna, G.; Luo, F.-T.; Green, S.A.; Liu, H. Highly Water-Soluble Neutral BODIPY Dyes with Controllable Fluorescence Quantum Yields. Org. Lett. 2011, 13, 438–441. [Google Scholar] [CrossRef]
- Liu, X.; Chi, W.; Qiao, Q.; Kokate, S.V.; Cabrera, E.P.; Xu, Z.; Liu, X.; Chang, Y.T. Molecular Mechanism of Viscosity Sensitivity in BODIPY Rotors and Application to Motion-Based Fluorescent Sensors. ACS Sens. 2020, 5, 731–739. [Google Scholar] [CrossRef]
- Purc, A.; Espinoza, E.M.; Nazir, R.; Romero, J.J.; Skonieczny, K.; Jezewski, A.; Larsen, J.M.; Gryko, D.T.; Vullev, V.I. Gating That Suppresses Charge Recombination-The Role of Mono-N-Arylated Diketopyrrolopyrrole. J. Am. Chem. Soc. 2016, 138, 12826–12832. [Google Scholar] [CrossRef]
- Mattiello, S.; Mecca, S.; Ronchi, A.; Calascibetta, A.; Mattioli, G.; Pallini, F.; Meinardi, F.; Beverina, L.; Monguzzi, A. Diffusion-Free Intramolecular Triplet–Triplet Annihilation in Engineered Conjugated Chromophores for Sensitized Photon Upconversion. ACS Energy Lett. 2022, 7, 2435–2442. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Guo, H.; Xie, L. Geometry relaxation-induced large Stokes shift in red-emitting borondipyrromethenes (BODIPY) and applications in fluorescent thiol probes. J. Org. Chem. 2012, 77, 2192–2206. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, J.; Lou, Z.; Li, S.; Wu, H.; Han, K.L. Switching of the triplet excited state of rhodamine/naphthaleneimide dyads: An experimental and theoretical study. J. Org. Chem. 2015, 80, 568–581. [Google Scholar] [CrossRef]
- Huang, L.; Le, T.; Huang, K.; Han, G. Enzymatic enhancing of triplet-triplet annihilation upconversion by breaking oxygen quenching for background-free biological sensing. Nat. Commun. 2021, 12, 1898. [Google Scholar] [CrossRef]
- Suresh, S.M.; Duda, E.; Hall, D.; Yao, Z.; Bagnich, S.; Slawin, A.M.Z.; Bassler, H.; Beljonne, D.; Buck, M.; Olivier, Y.; et al. A Deep Blue B,N-Doped Heptacene Emitter That Shows Both Thermally Activated Delayed Fluorescence and Delayed Fluorescence by Triplet-Triplet Annihilation. J. Am. Chem. Soc. 2020, 142, 6588–6599. [Google Scholar] [CrossRef]
- Wünderlich, D.; Scarlett, L.H.; Briefi, S.; Fantz, U.; Zammit, M.C.; Fursa, D.V.; Bray, I. Application of molecular convergent close-coupling cross sections in a collisional radiative model for the triplet system of molecular hydrogen. J. Phys. D Appl. Phys. 2021, 54, 115201. [Google Scholar] [CrossRef]
- Olesund, A.; Johnsson, J.; Edhborg, F.; Ghasemi, S.; Moth-Poulsen, K.; Albinsson, B. Approaching the Spin-Statistical Limit in Visible-to-Ultraviolet Photon Upconversion. J. Am. Chem. Soc. 2022, 144, 3706–3716. [Google Scholar] [CrossRef]
- Zhang, X.-F. BisBODIPY as PCT-based halogen free photosensitizers for highly efficient excited triplet state and singlet oxygen formation: Tuning the efficiency by different linking positions. Dyes Pigm. 2017, 146, 491–501. [Google Scholar] [CrossRef]
- Shokri, S.; Wiederrecht, G.P.; Gosztola, D.J.; Ayitou, A.J.-L. Photon Upconversion Using Baird-Type (Anti)Aromatic Quinoidal Naphthalene Derivative as a Sensitizer. J. Phys. Chem. C 2017, 121, 23377–23382. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Mu, Y.-d.; Guo, D.-c.; Hou, Y.-j.; Wang, S.-h. Facile electrospinning preparation and superior luminescence properties of BODIPY composite nanofibers. Text. Res. J. 2016, 87, 1795–1805. [Google Scholar] [CrossRef]
- Nepomnyashchii, A.B.; Broring, M.; Ahrens, J.; Bard, A.J. Synthesis, photophysical, electrochemical, and electrogenerated chemiluminescence studies. Multiple sequential electron transfers in BODIPY monomers, dimers, trimers, and polymer. J. Am. Chem. Soc. 2011, 133, 8633–8645. [Google Scholar] [CrossRef]
- Singh, V.K.; Yu, C.; Badgujar, S.; Kim, Y.; Kwon, Y.; Kim, D.; Lee, J.; Akhter, T.; Thangavel, G.; Park, L.S.; et al. Highly efficient organic photocatalysts discovered via a computer-aided-design strategy for visible-light-driven atom transfer radical polymerization. Nat. Catal. 2018, 1, 794–804. [Google Scholar] [CrossRef]
- Deng, F.; Sun, W.; Castellano, F.N. Texaphyrin sensitized near-IR-to-visible photon upconversion. Photochem. Photobiol. Sci. 2014, 13, 813–819. [Google Scholar] [CrossRef]
- Mattiello, S.; Monguzzi, A.; Pedrini, J.; Sassi, M.; Villa, C.; Torrente, Y.; Marotta, R.; Meinardi, F.; Beverina, L. Self-Assembled Dual Dye-Doped Nanosized Micelles for High-Contrast Up-Conversion Bioimaging. Adv. Funct. Mater. 2016, 26, 8447–8454. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Kim, M.K.; Kwon, J.; Shin, D.-Y.; Park, S.J.; Lee, C.-Y.; Kim, J.-J.; Hong, J.-I. Highly Efficient Light-Harvesting System Based on a Phosphorescent Acceptor Coupled with Dendrimer Donors via Singlet-Singlet and Triplet-Triplet Energy Transfer. Chem. Mater. 2007, 19, 3673–3680. [Google Scholar] [CrossRef]
- Li, J.K.; Zhang, M.Y.; Zeng, L.; Huang, L.; Wang, X.Y. NIR-Absorbing B,N-Heteroarene as Photosensitizer for High-Performance NIR-to-Blue Triplet-Triplet Annihilation Upconversion. Angew. Chem. Int. Ed. 2023, 62, e202303093. [Google Scholar] [CrossRef]
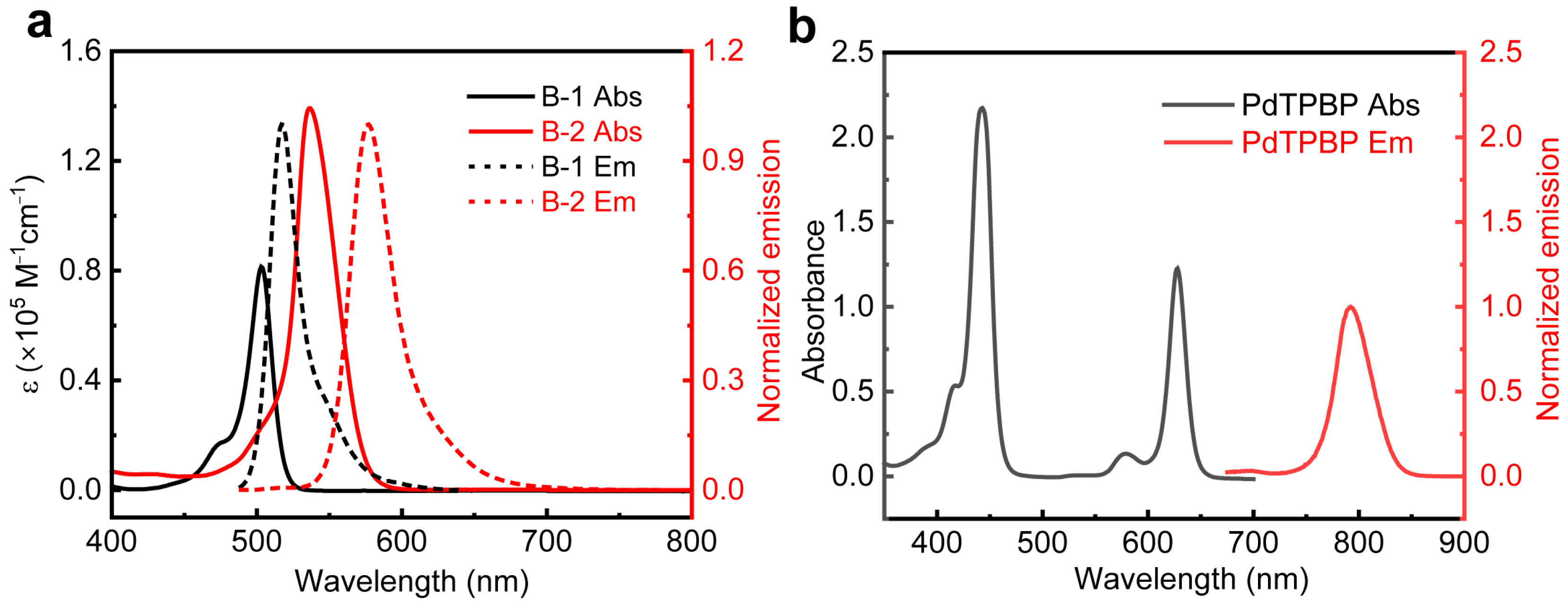
| Compound | λabs a (nm) | ε b | λemc (nm) | Φf1 d (%) | Φf2 e (%) | τf f(ns) | S1 g (eV) | ΔE2T1-S1 h (eV) |
|---|---|---|---|---|---|---|---|---|
| B-1 | 503 | 0.81 | 517 | 72 | 42 | 3.52 | 2.43 | 0.63 |
| B-2 | 536 | 1.39 | 577 | 92 | 86 | 3.25 | 2.21 | 0.83 |
| Compound | ηUC a | ΦTTET b (%) | ηTTA c (%) | Φf d (%) | Ith e | ksv f | kq g | τDF h (µs) | T1 i (eV) |
|---|---|---|---|---|---|---|---|---|---|
| B-1 | 4.0 | 49.1 | 88.0 | 34.3 | 76.9 | 0.57 | 0.23 | 445.5 | 1.53 |
| B-2 | 10.7 | 66.9 | 82.0 | 60.3 | 53.7 | 5.11 | 2.10 | 101.4 | 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zeng, L.; Jiang, L.; Zhang, M.; Chen, Y.; Huang, L. Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance. Molecules 2023, 28, 5474. https://doi.org/10.3390/molecules28145474
Gao M, Zeng L, Jiang L, Zhang M, Chen Y, Huang L. Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance. Molecules. 2023; 28(14):5474. https://doi.org/10.3390/molecules28145474
Chicago/Turabian StyleGao, Min, Le Zeng, Linhan Jiang, Mingyu Zhang, Yong Chen, and Ling Huang. 2023. "Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance" Molecules 28, no. 14: 5474. https://doi.org/10.3390/molecules28145474
APA StyleGao, M., Zeng, L., Jiang, L., Zhang, M., Chen, Y., & Huang, L. (2023). Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance. Molecules, 28(14), 5474. https://doi.org/10.3390/molecules28145474








