Effects of P:Ni Ratio on Methanol Steam Reforming on Nickel Phosphide Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimized Adsorbates and Their Binding Energies
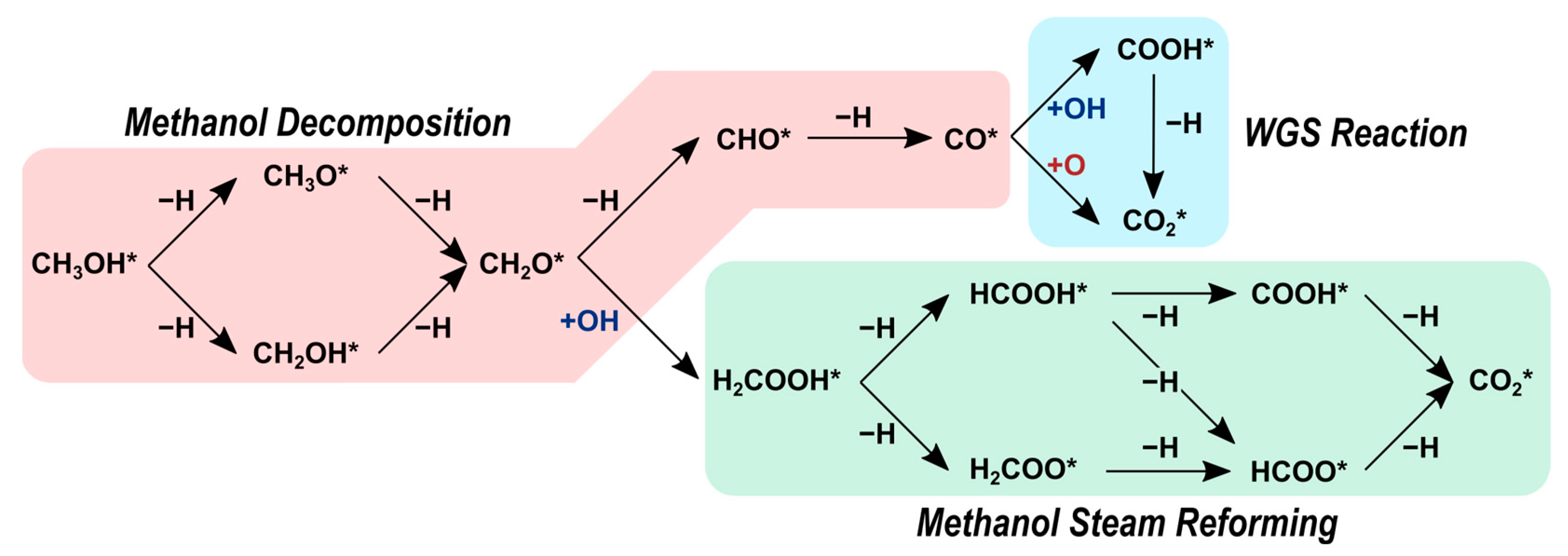
2.2. Reaction Pathways
2.2.1. H2O Dissociation
2.2.2. Methanol Decomposition
2.2.3. Methanol Steam Reforming
2.2.4. Water–Gas Shift Reaction
3. Computational Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Chaedir, B.A.; Sasmito, A.P.; Shamim, T. Progress on Open Cathode Proton Exchange Membrane Fuel Cell: Performance, Designs, Challenges and Future Directions. Appl. Energy 2021, 283, 116359. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.; Shi, C.; Chang, Y.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.; et al. Identifying A Universal Activity Descriptor and a Unifying Mechanism Concept on Perovskite Oxides for Green Hydrogen Production. Adv. Mater. 2023, 2305074. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, H.; Xu, W.; Peng, B.; Zhao, C.; Xie, M.; Lv, X.; Gao, Y.; Hu, K.; Fang, Y.; et al. Quasi-Topological Intercalation Mechanism of Bi0.67 NbS2 Enabling 100 C Fast-Charging for Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300790. [Google Scholar] [CrossRef]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.; Xu, H. The Dual-Site Adsorption and High Redox Activity Enabled by Hybrid Organic-Inorganic Vanadyl Ethylene Glycolate for High-Rate and Long-Durability Lithium–Sulfur Batteries. Small 2023, 19, 2206750. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D.; Ma, L. The Bio-Inspired Heterogeneous Single-Cluster Catalyst Ni100–Fe4S4 for Enhanced Electrochemical CO2 Reduction to CH4. Nanoscale 2023, 15, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for Methanol Steam Reforming—A Review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M. Steam Reforming of Methanol over a Cu/ZnO/Al2O3 Catalyst: A Kinetic Analysis and Strategies for Suppression of CO Formation. J. Power Sources 2002, 106, 249–257. [Google Scholar] [CrossRef]
- Agrell, J. Production of Hydrogen from Methanol over Cu/ZnO Catalysts Promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Luo, W.; Asthagiri, A. Density Functional Theory Study of Methanol Steam Reforming on Co(0001) and Co(111) Surfaces. J. Phys. Chem. C 2014, 118, 15274–15285. [Google Scholar] [CrossRef]
- Lin, S.; Johnson, R.S.; Smith, G.K.; Xie, D.; Guo, H. Pathways for Methanol Steam Reforming Involving Adsorbed Formaldehyde and Hydroxyl Intermediates on Cu(111): Density Functional Theory Studies. Phys. Chem. Chem. Phys. 2011, 13, 9622–9631. [Google Scholar] [CrossRef]
- Sutton, J.E.; Panagiotopoulou, P.; Verykios, X.E.; Vlachos, D.G. Combined DFT, Microkinetic, and Experimental Study of Ethanol Steam Reforming on Pt. J. Phys. Chem. C 2013, 117, 4691–4706. [Google Scholar] [CrossRef]
- Smith, G.K.; Lin, S.; Lai, W.; Datye, A.; Xie, D.; Guo, H. Initial Steps in Methanol Steam Reforming on PdZn and ZnO Surfaces: Density Functional Theory Studies. Surf. Sci. 2011, 605, 750–759. [Google Scholar] [CrossRef]
- Lin, S.; Xie, D.; Guo, H. Pathways of Methanol Steam Reforming on Pdzn and Comparison with Cu. J. Phys. Chem. C 2011, 115, 20583–20589. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef]
- Wu, H.; La Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.; Liotta, L. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A Comparative Discussion of the Catalytic Activity and CO2-Selectivity of Cu-Zr and Pd-Zr (Intermetallic) Compounds in Methanol Steam Reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef]
- Twigg, M.V. Deactivation of Copper Metal Catalysts for Methanol Decomposition, Methanol Steam Reforming and Methanol Synthesis. Top. Catal. 2003, 22, 191–203. [Google Scholar] [CrossRef]
- Kurtz, M.; Wilmer, H.; Genger, T.; Hinrichsen, O.; Muhler, M. Deactivation of Supported Copper Catalysts for Methanol Synthesis. Catal. Lett. 2003, 86, 77–80. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam Reforming and Dehydrogenation of Methanol: Difference in the Catalytic Functions of Copper and Group VIII Metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam Reforming of Methanol over Pd/ZnO: Effect of the Formation of PdZn Alloys upon the Reaction. Appl. Catal. A Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn Addition to Supported Pd Catalysts in the Steam Reforming of Methanol. Appl. Catal. A Gen. 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Iwasa, N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Al-Ali, L.I.; Elmutasim, O.; Al Ali, K.; Singh, N.; Polychronopoulou, K. Transition Metal Phosphides (TMP) as a Versatile Class of Catalysts for the Hydrodeoxygenation Reaction (HDO) of Oil-Derived Compounds. Nanomaterials 2022, 12, 1435. [Google Scholar] [CrossRef]
- Li, W.; Dhandapani, B.; Oyama, S.T. Molybdenum Phosphide: A Novel Catalyst for Hydrodenitrogenation. Chem. Lett. 1998, 27, 207–208. [Google Scholar] [CrossRef]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; Jiménez López, A. Studies of the Synthesis of Transition Metal Phosphides and Their Activity in the Hydrodeoxygenation of a Biofuel Model Compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Almithn, A.; Alhulaybi, Z. A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst. Catalysts 2022, 12, 1174. [Google Scholar] [CrossRef]
- Almithn, A.; Alghanim, S.N.; Mohammed, A.A.; Alghawinim, A.K.; Alomaireen, M.A.; Alhulaybi, Z.; Hossain, S.S. Methane Activation and Coupling Pathways on Ni2P Catalyst. Catalysts 2023, 13, 531. [Google Scholar] [CrossRef]
- Witzke, M.E.; Almithn, A.; Conrad, C.L.; Hibbitts, D.D.; Flaherty, D.W. Mechanisms and Active Sites for C–O Bond Rupture within 2-Methyltetrahydrofuran over Ni, Ni12P5, and Ni2P Catalysts. ACS Catal. 2018, 8, 7141–7157. [Google Scholar] [CrossRef]
- Witzke, M.E.; Almithn, A.; Conrad, C.L.; Triezenberg, M.D.; Hibbitts, D.D.; Flaherty, D.W. In Situ Methods for Identifying Reactive Surface Intermediates during Hydrogenolysis Reactions: C–O Bond Cleavage on Nanoparticles of Nickel and Nickel Phosphides. J. Am. Chem. Soc. 2019, 141, 16671–16684. [Google Scholar] [CrossRef] [PubMed]
- Waldt, C.; Montalvo-Castro, H.; Almithn, A.; Loaiza-Orduz, Á.; Plaisance, C.; Hibbitts, D. Role of Phosphorous in Transition Metal Phosphides for Selective Hydrogenolysis of Hindered C–O Bonds. J. Catal. 2023, 421, 403–418. [Google Scholar] [CrossRef]
- Öström, H.; Zhang, B.; Vallejo, T.; Merrill, B.; Huang, J.; Larue, J. Methanol Decomposition on Ni(111) and O/Ni(111). J. Chem. Phys. 2022, 156, 024704. [Google Scholar] [CrossRef]
- Wang, G.C.; Zhou, Y.H.; Morikawa, Y.; Nakamura, J.; Cai, Z.S.; Zhao, X.Z. Kinetic Mechanism of Methanol Decomposition on Ni(111) Surface: A Theoretical Study. J. Phys. Chem. B 2005, 109, 12431–12442. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Competitive Paths for Methanol Decomposition on Pt(111). J. Am. Chem. Soc. 2004, 126, 3910–3919. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Methanol Decomposition on Cu(111): A DFT Study. J. Catal. 2002, 208, 291–300. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Bolton, K.; Richards, T. DFT Study of the Adsorption and Dissociation of Water on Ni(111), Ni(110) and Ni(100) Surfaces. Surf. Sci. 2014, 627, 1–10. [Google Scholar] [CrossRef]
- Yang, H.; Whitten, J.L.; Friend, C.M. Adsorption of CH3O on Ni(111). Surf. Sci. 1994, 313, 295–307. [Google Scholar] [CrossRef]
- Schaff, O.; Hess, G.; Fritzsche, V.; Fernandez, V.; Schindler, K.-M.; Theobald, A.; Hofmann, P.; Bradshaw, A.M.; Davis, R.; Woodruff, D.P. The Structure of the Surface Methoxy Species on Ni(111). Surf. Sci. 1995, 331–333, 201–206. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio Molecular-Dynamics Simulation of the Liquid-Metal–Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kravchenko, P.; Plaisance, C.; Hibbitts, D. A New Computational Interface for Catalysis. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved Adsorption Energetics within Density-Functional Theory Using Revised Perdew-Burke-Ernzerhof Functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. “Special Points for Brillouin-Zone Integrations”—A Reply. Phys. Rev. B 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved Tangent Estimate in the Nudged Elastic Band Method for Finding Minimum Energy Paths and Saddle Points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Nudged Elastic Band Method for Finding Minimum Energy Paths of Transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; World Scientific: Singapore, 1998; pp. 385–404. [Google Scholar]
- Henkelman, G.; Jónsson, H. A Dimer Method for Finding Saddle Points on High Dimensional Potential Surfaces Using Only First Derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]



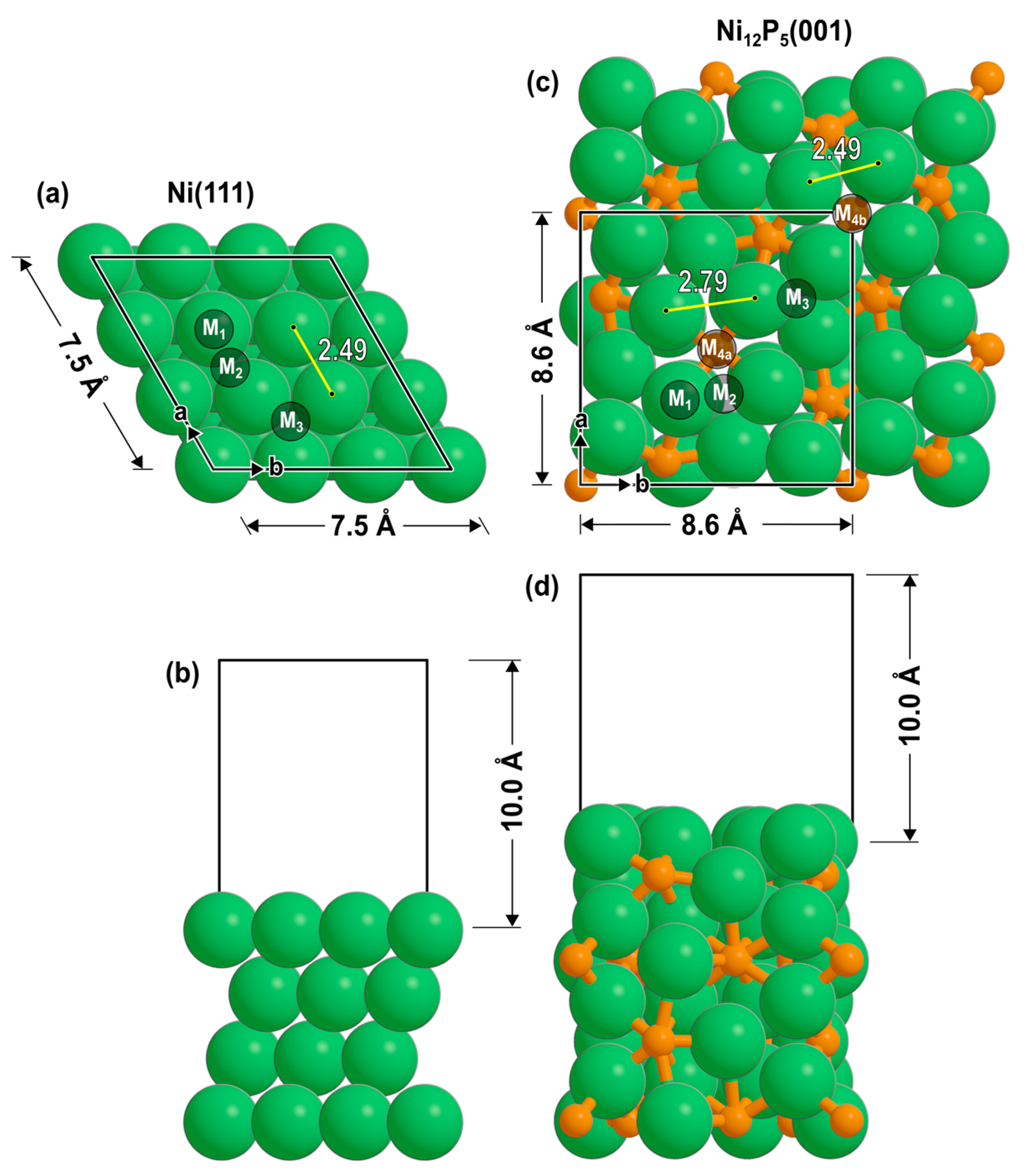
| Ni(111) | Ni12P5(001) | Ni2P(001) [32] | ||||
|---|---|---|---|---|---|---|
| Species | Adsorption Mode | ΔEads | Adsorption Mode | ΔEads | Adsorption Mode | ΔEads |
| kJ mol−1 | kJ mol−1 | kJ mol−1 | ||||
| H* | M3 | −258 | M4b | −294 | M3 | −224 |
| H2O* | M1 | −9 | M1 | −32 | M1 | −23 |
| OH* | M3 | −290 | M4b | −340 | M3 | −294 |
| O* | M3 | −506 | M4b | −584 | MP | −489 |
| CH3OH* | M1 | −11 | M1 | −36 | M1 | −24 |
| CH2OH* | M2 | −134 | M2 | −176 | M2 | −133 |
| CH3O* | M3 | −215 | M4b | −256 | M3 | −204 |
| CH2O* | M3 | −32 | M4b | −126 | M3 | −25 |
| CHO* | M3 | −178 | M4b | −262 | M2 | −161 |
| CO* | M3 | −150 | M4b | −203 | M1 | −109 |
| H2COOH* | M3 | −194 | M4a | −239 | M3 | −194 |
| HCOOH* | M1 | −13 | M1 | −47 | M1 | −24 |
| H2COO* | M3 | −346 | M4b | −495 | M3 | −360 |
| COOH* | M2 | −183 | M4b | −264 | M2 | −188 |
| HCOO* | M2 | −246 | M2 | −320 | M2 | −273 |
| CO2* | Parallel | −2 | Vertical | −2 | Parallel | −1 |
| No. | Reaction | Ni(111) | Ni12P5(001) | Ni2P(001) [32] | |||
|---|---|---|---|---|---|---|---|
| ΔHact | ΔHrxn | ΔHact | ΔHrxn | ΔHact | ΔHrxn | ||
| 1 | CH3OH → CH2OH + H | 141 | 25 | 81 | −4 | 114 | 63 |
| 2 | CH3OH → CH3O + H | 70 | −36 | 48 | 7 | 98 | 9 |
| 3 | CH2OH → CH2O + H | 47 | −30 | 57 | −98 | 82 | −8 |
| 4 | CH3O → CH2O + H | 82 | 42 | 75 | −20 | 73 | 41 |
| 5 | CH2O → CHO + H | 22 | −43 | 39 | −52 | 17 | −3 |
| 6 | CHO → CO + H | 11 | −128 | 37 | −105 | 19 | −60 |
| 7 | CH2O + OH → H2COOH | 42 | −18 | 18 | −4 | 5 | −40 |
| 8 | H2COOH → HCOOH + H | 63 | −50 | 54 | −56 | 33 | −38 |
| 9 | H2COOH → H2COO + H | 72 | 0 | 69 | −29 | 87 | 43 |
| 10 | HCOOH → COOH + H | 55 | −20 | 27 | −59 | 82 | 11 |
| 11 | HCOOH → HCOO + H | 25 | −58 | 15 | −108 | 61 | 20 |
| 12 | H2COO → HCOO + H | 23 | −124 | 64 | −61 | 13 | −64 |
| 13 | HCOO → CO2 + H | 80 | −24 | 89 | 43 | 39 | −33 |
| 14 | COOH → CO2 + H | 81 | −59 | 71 | −10 | 115 | −25 |
| 15 | CO + OH → COOH | 129 | 95 | - | - | 69 | 53 |
| 16 | CO + O → CO2 | 144 | 56 | 71 | 13 | 128 | 3 |
| 17 | H2O → OH + H | 74 | −25 | 49 | −45 | 91 | 5 |
| 18 | OH → O + H | 97 | −18 | 98 | −57 | 161 | 81 |
| 19 | OH + OH → H2O + O | 28 | −20 | 55 | 4 | 63 | 55 |
| 20 | 2H2O → H2O + OH + H | 68 | −4 | 35 | −64 | 68 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almithn, A. Effects of P:Ni Ratio on Methanol Steam Reforming on Nickel Phosphide Catalysts. Molecules 2023, 28, 6079. https://doi.org/10.3390/molecules28166079
Almithn A. Effects of P:Ni Ratio on Methanol Steam Reforming on Nickel Phosphide Catalysts. Molecules. 2023; 28(16):6079. https://doi.org/10.3390/molecules28166079
Chicago/Turabian StyleAlmithn, Abdulrahman. 2023. "Effects of P:Ni Ratio on Methanol Steam Reforming on Nickel Phosphide Catalysts" Molecules 28, no. 16: 6079. https://doi.org/10.3390/molecules28166079
APA StyleAlmithn, A. (2023). Effects of P:Ni Ratio on Methanol Steam Reforming on Nickel Phosphide Catalysts. Molecules, 28(16), 6079. https://doi.org/10.3390/molecules28166079







